Abstract
Tumor infiltrating lymphocytes (TILs) have been recognized in various cancers and may reflect a host immune response to malignant cells. TILs are a heterogeneous population of various types of mononuclear cells, including CD8 or CD4 + T cells and their subsets, B cells, myeloid derived suppressor cells (MDSC), macrophages, and other cells. Immunosuppressive factors in the tumor microenvironment (TME) that inhibit recruitment and function of TILs include immunosuppressive cells, cytokines secreted by tumor or mesenchymal cells, and co-inhibitory ligands expressed by tumor cells. Despite this complex interplay of immune cells and the TME, higher TIL density is associated with favorable prognosis in certain breast cancer subtypes, including HER2 overexpressing cancers, and “triple negative” cancers that do not express the estrogen and progesterone receptors or overexpress HER2. TILs infiltrating the tumor stroma (sTILs) are associated with higher rates of complete pathologic response to neoadjuvant chemotherapy, decreased recurrence and improved survival in early stage triple negative and HER2-positive breast cancer treated with adjuvant systemic therapy. An international working group has published guidelines on reporting TILs in pathology specimens. In this chapter we review the composition of TILs, mechanisms of immune evasion, recommendations for TILs measurement, and data supporting use of TILs as a prognostic and predictive biomarker in breast cancer.
Murali Janakiram and Hina Khan are contributed equally to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
It has been recognized for decades that some primary breast cancers are associated with infiltration by lymphocytes, often referred to as “tumor infiltrating lymphocytes” , or “TILs”. This phenomenon was first described in medullary carcinoma of the breast, an uncommon subtype of poorly differentiated invasive carcinoma characterized by dense lymphocytic infiltration, circumscription, syncytial growth and absence of estrogen receptor (ER), progesterone receptor (PR) expression and HER2 overexpression, and a relatively favorable prognosis (Moore and Foote 1949; Richardson 1956; Bloom et al. 1970). Recent reports have noted that lymphocytic infiltration to be more prevalent in HER2 overexpressing and “triple negative” invasive ductal carcinomas, and distinguished between lymphocytic infiltration of the tumor (iTILs) and stroma (sTILs) (Loi et al. 2013). In addition, there is a consistent body of evidence indicating a strong correlation between the presence of TILs, especially sTILs, in the primary tumor and a significantly reduced risk of breast cancer recurrence and mortality in both HER2 overexpressing (Dieci et al. 2015) and triple negative breast cancer (TNBC) (Loi et al. 2013; Dieci et al. 2015; Adams et al. 2014). An association between TILs in residual tumor after neoadjuvant chemotherapy and prognosis has also been reported (Dieci et al. 2014), and higher TIL density in diagnostic pre-treatment core biopsies is also predictive of pathologic complete response to neoadjuvant chemotherapy (Denkert et al. 2010, 2015; West et al. 2011; Ono et al. 2012; Mao et al. 2014). This strong association between TILs and clinical outcomes has led to an expert group providing guidelines for evaluating and scoring TILs in breast cancer, with the ultimate goal of capturing the prognostic information in an accurate and reproducible manner that provides sufficient analytic validity to permit further investigation and eventually clinical application (Salgado et al. 2015b). Although the infiltrating cells comprising the infiltrate have been dubbed “lymphocytes”, they are identified morphologically as mononuclear cells, and hence actually consist of a mixed population of cells including not only cytotoxic and suppressor T lymphocyte and B lymphocyte populations, but also natural killer (NK) cells, plasma cells, macrophages, dendritic cells, and myeloid derived progenitor cells (Fig. 12.1). With the emergence of immune checkpoint blockade as a new strategy to treat a wide variety of cancers, there has also been interest in more precisely characterizing the composition of the TIL population with the ultimate goal of developing predictive biomarkers that identify tumors more susceptible to eradication by immune checkpoint blockade or other immunotherapeutic approaches.
TILs are composed of a heterogeneous population of cells that may promote or suppress the development of cancer [Reproduced with permission from Salgado et al. (2015b)]
12.2 Characterization of TILs in Breast Cancer
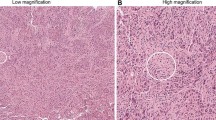
As described above, the International TILs Working Group is an expert panel that has provided recommendations for evaluation of TILs in breast cancer (Salgado et al. 2015b). The recommendations of the panel are summarized in Table 12.1, and several key recommendations are described herein. First, the panel recommended that all mononuclear cells within the border of the primary invasive tumor be identified as TILs in whole sections excluding areas with necrosis, crush artifact, or hyalinization; the panel did not recommend use of tissue microarrays (TMA) or evaluating only “hot spots” in either whole sections or TMAs. Although pretreatment core biopsies may also be used for TIL assessment prior to administration of neoadjuvant chemotherapy, there is limited information about analytic validity of assessing TILs in residual tumor after therapy. Second, the panel recommended distinguishing between iTILs and sTILs (Fig. 12.2), and to report primarily sTILs when assessing TIL status. The group defined iTILs as those in tumor nests having cell-to-cell contact with no intervening stroma and directly interacting with carcinoma cells. sTILs were defined as TILs dispersed in the stroma between the carcinoma cells that are not directly in contact with the malignant cells, and should be reported as percentage of stromal areas occupied by sTILs. The panel pointed out that trafficking between tumor and stromal microenvironment is likely a dynamic process captured in a static manner by histologic evaluation at a single time point, and hence distinguishing between iTILs and sTILs may be artifactual. Characterization of sTILs is more practical because of the greater abundance of sTILs relative to iTILs, greater ease in recognizing and enumerating sTILs, and no additional or more accurate prognostic information provided above and beyond that provided by sTILs enumeration. Third, the panel recommended that sTILs be characterized and reported in a continuous manner (ex. deciles of <10 %, 10–20 %, etc.), rather than a binary manner (ex. lymphocyte predominant breast cancer with at least 50 % sTILs), because very densely infiltrated tumors may be uncommon (<5–10 %). There is also a linear relationship between sTILs and prognosis without a prognostically relevant binary threshold.
Various levels of TIL infiltration in different breast cancer samples are shown. Top left sTILs < 1 % (20x)—The stroma is clearly visible in pink and is devoid of TILs, Top right sTILs—50 % (20x)—The stroma is visible and has a considerable infiltration of TILs, Bottom left sTILs—90 % (20x)—Intervening stroma is not seen and is nearly replaced by lymphocytes, Bottom right Intratumoral TILs (40x)—The lymphocytes are in direct contact with the tumor cells with no intervening stroma
The essential characteristics of a prognostic and/or predictive biomarker include analytic validity, clinical validity, and clinical utility. Although there is strong evidence supporting the association between sTILs and prognosis in early stage breast cancer treated with adjuvant chemotherapy and response to neoadjuvant cytotoxic therapy in TNBC, and hence clinical validity, the clinical utility of this information remains uncertain. For example, there is currently insufficient level of evidence to spare chemotherapy based on TIL assessment, or to select for patients most likely to benefit from immune checkpoint blockade. In addition, few studies have evaluated the intra and inter observer reproducibility, or analytic validity, of TILs assessment. For example, in the report by Adams et al., inter observer correlation was evaluated in a subset of 99 evaluable cases. Rates of agreement within 10 percentage points between two expert breast pathologists were 85 % (95 % confidence intervals [CI] 76–91 %) for sTILs and 97 % (95 % CI 91–99 %) for iTILs. If categorical cut points from the Kaplan-Meier analysis were used, the kappa statistic showed moderate agreement between the two pathologists (sTILs, 0.40; iTILs, 0.43) (Adams et al. 2014). Hence further studies or guidelines are required to study methods to improvise interobserver agreement in TIL evaluation in breast cancer.
12.3 TILs Are an Immunologic Response to Tumor Neoantigens
Tumors occurring in subjects harboring germ-line BRCA1 mutations are associated with more TILs than sporadic breast cancers (Lakhani et al. 1998). BRCA-mutation associated cancers have a higher mutational burden than sporadic cancers due to impaired homologous recombination and consequently less error free DNA repair. This results in a greater neoantigen burden and hence induces an immune response. Variability in the somatic mutational burden of cancer has been described, with tumors associated with exposure to tobacco and sunlight (e.g., lung cancer and melanoma, respectively) associated with the highest mutational burden, and breast cancer having a mutational burden in the intermediate range when considered in the context of all human cancers that have been characterized thus far (Alexandrov et al. 2013). Non-BRCA-associated TNBCs are also frequently characterized by defective DNA repair mechanisms due to germ-line defects in other DNA repair pathways (e.g., PALB2, RAD51) and BRCA1 promoter hypermethylation. Assays that identify tumors harboring “genomic scars” as a consequence of these deficiencies are likewise characterized by greater TIL density, providing additional evidence supporting the link between mutational burden and immune response (Telli et al. 2015). On the other hand, tumors harboring defective DNA repair mechanisms, whether due to germ-line or somatic alterations, are more sensitive to DNA damaging agents such as platinums, alkylating agents, and anthracyclines. Thus, it is unclear at this time as to whether the more favorable prognosis associated with TILs represents an effective immune response, greater sensitivity of tumors with high TILs to cytotoxic therapy, or both. Higher mutational burden has been shown to correlate with clinical response to anti-PD1 directed therapy in non-small cell lung cancer (Rizvi et al. 2015), suggesting that TILs may serve as a surrogate predictive biomarker for response to immune checkpoint blockade in a variety of cancers.
12.4 Composition of TILs and Subpopulations
The diagnosis of clinical cancer represents escape from cancer immunoediting, an immunologic process that reduces cancer burden through elimination or equilibrium (Dunn et al. 2004). Although the escape from or failure of this process results in the clinical detection of cancer, the association between TILs and prognosis suggests that host immunity is still relevant after cancer is diagnosed. TILs are composed of a heterogeneous population of cells having both immunostimulatory and immunosuppressive effects, and the balance of these effects contribute to tumor tolerance (Quezada et al. 2011).
The subpopulations of cells are shown in Fig. 12.1. Some cells in the TIL population suppress tumor progression, including CD8 + T cells, helper CD4 + T (Th1) cells, natural killer (NK) cells, whereas others promote tumor progression, including Th2 cells, myeloid derived stem cells, and T regulatory (Tregs) cells. Subpopulations of macrophage and dendritic cells can suppress (M1, DC1) or promote tumor (M2, DC2) tumors, while other cell populations may be either tumor suppressive or promoting, including B cells and Th17 cells (Salgado et al. 2015b). Although neutrophils are not considered in characterizing TILs, they may likewise have either tumor suppressing or promoting subpopulations (Sagiv et al. 2015).
TIL subpopulations may be evaluated using a variety of methodologies, including immunohistochemistry, RNA in situ hybridization, and flow cytometry. Gene expression profiling has identified tumor associated immune signatures that reflect the composition of the subpopulations. To date, classification of subpopulations has largely been described using immunohistochemistry including a panel of antibodies directed at CD4 , CD8 , CD25, and FOXP3+. Using this methodology, the major subtypes of immunosuppressive cells constituting TILs include T regs (CD4+ CD25+ FOXP3+) and myeloid derived suppressor cells (MDSC ) (Jiang and Shapiro 2014). Tregs produce RANKL which binds to RANK on human breast cancer cell lines and promotes lung metastases in mouse models (Tan et al. 2011). Depletion of Tregs with a vaccine and low dose cyclophosphamide results in increased cytolytic activity of T cells in Her-2 Neu transgenic mice (Weiss et al. 2012). MDSC suppress T cell proliferation through production of reactive oxygen species, disrupt binding of antigen specific peptides to CD8 T cells by inactivating tyrosinases in the TCR-CD8 (T cell receptor-CD8) complexes, and inhibit antigen presentation by tumor cells via nitration of tumor MHC class I expression (Jiang and Shapiro 2014). Hence these cell populations through pleiotropic effects can prevent or suppress an effective immune response.
12.5 The Tumor Microenvironment and TIL Function
TILs comprise only one component of the tumor microenvironment (TME), which also includes mesenchymal cells, and extracellular matrix/stroma. The extracellular matrix functions as a scaffold for tissue architecture, and also provides biochemical and biomechanical signals that influence cell growth, survival, migration and differentiation, as well as vascular development and immune function, and hence modulates the hallmarks of cancer (Pickup et al. 2014). Thus, crosstalk between TIL subpopulations and other components of the TME, mediated in part by chemokines and cytokines, results in a complex interplay that can influence the balance between tumor promotion and suppression, as described below.
Cytokines and chemokines: The stromal cells and other immune cells secrete various cytokines that profoundly influence different subpopulations of immune effector cells. For example, TGF-beta induces transcriptional repression of genes in CD8 + T cells resulting in impaired cytolytic activity (Thomas and Massague 2005). Products of altered steroid metabolism can accumulate and inhibit CCR7 thus preventing dendritic cell maturation and translocation into the lymphoid organs (Villablanca et al. 2010). Tumors secrete soluble ligands such as MHC class I polypeptide-related sequence (MIC) that deplete T cell receptors and attenuate response of specific effector T cells in response to tumor antigens (Groh et al. 2002). Thus cytokines secreted in the TME can inhibit presentation of antigens to T cells and can also inhibit responses of T cells to the tumor. Other cytokines, most notably interferon-gamma, play an important role in enhancing cell immunity.
Co-inhibitory and co-stimulatory receptors and ligands: T cell activation depends on recognition of antigens on host antigen presenting cells (APCs) and the presence of a simultaneous co-stimulatory or co-inhibitory signal delivered through the CD28 –B7 family of receptor-ligand interaction between T cells and APC or malignant cells. Certain B7 ligands, including PD-L1, B7-H3, B7x and HHLA2 inhibit T cell responses when expressed on APCs or tumor cells. Expression of these ligands in breast cancer and other cancers has been associated with unfavorable clinical features (Janakiram et al. 2012; 2014). For example, PD-L1 expression on human pancreatic and ovarian cancers is inversely correlated with CD8 + TILs (Ohaegbulam et al. 2015). Several studies also show an inverse correlation of another co-inhibitory ligand B7x and TILs in various cancers. Tumor cell B7x expression is inversely correlated with the intensity of TILs in renal cell carcinoma (Zhang et al. 2013), with the number of CD3+ and CD8+ TILs in uterine endometrioid carcinoma (Miyatake et al. 2007) and with the densities of CD3+ TILs in tumor nest and CD8+ TILs in tumor stroma in esophageal carcinoma (Chen et al. 2011). These results suggest that tumor-expressed B7x may be important in limiting TILs infiltration. The expression of coinhibitory receptor PD-1 on TILs has also been shown to be associated with decreased overall survival in breast cancer (Sun et al. 2014; Muenst et al. 2013). In summary, TILs recruitment and function is influenced by a complex interplay of neoantigens and co-inhibitory ligands on the tumor, coinhibitory receptors on TILs, subpopulation of cells in the infiltrate and the tumor microenvironment.
12.6 Clinical Validity of TILs as a Prognostic Biomarker in Patients with Breast Cancer Treated with Adjuvant Chemotherapy
The results of studies evaluating the association between TILs and prognosis in operable breast cancer are summarized in Table 12.2.
Relationship between TILs and Breast Cancer Subtype: Loi et al. (2013) first described the variability of TILs by breast cancer subtype, and the strong association between TILs and prognosis in TNBC. TILs were evaluated independently by two expert pathologists in primary tumor specimens from 2009 patients with axillary node-positive breast cancer enrolled on the BIG 02-98 adjuvant phase III trial comparing anthracycline chemotherapy given without a taxane (doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil (CMF) or doxorubicin plus cyclophosphamide followed by CMF), concurrently or sequentially with a taxane (doxorubicin plus docetaxel followed by CMF or doxorubicin followed by docetaxel followed by CMF). iTILs and sTILs were found to be significantly higher in TNBC and HER2-positive breast cancer compared with ER-positive, HER2-negative breast cancer. In addition, there was no significant prognostic association in the entire population (n = 2009) or ER-positive, HER2-negative population (n = 1079).
Relationship between TILs and Prognosis in Triple Negative Breast Cancer: Although no association was found between TIL and prognosis in the overall population and in ER-positive, HER2-negative disease in analysis of the BIG 02-98 specimens reported by Loi et al. (Loi et al. 2013), each 10 % increase in iTILs and sTILs in the TNBC population was associated with 17 and 15 % reduced risk of relapse (adjusted p = 0.1 and p = 0 0.025), respectively, and 27 and 17 % reduced risk of death irrespective of chemotherapy type used (adjusted p = 0.035 and p = 0.023), respectively. This report therefore provided the first evidence indicating the strong associated between TILs and prognosis in TNBC. Adams et al. (2014) reported a confirmatory analysis that focused on 481 patients with stages I-III TNBC enrolled on two large adjuvant phase III trials (ECOG 2197 and ECOG 1199) in which all patients received anthracycline-cyclophosphamide-containing, usually in combination with a taxane. In contrast to the analysis by Loi et al., TILs were read independently rather than in tandem by two expert pathologists. Similar to the report by Loi et al., however, among the 481 tissue samples analyzed; for every 10 % increase in sTIL there was a 14 % reduction in risk of recurrence or death, 18 % reduction in risk of distant recurrence and a 19 % reduction in risk of death was seen. These two independent reports therefore demonstrated that sTILs were a strong and independent prognostic marker for disease-free survival (DFS) and overall survival (OS) in patients with stages I-III TNBC treated with adjuvant anthracycline-containing chemotherapy.
The relationship between TILs and prognosis was also evaluated retrospectively using breast cancer specimens obtained from two randomized trials comparing adjuvant chemotherapy with no chemotherapy in 817 patients with node-positive and node-negative breast cancer (Dieci et al. 2014). In the TNBC subgroup, both iTILs and sTILs were significantly associated with DFS, with each 10 % increase associated with 14 and 13 % reduction in risk of relapse (HR: 0.86, 95 % CI: 0.78–0.94 and HR: 0.87, 95 % CI: 0.80–0.94), respectively. TILs were prognostic in both the chemotherapy treated and untreated population, with no statistical interaction observed. The FinHer study was another phase III multicenter adjuvant trial that included 1010 patients with high-risk node-negative or node-positive breast cancer; in this cohort each 10 % increase in TILs was significantly associated with a 13 % decrease in risk of distant recurrence (HR: 0.77, 95 % CI: 0.61–0.98, p = 0.02) in 134 TIL evaluable primary TNBC cases (Loi et al. 2014b). Thus, several reports have confirmed the prognostic role of increased TIL in patients with operable TNBC treated with or without adjuvant chemotherapy.
HER2-Positive Breast Cancer: The association between TILs and prognosis was also demonstrated in HER2-positive operable breast cancer. As previously described, the FinHer study was a multicenter phase III trial that included not only patients with TNBC, but also patients with HER2 overexpressing breast cancer who received adjuvant chemotherapy alone or in combination with trastuzumab. In 209 patients with HER2-positive breast cancer treated with adjuvant trastuzumab in the FinHER study, a 10 % increase in sTILs was associated with an increase in distant disease free survival (HR = 0.77; 95 % CI: 0.61–0.98). The association between TILs and prognosis was also analyzed in the N9831 study, which compared adjuvant chemotherapy alone or in combination with trastuzumab in HER2 overexpressing operable breast cancer (Perez et al. 2014). Lymphocyte predominant breast cancers (LPBC) with high sTILs (>60 %), which accounted for 9.9 % (n = 94) of the population, was independently associated with improved recurrence-free survival (RFS) in patients treated with chemotherapy alone, but not in the chemotherapy plus trastuzumab group, and did not predict benefit from trastuzumab. In patients treated with chemotherapy alone, the 10 year RFS rates were 90.9% and 64.5 % for LPBC and non-LPBC groups, respectively (HR: 0.23; 95 % CI: 0.073–0.73). Subgroup analysis from the BIG-02-98 adjuvant phase III trial of lymph node positive breast cancer patients also showed a notable benefit of increasing TILs (10 % increments) in the HER2-positive cohort treated with anthracycline-only chemotherapy without trastuzumab, although this was not seen in anthracycline and docetaxel arm. It is unclear currently why such an interaction should be present with the type of chemotherapy regimen, although a higher dose of anthracycline could be responsible for the immune mediated response. Based on all these studies, a higher TILs infiltration is predictive of outcome in HER2+ breast cancer especially in the anthracycline only treated subgroup.
ER-Positive, HER2-Negative Breast Cancer: Data on the prognostic effect of TIL in the hormone receptor positive breast cancer groups is limited. Recent preliminary data from two ongoing randomized adjuvant trials has shown that there is no prognostic impact of TIL in the ER+/HER2– subgroup (Maria Vittoria Dieci et al. 2014).
12.7 Clinical Validity of TILs as a Prognostic and Predictive Biomarker in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy
Neoadjuvant chemotherapy of localized breast cancer leads to clinical responses in as many as 70–90 % of patients. However, pathological complete response (pCR), defined as a complete or near complete absence of residual tumor, is only seen in 10–25 % of patients (Fisher et al. 1998; Smith et al. 2002). pCR is a short term surrogate associated with a long-term favorable prognosis, especially in HER2-positive and TNBC (Cortazar and Geyer 2015), and is now accepted by regulatory agencies such as the United States Food and Drug Administration for accelerated approval of new agents in patients with localized breast cancer who are candidates for neoadjuvant chemotherapy (Prowell and Pazdur 2012). Patients with residual disease have a variable prognosis, but extensive residual disease in patients with TNBC and HER-positive breast cancer appear to have a high risk of recurrence (Symmans et al. 2007). Hence there is an interest in determining the relationship between TILs in pretreatment core biopsies as a predictive biomarker for pCR in patients with neoadjuvant therapy, and as a prognostic biomarker in patients with residual disease after neoadjuvant therapy because of the variable prognosis for this population. The results of these reports are summarized in Table 12.3.
TILs and Predicting Response to Neoadjuvant Chemotherapy: Lymphocyte infiltration was analyzed in 1058 pre-treatment cancer tissues from the GeparDuo and GeparTrio cohorts, both of which were phase III, randomized trials assessing responses to combination neoadjuvant chemotherapy regimens. The presence of intratumoral lymphocytes, defined as >10 % stromal area infiltrated with lymphocytes, was an independent parameter for pCR in both the cohorts (Denkert et al. 2010). The pCR rates were 42 % and 40 %, respectively. Tumors with low TIL had pCR rates of 3 and 7 % respectively. In the GeparSixto neoadjuvant study assessing addition of carboplatin to an anthracycline-taxane combination in 580 patients, pCR rates were 76.2 % for LPBC (defined as >60 % of either intratumoral or stromal TILs) compared to 52.2 % for non-LPBC (p = 0.01) in those with TNBC (Denkert et al. 2015).
In a meta-analysis including 13 neoadjuvant studies and 3251 patients, TNBC with higher TILs in pretreatment biopsy correlated with higher pCR rates to neoadjuvant chemotherapy (Mao et al. 2014). Greater TIL density was associated with a higher pCR rate for neoadjuvant chemotherapy (OR: 3.93, 95 % CI: 3.26–4.73, p < 0.001), including iTILs (OR: 4.15, 95 % CI: 2.95–5.84, p < 0.001) or sTILs (OR: 3.58, 95 % CI: 2.50–5.13, p < 0.001). Pretreatment TILs had predictive values in ER negative, triple negative and HER2 positive breast cancer patients, but not in ER-positive disease. Therefore, TIL analysis on initial tumor samples serves as an important predicting factor for pathologic response in TNBC.
In a study of 180 stage II and III breast cancer patients, tumors with Foxp3 and CD8 infiltrates were associated with a high-pCR rate (p < 0.001 and p = 0.007, respectively) in those who received neo-adjuvant weekly paclitaxel followed by 5-fluourouracil, epirubicin and cyclophosphamide (Oda et al. 2012). Foxp3 infiltrate was a significant independent predictor of pCR (p = 0.014), but CD8 infiltrate was not. In another study with 153 tumor samples, high CD8+ TILs in pretreatment biopsy was found to be an independent predictor of response to neoadjuvant chemotherapy (Seo et al. 2013). These results demonstrate that subpopulations of lymphocytes may also be predictive of response to neoadjuvant chemotherapy, although further studies in larger populations are needed in order to determine whether this provides more accurate prognostic and predictive information than simply evaluating sTILs by conventional hematoxylin and eosin staining.
TILs and Predicting Response to Trastuzumab: In the HER2-population, the response to trastuzumab and its association with TILs has been investigated in the neo-adjuvant setting. In the GeparQuattro trial, 156 patients with HER2+ breast cancer received neoadjuvant trastuzumab with chemotherapy (4 cycles of epirubicin/cyclophosphamide with docetaxel with or without capecitabine); each 10 % increment in TILs was associated with higher rates of pCR (adjusted OR: 1.14, 95 % CI: 1.01–1.29) (Loi et al. 2014a). The neoadjuvant trial GeparSixto, investigated the effect of adding carboplatin to an neoadjuvant anthracycline-taxane combination in 580 patients with triple negative or HER2+ breast cancer (Denkert et al. 2015); trastuzumab and lapatinib were also given in patients with HER2+ disease, and bevacizumab to patients with TNBC, which included 25 % of patients who had LPBC (defined as >60 % of either iTILs or sILs). Overall, the pCR rate was significantly higher in the LPBC compared with the non-LPBC group (59.9 vs. 33.8 %, p = 0.001). pCR rate were significantly higher for the LPBC group in the absence of platinum (46.6 vs. 33.5 % p = 0.05) and presence of platinum (>75 vs. 38.1 %, p < 0.0005). Higher TIL density has also been associated with higher pCR rates after neoadjuvant HER2-directed therapy plus chemotherapy (Salgado et al. 2015a; Denkert et al. 2014, 2015).
In a pooled meta-analysis of 13 published studies with 3555 patients, high level of TILs in pretreatment biopsy indicated higher pCR rates after neoadjuvant chemotherapy in TNBC and HER2+ breast cancer. The correlation of pCR with TILs was not seen in hormone receptor positive [HR+/HER2−] disease (Mao et al. 2014). High CD8 + T-lymphocytes in samples pre- (OR: 3.36; 95 % CI: 1.15–9.85) or post-neoadjuvant chemotherapy (OR: 4.71; 95 % CI: 1.29–17.27) was associated with a higher pCR. In the HER2+ group, high TILs not only predict a favorable response to neoadjuvant trastuzumab, but also to chemotherapy. Study of TILs and its response to chemotherapy in 368 pretreatment tissues from two ER negative cohorts (EORTC 10994 and BIG 00-01) (West et al. 2011), showed that high level of CD8 + TIL was an independent predictor of anthracycline response.
TILs and Predicting Response to Endocrine Therapy: The status of TILs following endocrine therapy is not clearly defined. In a study of patients with ER+ breast cancer treated with neoadjuvant steroidal aromatase inhibitor (AI) therapy, changes in CD8+ T cells/Foxp3+ or T regulatory cells ratio before and after therapy correlated with response. A significant increase in the CD8+/Treg ratio was detected after hormonal therapy in responders (p = 0.028) but not in nonresponders (Chan et al. 2012). Thus, the CD8+/Treg ratio in surgical pathology specimens can be a potential surrogate marker for predicting responses to neoadjuvant endocrine therapy.
TILs in Residual Cancer after Neoadjuvant Chemotherapy and Prognosis: In a retrospective study of 304 TNBC patients with residual disease after primary neoadjuvant chemotherapy, the presence of TIL in residual tumor was associated with better prognosis (Dieci et al. 2014). Both sTILs and iTILs were strong prognostic factors for metastases free survival and OS. The 5-year OS rate was 91 % for high TILs and 55 % for low TILs subgroup (HR: 0.19, 95 % CI: 0.06–0.61). The prognostic impact of TILs was most significant in patients with large tumor burden (>2 cm) or lymph node metastases.
12.8 Conclusion
The key points described in this chapter are summarized in Table 12.4. First, TIL density is a prognostic biomarker, and also a predictive biomarker for response to neoadjuvant chemotherapy in TNBC and HER2 positive breast cancer. Second, characterization of sTIL density is a more practical and reproducible biomarker than iTILs, and expert-based guidelines have been developed for characterization of TIL density. Third, TIL recruitment and function is influenced by various factors in the microenvironment. Further research is needed to evaluate in detail the composition of TIL, and their subclasses in the tumor microenvironment. In case of T cells their composition, T cell receptor repertoire, neoantigens being identified by the T cells and the presence of costimulatory or coinhibitory molecules needs to be further elucidated. Additional research is also needed in order to determine whether TILs provide prognostic information in metastatic breast cancer, or predict better response to vaccines or immune checkpoint blockade.
References
Adams S, Gray RJ, Demaria S et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32(27):2959–2966
Alexandrov LB, Nik-Zainal S, Wedge DC et al (2013) Signatures of mutational processes in human cancer. Nature 500(7463):415–421
Aruga T, Suzuki E, Saji S et al (2009) A low number of tumor-infiltrating FOXP3-positive cells during primary systemic chemotherapy correlates with favorable anti-tumor response in patients with breast cancer. Oncol Rep 22(2):273–278
Bloom HJ, Richardson WW, Field JR (1970) Host resistance and survival in carcinoma of breast: a study of 104 cases of medullary carcinoma in a series of 1,411 cases of breast cancer followed for 20 years. Br Med J 3(5716):181–188
Chan MS, Wang L, Felizola SJ et al (2012) Changes of tumor infiltrating lymphocyte subtypes before and after neoadjuvant endocrine therapy in estrogen receptor-positive breast cancer patients—an immunohistochemical study of Cd8+ and Foxp3+ using double immunostaining with correlation to the pathobiological response of the patients. Int J Biol Markers 27(4):e295–e304
Chen LJ, Sun J, Wu HY et al (2011) B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother 60(7):1047–1055
Cortazar P, Geyer CE Jr (2015) Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol 22(5):1441–1446
Denkert C, Loibl S, Noske A et al (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(1):105–113
Denkert C, Loibl S, Salat C et al (2014) Abstract S1-06: Increased tumor-associated lymphocytes predict benefit from addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer in the GeparSixto trial (GBG 66). Cancer Res 73(24 Supplement):S1-06–S01-06
Denkert C, von Minckwitz G, Brase JC et al (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without Carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 33(9):983–991
Dieci MV, Criscitiello C, Goubar A et al (2014) Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 25(3):611–618
Dieci MV, Mathieu MC, Guarneri V et al (2015) Prognostic and predictive value of tumor infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 26(8):1698–1704
Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22:329–360
Fisher B, Bryant J, Wolmark N et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16(8):2672–2685
Groh V, Wu J, Yee C, Spies T (2002) Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419(6908):734–738
Issa-Nummer Y, Darb-Esfahani S, Loibl S et al (2013) Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer—a substudy of the neoadjuvant GeparQuinto trial. PLoS ONE 8(12):e79775
Janakiram M, Abadi YM, Sparano JA, Zang X (2012) T cell coinhibition and immunotherapy in human breast cancer. Discov Med 14(77):229–236
Janakiram M, Chinai J, Fineberg S et al (2014) Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res 21(10):2359–2366
Jiang X, Shapiro DJ (2014) The immune system and inflammation in breast cancer. Mol Cell Endocrinol 382(1):673–682
Ladoire S, Arnould L, Apetoh L et al (2008) Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 14(8):2413–2420
Lakhani SR, Jacquemier J, Sloane JP et al (1998) Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst 90(15):1138–1145
Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G (2013) Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 16(1):32–39
Liu F, Li Y, Ren M et al (2012) Peritumoral FOXP3(+) regulatory T cell is sensitive to chemotherapy while intratumoral FOXP3(+) regulatory T cell is prognostic predictor of breast cancer patients. Breast Cancer Res Treat 135(2):459–467
Liu S, Foulkes WD, Leung S et al (2014) Prognostic significance of FOXP3 + tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res 16(5):432
Loi S, Sirtaine N, Piette F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31(7):860–867
Loi S, Michiels S, Salgado R et al (2014a) Tumor infiltrating lymphocytes (TILs) indicate trastuzumab benefit in early-stage HER2-positive breast cancer (HER2 + BC). Cancer Res 73(24 Supplement)
Loi S, Michiels S, Salgado R et al (2014b) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25(8):1544–1550
Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K (2014) The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS ONE 9(12):e115103
Maria Vittoria Dieci AG, Marie-Christine Mathieu, Valentina Guarneri, Pier Franco Conte, Suzette, Delaloge FA (2014) Prognostic and predictive value of tumor infiltrating lymphocytes (TIL) in two phase III randomized adjuvant breast cancer (BC) trials. J Clin Oncol 32(5s) [suppl; abstr 11087]
Miyatake T, Tringler B, Liu W et al (2007) B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid adenocarcinomas and inversely correlated with tumor T-cell infiltration. Gynecol Oncol 106(1):119–127
Moore OS Jr, Foote FW Jr (1949) The relatively favorable prognosis of medullary carcinoma of the breast. Cancer 2(4):635–642
Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE (2013) The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 139(3):667–676
Oda N, Shimazu K, Naoi Y et al (2012) Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Res Treat 136(1):107–116
Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X (2015) Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 21(1):24–33
Ono M, Tsuda H, Shimizu C et al (2012) Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 132(3):793–805
Perez EA BK, Anderson SK, Thompson EA, Badve SS, Bailey H, Baehner FL (2014) Stromal tumor-infiltrating lymphocytes(S-TILs). In: The alliance N9831 trial S-TILs are associated with chemotherapy benefit but not associated with trastuzumab benefit. Paper presented at the San Antonio Breast Cancer Symposium
Pickup MW, Mouw JK, Weaver VM (2014) The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15(12):1243–1253
Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 366(26):2438–2441
Quezada SA, Peggs KS, Simpson TR, Allison JP (2011) Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev 241(1):104–118
Richardson WW (1956) Medullary carcinoma of the breast; a distinctive tumour type with a relatively good prognosis following radical mastectomy. Br J Cancer 10(3):415–423
Rizvi NA, Hellmann MD, Snyder A et al (2015) Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128
Sagiv JY, Michaeli J, Assi S et al (2015) Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 10(4):562–573
Salgado R, Denkert C, Campbell C et al (2015a) Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in her2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the neoaltto trial. JAMA Oncology 1(4):448–454
Salgado R, Denkert C, Demaria S et al (2015b) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271
Seo AN, Lee HJ, Kim EJ et al (2013) Tumour-infiltrating CD8 + lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 109(10):2705–2713
Smith IC, Heys SD, Hutcheon AW et al (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20(6):1456–1466
Sun S, Fei X, Mao Y et al (2014) PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother 63(4):395–406
Symmans WF, Peintinger F, Hatzis C et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422
Tan W, Zhang W, Strasner A et al (2011) Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470(7335):548–553
Telli ML, Jensen KC, Vinayak S et al (2015) Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J Clin Oncol 33(17):1895–1901
Thomas DA, Massague J (2005) TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8(5):369–380
Villablanca EJ, Raccosta L, Zhou D et al (2010) Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med 16(1):98–105
Weiss VL, Lee TH, Song H et al (2012) Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T Cells. PLoS ONE 7(2):e31962
West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH (2011) Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 13(6):R126
Yamaguchi R, Tanaka M, Yano A et al (2012) Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 43(10):1688–1694
Zhang L, Wu H, Lu D et al (2013) The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene 32(46):5347–5358
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Janakiram, M., Khan, H., Fineberg, S., Zang, X., Sparano, J.A. (2016). Tumor Infiltrating Lymphocytes as a Prognostic and Predictive Biomarker in Breast Cancer. In: Badve, S., Gökmen-Polar, Y. (eds) Molecular Pathology of Breast Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-41761-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-41761-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41759-2
Online ISBN: 978-3-319-41761-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)