Abstract
Mainland sanctuaries, where introduced mammalian predators are controlled or excluded, have the potential to improve the conservation status of New Zealand lizards. This is due to the reliance of a large number of species on habitats unavailable on offshore islands. However, despite considerable predator control efforts, lizard populations are still in decline, even in some mainland sanctuaries. The main cause of this failure appears to be that predator control is hard to sustain and largely targeted at protecting bird populations, which require lower levels of predator suppression than lizard populations. Even fenced, mainland, predator-exclusion sites are prone to reinvasions, particularly of mice, which are difficult to exclude at the outset. Episodic irruptions of mice within fenced sanctuaries, and other mammalian predator species in unfenced sanctuaries, can quickly decrease lizard numbers. Small lizard populations are particularly vulnerable. We discuss two case studies to illustrate population dynamics and limitations to understanding mechanisms underlying patterns of population declines in New Zealand skinks: ornate skinks (Oligosoma ornatum) in a fenced mainland site and speckled skinks (O. infrapunctatum) in an unfenced mainland site. We also speculate about the effects on lizards of native and non-native birds and introduced social insects, including wasps and ants. Understanding biological interactions and obtaining more species- and situation-specific data for lizards will provide information on limits to recovery, detection time frames after management actions, risks and benefits of habitat enhancements and density targets for introduced species where total eradication is impractical.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
The conservation of New Zealand’s 100+ species of native lizards is reliant on the control or elimination of introduced mammals (Hitchmough et al. 2016a, b). In particular, this applies to rodents, mustelids and cats, which are known to both prey upon lizards directly and to compete with them for resources (Towns et al. 2001; Innes et al. 2010; Towns et al. 2016a). On offshore islands, it is often possible to eradicate the entire suite of introduced mammals, which usually allows native lizards to recover or be restored via translocations (Towns and Broome 2003; Towns et al. 2016a). However, islands represent only a small proportion of suitable habitats for lizards, and therefore conservation on the mainland is crucial for maintaining the full diversity of New Zealand lizards and restoring viable populations (Nelson et al. 2015).
Mainland sanctuaries are areas of protected native habitats that mimic islands in many aspects; they are surrounded by fences designed to exclude introduced mammals or threaded with a network of predator traps or poisoning arrays, which protect both their core areas from introduced predators and their boundaries from reinvasion (Saunders 2000). By the end of 2009, 8,396 ha, spanning 28 conservation areas, had been enclosed by 113 km of pest-proof fences and cleared of most mammalian pests (Burns et al. 2012). In addition, by 2011, a larger area (64,000 ha) was managed in unfenced mainland sanctuaries where at least three species of animal pest were controlled. This is an area even greater than the ~37,000 ha of pest-free habitat on offshore islands around the New Zealand coast (Innes and Saunders 2011).
Unfortunately, in predator control operations on the mainland, it is not always possible to eradicate all introduced mammals, even in fully fenced sanctuaries. Not only do these predators and/or competitors remain or reinvade the protected area (fenced or unfenced), but their potential effect might vary from year to year (e.g. Long et al. 2014). Indeed, some invasive mammal species are released from predation or competitor pressure by the removal of other mammalian pests through meso-predator (Crooks and Soulé 1999) or competitor release (Caut et al. 2007; Norbury et al. 2013). For example, mice (Mus musculus) experience competitor and predator release from rats (Rattus spp.), so if rats are removed, mice can reach plague numbers or reinvade sanctuaries. In Tāwharanui Open Sanctuary (Auckland), an area in which rats, but not mice, were effectively controlled, mice attained the highest densities (190 per 100 trap nights) ever recorded in New Zealand (Goldwater et al. 2012). Mice prey on lizards, and several studies have concluded that predation by mice can limit New Zealand lizard populations (Newman 1994; Hoare et al. 2007a; Wedding 2007; Norbury et al. 2013, 2014). At two coastal pebble beaches in the Tāwharanui Regional Park, shore skink (Oligosoma smithi) populations increased following the elimination of all introduced mammals, but when mice reinvaded and their populations irrupted, skink numbers declined (Graham Ussher, pers. comm.).
Conservationists are aware of these issues and often aim to eradicate all introduced mammalian predators and competitors, control subsequent incursions and manage pest population numbers via targeted poisoning or trapping operations. The success of predator control in a sanctuary is often demonstrated by subsequent growth of bird populations (Brown et al. 2015). However, birds vary in their sensitivity to different species and/or abundance of predatory mammals, and little is known about the thresholds for acceptable levels of pest mammals, especially mice. This is the case even for relatively well-studied bird populations (e.g. Long et al. 2014; Brown et al. 2015; Norbury et al. 2015). Empirical data on the level of predator control required for recovery of lizard populations in New Zealand are even more limited; there is insufficient data to assign a threat status to approximately 5 % of lizard species (Hitchmough et al. 2016a). Even for those species whose population sizes and trends are better known, understanding of the relationships between their population dynamics and specific mammalian predation pressure is lacking.
Quantifying the responses of indigenous biota of all types, including lizards, to conservation management is often hindered by limited resources, inadequate monitoring techniques and the complexity of their ecological interactions (reviewed in Norbury et al. 2015). On islands where mammalian pests have been eradicated, increases in the populations of endemic lizards demonstrate that the recovery of remnant populations is possible, although in some cases, these lizard populations grew from previously undetected source populations, highlighting the difficulty in monitoring small and cryptic species of reptiles at low densities (e.g. Hoare et al. 2007b). In addition, the time taken for recovery is likely determined by the life history characteristics of the species, as well as the size of the remnant population. For example, population growth estimates of translocated reptiles demonstrate that it will take decades to confirm success, because reproductive output can be as low as 7 % per annum for some species (e.g. Whitaker’s skink, O. whitakeri; Towns and Ferreira 2001). Recent studies on the mainland also demonstrate that lizard populations can respond positively to control of mammalian predators (e.g. Reardon et al. 2012; Jones et al. 2013; Norbury et al. 2013). However, whether lizard populations are able to recover with low levels, or occasional incursions, of predatory mammals in mainland sanctuaries is likely to be species specific due to life history characteristics and behaviours and the size of the lizard population.
In general terms, over half the studies that examined the response of native New Zealand species (both plants and animals) to predator control demonstrated that unless pest densities were reduced below a key threshold level (which varied across species), populations did not recover (Norbury et al. 2015). Repeated incursions of predatory mammals into a sanctuary are likely to maintain lizard populations at low levels or create new bottlenecks. This, in turn, increases their vulnerability to stochastic events and to Allee and genetic effects related to small population sizes, such as reduced diversity and inbreeding (e.g. Towns and Ferreira 2001; Miller et al. 2009). Species with a low reproductive output and that are large-bodied, nocturnal and terrestrial are the least likely to be robust to fluctuations in mammal numbers. Indeed, these are over-represented in the extinction record (Towns and Daugherty 1994; Tingley et al. 2013).
At present, conservation in mainland sanctuaries predominantly targets birds, but where attention is given to reptiles, the typical goal is to support the recovery of existing resident species and then restore locally extinct lizard populations. The strength of mainland sanctuaries is that they have the potential to provide habitat not available on islands, and they are therefore key to the survival of some species (Nelson et al. 2015). However, basic questions still exist: Is complete eradication of introduced mammalian predators in mainland sanctuaries required for the recovery of lizard populations (both existing and reintroduced)? If not, what threshold level of predator control is required for healthy/stable lizard populations? How does this vary with different species of mammalian predators? Which species of mammal present the greatest risks for lizards? In this chapter, we discuss these issues for lizard conservation on the mainland using two new case studies that span more than a decade. A case study approach is used because comparable data across mainland sanctuaries are not available. Population studies of two ‘at risk-declining’ species (New Zealand Threat Classification, Hitchmough et al. 2016a) were compared: ornate skinks (O. ornatum) in ZEALANDIA, a fenced sanctuary where the skink population is recovering, and speckled skinks (O. infrapunctatum) adjacent to the Rotoiti Nature Recovery Project, an unfenced area with mammalian predator control. We demonstrate the difficulty of understanding the mechanisms driving lizard population dynamics in mainland sanctuaries and identify the issues that confound our understanding of the effects of predatory mammals and that warrant further research.
12.2 Case Study: Ornate Skinks in ZEALANDIA
ZEALANDIA is a 225 ha wildlife sanctuary in Wellington. Its population of ornate skinks (O. ornatum, Romijn 2013; Fig. 12.1) is protected by a perimeter fence, erected in 1999, designed to exclude introduced mammals. The only introduced mammals remaining within its borders are mice. Estimates of mouse abundance were initially at densities that would likely have been detrimental to lizards (e.g. 122.8 mice per 100 corrected trap nights in March 2004 for one transect line; Raewyn Empson, unpublished data; Newman 1994), but since October 2004, mice have been controlled annually (McKenzie 2007). This has resulted in lower mouse densities (mean annual peak of <22 per 100 trap nights in May–July 2009–2015 over three trap lines; mean of averaged abundances for three lines in Nov 2004–Nov 2015 was 6.6 mice per 100 trap nights compared to 68.8 mice per 100 trap nights before control started; Raewyn Empson, unpublished data; Fig. 12.2). However, peak densities for individual trap lines in May–July ranged from 0 to 59.3 mice per 100 trap nights, so in acknowledgement of the risk of high numbers of mice to some fauna, a small mouse exclosure (1 ha) was created in 2006 (McKenzie 2007; Butler et al. 2014).
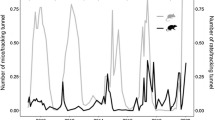
Ornate skink (Oligosoma ornatum) captures in pitfall traps for each year at ZEALANDIA, Wellington. Circles represent captures in the main sanctuary, and open triangles represent captures in the mouse exclosure. Note that two replicate sites were surveyed in both the main sanctuary and in the mouse exclosure. Crosses represent the index of abundance for peak mouse numbers on a trap line adjacent to pitfall traps in the main sanctuary. Peak mouse numbers generally occurred in May, but in 2010, mouse numbers peaked in July. Skink capture rates increased significantly both in the main sanctuary and the mouse exclosure up until 2013, when skink numbers plateaued. Higher capture rates occurred in the mouse exclosure in 2013 (Romijn 2013)
Ornate skinks are one of 61 currently known endemic extant species of New Zealand skink (Chapple et al. 2009; Hitchmough et al. 2016a, b). Although they remain widespread, and occasionally locally abundant, across much of the North Island (Chapple and Hitchmough 2016), ornate skinks are ranked as ‘at risk—declining’ under the New Zealand Threat Classification System due to a loss of local populations on the mainland, and there is an expectation that their long-term future is dependent on islands remaining free of mammalian predators (Hitchmough et al. 2016a). They are medium-sized lizards (maximum SVL 87 mm; Romijn 2013) that have a wide activity period (but more actively forage during dawn and dusk) and eat a range of invertebrates (Porter 1987; Towns 1999). Ornate skinks are usually found in leaf litter or under logs and rocks and in forests, shrublands and heavily vegetated coastlines throughout the North Island and many of its offshore islands (excluding the Poor Knights Islands which have a related local endemic species, O. roimata; Patterson et al. 2013; Chapple and Hitchmough 2016). Their reproductive output is high relative to other New Zealand skinks, with females giving birth to up to five offspring per year (Cree 1994; Cree and Hare 2016). Both males and females are sexually mature in their second year.
Surveys of lizards in ZEALANDIA only began after the perimeter fence was erected, and mice had reached high densities, with ornate skinks first observed in 2001 (Raewyn Empson, unpublished data). In 2006–2015, pitfall trapping for skinks was conducted during eight summer sessions to evaluate population characteristics and identify the effect of mice on ornate skink populations (Romijn 2013). Ornate skink populations were monitored in two locations within the sanctuary, each subject to different predator regimes: (1) the main area of the sanctuary where mice were present and (2) in the mouse exclosure, where mice were excluded. Although mice occasionally reinvaded the mouse exclosure (assumed to be transported by birds), they were removed by trapping when detected. All sampling sites were at similar altitudes in similar regenerating, forested vegetation dominated by native species along the west facing slopes of Te Mahanga Stream. Ornate skinks were monitored using pitfall traps, placed at 2 m intervals in a grid of nine traps, in two replicate sites within each predator regime (Hare 2012; Romijn 2013). Wire mesh was placed inside each trap to provide some protection for skinks should a mouse enter the trap. A large leaf was placed on top of the mesh to provide cover. Each trap was baited with a piece of canned pear, replaced daily through a nine-night trapping period (Romijn 2013).
Overall, 148 ornate skinks were captured (6.4 skinks per 100 trap nights). The mean capture rates of ornate skinks increased significantly (Romijn 2013) in both the main sanctuary (from 3.1 skinks per 100 trap nights in 2006 to 6.2 per 100 trap nights in 2015) and the mouse exclosure (from 0 skinks per 100 trap nights in 2006 to 13.0 per 100 trap nights in 2015). However, the highest capture rates were in 2013 in both areas (10.5 per 100 trap nights in the main sanctuary and 17.9 per 100 trap nights in the mouse exclosure). After 2013, numbers of ornate skinks levelled off in both locations, although captures in the mouse exclosure were higher than in the main sanctuary (Fig. 12.2). Mouse trapping rates were higher in the main sanctuary in 2014/2015, but this does not account for the unexpected apparent levelling off of skink numbers in the mouse exclosure (Raewyn Empson, unpublished data). The growth of the skink population was presumably achieved by maintaining an annual mouse control programme, with skink abundances peaking when mice were contained to very low levels (~10 mice per 100 trap nights) in consecutive years (Fig. 12.2). Data collected in 2013 indicated that males were larger (SVL) in the mouse exclosure compared to those in the main sanctuary, but sex ratios were not significantly different between the two sites (Romijn 2013).
The increase in captures of ornate skinks at ZEALANDIA until 2013 most likely represents, at least in part, a growing skink population that had either been totally, or partially, released from predation by introduced mammalian predators. The population growth rate was slower, and males were smaller, where skinks coexisted with mice; both factors could have long-term consequences for the population (Romijn 2013). These data are consistent with patterns of, and limits to, recovery of skinks elsewhere (e.g. Towns 1991, 1994; Newman 1994; Towns and Ferreira 2001). A plateau in captures since 2013 at both sites suggests that the population is now limited by other factors, though it seems unlikely that numbers have reached carrying capacity based on capture rates (e.g. Towns 1994; Romijn 2013).
It is possible that the recovery of skinks in ZEALANDIA has been slowed by the predation pressure of tuatara (Sphenodon punctatus) and birds. Tuatara were reintroduced to ZEALANDIA in 2005, with 60 placed in the mouse exclosure (McKenzie 2007). However, tuatara are not thought to be significant predators of skinks as both species co-occur on numerous islands in high densities (e.g. Phillpot 2000), and skinks are a rare occurrence in diet studies of tuatara (Walls 1981). Five volant bird species that are known to eat lizards are present in the sanctuary: kingfisher (Todiramphus sanctus), morepork (Ninox novaeseelandiae), New Zealand falcon (Falco novaeseelandiae), blackbird (Turdus merula) and the common starling (Sturnus vulgaris) (Van Winkel and Ji 2012). Of these, the kingfisher is likely to be a major predator as lizards have been one of the most frequent prey items brought to the nest (Hayes 1991). However, whether increasing predation by kingfishers has slowed population growth of skinks in ZEALANDIA is not known. North Island robins (Petroica longipes) and the North Island saddleback (Philesturnus rufusater) may also prey on skinks. These birds hunt for invertebrates in leaf litter and have been introduced to ZEALANDIA in the context of ecological restoration. Finally, two species of flightless bird had access to the main sanctuary but neither are not thought to have impacted skink numbers; although North Island weka (Gallirallus australis grayi) are known to have detrimental effects on lizard populations (Towns et al. 2002), only nine birds, which had apparently disappeared by 2010, were released into the northern part of the sanctuary (Raewyn Empson, unpublished data), so it seems unlikely that they would have had a significant effect. Little spotted kiwi (Apteryx owenii) would probably eat lizards they could catch, but these are unlikely to have population-level effects (reviewed in Romijn 2013).
Studies have shown that where management actions have led to an increase in bird density, there can be detrimental effects on other indigenous fauna. Watts et al. (2011) attributed declines in beetle species richness and abundance at ZEALANDIA to an increase in both mice and native birds. Sinclair et al. (2005) suggested that the decrease in invertebrate diversity on Kapiti Island after the eradication of rats was in part due to increased native bird predation. New introductions, and increased population sizes of birds and tuatara in the sanctuary, may now be the factors limiting population growth of skinks. However, further monitoring will be needed to determine the population dynamics between lizard populations and their native predators, as the population of ornate skinks now observed may be indicative of an equilibrium (or oscillation) between predator and prey.
12.3 Case Study: Speckled Skinks in the Rotoiti Nature Recovery Project
The Rotoiti Nature Recovery Project (RNRP), within New Zealand’s Nelson Lakes National Park in the South Island, is a Department of Conservation ‘mainland island’, established to facilitate the recovery of birds. Mammalian predators (mustelids, cats, possums and rats) are controlled within, and on the periphery of, the national park using trapping and poisoning, but there is no barrier fence protecting the approximately 5000 ha area from reinvasion (Saunders 2000; Department of Conservation 2015). Predator numbers, including those not specifically targeted, decline during control pulses, but the effectiveness of control varies by year, and therefore targets for tracking rates (e.g. less than 5 % tracking rates for rats) are not always met (Long et al. 2014; Dumont 2015).
Speckled skinks (O. infrapunctatum; Fig. 12.3) on the periphery of the RNRP have experienced predator control since 2001 (Dumont 2015). The species is also found in the Nelson-Marlborough region and Westland (there are also seven putative undescribed taxa within the O. infrapunctatum species complex; Hitchmough et al. 2016b; Chapple and Hitchmough 2016). Like the ornate skink, speckled skinks are listed on the New Zealand Threat Classification list as ‘at risk—declining’, because they have been reduced to sparsely distributed, declining populations (Dumont 2015; Hitchmough et al. 2016a). Speckled skinks are medium to large (maximum SVL of 75–106 mm depending on location) endemic lizards that inhabit densely vegetated grassland, shrubland or fern-land below 900 m (Whitaker 2000). Speckled skinks are active during the day and presumably consume a diet of invertebrates and fruit, akin to other skinks (Hare et al. 2016). Little is known about the reproductive rate of speckled skinks, but if other comparable species are used as a guide, then five offspring per female per year may be possible (Cree 1994; Dumont 2015).
Speckled skink populations were monitored between 2002 and 2011 in an area of predator control at the periphery of the RNRP zone and at an adjacent farm (not subject to predator control) to determine whether introduced mammalian predator control as part of the RNRP supported recovery of skink populations (Dumont 2015). A total of 38 pitfall traps were established in locations favourable to skinks within the RNRP zone, divided equally among two sites. Trapping effort varied throughout the study but was always conducted to maximise capture rates. A smaller amount of effort was placed on trapping skinks at the adjacent farm with no predator control, but skinks were reported as abundant in the 1960–1970s. Monitoring at the farm included an array of 50 traps divided across two sites in a grid pattern (Dumont 2015).
A total of 76 speckled skinks were captured throughout the study, with the majority being adults (96 %) captured within the RNRP sites (Fig. 12.4). Skink captures declined through time at both sites to the point where they were only barely detectable by 2012. Throughout the same period, hedgehogs (Erinaceus europaeus), predators not specifically targeted by the predator control programme, were the only introduced mammal species to decrease in abundance. Speckled skink capture rates have been higher during periods of increased cat control (Dumont 2015), but the significance of cat trapping data is unclear; higher catch rates may mean fewer cats, but might also mean more cats because catch rates may simply be an index of the number of cats dispersing into the area.
Speckled skink (Oligosoma infrapunctatum) captures at Rotoiti Nature Recovery Project zone, where mammalian predators are controlled, in 2002–2011 (triangles), and on an adjacent farm, with no mammalian control, in 2010–2011 (circles) (Dumont 2015). The open circle in 1995 indicates the capture rate at the farm site prior to this study. Skink captures declined significantly through time at both sites
This long-term study demonstrates that the current level of mammalian predator control is insufficient to protect or facilitate recovery of the speckled skink population adjacent to the RNRP zone. Dumont (2015) also collected data on two other skink species: spotted skinks, O. lineoocellatum, and northern grass skinks, O. polychroma. The life history characteristics and distribution of the latter, in particular, predict that it should be less vulnerable to predation or have a greater capacity to rebound, but the pattern of decline was common to all three species at both sites (Dumont 2015). Data for lizard populations in the RNRP zone are consistent with other studies in showing a lack of effectiveness of low- to medium-intensity predator control (Hoare et al. 2007a; Wilson et al. 2007; Reardon et al. 2012). However, introduced wasps place an additional pressure on lizards in the RNRP zone. These are present in high numbers and are likely to present a predation and competition threat for lizards (Rod Hitchmough, unpublished data).
12.4 Discussion
12.4.1 Conservation of Skinks in Mainland Sanctuaries
The study of ornate skinks within a fenced sanctuary at ZEALANDIA indicated that populations of this species may be able to increase in density only if mice are successfully controlled to low levels over consecutive years (e.g. average annual abundance approximately ≤10 mice per 100 trap nights). In contrast, research as part of the RNRP demonstrated that mammalian predator control targeted at bird recovery was insufficient to reverse declines in speckled skink populations on the periphery of the trapped area.
While populations of skinks may be able to persist and even increase in the presence of mammalian predators, as was the case when mouse density was low at ZEALANDIA, there are likely to be negative consequences for population structure. For example, mice may selectively eat larger individuals or have greater opportunity to capture these because larger individuals are limited in their ability to access smaller crevices, reducing escape prospects. Indeed, male ornate skinks in the ZEALANDIA mouse exclosure in our study were larger than those outside the exclosure. Changes in the average size of mature individuals may have a negative effect on fitness (Newman 1994). In addition, there may be unmeasured sublethal effects, such as a reduced ability to forage or thermoregulate if mice force lizards to change their behaviour to reduce predation (e.g. Herczeg et al. 2008; Hare et al. 2016; Hare and Cree 2016). Combined with direct predation effects, these factors could contribute to reduced recruitment.
Mice populations that occur in a sanctuary are protected from competition with other introduced mammals, and as a result, their populations can experience episodic outbreaks (Goldwater et al. 2012). Thus, there is a greater risk of successive population bottlenecks and other small population consequences for skink populations. These effects are likely to be similar to those experienced by birds in beech forests during masting events (Brown et al. 2015). Small populations are especially vulnerable to demographic and genetic stochasticity, environmental variability and catastrophic events (e.g. Miller et al. 2009; Towns et al. 2016, but see also O’Donnell et al. 2016). In contrast, larger populations are more robust to variation in reproductive output among cohorts, sex ratio imbalance and inbreeding. The minimum size of a population to avoid bottleneck effects in New Zealand lizards is not known, but it is possible that inbreeding depression, as a consequence of passing through a bottleneck, may be one factor preventing population recovery, even in the absence of predators. This is an area in need of further study.
The ornate skink case study also hints at the possibility that increases in non-mammalian predators restrict expansion of skink populations, because skink numbers apparently levelled off after 2013, even without mice. This suggests that other factors at this protected site were in play. In mainland sanctuaries with effective mammalian predator control, native predators increase in abundance. This is celebrated as a success for the sanctuary and rightly so. However, little is known about the ability of native predators to inhibit the recovery of remnant lizard populations. In addition, sanctuaries without introduced mammals also support large numbers of non-native birds, which either nest or roost in these safe sites. Some species are known to prey on lizards (Thompson 2000; Van Winkel and Ji 2012; Hare et al. 2016). Numerically, non-native birds that target small food items could have an enormous, yet largely unquantified, impact on lizard populations through direct predation and competition for food. Evidence from islands (e.g. Korapuki Island, Towns et al. 2016b) demonstrates that lizard populations can recover in the presence of native and non-native bird predators, but evidence on the mainland is limited. New Zealand geckos and skinks evolved for millions of years in the presence of a diverse range of predatory terrestrial and volant bird species, including both diurnal (e.g. kingfishers) and nocturnal species (e.g. owls). In contrast, introduced mammalian predators, against which native lizards appear to lack defences, pose a novel threat; these predators are more likely to hunt using olfactory cues rather than the visual and auditory cues used by avian predators. Finally, larger populations of lizards are unlikely to experience the same negative impacts of bird predation.
Predator control at an unfenced site on the periphery of the RNRP zone was insufficient for protection of lizards. Our research on speckled skinks supported the generally well-held view that terrestrial, large-bodied lizards are extremely vulnerable to mammalian predation (e.g. Whitaker’s skink; Hoare et al. 2007a). To date, predator control in unfenced sites on the mainland has only been successful for lizard recovery when it has been specifically targeted for lizard recovery, and even so, skinks only recovered in core areas of predator control and not in the peripheral areas (e.g. grand, O. grande, and Otago, O. otagense, skinks; Reardon et al. 2012). Intensive small-scale control of predatory mammals, targeted for lizard recovery, is needed to prevent further species loss, particularly where species survival and medium-term persistence is possible with small, isolated populations. Such minimum scale and minimum cost management is vital in order to gain some level of security from extinction in the short term. However, better techniques that are applicable for the sustainable management of lizards at larger habitat/landscape scales, and which are robust to outbreak or reinvasion problems, are required.
Smaller and more common species of skinks within the RNRP were also found to be in decline (Dumont 2015). Nonetheless, control measures such as fencing and trapping can increase capture rates for some common species (e.g. McCann’s skink O. maccanni, southern grass skink O. polychroma; Wilson et al. 2007, 2017). This is probably because the more common species often have life history characteristics, such as higher reproductive productivity (Cree 1994; Cree and Hare 2016) that can enable populations to bounce back after episodic predator increases have been contained (O’Donnell and Hoare 2012). However, the ability of some skink species to respond to predator control does not mean that they are immune from local extinction. Population recovery may be constrained by small population size and distribution at the time of the bottleneck, relatively low reproductive output (Cree 1994; Cree and Hare 2016) and other habitat (e.g. variation in population size with habitat, overlapping retreat sites with mammals or burning and cultivation regimes) or time variables (e.g. seasonal and long-term climatic variation) that could have additive or synergistic effects on limits to recovery. Analogies of how the recovery of a skink population may be limited on the mainland, even with predator control, can be drawn from density estimates from islands that have never had mammalian predators or competitors and where skink populations are presumably at carrying capacity and can reach extremely high numbers (e.g. estimated population density of 3700/ha for spotted skinks, O. lineoocellatum on North Brother Island; Phillpot 2000). Reliable information on long-term responses of populations of more common (and smaller-bodied) species in areas of mammalian predator control is needed. This will enable managers to identify threshold population sizes (or tracking indices) of mammal species that allow net population growth of lizards. Such information is crucial for decision-making on levels of mammalian predator control if it is not possible to eradicate predatory mammals from an ecosystem.
12.4.2 Conservation of Geckos in Sanctuaries
The implications of predator control for geckos are less clear than they are for skinks. We assume that gecko responses will be similar to those of skinks, but their recovery is likely to be slower because geckos have a lower reproductive output (maximum of two offspring per adult female per annum for small, warm-climate species, decreasing to one or two every second year for larger or cooler-climate species), and longer time to maturity (ranging from 2 to 8 years depending on temperature and body size) (Cree 1994; Cree and Hare 2016).
Data on gecko population dynamics in New Zealand are limited and, for the mainland, predominantly based on sightings per unit search effort, although recent work into use of artificial cover objects has improved detection (Bell 2009). Small capture numbers and too few recaptures to adequately estimate population size present difficulties for managing these populations (e.g. Hoare et al. 2013). Trends over time are largely based on capture data from islands after mammal eradications since there were no, or few, gecko sightings prior to eradication but then more reliable sightings post-eradication. The behaviour of some species has allowed them to survive without detection since they spend more time in trees or on cliffs, thereby also avoiding largely ground-based mammalian predators (Hoare et al. 2007b). Additional detection difficulties arise as a result of the cryptic colouration and behaviour of geckos and their use of largely inaccessible arboreal habitats. Low reproductive rates (Cree 1994) can also delay detection in the period immediately after eradication of mammals as populations take time to recover. For example, despite pre-eradication surveys, it took 17 years after mammalian predators were eradicated from Kapiti Island, off the North Island coast, before gold-striped geckos (Woodworthia chrysosiretica) were first observed (Richard Romijn, unpublished data). Similarly, 12 years after eradication of all pest mammals from Codfish Island/Whenua Hou, 300 h of targeted spotlighting detected only three jewelled geckos (Naultinus gemmeus). Cloudy geckos (Mokopirirakau nebulosus) were not encountered during the targeted search, though they have been observed occasionally since the searches, confirming their persistence on the island (James Reardon, unpublished data).
Mainland examples of gecko population trends are scarce but important to note. For example, a Duvaucel’s gecko (Hoplodactylus duvaucelii) was discovered on Maungatautari, a fenced mountain sanctuary in the Waikato region, North Island, 4 years after introduced mammals were eradicated (Morgan-Richards et al. 2016). It is possible that this observation resulted from behavioural changes by this species, rather than a population-level response to eradication of mammals due to the K-selected life history characteristics of Duvaucel’s gecko (Cree 1994; Hoare et al. 2007b). The effects of mammalian predator control on skinks likely also apply to geckos. In other words, total, or at least very strict, control of mammalian predators to low levels over sustained periods is probably required for gecko recovery.
12.4.3 Research Directions and Management Challenges
To limit or mitigate the effects of introduced mammalian predators on lizards, basic biological information, and how this might vary across the distribution of each species, is needed. However, this is lacking for most species. Distributional information is limited for all New Zealand lizard species, and extant population information often hints at a larger former distribution (e.g. Whitakers skink, Worthy 1987; Hoare et al. 2007a; Worthy 2016). For example, where lizards have apparent specific ecotypes, such as those of alpine specialists, it is unclear whether they are relicts or simply represent edge habitat of their former (pre-mammal) ranges (DOC 2016), a pattern that has been found in other groups of animals (Beauchamp and Worthy 1988). The distributions of the Cascade gecko (Mokopirirakau ‘Cascades’) and the Takitimu gecko (M. cryptozoicus), both once assumed to be alpine specialists, also include sightings in locations well below subalpine altitudes. This provides considerable evidence for previously broader altitudinal distributions. Ecological research into climate-induced physiological limitations that will enhance knowledge of potential distributions and guide management for detection and restoration is needed (Chapple and Hitchmough 2016; Hare and Cree 2016). In addition, more robust data on life history characteristics, and intrinsic rates of increase, would help inform the time needed to detect species before attempts are made to reintroduce missing species (Cree and Hare 2016). In most instances, data are likely to be both species and situation dependent, requiring more research effort into the biology of New Zealand lizards than has been the case to date.
The potential rise in predation on lizards by increased populations of predatory birds (both native and non-native) as a result of successful control of mammalian predators is not the only barrier to lizard recovery, particularly in mainland sanctuaries. An additional challenge to the recovery of lizards, albeit at this point only speculative, is the potential impact from increased abundance and distribution of introduced social insects including wasps and Argentine ants ( Linepithema humile ), either from direct effects on survival or indirect effects on food sources. Invertebrates are strongly negatively affected by wasps (Lester et al. 2013), and losses of lizards in captivity have been attributed to wasps and ants (Rod Hitchmough, unpublished data). Since both lizards and introduced wasps also consume nectar and invertebrates, it seems reasonable to expect there are downstream effects on lizards in areas with large wasp populations (Towns 2002). Wasps have increased in abundance and diversity in the last 90 years (Lester et al. 2013) and are particularly problematic in honeydew beech forest systems (e.g. Beggs 2001). The RNRP zone, where our case study on speckled skinks was based, has a serious problem with high wasp densities, dating from the invasion of common wasps (Vespula vulgaris) in the 1980s. Wasp poisoning is underway in the RNRP zone and other sites around New Zealand (Harper et al. 2016). Identifying the detrimental effects of wasps on lizard populations will be problematic due to concurrent mammalian predator control, and the nature of trying to assess effects in complex natural systems under adaptive management. A significant advancement in recent times has been the apparently successful control of wasps using toxic bait stations, which potentially offers an effective and sustainable management tool (Harper et al. 2016). This presents an opportunity to conduct controlled experiments to test the impacts of wasps and ants on lizards. These would be best done in areas adjacent to current mainland island conservation projects so that the level of any observed effects can be separated from those caused by mammalian predators.
Research to date suggests that predation by mammalian predators is by far the most important factor limiting lizard populations (e.g. Lettink et al. 2010), highlighting the importance of mammalian predator control. Nonetheless, other management actions also offset the causes of decline in lizard populations. These include wasp and ant control and habitat manipulation to increase structural complexity, thereby improving survival by providing protection from extreme environments and predators (Sinclair et al. 1998; Norbury et al. 2015). However, because mice are likely to remain in most mainland sanctuaries, increasing habitat and refuges for terrestrial lizards also benefits small rodents, thereby potentially increasing predation risk. All activities regarded as habitat enhancements should be confirmed via an experimental approach rather assuming that they are, in fact, improvements. Finally, developing effective methods of lizard conservation in mainland sanctuaries would not only aid the recovery of the full range of native species in such areas but could also contribute to the development of novel, or more refined, solutions to maintaining stable populations of lizards. This could be particularly valuable in other areas of the mainland where predator control is currently not feasible or performed at an inadequate scale. Biodiversity in mainland sanctuaries could be enormously improved if lizard conservation was afforded the same attention as that of birds.
References
Beauchamp AJ, Worthy TH (1988) Decline in the distribution of takahe (Porphyrio mantelli): a re-examination. J R Soc N Z 18(1):103–118
Beggs JR (2001) The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abundant carbohydrate resource. Biol Conserv 99:17–28
Bell TP (2009) A novel technique for monitoring highly cryptic lizard species in forests. Herpetol Conserv Biol 4(3):415–425
Brown K, Elliot G, Innes J, Kemp J (2015) Ship rat, stoat, and possum control on mainland New Zealand: an overview of techniques, successes and challenges. Department of Conservation, Wellington, 36p
Burns B, Innes J, Day T (2012) The use and potential of pest-proof fencing for ecosystem restoration and fauna conservation in New Zealand. In: Somers MJ, Hayward MS (eds) Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? Springer, New York, pp 65–90
Butler D, Lindsay T, Hunt J (2014) Paradise saved: the remarkable story of New Zealand’s wildlife sanctuaries and how they are stemming the tide of extinction. Random House, Auckland, 320p
Caut S, Casanovas JG, Virgos E, Lozano J, Witmer GW, Courchamp F (2007) Rats dying for mice: modelling the competitor release effect. Austral Ecol 32:858–868
Chapple DG, Hitchmough RA (2016) Biogeography of New Zealand lizards. Chap. 5. In: Chapple DG (ed) New Zealand lizards. Springer, Cham
Chapple DG, Daugherty CH, Ritchie PA (2009) Origin, diversification, and systematics of the New Zealand skink fauna (Reptilia: Scincidae). Mol Phylogenet Evol 52:470–487
Cree A (1994) Low annual reproductive output in female reptiles from New Zealand. N Z J Zool 21:351–372
Cree A, Hare KM (2016) Reproduction and life history of New Zealand lizards. Chap. 7. In: Chapple DG (ed) New Zealand lizards. Springer, Cham
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566
Department of Conservation (2015) http://www.doc.govt.nz/our-work/mainland-islands/rotoiti/. Accessed 11 Nov 2015
Department of Conservation (2016) http://www.doc.govt.nz/our-work/reptiles-and-frogs-distribution/atlas/. Accessed 21 Jan 2016
Dumont CT (2015) An investigation into declining skink populations and their behavioural responses to introduced mammalian predators. Masters of Science thesis, University of Canterbury, Christchurch, 154p
Goldwater N, Perry GLW, Clout MN (2012) Responses of house mice to the removal of mammalian predators and competitors. Austral Ecol 37:971–979
Hare KM (2012) Herpetofauna: pitfall trapping version 1.0. Inventory and monitoring toolbox: herpetofauna DOCDM-760240, Department of Conservation, Wellington, 22p
Hare KM, Cree A (2016) Thermal and metabolic physiology of New Zealand lizards. Chap. 9. In: Chapple DG (ed) New Zealand lizards. Springer, Cham
Hare KM, Chapple DG, Towns DR, van Winkel D (2016) The ecology of New Zealand’s lizards. Chap. 6. In: Chapple DG (ed) New Zealand lizards. Springer, Cham
Harper GA, Joice N, Kelly D, Toft R, Clapperton BK (2016) Effective distances of wasp (Vespula vulgaris) poisoning using clustered bait stations in beech forest. N Z J Ecol 40:65–71
Hayes LM (1991) Behaviour of New Zealand kingfishers feeding chicks. Notornis 38:73–79
Herczeg G, Herrero A, Saarikivi J, Gonda A, Jantti M, Merila J (2008) Experimental support for the cost–benefit model of lizard thermoregulation: the effects of predation risk and food supply. Oecologia 155(1):1–10
Hitchmough R, Barr B, Monks J, Lettink M, Reardon J, Tocher M, van Winkel D, Rolfe J (2016a) Conservation status of New Zealand reptiles, 2015, New Zealand threat classification series. Department of Conservation, Wellington
Hitchmough RA, Patterson GB, Chapple DG (2016b) Putting a name to diversity: taxonomy of the New Zealand lizard fauna. Chap. 4. In: Chapple DG (ed) New Zealand lizards. Springer, Cham
Hoare JM, Adams LK, Bull LS, Towns DR (2007a) Attempting to manage complex predator-prey interactions fails to avert imminent extinction of a threatened New Zealand skink population. J Wildl Manage 71:1576–1584
Hoare JM, Pledger S, Nelson NJ, Daugherty CH (2007b) Avoiding aliens: behavioural plasticity in habitat use enables large, nocturnal geckos to survive Pacific rat invasions. Biol Conserv 136:510–519
Hoare JM, Melgren P, Chavel EE (2013) Habitat use by southern forest geckos, Mokopirirakau “Southern Forest” in the Catlins, Southland. N Z J Zool 40(2):129–136
Innes J, Saunders A (2011) Eradicating multiple pests: an overview. In: Veitch CR, Clout MN, Towns DR (eds) Island invasives: eradication and management. IUCN, Gland, pp 177–181
Innes J, Kelly D, McC Overton J, Gillies C (2010) Predation and other factors currently limiting New Zealand forest birds. N Z J Ecol 34(1):86–114
Jones C, Norbury G, Bell T (2013) Impacts of introduced European hedgehogs on endemic skinks and weta in tussock grassland. Wildl Res 40:36–44
Lester PJ, Beggs JR, Brown RL, Edwards ED, Groenteman R, Toft RJ, Twidle AM, Ward DF (2013) The outlook for control of New Zealand’s most abundant, widespread and damaging invertebrate pests: social wasps. N Z Sci Rev 70(4):56–62
Lettink M, Norbury G, Cree A, Seddon PJ, Duncan RP, Schwarz CJ (2010) Removal of introduced predators, but not artificial refuge supplementation, increases skink survival in coastal duneland. Biol Conserv 143:72–77
Long J, Waite J, Joice N, Grose T (2014) Rotoiti nature recovery project annual report 2013–14, Nelson lakes mainland island, Nelson Lakes National Park, 85p
McKenzie KL (2007) Returning tuatara (Sphenodon punctatus) to the New Zealand mainland. Master of Science thesis, Victoria University of Wellington, Wellington, 82p
Miller KA, Chapple DG, Towns DR, Ritchie PA, Nelson NJ (2009) Assessing genetic diversity for conservation management: a case study of a threatened reptile. Anim Conserv 12:163–171
Morgan-Richards M, Rheyda Hinlo M, Smuts-Kennedy C, Innes J, Ji W, Barry M, Brunton D, Hitchmough RA (2016) Identification of a rare gecko from North Island New Zealand, and genetic assessment of its probable origin: a novel mainland conservation priority? J Herpetol 50:77–86
Nelson NJ, Hitchmough R, Monks JM (2015) New Zealand reptiles and their conservation. In: Stow A, Maclean N, Holwell GI (eds) Austral Ark: the state of wildlife in Australia and New Zealand. Cambridge University Press, Cambridge, pp 382–404
Newman DG (1994) Effect of a mouse Mus musculus eradication programme and habitat change on lizard populations on Mana Island, New Zealand, with special reference to McGregor’s skink, Cyclodina macgregori. N Z J Zool 21:443–456
Norbury G, Byrom A, Pech R, Smith J, Clarke D, Anderson D, Forrester G (2013) Invasive mammals and habitat modification interact to generate unforeseen outcomes for indigenous fauna. Ecol Appl 23:1707–1721
Norbury G, van den Munckhof M, Neitzel S, Hutcheon A, Reardon J, Ludwig K (2014) Impacts of invasive house mice on post-release survival of translocated lizards. N Z J Ecol 38:322–327
Norbury GL, Pech RP, Byrom AE, Innes J (2015) Density-impact functions for terrestrial vertebrate pests and indigenous biota: guidelines for conservation managers. Biol Conserv 191:409–420
O’Donnell CFJ, Hoare JM (2012) Monitoring trends in skink sightings from artificial retreats: influences of retreat design, placement period, and predator abundance. Herpetol Conserv Biol 7(1):58–66
O’Donnell CFJ, Richter S, Dool S, Monks JM, Kerth G (2016) Genetic diversity is maintained in the endangered New Zealand long-tailed bat (Chalinolobus tuberculatus) despite a closed social structure and regular population crashes. Conserv Genet 17(1):91–102
Patterson GB, Hitchmough RA, Chapple DG (2013) Taxonomic revision of the ornate skink (Oligosoma ornatum; Reptilia: Scincidae) species complex from northern New Zealand. Zootaxa 3736(1):54–68
Phillpot P (2000) The skinks of North Brother Island: abundance, habitat use and species interactions. Master of Science thesis, Victoria University of Wellington, 138p
Porter R (1987) An ecological comparison of two Cyclodina skinks (Reptilia: Lacertilia) in Auckland, New Zealand. N Z J Zool 14:493–507
Reardon JT, Whitmore N, Holmes KM, Judd LM, Hutcheon AD, Norbury G, Mackenzie DI (2012) Predator control allows critically endangered lizards to recover on mainland New Zealand. N Z J Ecol 36:141
Romijn RL (2013) Can skinks recover in the presence of mice? Bachelor of Science (Honours) thesis, Victoria University of Wellington, Wellington, 51p
Saunders A (2000) A review of Department of Conservation mainland restoration projects and recommendations for further action. Department of Conservation, Wellington, 219p
Sinclair ARE, Pech RP, Dickman CR, Hik D, Mahon P, Newsome AE (1998) Predicting effects of predation on conservation of endangered prey. Conserv Biol 12(3):564–575
Sinclair L, McCartney J, Godfrey J, Pledger S, Wakelin M, Sherley G (2005) How did invertebrates respond to eradication of rats from Kapiti Island, New Zealand? N Z J Ecol 32:293–315
Thompson MB (2000) Oligosoma spp. (New Zealand skinks) predation. Herpetol Rev 31:175
Tingley R, Hitchmough RA, Chapple DG (2013) Life-history traits and extrinsic threats determine extinction risk in New Zealand lizards. Biol Conserv 165:62–68
Towns DR (1991) Response of lizard assemblages in the Mercury Islands, New Zealand, to removal of an introduced rodent: the kiore (Rattus exulans). J R Soc N Z 21:119–136
Towns DR (1994) The role of ecological restoration in the conservation of Whitaker’s skink (Cyclodina whitakeri), a rare New Zealand lizard (Lacertilia: Scincidae). N Z J Zool 21:457–471
Towns DR (1999) Cyclodina spp. skink recovery plan, 1999–2004, Threatened species recovery plan 27. Department of Conservation, Wellington, 75p
Towns DR (2002) Interactions between geckos, honeydew scale insects and host plants revealed on islands in northern New Zealand, following eradication of introduced rats and rabbits. In: Veitch CR, Clout MN (eds) Turning the tide: the eradication of invasive species. IUCN, Gland, pp 329–335
Towns DR, Broome KG (2003) From small Maria to massive Campbell: forty years of rat eradications from New Zealand Islands. N Z J Zool 30:377–398
Towns DR, Daugherty CH (1994) Patterns of range contractions and extinctions in the New Zealand herpetofauna following human colonisation. N Z J Zool 21(4):325–339
Towns DR, Ferreira S (2001) Conservation of New Zealand lizards (Lacertilia: Scincidae) by translocation of small populations. Biol Conserv 98(2):211–222
Towns DR, Daugherty CH, Cree A (2001) Raising the prospects of a forgotten fauna: a review of 10 years of conservation effort for New Zealand reptiles. Biol Conserv 99:3–16
Towns DR, Neilson KA, Whitaker AH (2002) North Island Oligosoma spp. skink recovery plan, 2002–2012, Threatened species recovery plan 48. Department of Conservation, Wellington, 62p
Towns DR, Hitchmough RA, Perrott J (2016a) Conservation of New Zealand lizards: a fauna not forgotten but undervalued? Chap. 11. In: Chapple DG (ed) New Zealand lizards. Springer, Cham
Towns DR, Borrelle SB, Thoresen J, Buxton RT, Evans A (2016b) Mercury Islands and their role in understanding seabird island restoration. N Z J Ecol 40(2):235–249
Towns DR, Miller KA, Nelson NJ, Chapple DG (2016) Will translocations to islands reduce extinction risk for reptiles? Case studies from New Zealand. Biol Conserv doi:10.1016/j.biocon.2016.04.024
Van Winkel D, Ji W (2012) Evidence of lizard predation by New Zealand kingfishers (Todiramphus sanctus vagans) and potential implications for threatened species translocations. N Z J Zool 39(3):201–208
Walls GY (1981) Feeding ecology of the tuatara (Sphenodon punctatus) on Stephens Island, Cook Strait. N Z J Ecol 4:89–97
Watts CH, Armstrong DP, Innes J, Thornburrow D (2011) Dramatic increases in weta (Orthoptera) following mammal eradication on Maungatautari—evidence from pitfalls and tracking tunnels. N Z J Ecol 35:261–272
Wedding CJ (2007) Aspects of the impacts of mouse (Mus musculus) control on skinks in Auckland, New Zealand. Master of Science thesis, Massey University, Auckland, 133p
Whitaker T (2000) Lizards of Nelson and Marlborough: a field key. Nelson/Marlborough Conservancy, Department of Conservation, Wellington, 20p
Wilson DJ, Clarke DA, Mulvey RL, Reardon JT (2017) Assessing and comparing population densities and indices of skinks under three predator management regimes. N Z J Ecol 41(1)
Wilson DJ, Mulvey RL, Clark RD (2007) Sampling skinks and geckos in artificial cover objects in a dry mixed grassland-shrubland with mammalian predator control. N Z J Ecol 31:169–185
Worthy TH (1987) Osteological observations on the larger species of the skink Cyclodina and the subfossil occurrence of these and the gecko Hoplodactylus duvaucelii in the North Island, New Zealand. N Z J Zool 14:219–229
Worthy TH (2016) A review of the fossil record of New Zealand lizards. Chap. 3. In: Chapple DG (ed) New Zealand lizards. Springer, Cham
Acknowledgements
Thanks to the friends of Rotoiti volunteers, Diana McMahon, Eric Dumont, Sirin Gnadeberg and Richard Meutstege; Ingrid McConchie for site access and Genevieve Taylor, Kimberly Parlane, Tamsin Bruce, Sally Leggett, Petrina Carter, Grant Harper, Elena Moltchanova, Laura Azzani, Matt Hanson, the Department of Conservation and friends of Rotoiti for project support. Our work was conducted under the following permits: Department of Conservation (NM–29621–FAU, WE/31544-FAU, WE112/RES, WE/297/RES, WE/340/RES, WE/33952/CAP), University of Canterbury Animal Ethics (2010/28R) and Victoria University of Wellington (2003R16-06, 2005R11-08, 2008R14, 2012R11). The Todd Foundation Award for Excellence, Federation of Graduate Women Trust Award, University of Canterbury Alumni Association Scholarship, the BAYER Boost Scholarship and the University of Canterbury part funded this research. We thank David Towns for reviewing an earlier version of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Nelson, N.J. et al. (2016). Lizard Conservation in Mainland Sanctuaries. In: Chapple, D. (eds) New Zealand Lizards. Springer, Cham. https://doi.org/10.1007/978-3-319-41674-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-41674-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41672-4
Online ISBN: 978-3-319-41674-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)