Abstract
This article presents the results of a pilot dose survey including fifty patients who underwent combined screening: full field digital mammography (FFDM) plus digital breast tomosynthesis (DBT). The study also aimed to demonstrate the different dosimetric outcome from using different glandularity assumptions and dosimetry methods. The mean glandular dose to each patient was computed using Dance’s method with UK glandularity assumption. The calculations were repeated using Wu/Boone’s method with the “50–50” breast assumption and the results compared to those using Dance’s method. For the typical breasts, the dose from combined examination was around 9.56 mGy: 4.26 mGy from two-view FFDM and 5.30 mGy from two-view DBT. Adopting UK glandularity assumption was believed to more realistically reflect the population dose. The comparison between Dance’s and Wu/Boone’s methods indicated that the latter tended to show lower dose values with mean differences of −3.6 % for FFDM and −5.5 % for DBT.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Clinical studies [1–3] have shown that both the sensitivity and specificity of mammography screening would be generally improved when digital breast tomosynthesis (DBT) was used in combination to full field digital mammography (FFDM) (referred as combined mammography screening). It has been questioned whether the extra radiation dose associated with combined imaging would be adequately justified for population-based screening, particularly as the effect on breast cancer mortality is as yet unproven. Therefore, BreastScreen Australia has yet to recommend the inclusion of DBT in mammography screening awaiting further national clinical evidence and a better knowledge about the radiation dose from combined examinations to ensure patient safety.
The situation in Australia is additionally complicated as there is yet no national consensus regarding the standard method to determine radiation dose for DBT exposures nor in fact for patient dose surveys in mammography. At present, there are two main schools of mammographic dosimetry formalism respectively advocated by Europe and North America: The former is based on the works by Dance [4, 5] (referred to as the Dance’s method) which incorporates both FFDM and DBT methodologies and the latter is from the original works by Wu [6, 7], later extended by Boone [8] who worked on FFDM methodologies, and the work of Sechopoulos [9, 10] which developed into the American Association of Physicists in Medicine (AAPM) protocol for DBT dosimetry [11] (overall referred to as the Wu/Boone’s method). All systems rely on Monte Carlo simulation techniques to estimate the mean glandular dose, a dose metric to characterize the risk of radiation-induced breast cancer, but with different simulation details leading to the expectation of different dosimetric outcomes.
Importantly the assumptions of breast density, used in dose estimation, need to be examined. For dosimetry, breast density is referred as “glandularity” which describes the proportional mass content of fibro-glandular tissues in the tissue core of the simulated breast model, excluding a relatively thin layer of adipose tissue on the surface. In Dance’s method, polynomial functions were derived from the screening data in the United Kingdom (UK) to estimate the average glandularity of the imaged breasts by their compressed thicknesses (referred as UK glandularity assumption) for two age groups of women and such estimations can be directly incorporated into dose calculations [12]. Despite the potential errors, this approach is believed to be able to more realistically reflect the mean glandular dose to the screening population. In comparison, the Wu/Boone’s method does not provide a specific guideline and assumes equal proportion of fibro-glandular and adipose tissues (referred as 50–50 breast assumption) in any dose calculation. This specific composition was conventionally believed to represent an average breast but recent works by Yaffe et al. [13] and Vedantham et al. [14] have added weight to previous work casting doubt on the validity of the 50–50 breast assumption by demonstrating that the fibro-glandular tissue composition is more typically in the range of around 20 %, depending on the definition of fibro-glandular tissue composition employed and the profile of women surveyed.
The study below presents the pilot dose data from combined mammography screening in Australia. It also aims to investigate the dosimetric outcome as a result of using different dosimetry methods and different assumptions on glandularity. The outcome of this study will be valuable when evaluating the introduction of DBT into the screening practice.
2 Methods and Materials
This pilot dose survey utilized patient data generated by a Hologic Selenia Dimensions (Hologic, Bedford, USA) mammographic unit with DBT functionality. Fifty asymptomatic female patients (average age of 54) who attended for screening for breast cancer in a private centre were randomly sampled from PACS records. Combined examinations, which composed of both FFDM and DBT acquisitions under the same compressed breast thickness, were performed at two views [cranio-caudal (CC) and mediolateral oblique (MLO)] on both breasts. Eight images were acquired for each patient, constituting a total of 400 images in this study. All examinations were performed using the automatic exposure control (AEC) setting: “Autofilter” that automatically selects suitable tube voltage (kV), anode/filter combinations and current-time product (mAs) depending on the compressed thickness of the breast and its attenuation to x-ray determined by the pre-pulse x-ray exposure.
From the patients’ images, the acquisition parameters and characteristic of the breasts were extracted from the relevant DICOM tags. The volumetric breast density was also estimated for each patient using commercial software: QuantraTM (Version 2.0). The radiation output and the beam qualities of the mammographic unit were measured using a dedicated mammography ionization chamber (Model 10 × 6-6M; Radcal, Monrovia, USA) and aluminum foils of high purity, respectively.
Combining the measurement results and the data collected for each patient, the associated mean glandular dose was computed using Dance’s method for both FFDM and DBT acquisitions with the UK glandularity assumptions to study the typical dose from a combined examination. Subsequently, the calculations were repeated with the 50–50 breast assumption to study its impact on the dosimetric outcome and to allow direct comparison with the results calculated with Wu/Boone’s methods.
3 Results and Discussion
3.1 Radiation Dose from Combined Examination
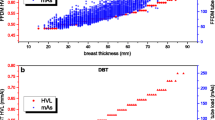
The demographics of the sampled patients were presented in Fig. 1, illustrating the distribution of compressed breast thickness. The average compressed thicknesses for CC and MLO view are 52.83 ± 15.18 mm and 52.89 ± 17.16 mm. Owing to the similar distributions of thickness, the respective radiation doses from these two views were not differentiated. The radiation dose from FFDM and DBT was presented in Table 1, as a function of compressed breast thickness and upon the UK glandularity assumption. On average, DBT delivers approximately 27 % more radiation dose to the imaged breast than FFDM which is comparable with the published results in the literature [15]. For the median compressed thickness between 50 and 60 mm, the mean glandular dose to the imaged breasts from a combined examination is approximately 9.56 mGy: 4.26 mGy from two-view FFDM and 5.30 mGy from two-view DBT.
The combined examinations have been shown to improve the effectiveness of mammography screening but at the expense of roughly doubling the radiation dose as demonstrated in the present survey. It is clear that the total dose to patients undergoing mammography screening will be determined by the clinical role of DBT in the future – whether it will be used solely or adjunct to FFDM and whether one-view DBT is adequate for accurate screening and diagnosis. In that regard, the clinical usefulness of synthetic 2D images reconstructed from DBT slice images would also require further investigation.
3.2 Impact of Glandularity Assumption
When compared to the results based upon the UK glandularity assumption (Table 1), using the 50–50 breast assumption would overestimate the dose for thinner breasts (thickness: 20 to 40 mm) and underestimate the values for the thicker ones (thickness: 50 to 100 mm) (Table 2). These results were expected as breast glandularity was assumed to decline in a polynomial fashion with compressed breast thickness (left y-axis of Fig. 2): the thinner breasts would have glandularity greater than 50 % and vice versa for thicker ones as indicated by the dotted line in Fig. 3. Two polynomial curves are presented, representing the estimations for age groups: 40 to 49 (upper curve) and 50 to 64 (lower curve).
The breast glandularity by mass proportion (left y-axis values) predicted by the UK glandularity assumption: two polynomial curves are presented, representing the estimations for age groups: 40 to 49 (upper curve) and 50 to 64 (lower curve). Also presented in the graph are the volumetric estimations by QuantraTM (right y-axis values)
The present results are limited by the relatively small sample size in the survey and thus the glandularity distribution might not be representative owing to the variation in average glandularity within a sampled breast thickness and age group. Also, as discussed by Beckett and Kotre [16], the estimated glandularity from the UK assumption in theory would only be referring to the column of tissues above the AEC chamber and might not be directly comparable to the volumetric glandularity reported by automated software (e.g. QuantraTM), in addition to the different definitions used which will be detailed next. This was evident in the right y-axis values of Fig. 2 which demonstrated that the individual volumetric glandularity, independently measured by QuantraTM, in general deviated from the polynomial relationships with compressed thickness.
Incorporating individual glandularity information into dose calculation is not straight-forward in clinical settings as the information is not always available. Moreover, the values reported by radiologists or automated software are based on tissue area or volumetric proportion as opposed to the mass proportion used in context of dosimetry. Additionally, the former estimates often include the subcutaneous adipose tissue, which is however excluded in dosimetry. Conversion between the two definitions is possible but access to the images would be necessary for further image processing. Commercial software is now available to perform “personalized” dose estimation taking into account the volumetric glandularity measured, and its impact on clinical practice would require further investigation [17].
3.3 Comparison of Dosimetry Methods
Linear relationships were observed between the dosimetry methods for both FFDM and DBT acquisitions (Fig. 3). Moreover, high correlations were also demonstrated with respective Pearson coefficients of 0.999 and 0.957. Further, compared to Dance’s methodology, the Wu/Boone method tended to show lower dose values with the maximum difference of −10.8 % (mean = −3.6 %) for FFDM and −11.0 % (mean = −5.5 %) for DBT acquisitions. From the paired t-test, these differences were statistically significant (p < 0.0001).
This difference is mainly attributed to the different simulated breast models adopted in the two methods. In Dance’s work, the breast was simulated as a symmetric semi-circular cylinder with an outer layer of adipose tissue (a surrogate of subcutaneous adipose tissue and skin) of thickness 4 mm enclosing a central region comprising a uniform mixture of breast tissues. Wu/Boone have utilized a similar breast model except with an outer adipose layer of 5 mm. This slight thickness variation of the outermost adipose would constitute a prominent dosimetric difference especially for the low x-ray energies utilized in mammography. This was evident that the largest deviations were from acquisitions with low kV settings. Other contributing factors include the different photon interaction cross-sectional areas (maximum alternations up to 10 %) as well as the difference in the irradiation geometry (maximum alternations up to 2.3 %). These small but significant differences must be recognized by users when interpreting dose values in reports and literature for risk assessment.
4 Conclusion
It was revealed in the pilot survey that the mean glandular dose to the imaged breasts from two-view combined examinations, as measured by mean glandular dose, would range from 5.90 to 23.14 mGy. For the median breasts in this survey, the total dose was approximately 9.56 mGy: 4.26 mGy from two-view FFDM and 5.30 mGy from two-view DBT. It was further demonstrated that these dose values would vary with the glandularity assumptions as well as the dosimetry methods and such dosimetric differences must be well recognized.
The results from this pilot study are inherently limited by its sample size and the fact that only one mammography unit is included. These limitations will be addressed in a follow-up study involving a larger sample size and multiple clinics using mammography units from different vendors.
References
Rose, S.L., et al.: Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am. J. Roentgenol. 200, 1401–1408 (2013)
Ciatto, S., et al.: Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 14, 583–589 (2013)
Skaane, P., et al.: Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 267, 47–56 (2013)
Dance, D.R., Young, K.C., van Engen, R.E.: Further factors for the estimation of mean glandular dose using the United Kingdom, European and IAEA breast dosimetry protocols. Phys. Med. Biol. 54, 4361–4372 (2009)
Dance, D.R., Young, K.C., van Engen, R.E.: Estimation of mean glandular dose for breast tomosynthesis: factors for use with the UK, European and IAEA breast dosimetry protocols. Phys. Med. Biol. 56, 453–471 (2011)
Wu, X., Barnes, G.T., Tucker, D.M.: Spectral dependence of glandular tissue dose in screen-film mammography. Radiology 179, 143–148 (1991)
Wu, X., Gingold, E.L., Barnes, G.T., Tucker, D.M.: Normalized average glandular dose in molybdenum target-rhodium filter and rhodium target-rhodium filter mammography. Radiology 193, 83–89 (1994)
Boone, J.M.: Normalized glandular dose (DgN) coefficients for arbitrary X-ray spectra in mammography: computer-fit values of Monte Carlo derived data. Med. Phys. 29, 869–875 (2002)
Sechopoulos, I., Suryanarayanan, S., Vedantham, S., D’Orsi, C., Karellas, A.: Computation of the glandular radiation dose in digital tomosynthesis of the breast. Med. Phys. 34, 221–232 (2007)
Sechopoulos, I., D’Orsi, C.: Glandular radiation dose in tomosynthesis of the breast using tungsten targets. J. Appl. Clin. Med. Phys. 9, 161–171 (2008)
Sechopoulos, I., et al.: Radiation dosimetry in digital breast tomosynthesis: report of AAPM tomosynthesis subcommittee task group 223. Med. Phys. 41, 091501 (2014)
Dance, D.R., Skinner, C.L., Young, K.C., Beckett, J.R., Kotre, C.J.: Additional factors for the estimation of mean glandular breast dose using the UK mammography dosimetry protocol. Phys. Med. Biol. 45, 3225–3240 (2000)
Yaffe, M.J., et al.: The myth of the 50–50 breast. Med. Phys. 36, 5437–5443 (2009)
Vedantham, S., Shi, L., Karellas, A., O’Connell, A.M.: Dedicated breast CT: fibroglandular volume measurements in a diagnostic population. Med. Phys. 39, 7317–7328 (2012)
Dance, D.R., Strudley, C.J., Young, K.C., Oduko, J.M., Whelehan, P.J., Mungutroy, E.: Comparison of breast doses for digital tomosynthesis estimated from patient exposures and using PMMA breast phantoms. In: Maidment, A.D., Bakic, P.R., Gavenonis, S. (eds.) IWDM 2012. LNCS, vol. 7361, pp. 316–321. Springer, Heidelberg (2012)
Beckett, J.R., Kotre, C.J.: Dosimetric implications of age related glandular changes in screening mammography. Phys. Med. Biol. 45, 801–813 (2000)
Tromans, C.E., Highnam, R., Morrish, O., Black, R., Tucker, L., Gilbert, F., Brady, S.M.: Patient specific dose calculation using volumetric breast density for mammography and tomosynthesis. In: Fujita, H., Hara, T., Muramatsu, C. (eds.) IWDM 2014. LNCS, vol. 8539, pp. 158–165. Springer, Heidelberg (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Tse, J., Fulton, R., Rickard, M., Brennan, P., McLean, D. (2016). A Pilot Study on Radiation Dose from Combined Mammography Screening in Australia. In: Tingberg, A., Lång, K., Timberg, P. (eds) Breast Imaging. IWDM 2016. Lecture Notes in Computer Science(), vol 9699. Springer, Cham. https://doi.org/10.1007/978-3-319-41546-8_42
Download citation
DOI: https://doi.org/10.1007/978-3-319-41546-8_42
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41545-1
Online ISBN: 978-3-319-41546-8
eBook Packages: Computer ScienceComputer Science (R0)