Abstract
Several animal and plant lineages exhibit pronounced sexual size dimorphism (SSD). Here, we review the evolution of female-biased, extreme SSD (hereafter eSSD; females at least twice male size) in two model spider clades, Nephilidae and Argiopinae. Although these two clades exhibit comparable levels of eSSD, we show that the phenomenon takes different evolutionary pathways. In nephilids, no correlation between male and female size changes is detected while this correlation is maintained in argiopines. In nephilids, sizes in both sexes increase through evolutionary time, but female sizes rise faster, which maintains eSSD. In contrast, argiopines exhibit no directional size change in either sex, and eSSD slowly declines. Model fitting analyses reveal that in nephilids, female size and eSSD adhere to Brownian motion, but male body size evolves toward an optimum between 3.5 and 5.7 mm. In contrast, no directional trends can be detected in argiopines with Brownian motion as the best-fit model. Finally, phylogenetic allometric analyses reveal no relationships between male and female sizes in nephilids, while argiopine size evolution is isometric. The sole agreement between the clades seems to be falsification of both Rensch’s rule and its converse. However, to establish pervasive patterns in spider size evolution, studies on other comparable lineages are essential. We point toward candidate clades and pose open questions in eSSD research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Sexual size dimorphism (SSD ) describes a morphological condition where male and female sizes differ significantly within a species. Vertebrate cases with males as the larger sex are well known (e.g., humanoids, elephant seals, or elephants) and readily explained by male–male competition mechanisms (Trivers 1972; Isaac 2005). However, female-biased sexual size dimorphism may account for more dramatic size differences (Blanckenhorn 2005; Fairbairn et al. 2007) and its evolution is also more difficult to interpret. Notable examples of large females and small males include widow spiders, barnacles, anglerfish, queen ants, and marine echiuran worms. In spiders, females may be over 100 times the male’s weight (Kuntner et al. 2012), but the record-holding animal is the octopus where females outweigh males by more than 10,000 fold (Norman et al. 2002). Because cases of extreme, female-biased SSD (hereafter eSSD, where females are at least twice male size) are rather rare and taxonomically scattered, the phenomenon has intrigued early evolutionists (Darwin 1871). Nevertheless, eSSD has not been subjected to rigorous modern evolutionary research, and the phenomenon continues to receive mere occasional bursts of interest (reviewed in Fairbairn et al. 2007). We consequently lack solid comparative data that would explain common mechanisms responsible for the repeated origin, convergence, and maintenance of eSSD in several lineages.
Most studies have investigated intraspecific patterns of SSD on selected model species (Fairbairn 2005; Cox and Calsbeek 2009; Blanckenhorn et al. 2011). These works largely corroborate the differential equilibrium model of SSD evolution that invokes opposing selection pressures on the sexes (Blanckenhorn 2005). However, having ignored the phylogenetic basis of SSD, these single-species studies offer only limited explanations of processes that lead to SSD because they look at individual fitness costs and benefits in phylogenetic isolation. To gain a more complete picture of the phenomenon, comparative studies are therefore needed, but these are relatively rare (Hormiga et al. 2000; Teder and Tammaru 2005; Foellmer and Moya-Laraño 2007; Webb and Freckleton 2007; Cheng and Kuntner 2014; Kuntner and Elgar 2014; Teder 2014) or cannot be directly compared. In this review, we revisit the evolution of eSSD in spiders within the context of convergent evolution that looks for common themes in phylogenetically independent lineages.
Orb web spiders (Araneoidea ) represent an ideal animal group for comparatively testing evolutionary hypotheses regarding the origin and maintenance of eSSD, as eSSD has evolved at least four, but more likely up to nine times independently in this group of spiders (Hormiga et al. 2000; Kuntner et al. 2015). Yet most araneoid lineages remain sexually size monomorphic. Originally, cases of eSSD were referred to as male dwarfism (Vollrath and Parker 1992), but authors have more recently argued that eSSD is generally better explained through female size increase rather than male size decrease (Coddington et al. 1997; Hormiga et al. 2000). However, although such interpretation, if general, would elegantly explain eSSD in spiders as female gigantism due to selection for increased fecundity (Head 1995; Higgins 2002), analyses at a finer taxonomic scale reveal that the origin and shifts of eSSD are much more complex (Kuntner and Coddington 2009; Kuntner and Elgar 2014). These studies have linked eSSD to a combination of sexual and natural selection components, but as we show here, interspecific patterns of eSSD commonly show no directional trends (Cheng and Kuntner 2014).
Our recent work demonstrates that studying the species-level lineages provides increased resolution compared with studies at higher taxonomic levels. Our understanding of the evolutionary mechanisms of eSSD in spiders, however, is confined to only two clades of a comparable evolutionary age: the family Nephilidae (Kuntner et al. 2008, 2013; Kuntner and Coddington 2009; Higgins et al. 2011; Kuntner and Elgar 2014) and the araneid subfamily Argiopinae (Cheng and Kuntner 2014, 2015). As we show in this review, these clades show, against all predictions, two strikingly differing patterns which suggest that eSSD in these clades arose and is maintained through different mechanisms.
2 Studied Clades
We studied independent evolution of eSSD in two orb-weaving spider clades: Nephilidae and Argiopinae (Fig. 8.1). Both clades have over 50 species, are distributed worldwide, and have been extensively studied (e.g., reviews by Kuntner et al. 2008, 2013; Cheng et al. 2010; Walter and Elgar 2012; Cheng and Kuntner 2014; Schneider et al. 2015). Their SSD is pronounced and varies within the clades (Cheng and Kuntner 2014; Kuntner and Elgar 2014), with most species showing eSSD . Recent research robustly resolved species-level phylogenies for these groups (Kuntner et al. 2013; Cheng and Kuntner 2014) and used them to reconstruct sex-specific trends in evolution of size (Cheng and Kuntner 2014; Kuntner and Elgar 2014). We here briefly recap these patterns by first bringing them to the same scale to make them fully comparable, then adding analyses that were reported in one but not both prior studies.
Representatives of the two clades (Nephilidae and Argiopinae) investigated for evolution of extreme female-biased sexual size dimorphism (eSSD). The left image shows a small orange Nephila pilipes male climbing on the body of a large female; right image shows a small male Argiope australis hanging in the web above a large female. Image copyright M. Kuntner
3 Macroevolutionary Patterns
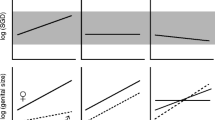
Figure 8.2 plots macroevolutionary patterns of sex-specific size changes and the evolution of eSSD in the two investigated clades with comparable ages, 40–50 Myr (Kuntner et al. 2013; Cheng and Kuntner 2014). The upper graphs show female and the middle ones show male body size changes reconstructed across the phylogenies of both lineages, with the x axis scaled to cladogenetic events (Cheng and Kuntner 2014; Kuntner and Elgar 2014). The bottom graphs show the reconstructed changes in eSSD, plotted as sexual size dimorphism index (SDI = [female body length/male body length] − 1) (Lovich and Gibbons 1992; Cheng and Kuntner 2014). In nephilids, both female (Linear regression, P = 0.003) and male sizes (Linear regression, P = 0.001) rise significantly through evolutionary time. However, the female slope rises more steeply than the male slope (Linear regression, P = 0.015; Kuntner and Elgar 2014), and these combined maintain eSSD as shown by the SDI slope in Fig. 8.2 that stagnates (Linear regression, P = 0.346). The nephilid pattern has been labeled as sexually dimorphic gigantism (Kuntner and Elgar 2014), meaning that both sexes increase in size, but the females more so than the males. On the other hand, Fig. 8.2 shows that the argiopine sex-specific sizes show no trends across cladogenetic events (Cheng and Kuntner 2014): Linear regressions, P female size = 0.511; P male size = 0.280. Therefore, size evolution in argiopines is nondirectional (Cheng and Kuntner 2014). However, the new pattern reported here is that SDI in this clade decreases through evolutionary time (Linear regression, P = 0.019).
Macroevolutionary patterns of sex-specific size changes and the evolution of eSSD in the two investigated clades. SDI = sexual dimorphism index. Time on X corresponds to cladogenetic events from the phylogenies. The original analyses (Cheng and Kuntner 2014; Kuntner and Elgar 2014) were here brought to the same scale to be comparable. Asterisks mark significance
4 Correlation Between Male and Female Size Evolution
Phylogenetically independent contrasts (PIC) analyses of sex-specific size data also establish strikingly contrasting patterns in the two clades. As reported in prior studies on nephilids (Kuntner and Coddington 2009; Kuntner and Elgar 2014), female and male sizes in this clade show no phylogenetic correlation (r 2 = 0.05, t = 1.24, F 1,27 = 1.5, 2-tailed P = 0.23). This independence between male and female size evolution implies a broken genetic linkage between the sexes and suggests that the sexes can independently respond to different selection regimes (see below). In contrast, argiopines show correlated phylogenetic changes in male and female sizes (r 2 = 0.36, t = 4.90, F 1,38 = 24.00, 2-tailed P = 0.008) (Cheng and Kuntner 2014). In argiopines, male size is linked to female size and vice versa so that selection on the size of any sex results in size changes in the other (Cheng and Kuntner 2015).
5 Detecting Evolutionary Signal
Our prior work on argiopines performed model fitting to sex-specific size data and showed no evolutionary trends for either sex and also no evolutionary trend for SSD (Cheng and Kuntner 2014). Although we would expect models that reveal selection for size (either optimum size or directional size), those analyses could not reject the Brownian motion model for size and SSD (Table 8.1). Here, we also performed model fitting to the nephilid size and SSD data following the methodology from the argiopine study (Cheng and Kuntner 2014). We tested the fit of Brownian motion (BM), Brownian motion with a directional trend (Trend), and the single-optimum Ornstein–Uhlenbeck (OU) on the nephilid tree (Kuntner et al. 2013; Kuntner and Elgar 2014) pruned for those taxa for which we had no data for one sex. We first estimated the log-likelihood value of each model, and then selected the best-fit model using the likelihood ratio test. For model fitting methodology and model assumptions, see Cheng and Kuntner (2014) and the literature cited there.
The results cannot reject the BM model for nephilid female body size changes, while the OU model best explains nephilid male size changes (Table 8.1). This suggests that nephilid male evolution is directed toward a body size optimum . Although our analyses cannot unequivocally establish this optimum, we find it logical that it should be on the trend line of the graph in Fig. 8.2, i.e., between 3.5 and 5.7 mm. While for SDI (and thus eSSD ) we cannot reject BM, the OU model shows only a marginally nonsignificant result (Table 8.1).
6 Allometric Patterns
Allometric analyses of male (on y axis) and female sizes (on x axis) provide a different aspect of understanding SSD evolution (Fairbairn 1997). An isometric slope would imply that size changes of males and females are comparable, and that SSD is maintained. In organisms with a male-biased SSD, a positive allometric slope (β > 1) is usually detected, meaning that SSD increases as male body size increases (Rensch 1950). While this pattern, known as Rensch’s rule , is common in birds and mammals, its opposite is predicted to hold in animals with a female-biased SSD, including spiders (Abouheif and Fairbairn 1997; Fairbairn 1997). The converse of Rensch’s rule predicts a negative allometric slope (β < 1) meaning that SSD increases as female body size increases (Abouheif and Fairbairn 1997; Foellmer and Moya-Laraño 2007; Cheng and Kuntner 2014). Note that by convention allometric analyses of sex-specific sizes plot male size on y and female size on x axes (Fairbairn 1997).
In spiders, the literature therefore expects to find the converse pattern of Rensch’s rule (Abouheif and Fairbairn 1997). However, against these predictions, a comparative study at higher phylogenetic levels in spiders failed to find any departure from isometry (Foellmer and Moya-Laraño 2007). Likewise, our study established that in argiopines size patterns do not depart from isometry (Cheng and Kuntner 2014). These same patterns were detected when analyzing tip data and phylogenetically controlled (PIC) data shown in Fig. 8.3. Here, we reanalyzed the nephilid size data in a comparable way using major axis regression following Cheng and Kuntner (2014). When analyzing raw size data from the tips of the phylogeny, we detected a negative allometry resembling the converse pattern of Rensch’s rule (MA slope = 0.365 (0.147–0.619), P = 0.009). However, when analyzing PIC data, we detected no relationship between male and female size (Fig. 8.3; MA slope = 0.775 (−0.384 to −8.695), P = 0.139). Although this result defies expected allometric patterns from the literature, it is consistent with the above reported lack of any correlation between female and male size evolution in nephilids.
The results from allometric analyses of size data using major axis regression on phylogenetically independent contrast data. The nephilid analysis is new and follows the methodology described in the argiopine study (Cheng and Kuntner 2014). Red lines are allometric slopes; gray lines are 95 % confidence intervals of the slope; and dashed lines indicate a slope equal to one. The nephilid allometric slope does not significantly depart from zero, implying that male and female sizes evolve independently. The allometric slope for argiopines, on the other hand, is not significantly different from one, implying isometric evolution
Our results in the two clades, combined with those from Foellmer and Moya-Laraño (2007), provide no comparative evidence for Rensch’s rule or its converse in spiders.
7 Evolutionary Pressures
The correlated isometric evolution in size between the sexes in argiopines in contrast to nephilids suggests that the species from each clade either respond differently to the same sex-specific selection pressures, or that these are different in each clade. In argiopines, any sex-specific tendency in size changes must also affect the changes in the opposite sex. In nephilids, on the other hand, one sex is free to directly respond to selection pressures for size changes without affecting the opposing sex. This key difference has direct implications for establishing which selection pressures drive size changes in each sex and consequently eSSD .
Female gigantism in spiders is usually attributed to selection for increased female fecundity (i.e., fecundity selection ; Head 1995; Kuntner and Elgar 2014). However, our model fitting results detect random fluctuations in female size rather than a trend toward large size as would be expected under the fecundity model. Nevertheless, female size is on the rise in macroevolutionary time in nephilids and this contributes to the eSSD observed in that clade (Fig. 8.2). This is not the case in argiopines, where eSSD has been suggested to be phylogenetically undetectable, perhaps a consequence of ecological factors operating at the population level (Cheng and Kuntner 2014). Female fecundity has also been questioned as the driver of sexual shape dimorphism in argiopines (Cheng and Kuntner 2015).
Several hypotheses explain the selection pressures that prevent male sizes from following those of females. In size dimorphic spiders, small male sizes have been proposed to be advantageous in mate searching, either to reduce male mortality during risky search for sedentary females (Vollrath and Parker 1992; Walker and Rypstra 2003; Kasumovic et al. 2007), or because maturation at small sizes may give males an advantage in scramble competition for virgin females (Danielson-François et al. 2012; Neumann and Schneider 2015). Small male size may also be advantageous during episodes of sexual conflict , perhaps because smaller males more easily evade sexual cannibalism (Elgar 1991). Gravity selection , likewise, may operate strongly in those species that need to climb and selects for males of small or optimal size (Moya-Laraño et al. 2002, 2009; Corcobado et al. 2010; but see Brandt and Andrade 2007). On the other hand, male–male contests favor larger male size and across the nephilid taxa, this may be the most pervasive selection trend (Kuntner and Elgar 2014).
A combination of these evolutionary pressures on males may operate in nephilid and argiopine spiders. We hypothesize that selection for male size in argiopines affects the female size as well, as the comparative results suggest that they coevolve. Thus, male–male competition that selects for larger males pushes size in both sexes into the same direction as fecundity that selects for larger females. In contrast, mate searching-related mechanisms work in the opposite direction, again affecting both sexes. In argiopines, the net result is an evolutionary decrease in eSSD . In nephilids, on the other hand, selection for small male size may not affect the females at all. Here, we hypothesize that nephilid females freely respond to selection for large size whereas males experience trade-offs that result in selection for optimal size. Such sexually decoupled size evolution is responsible for maintenance of eSSD in this clade.
8 Outlook
To establish pervasive patterns in spider size evolution, studies on other comparable lineages are essential. This section briefly points toward promising candidate clades that (i) show comparable levels in eSSD to nephilids and argiopines; (ii) and whose eSSD is either convergent to the one in nephilids and argiopines or alternatively shows a homologous origin to one of these clades, but with subsequent modification (Hormiga et al. 2000; Kuntner et al. 2015).
Among araneid candidates that exhibit nonhomologous origin of eSSD to nephilids and argiopines are bark spiders (genus Caerostris), and the groups Gasteracanthinae (Gasteracantha, Micrathena, and other genera) and Mastophorinae; among theridiids are widows (genus Latrodectus), and the genera Tidarren and Echinotheridion; a lone size dimorphic tetragnathid clade is the genus Opadometa. There are further cases of eSSD in crab spiders (Thomisidae) and of more moderate SSD in raft spiders (genus Dolomedes, Pisauridae). Studies repeatedly suggest that levels of SSD in these lineages may relate to unusual traits in their sexual biology, physiology, and web ecology (Michalik et al. 2005, 2010; Agnarsson et al. 2010; Kuntner and Agnarsson 2010; Gregorič et al. 2011a, b; Schwartz et al. 2013; Kuntner et al. 2015; Kralj-Fišer et al. 2016).
In addition, we find the following two araneid clades particularly important as they seem to share a phylogenetically deep origin of SSD with Argiope (Cheng and Kuntner 2014): tent spiders—genus Cyrtophora whose SSD varies from moderate to extreme, and scorpion-tailed spiders—genus Arachnura that may represent the most extreme cases of SSD in spiders.
Animal lineages with extremely female size-biased species are rather exceptional, but we argue that these phylogenetic outliers are important. However, in addition to studying the highly eSSD clades such as those proposed above, comparative research should also involve their monomorphic relatives, so phylogenetic insight is essential.
We also call for detailed genetic studies of eSSD in spiders. If the same sets of genes determine size in both sexes, then it is logical to predict more or less equal sizes in males and females. The known eSSD cases thus require a genetic explanation, but such work has not been done. It would be revealing to begin to understand the genetic differences between a lineage with a demonstrated correlated size evolution (e.g., argiopines) and another with a broken correlational patterns (e.g., nephilids).
In extremely sexually size dimorphic clades, one would expect to detect sex-specific size optima due to a combination of natural and sexual selection pressures. Fecundity selection is expected to strongly favor large female size in egg-laying animals, while pressures that relate to mate searching are predicted to maintain small male sizes (Blanckenhorn 2005). The combined fecundity and gravity hypotheses predict a trend in increased female size and an optimal (smallish) size to be maintained in males. This pattern is here found in nephilids, but not in argiopines. Experimental research should investigate the validity of the optimal nephilid male size, here hypothesized between 3.5 and 5.7 mm. Does it relate to gravity, sexual conflict , mate searching, differential mortality, other causes, or a combination of these?
9 Summary
Although nephilids and argiopines exhibit comparable levels of eSSD (Fig. 8.1), we demonstrate clear differences in evolutionary patterns that result in eSSD and maintain it. In nephilids, the correlation in sex-specific size changes is broken while this correlation is maintained in argiopines. In nephilids, the size in both sexes significantly increases in evolutionary time, but females grow faster and this difference maintains eSSD. In contrast, argiopines exhibit no direction of size change in either sex, and eSSD slowly declines. Model fitting analyses reveal that in nephilids, female size and eSSD do not depart from Brownian motion, but male size tends toward an optimum. In contrast, no directional trends can be detected in argiopines where Brownian motion best fits the data. Finally, phylogenetic allometric analyses reveal no relationships between male and female sizes in nephilids, but in contrast, argiopine size evolution is isometric. The sole similarity between the clades seems to be falsification of both Rensch’s rule and its converse.
References
Abouheif E, Fairbairn DJ (1997) A comparative analysis of allometry for sexual size dimorphism: assessing Rensch’s rule. Am Nat 149:540–562. doi:10.1086/286004
Agnarsson I, Kuntner M, Blackledge TA (2010) Bioprospecting finds the toughest biological material: extraordinary silk from a giant riverine orb spider. PLoS ONE 5:e11234. doi: 10.1371/journal.pone.0011234
Blanckenhorn W (2005) Behavioral causes and consequences of sexual size dimorphism. Ethology 111:977–1016. doi:10.1111/j.1439-0310.2005.01147.x
Blanckenhorn WU, Stillwell RC, Young KA, Fox CW, Ashton KG (2011) When Rensch meets Bergmann: does sexual size dimorphism change systematically with latitude? Evolution 60:2004–2011. doi:10.1111/j.0014-3820.2006.tb01838.x
Brandt Y, Andrade MCB (2007) Testing the gravity hypothesis of sexual size dimorphism: are small males faster climbers? Funct Ecol 21:379–385. doi:10.1111/j.1365-2435.2007.01243.x
Cheng RC, Kuntner M (2014) Phylogeny suggests non-directional and isometric evolution of sexual size dimorphism in argiopine spiders. Evolution 68:1–31. doi:10.1111/evo.12504
Cheng RC, Kuntner M (2015) Disentangling the size and shape components of sexual dimorphism. Evol Biol 42:223–234. doi:10.1007/s11692-015-9313-z
Cheng RC, Yang EC, Lin CP, Herberstein ME, Tso IM (2010) Insect form vision as one potential shaping force of spider web decoration design. J Exp Biol 213:759–768. doi:10.1242/jeb.037291
Coddington JA, Hormiga G, Scharff N (1997) Giant female or dwarf male spiders? Nature 385:687–688
Corcobado G, Rodríguez-Gironés MA, De Mas E, Moya-Laraño J (2010) Introducing the refined gravity hypothesis of extreme sexual size dimorphism. BMC Evol Biol 10:236. doi:10.1186/1471-2148-10-236
Cox RM, Calsbeek R (2009) Sex-specific selection and intraspecific variation in sexual size dimorphism. Evolution 64:798–809. doi:10.1111/j.1558-5646.2009.00851.x
Danielson-François A, Hou C, Cole N, Tso IM (2012) Scramble competition for moulting females as a driving force for extreme male dwarfism in spiders. Anim Behav 84:937–945. doi:10.1016/j.anbehav.2012.07.018
Darwin C (1871) The descent of man, and selection in relation to sex. John Murray, London
Elgar MA (1991) Sexual cannibalism, size dimorphism, and courtship in orb-weaving spiders (Araneidae). Evolution 45:444–448
Fairbairn DJ (1997) Allometry for sexual size dimorphism: patterns and process in the coevolution of body size in males and females. Annu Rev Ecol Syst 28:659–687. doi:10.1146/annurev.ecolsys.28.1.659
Fairbairn DJ (2005) Allometry for sexual size dimorphism: testing two hypotheses for Rensch’s rule in the water strider Aquarius remigis. Am Nat 166(Suppl):S69–S84. doi:10.1086/444600
Fairbairn DJ, Blanckenhorn WU, Székely T (2007) Sex, size, and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford
Foellmer MW, Moya-Laraño J (2007) Sexual size dimorphism in spiders: patterns and processes. In: Fairbairn DJ, Blanckenhorn WU, Székely TS (eds) Sex, size, and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford, pp 71–82
Gregorič M, Agnarsson I, Blackledge TA, Kuntner M (2011a) How did the spider cross the river? Behavioral adaptations for river-bridging webs in Caerostris darwini (Araneae: Araneidae). PLoS ONE 6:e26847. doi:10.1371/journal.pone.0026847
Gregorič M, Agnarsson I, Blackledge TA, Kuntner M (2011b) Darwin’s bark spider: giant prey in giant orb webs (Caerostris darwini, Araneae: Araneidae)? J Arachnol 39:287–295. doi:10.1636/CB10-95.1
Head G (1995) Selection on fecundity and variation in the degree of sexual size dimorphism among spider species (Class Araneae). Evolution 49:776–781. doi:10.2307/2410330
Higgins L (2002) Female gigantism in a New Guinea population of the spider Nephila maculata. Oikos 99:377–385. doi:10.1034/j.1600-0706.2002.990220.x
Higgins L, Coddington J, Goodnight C, Kuntner M (2011) Testing ecological and developmental hypotheses of mean and variation in adult size in nephilid orb-weaving spiders. Evol Ecol 25:1289–1306. doi:10.1007/s10682-011-9475-9
Hormiga G, Scharff N, Coddington JA (2000) The phylogenetic basis of sexual size dimorphism in orb-weaving spiders (Araneae, Orbiculariae). Syst Biol 49:435–62. doi:10.1080/10635159950127330
Isaac JL (2005) Potential causes and life-history consequences of sexual size dimorphism in mammals. Mamm Rev 35:101–115
Kasumovic MM, Bruce MJ, Herberstein ME, Andrade MCB (2007) Risky mate search and mate preference in the golden orb-web spider (Nephila plumipes). Behav Ecol 18:189–195. doi:10.1093/beheco/arl072
Kralj-Fišer S, Čandek K, Lokovšek T, Čelik T, Cheng RC, Elgar MA, Kuntner M (2016) Mate choice and sexual size dimorphism, not personality, explain female aggression and sexual cannibalism in raft spiders. Anim Behav 111:49–55. doi:10.1016/j.anbehav.2015.10.013
Kuntner M, Agnarsson I (2010) Web gigantism in Darwin’s bark spider, a new species from Madagascar (Araneidae: Caerostris). J Arachnol 38:346–356. doi:10.1636/B09-113.1
Kuntner M, Coddington JA (2009) Discovery of the largest orbweaving spider species: the evolution of gigantism in Nephila. PLoS ONE 4:2–6. doi:10.1371/journal.pone.0007516
Kuntner M, Elgar MA (2014) Evolution and maintenance of sexual size dimorphism: aligning phylogenetic and experimental evidence. Front Ecol Evol 2:1–8. doi:10.3389/fevo.2014.00026
Kuntner M, Coddington JA, Hormiga G (2008) Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): testing morphological and ethological homologies. Cladistics 24:147–217
Kuntner M, Zhang S, Gregorič M, Li D (2012) Nephila female gigantism attained through post-maturity molting. J Arachnol 40:345–347. doi:10.1636/B12-03.1
Kuntner M, Arnedo MA, Trontelj P, Lokovšek T, Agnarsson I (2013) A molecular phylogeny of nephilid spiders: Evolutionary history of a model lineage. Mol Phylogenet Evol 69:961–979. doi:10.1016/j.ympev.2013.06.008
Kuntner M, Agnarsson I, Li D (2015) The eunuch phenomenon: adaptive evolution of genital emasculation in sexually dimorphic spiders. Biol Rev 90:279–296. doi:10.1111/brv.12109
Lovich JE, Gibbons JW (1992) A review of techniques for quantifying sexual size dimorphism. Growth Dev Aging 56:269–281
Michalik P, Knoflach B, Thaler K, Alberti G (2005) The spermatozoa of the one-palped spider Tidarren argo (Araneae, Theridiidae). J Arachnol 33:562–568. doi:10.1636/04-65.1
Michalik P, Knoflach B, Thaler K, Alberti G (2010) Live for the moment: adaptations in the male genital system of a sexually cannibalistic spider (Theridiidae, Araneae). Tissue Cell 42:32–36. doi:10.1016/j.tice.2009.06.004
Moya-Laraño J, Halaj J, Wise DH (2002) Climbing to reach females: Romeo should be small. Evolution 56:420–425. doi:10.1111/j.0014-3820.2002.tb01351.x
Moya-Laraño J, Vinković D, Allard CM, Foellmer MW (2009) Optimal climbing speed explains the evolution of extreme sexual size dimorphism in spiders. J Evol Biol 22:954–63. doi:10.1111/j.1420-9101.2009.01707.x
Neumann R, Schneider JM (2015) Differential investment and size-related mating strategies facilitate extreme size variation in contesting male spiders. Anim Behav 101:107–115. doi:10.1016/j.anbehav.2014.12.027
Norman MD, Paul D, Finn J, Tregenza T (2002) First encounter with a live male blanket octopus: The world’s most sexually size-dimorphic large animal. New Zeal J Mar Freshw Res 36:733–736. doi:10.1080/00288330.2002.9517126
Rensch B (1950) Die Abhängigkeit der relativen Sexualdifferenz von der Körpergrösse. Bonner Zool Beiträge 1:58–69
Schneider J, Uhl G, Herberstein ME (2015) Cryptic female choice within the genus Argiope: a comparative approach. In: Cryptic Female Choice in Arthropods. Springer, Berlin, pp 55–77
Schwartz SK, Wagner WE, Hebets EA (2013) Spontaneous male death and monogyny in the dark fishing spider. Biol Lett 9:20130113. doi:10.1098/rsbl.2013.0113
Teder T (2014) Sexual size dimorphism requires a corresponding sex difference in development time: a meta-analysis in insects. Funct Ecol 28:479–486. doi:10.1111/1365-2435.12172
Teder T, Tammaru T (2005) Sexual size dimorphism within species increases with body size in insects. Oikos 108:321–334. doi:10.1111/j.0030-1299.2005.13609.x
Trivers RL (1972) Parental investment and sexual selection. Sex Sel Descent Man, 1871–1971 136–179. doi:10004055386
Vollrath F, Parker GA (1992) Sexual dimorphism and distorted sex ratios in spiders. Nature 355:156–159. doi:10.1038/355242a0
Walker SE, Rypstra AL (2003) Sexual dimorphism and the differential mortality model: is behaviour related to survival? Biol J Linn Soc 78:97–103. doi:10.1046/j.1095-8312.2003.00134.x
Walter A, Elgar MA (2012) The evolution of novel animal signals: silk decorations as a model system. Biol Rev 87:686–700. doi:10.1111/j.1469-185X.2012.00219.x
Webb TJ, Freckleton RP (2007) Only half right: species with female-biased sexual size dimorphism consistently break Rensch’s rule. PLoS ONE. doi:10.1371/journal.pone.0000897
Acknowledgments
We thank Pierre Pontarotti for inviting us to contribute to this volume, an anonymous reviewer for helpful suggestions on how to improve our presentation, as well as Ingi Agnarsson, Matjaž Gregorič, Simona Kralj-Fišer, Klemen Čandek, Shakira Quiñones-Lebrón, and Javad Malekhosseini for valuable feedback on early drafts.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kuntner, M., Cheng, RC. (2016). Evolutionary Pathways Maintaining Extreme Female-Biased Sexual Size Dimorphism: Convergent Spider Cases Defy Common Patterns. In: Pontarotti, P. (eds) Evolutionary Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-41324-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-41324-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41323-5
Online ISBN: 978-3-319-41324-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)