Abstract
Hypoxic-ischemic (HI) injury is an important cause of death and disabilities. Despite all improvements in neonatal care, the number of children who suffer some kind of injury during birth has remained stable in the last decade. A great number of studies have shown alterations in neural cells and many animal models have been proposed in the last 5 decades. Robinson et al. (2005) proposed an HI model in which the uterine arteries are temporarily clamped on the 18th gestation day. The findings were quite similar to the ones observed in postmortem studies. The white matter is clearly damaged, and a great amount of astrogliosis takes place both in the gray and white matters. Motor changes were also found but no data regarding the cerebellum, an important structure related to motor performance, was presented. Using this model, we have shown an increased level of iNOS at P0 and microgliosis and astrogliosis at P9, and astrogliosis at P23 (up to 4 weeks from the insult). NO is important in migration, maturation, and synaptic plasticity, but in exacerbated levels it may also contribute to cellular and tissue damage. We have also evaluated oligodendroglia development in the cerebellum. At P9 in HI animals, we found a decrease in the number of PDGFRα+ cells and an apparent delay in myelination, suggesting a failure in oligodendroglial progenitors migration/maturation and/or in the myelination process. These results point to an injury in cerebellar development that might help to explain the motor problems in HI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
General Considerations
Hypoxic-ischemic (HI) brain injury is an important cause of death and disabilities around the world, both in developing and developed countries (Vannucci and Vannucci 2005; Volpe 2009). According to WHO, about one million deaths occur yearly due to birth issues (Lawn et al. 2005). Despite all efforts at neonatal care in recent decades, the number of children who suffer injury during birth has remained stable during the last decade (Nelson et al. 2003). After perinatal insults, infant brains suffer oligodendrocyte loss, hypomyelination, astrogliosis (Marín-Padilla 1997), and perturbed cortical development (Marín-Padilla 1999). The mechanisms underlying these pathological changes remain unclear.
Cerebral palsy (CP), a chronic debilitating disorder of impaired motor development, is strongly associated with perinatal brain injury (Kuban and Leviton 1994; Volpe 2001, 2003). Various perinatal brain insults have been associated with CP, including prematurity and chorioamnionitis (Perlman et al. 1996; Verma et al. 1997; Spinillo et al. 1998; Wu and Colford 2000; Terzidou and Bennett 2001). Although full term infants can develop CP, it occurs more frequently in premature infants (Cummins et al. 1993).
Because various insults at different gestational stages induce elevated levels of cytokines and disrupt brain development, it has been proposed that aberrant cytokine expression underlies perinatal brain injury (Adlinolfi 1993). The pathogenesis of perinatal brain insults is, however, likely to involve numerous pathways associated with cytokines and oxygen-free radical species (Haynes et al. 2003; Folkerth et al. 2004), and their relative contributions have yet to be defined.
Perinatal brain injury invariably involves the gray and white matters, with the balance between them depending on the stage of cerebral developmental and vessel maturation. In order to study HI insult and its mechanisms of damage, several animal models have been proposed. Each has focused on a particular developmental stage, trying to mimic one of the many types of brain injury that occurs in humans (Fig. 1).
Timeline of brain development at the cellular level and the temporal relationship of rat and mouse versus human brain development. Arrows point to ages that HI insult in rats/mouse in prenatal life (light gray), at birth (medium gray) and postnatal life (dark gray) are most often performed. Light gray boxes summarize the major effects observed in rodent HI models with the numbers indicating representative references: 1—Tashima et al. (2001); 2—Grojean et al. (2003); 3—Loeliger et al. (2003); 4—Dieni and Rees (2003); 5—Robinson et al. (2005); 6—Olivier et al. (2005)
The first model was proposed by Levine (1960) using adult rats with a permanent ligation of the carotid artery. This model was particularly useful to study stroke. Rice et al. (1981) adapted this model in postnatal day (P)7 rats with the carotid ligation either permanent or temporary, creating one of the most used models in HI field. This age was chosen because it is comparable to newborn humans regarding several parameters, including cell proliferation rate, cell migration, and establishment of layering patterns in the cortex. This model has been useful for understanding several mechanisms of HI injury. However, it excludes close interaction between mother and fetus.
To include the relationship between mother and fetus, Wigglesworth proposed a model of growth restriction in 1964, in which one uterine artery was permanently ligated on embryonic day (E)17, inducing ischemia and probably hypoxia, yet the purpose of the study was exactly to show ischemia. Pups from the ligated uterine horn exhibited growth restriction at birth, in both rats and pigs (Wigglesworth 1964; Minkowski et al. 1981; Morand et al. 1982; Chanez et al. 1993; Jensen et al. 1996; Sadiq et al. 1999).
In a growth restriction model, Olivier et al. (2005) found damage to white matter like that in humans who suffer perinatal hypoxia. The growth-restricted animals did not recover weight, even in adulthood. Moreover, there were diffuse white matter lesions, increased cell death, and macrophage invasion, indicating increased inflammation. At P7, they observed a loss of pre-oligodendrocytes and deficient myelination. Those characteristics resemble what is seen in preterm infants with birth complications.
Another group in 2005 presented an HI model in which all four uterine arteries were clamped for 15, 30, or 45 min on gestation day 18 (Robinson et al. 2005). The results were similar to those of the growth restriction model. Additionally, only 45 min of HI mimicked the neuropathology of what is seen in humans (Marín-Padilla 1997, 1999): white matter astrogliosis, oligodendrocyte death, axonal injury, and altered cortical cerebral layering. Robinson et al. 2005 also described increased proinflammatory cytokines both in amniotic fluid and frontal lobe of the fetuses 4 and 24 hours after the insult. Motor performance also declined, for locomotion diminished in the open field test and steps shortened in the stride length test in adult animals.
The authors pointed out that this walking pattern is characteristic of children who develop cerebral palsy and its spastic gait. This systemic rodent prenatal HI insult accurately models human perinatal brain injury in several important ways, including functional association of altered brain development with motor delay, and consequently provides novel insights into the pathogenesis of human perinatal brain insults. As the cerebellum has the major importance in motor learning, we wish to obtain information concerning the effects of HI using a rodent systemic prenatal model. After Robinson et al. 2005 found that 45 min is the time that mimics human pathology, we ligated the four uterine arteries for this period.
Nitric Oxide Synthase Levels and Distribution Were Impaired in a Prenatal Systemic HI Model
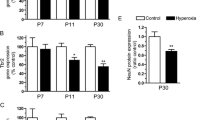
Enhancement of nitric oxide synthase (NOS) isoform expression has been reported in CNS areas after HI events (Kaur et al. 2006; Vexler and Yenari 2009). NO overproduction contributes to excitotoxicity, resulting in cell death and axonal damage (see Chapter “Glial Cells and Integrity of the Nervous System”). We measured the levels of neuronal (nNOS) and inducible (iNOS) isoforms at P0 (day of birth, i.e., 5 days after the HI insult). There was no difference in the level of nNOS protein in the cerebellum of HI animals compared to SHAM controls, as shown in Fig. 2a. However, the level of iNOS was significantly increased in HI animals (Fig. 2b).
Increase in iNOS following HI injury. Both nNOS and iNOS levels are shown in the rat cerebellum at birth (P0) in SHAM and HI group. Data are represented as means ± SEM in arbitrary units (AU), resulting from 3 independent experiments. a nNOS–SHAM = 14.9 ± 2.7; HI = 13.4 ± 2.7, p = 0.7036. No significant difference was observed between groups (p > 0.05). b iNOS–SHAM = 15.6 ± 4.5; HI = 52.6 ± 9.1, p = 0.0220. HI group presents a significant increase in iNOS levels (p < 0.05)
Glial cells have been suggested as the major source of this NO overproduction (Kashiwagi et al. 2003). NADPH-d histochemistry labels the NOS family (all three isoforms), the enzymes responsible for NO production. The number of NADPH-d+ cells is significantly increased in cerebellar white matter of young rats (Savignon et al. 2012). At P9 there were no differences in the number of NADPH-d+ cells in the cerebellar white matter comparing non-manipulated (NM), SHAM, and HI animals. However, at P23, the number of NADPH-d+ cells decreased in NM and SHAM animals, remaining significantly higher in HI animals (as discussed in Fig. 5 of Savignon et al. 2012).
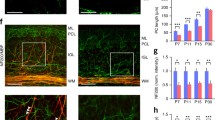
We identified NADPH-d+ cells in the white matter using specific markers for macrophage/microglia (ED1) or astrocytes (GFAP). At P9, both SHAM and HI animals presented NADPH-d+/ED1+ cells (Fig. 3a, b—arrows) and NADPH-d+/GFAP+ cells (Fig. 3c, d—arrows). In both groups, the morphology of NADPH-d+/ED1+ cells is typical of reactive microglia, i.e., small and rounded cells. At P23, both groups still presented NADPH-d+/ED1+ cells in the white matter (Fig. 3e, f—arrows), with the same morphology as in P9. However, at P23, HI animals still presented NADPH-d+/GFAP+ cells similar to reactive astrocytes (Fig. 3h—arrows), whereas SHAM animals did not present NADPH-d+/GFAP+ cells morphologically similar to reactive astrocytes, but instead showed typical GFAP+ astroglia (Fig. 3g—arrowheads), indicating that the insult has long-term effects on tissue (Savignon et al. 2012).
NOS activity remains associated with reactive astrocyte end feet at blood vessels in P23 HI cerebellum. Double labeling with NADPH-d histochemistry (dark-blue) and microglia or astroglia immunoidentification (brown) in the vermis region of the cerebellar white matter (0.5 mm mediolateral distance) during development. a–d P9; e–h P23. a, c, e and g (SHAM); b, d, f and h (HI); a–b and e–f double labeled with anti-ED1 antibody; c, d and g, h double labeled with anti-GFAP antibody. In both groups at P9, we can observe small, rounded NADPH-d+/ED1+ cells (a and b) or NADPH-d+/GFAP+ cells (c and d), as indicated by arrows. In d, observe a blood vessel, transversally cut, which presents NADPH-d staining, surrounded by GFAP+ astrocytic endfeet (asterisk). At P23, observe small rounded NADPH-d+/ED1+ cells in both groups, as indicated by arrows. HI animals display NADPH-d+/GFAP+ cells with typical reactive astrocyte morphology (arrows). SHAM animals do not present NADPH-d+/GFAP+ cells resembling reactive astrocytes. Arrowheads point to typical GFAP+ astrocytes, with no NADPH-d labeling. Notice the presence of NADPH-d+ blood vessels (asterisks in g and h) that are surrounded by GFAP+ astrocytic processes in HI animals (h) but not in SHAM animals (g). Calibration bar: 50 μm. Reproduced from Savignon et al. (2012)
These results, mainly those found at P9, were not a complete surprise since the surgery procedure and anesthesia may account for an inflammation component or other damage. It is worth noting that microglia/astrocytes preferentially express the iNOS isoform when reactive, as in cases of injury and inflammation, typifying what is called microgliosis and astrogliosis (see Chapter “Glial Cells and Integrity of the Nervous System”). Thus, we have shown that the cerebellar tissue presents an environment hostile to other cells such as oligodendrocyte progenitors. It has been shown in the last two decades that NO is important in migration, maturation, and synaptic plasticity of a variety of cerebellar cells. However, it is also a contributing factor to cellular and tissue damages if that production is greatly increased, as it occurs during inflammation.
Oligodendroglia Loss in the Cerebellum
Neurons, oligodendrocytes, and particularly their progenitors are most affected by HI (Back et al. 2002a, b). As mentioned in Chapter “Oligodendrocytes: Functioning in a Delicate Balance Between High Metabolic Requirements and Oxidative Damage”, oligodendroglia progenitors do not have a mature enzymatic system to deal with the substantial free radicals delivered in HI events, particularly by microglia (Thorburne and Juurlink 1996; Le Mellédo et al. 2004). NO produced by glia expressing iNOS (You and Kaur 2000; Park et al. 2002) may also be responsible for this vulnerability. It has been demonstrated that both neurons and oligodendrocytes release considerable glutamate to the extracellular compartment, and this together with increasing NO, may cause excitotoxicity and cell death (Back et al. 2007). Activated microglia express glutamate receptors (Gottlieb and Matute 1997) and may be modulated by the excess extracellular glutamate, producing more NO.
Oligodendrocytes are derived from various subpopulations of progenitors (see Chapter “Glial Cells and Integrity of the Nervous System” for further reading on oligodendrocyte development ). In the subventricular layer one arises to populate forebrain (cortex) and midbrain (thalamus and hypothalamus), while another in the ceiling of the fourth ventricle populates hindbrain (cerebellum, pons, and brain stem). There is some disagreement regarding the timing of these events. In the forebrain it is early and in the hippocampus and cerebellum it is quite late.
Reynolds and Wilkin (1988) showed the sequential changes in oligodendroglia during development, beginning as nondifferentiated cells in the superior medullary vellum (SMV) and the base of cerebellum, which then populate the whole organ. Others described the phenotypic and antigenic changes that oligodendroglia undergo during differentiation both in vitro as in vivo (Pfeiffer et al. 1993; Baumann and Pham-Dinh 2001).
Oligodendroglial progenitors express alpha-receptor to platelet-derived growth factor (PDGFRα) (Baumann and Pham-Dinh 2001). Data from our laboratory showed that in both HI and control animals the density of PDGFRα+ progenitors at P2 is about 20 cells/100 μm2 (unpublished data), escalating to about 50 cells per field at P9, and returning at P23 to the same levels as at P2.
At P2, there were no differences in the number of PDGFRα+ cells in cerebellar white matter in both groups (Fig. 4a, b). This was not a complete surprise, since rodent cerebellum develops rapidly postnatally. At P9, there was a significant increase in PDGFRα+ cells in both groups when compared to P2 (uANOVA; F = 126.34, p < 0.001). However, HI animals showed a significant lower number in this progenitor subpopulation compared to SHAM animals (Fig. 4c, d), indicating that a prenatal HI event somehow affected the proliferation rate and/or survival of oligodendroglial progenitors. At P23, in both groups a significant reduction in PDGFRα+ counting was observed in both groups (Fig. 4e, f). This was expected, since the rate of proliferation diminishes and the progenitors start to differentiate, downregulate PDGFRα, and form myelin. Figure 4g depicts the cell counting results for each group.
Number of PDGFRα+ cells in the cerebellar white matter of SHAM and HI animals at P2, P9 and P23. At P2, we have not observed differences in the number of PDGFRα+ cells in cerebellar white matter (a, b). At P9 (c, d) there is a significant increase in the number of PDGFRα+ cells in both groups when compared to P2 (uANOVA; F = 126.34, p < 0.001). However, HI animals have a lower number of PDGFRα+ cells than SHAM at P9 (d, but better shown in g). At P23, a significant reduction in PDGFRα+ counting was observed in both groups (e, f) when compared to P9. g Depicts the cell counting for each group, with density measured as number per 100 μm2. Calibration bar: 50 μm
Myelin basic protein (MBP ), a marker of mature oligodendrocyte and myelin (see Chapters “Glial Cells and Integrity of the Nervous System” and “Oligodendrocytes: Functioning in a Delicate Balance Between High Metabolic Requirements and Oxidative Damage”), was also impaired in the prenatal HI systemic model. At P9 in SHAM animals, MBP+ fibers were observed close to the calbindin-positive Purkinje cell layer (arrows in Fig. 5a), whereas in HI animals those MBP + fibers were clearly located in the main white matter tracts (Fig. 5b), suggesting an apparent delay in myelination in the cerebellum. As development proceeds, oligodendrocytes/myelin were found in all extents of the granular layer in both groups. Yet, it appears that some failure occurred in the oligodendroglial progenitors migration/maturation and/or in the myelination process, since we found non-myelinated gaps in the granular layer (asterisks in Fig. 5d). This occurred only in HI animals. This pattern was maintained in HI animals until adulthood (Fig. 5f). Together, these results point to an injury in cerebellar oligodendroglia development that might help to explain the motor problems observed in HI animals.
Myelination is delayed in HI animals. Myelin basic protein (MBP ) is labeled in red and calbindin, in Purkinje cells, in green. At P9, there is an apparent delay in myelination in the cerebellum. In SHAM animals, some MBP+/Calbindin+ axons are close to Purkinje cell layer (arrows in a), while in HI animals those axons are not (b). From P23 (c, d) until adulthood (e, f), MBP+/Calbindin+ occupy all of the granular layer (arrows) in both SHAM and HI animals. Calibration bar: 100 μm
Concluding Remarks
Multiple types of injury resulting from preterm birth in humans, including systemic HI, converge to hinder brain cell survival, particularly for immature oligodendrocytes and cerebral neurons (Volpe 2009). Impaired brain cell survival and differentiation continue for a prolonged period after the initial injury in animal models (Robinson et al. 2005; Mazur et al. 2010). At the time of the HI insult and in the days following, when the levels of cytokine and other inflammatory modulators are still elevated (Robinson et al. 2005), several glial and neuronal progenitor populations are entering the cerebellum parenchyma through the prospective cerebellar white matter. These progenitors, especially of oligodendrocytes, are more vulnerable to HI events because they lack the enzymatic complexes capable of dealing with the great amount of free radicals produced during HI. NO forms free radicals if produced in large amounts and is toxic to oligodendrocyte progenitors. In addition, elevated NO may trigger N-methyl-d-aspartate (NMDA)-mediated intracellular Ca++-influx and CREB-mediated transcription of apoptotic proteins such as Bax, Bad, and Bcl-xl, causing neuronal death (Zubrow et al. 2002a, b; Mishra et al. 2006).
Our results showed that in this systemic model of prenatal HI, oligodendroglial differentiation in the cerebellum was impaired, with a reduction in the number of PDGFRα-cells (oligodendrocyte progenitors) and mature oligodendroglial cells, as demonstrated by reduced MBP immunostaning. This supports this model for use in devising new therapeutic strategies for HI insults.
Abbreviations
- CNS:
-
Central nervous system
- CP:
-
Cerebral palsy
- CREB:
-
cAMP response element-binding protein
- ED1:
-
Antibody that labels macrophage/microglia
- GFAP:
-
Glial fibrillary acid protein
- HI:
-
Hypoxia ischemia
- MBP:
-
Myelin basic protein
- NADPH-d:
-
Nicotinamide adenine dinucleotide phosphate reduced diaphorase
- NADPH-d+:
-
Nicotinamide adenine dinucleotide phosphate reduced diaphorase positive
- NM:
-
Non-manipulated
- NMDA:
-
N-methyl-d-aspartate
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- nNOS:
-
Neuronal nitric oxide synthase
- iNOS:
-
Inducible nitric oxide synthase
- PDGFRα:
-
Platelet derived growth factor receptor alpha
- P0:
-
Postnatal day 0, here considered as the day of birth
- P2, 7, 9, 23:
-
Postnatal day 2, 7, 9, and 23
- SHAM:
-
Surgical control
- SMV:
-
Superior Medullary Vellum
- uANOVA:
-
Univariate analises of variance
- WHO:
-
World health organization
References
Adlinolfi M (1993) Infectious diseases in pregnancy, cytokines and neurological impairment: an hypothesis. Dev Med Child Neurol 35:549–553
Back SA, Han BH, Luo NL et al (2002a) Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci 22:455–463
Back SA, Luo NL, Borenstein NS et al (2002b) Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol 61:197–211
Back SA, Craig A, Kayton RJ et al (2007) Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab 27:334–347. doi:10.1038/sj.jcbfm.9600344
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Chanez C, Rabin O, Heroux M, Giguere JF (1993) Cerebral amino acid changes in an animal model of intrauterine growth retardation. Metab Brain Dis 8:61–72
Cummins SK, Nelson KB, Grether JK, Velie EM (1993) Cerebral palsy in four northern California counties, births 1983 through 1985. J Pediatr 123:230–237
Dieni S, Rees S (2003) Dendritic morphology is altered in hippocampal neurons following prenatal compromise. J Neurobiol 55:41–52. doi:10.1002/neu.10194
Folkerth RD, Haynes RL, Borenstein NS et al (2004) Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol 63:990–999
Gottlieb M, Matute C (1997) Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab 17:290–300. doi:10.1097/00004647-199703000-00006
Grojean S, Schroeder H, Pourié G et al (2003) Histopathological alterations and functional brain deficits after transient hypoxia in the newborn rat pup: a long term follow-up. Neurobiol Dis 14:265–278. doi:10.1016/S0969-9961(03)00082-2
Haynes V, Elfering SL, Squires RJ et al (2003) Mitochondrial nitric-oxide synthase: role in pathophysiology. IUBMB Life 55:599–603. doi:10.1080/15216540310001628681
Jensen A, Klönne HJ, Detmer A, Carter AM (1996) Catecholamine and serotonin concentrations in fetal guinea-pig brain: relation to regional cerebral blood flow and oxygen delivery in the growth-restricted fetus. Reprod Fertil Dev 8:355–364
Kashiwagi K, Iizuka Y, Mochizuki S et al (2003) Differences in nitric oxide production: a comparison of retinal ganglion cells and retinal glial cells cultured under hypoxic conditions. Brain Res Mol Brain Res 112:126–134
Kaur C, Sivakumar V, Foulds WS (2006) Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci 47:1126–1141. doi:10.1167/iovs.05-0518
Kuban KC, Leviton A (1994) Cerebral palsy. N Engl J Med 330:188–195. doi:10.1056/NEJM199401203300308
Lawn J, Shibuya K, Stein C (2005) No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ 83:409–417
Le Mellédo J-MM, Mahil N, Baker GB (2004) Nitric oxide: a key player in the relation between cardiovascular disease and major depressive disorder? J Psychiatry Neurosci 29:414–416
Levine S (1960) Anoxic-ischemic encephalopathy in rats. Am J Pathol 36:1–17
Loeliger M, Watson CS, Reynolds JD et al (2003) Extracellular glutamate levels and neuropathology in cerebral white matter following repeated umbilical cord occlusion in the near term fetal sheep. Neuroscience 116:705–714
Marín-Padilla M (1997) Developmental neuropathology and impact of perinatal brain damage. II: white matter lesions of the neocortex. J Neuropathol Exp Neurol 56:219–235
Marín-Padilla M (1999) Developmental neuropathology and impact of perinatal brain damage. III: gray matter lesions of the neocortex. J Neuropathol Exp Neurol 58:407–429
Mazur M, Miller RH, Robinson S (2010) Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury. J Neurosurg Pediatr 6:206–221. doi:10.3171/2010.5.PEDS1032
Minkowski A, Chanez C, Priam M et al (1981) Long lasting effects of intrauterine malnutrition on neurotransmitters metabolism in the brain of developing rats. Prog Clin Biol Res 77:643–660
Mishra O, Mishra R, Ashraf Q, Delivoria-Papadopoulos M (2006) Nitric oxide-mediated mechanism of neuronal nitric oxide synthase and inducible nitric oxide synthase expression during hypoxia in the cerebral cortex of newborn piglets. Neuroscience 140:857863. doi:10.1016/j.neuroscience.2006.02.060
Morand O, Chanez C, Masson M et al (1982) Alteration in fatty acid composition of neurons, astrocytes, oligodendrocytes, myelin and synaptosomes in intrauterine malnutrition in rat. Ann Nutr Metab 26:111–120
Nelson KB, Grether JK, Dambrosia JM et al (2003) Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res 53:600–607. doi:10.1203/01.PDR.0000056802.22454.AB
Olivier P, Baud O, Evrard P et al (2005) Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol 64:998–1006
Park SY, Lee H, Hur J et al (2002) Hypoxia induces nitric oxide production in mouse microglia via p38 mitogen-activated protein kinase pathway. Brain Res Mol Brain Res 107:9–16
Perlman JM, Risser R, Broyles RS (1996) Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics 97:822–827
Pfeiffer SE, Warrington AE, Bansal R (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol 3:191–197
Reynolds R, Wilkin GP (1988) Development of macroglial cells in rat cerebellum. II. An in situ immunohistochemical study of oligodendroglial lineage from precursor to mature myelinating cell. Development 102:409–425
Rice JE, Vannucci RC, Brierley JB (1981) The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9:131–141. doi:10.1002/ana.410090206
Robinson S, Petelenz K, Li Q et al (2005) Developmental changes induced by graded prenatal systemic hypoxic-ischemic insults in rats. Neurobiol Dis 18:568–581. doi:10.1016/j.nbd.2004.10.024
Sadiq HF, Das UG, Tracy TF, Devaskar SU (1999) Intra-uterine growth restriction differentially regulates perinatal brain and skeletal muscle glucose transporters. Brain Res 823:96–103
Savignon T, Costa E, Tenorio F et al (2012) Prenatal hypoxic-ischemic insult changes the distribution and number of NADPH-diaphorase cells in the cerebellum. PLoS ONE 7:e35786. doi:10.1371/journal.pone.0035786
Spinillo A, Capuzzo E, Stronati M et al (1998) Obstetric risk factors for periventricular leukomalacia among preterm infants. Br J Obstet Gynaecol 105:865–871
Tashima L, Nakata M, Anno K et al (2001) Prenatal influence of ischemia-hypoxia-induced intrauterine growth retardation on brain development and behavioral activity in rats. Biol Neonate 80:81–87
Terzidou V, Bennett P (2001) Maternal risk factors for fetal and neonatal brain damage. Biol Neonate 79:157–162
Thorburne SK, Juurlink BH (1996) Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 67:1014–1022
Vannucci RC, Vannucci SJ (2005) Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci 27:81–86. doi:10.1159/000085978
Verma U, Tejani N, Klein S et al (1997) Obstetric antecedents of intraventricular hemorrhage and periventricular leukomalacia in the low-birth-weight neonate. Am J Obstet Gynecol 176:275–281
Vexler ZS, Yenari MA (2009) Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci 31:378–393. doi:10.1159/000232556
Volpe JJ (2001) Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50:553–562. doi:10.1203/00006450-200111000-00003
Volpe JJ (2003) Cerebral white matter injury of the premature infant-more common than you think. Pediatrics 112:176–180
Volpe J (2009) Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol 24:10851104. doi:10.1177/0883073809338067
Wigglesworth (1964) Experimental growth retardation in the foetal rat. J Pathol Bacteriol 88:1–13
Wu YW, Colford JM (2000) Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA 284:1417–1424
You Y, Kaur C (2000) Expression of induced nitric oxide synthase in amoeboid microglia in postnatal rats following an exposure to hypoxia. Neurosci Lett 279:101–104
Zubrow AB, Delivoria-Papadopoulos M, Ashraf QM et al (2002a) Nitric oxide-mediated expression of Bax protein and DNA fragmentation during hypoxia in neuronal nuclei from newborn piglets. Brain Res 954:60–67
Zubrow AB, Delivoria-Papadopoulos M, Ashraf QM et al (2002b) Nitric oxide-mediated Ca2+/calmodulin-dependent protein kinase IV activity during hypoxia in neuronal nuclei from newborn piglets. Neurosci Lett 335:5–8
Acknowledgments
We would like to thank Jorge Pereira das Neves for technical assistance and Mariana Soares Magalhães for animal care.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Barradas, P.C. et al. (2016). Prenatal Systemic Hypoxia-Ischemia and Oligodendroglia Loss in Cerebellum. In: von Bernhardi, R. (eds) Glial Cells in Health and Disease of the CNS. Advances in Experimental Medicine and Biology, vol 949. Springer, Cham. https://doi.org/10.1007/978-3-319-40764-7_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-40764-7_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40762-3
Online ISBN: 978-3-319-40764-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)