Abstract
The Drosophila salivary glands (SGs) are historically well known for their polytene chromosomes and became a tissue of choice to study sequential gene activation by the steroid hormone ecdysone. The widely accepted and most well documented function of the Drosophila salivary gland is the production of a secretory glue released during pupariation to fix the freshly formed puparia to a substrate. Besides fulfilling this function, which is tightly associated with the enormous production and exocytosis of a small group of secretory glycoproteins (Sgs proteins), the same SGs display also massive apocrine secretion 8–10 h after puparium formation (APF). A detailed analysis of the apocrine activity provided compelling evidence that this is non-vesicular transport and secretory mechanism which substantially differs from canonical exocytosis taking place 14–16 h prior to apocrine release. From the point of view of Drosophila fast development, this is significant time gap between two different cellular activities. This system offers a unique opportunity to dissect the molecular mechanistic aspects of the apocrine transport and secretory machinery using specific genetic tools available in the fruitfly. Although these obviously different cellular activities serve two very different purposes, in both cases the SG behaves as a distinct and also typical exocrine organ capable of two independent and separated functions, one in the late larva, the second in the late prepupa. A comparison of the secretory material and its properties from the exocytotic Sgs proteins and the apocrine secretion reveals the unexpected capabilities of this organ in reprogramming its function for two deeply different roles.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The larval salivary glands (SGs) of the fruit fly Drosophila are a single layer of unbranched, tubular epithelial tissue of ectodermal origin. The SG is the largest secretory organ in Drosophila, and is composed of just two principal cell types: duct cells and secretory cells. During embryogenesis, the future larval salivary glands arise from a contiguous primordia on the ventral ectodermal surface of parasegment 2 (Skaer 1993; Andrew et al. 1994; Campos-Ortega and Hartenstein 1997; Henderson and Andrew 2000; Bradley et al. 2001; Myat 2005; Vining et al. 2005; Kerman et al. 2006). Once specified, salivary gland cells do not undergo further rounds of cell division or cell death, with each lobe having approximately 130–145 large polarized epithelial cells specialized for secretion (Poulson 1937; Makino 1938; Sonnenblick 1940, 1950; Skaer 1993; Campos-Ortega and Hartenstein 1997). The absence of mitotic activity after the formation of the lateroventral ectodermal placodes suggests that the cells participating in the formation of these plates, have already been determined to become salivary gland cells. Despite the absence of cell divisions, the glands continue to grow, initially during the embryonic stage and mainly during larval development due to an increase in cell volume. This is accompanied by chromosomal replication without subsequent separation of the homologues (endoreduplication), and, as a consequence, the chromosomes become multistranded (polytene). Within individuals the gland lobes usually have an asymmetric cell number. Although the most frequently cited average number of gland cells per lobe is 128 (Grob 1952; Schnitter 1961; Hadorn and Faulhaber 1962; Gloor 1962; Berendes and Ashburner 1978; Ashburner et al. 2005), our own replicate observations have shown that the number of secretory gland is distributed about a mode of 134 cells. Only the few cells located at the junction between the duct and the start of the gland’s cells start to divide during the second instar; these will form the ring of the prospective imaginal gland cells. The larval duct is composed of about 55 cuboidal epithelial cells that form simple tubes which connect the secretory cells to the larval mouth by a Y-shaped tubular conduit (Berendes and Ashburner 1978; Abrams et al. 2003). Despite fact that all of the gland’s secretory cells appear to be identical during nearly all of the larvae’s life, three structurally different secretory cell subtypes - corpuscular, transitional and columnar – can be recognized in the late 3rd larval instar (von Gaudecker 1972; Lane et al. 1972; Farkaš and Šuťáková 1998).
At about 4–6 h after the appearance of the ectodermal placodes the embryonic SGs display signs of secretory activity. The lumen of the gland contains a substance that readily absorbs hematoxylin and stains metachromatically with toluidine blue (Poulson 1950; Sonnenblick 1950). Myat and Andrew (2002) observed that this secretory activity of embryonic SGs is controlled by the crb and hkb genes. This embryonic secretion appears to be cyclic and there is good reason to believe that it provides for repeated excretion of luminal proteins that are required for the assembly of the extracellular matrix on the apical surface during tube expansion in manner similar to the tube expansion in the embryonic tracheal system (Tsarouhas et al. 2007; Jayaram et al. 2008; Wang et al. 2009; Armbruster and Luschnig 2012; Burgess et al. 2012).
In the first instar larva, the salivary glands are located on either side of the body, with both gland lobes usually confined to the first two thoracic segments just below the muscles of the body wall. The gland cells are uniform in size (Fig. 15.1). During the second instar the glands continue to grow until their lobes extend into to first abdominal segment. The cells remain uniform in size but their shape in cross section becomes more conical. At the beginning of the third instar there develops an anterior-posterior gradient in cell size, which is accompanied by a differential increase in nuclear volume, probably reflecting a differential increase in the level of polyteny of the chromosomes (Bodenstein 1950; Berendes 1965; Berendes and Ashburner 1978). During larval growth, SGs are thought to produce digestive enzymes released into the alimentary tract (Hsu 1948; Gregg et al. 1990); however, for unknown reasons there has been very little attention paid to their identity and characteristic. Thus, the only major and unambiguously documented function of the larval salivary glands is to produce a large amount of mucinous glue-containing secretory granules during the second half of the last instar that, when released during pupariation, serves to affix the freshly formed puparia to a substrate in an upright position (Fraenkel 1952; Fraenkel and Brookes 1953). In anticipation of this function, at about the middle of the third instar globular, highly refractile granules appear in the cytoplasm of the posterior gland cells (Ross 1939; Painter 1945; Bodenstein 1950; Berendes 1965). The cytoplasm which was strongly basophilic during the first and second instars, looses its basophilia when the granules appear (Lesher 1951a, b, 1952). These granules, the number of which gradually increases in a posterior to anterior direction, display a strong periodic acid-Schiff (PAS) positive reaction (Lesher 1952; Berendes 1965; Poels 1970; von Gaudecker and Schmale 1974; Kolesnikov and Zhimulev 1975). These glue secretory granules (Fig. 15.2) are produced mainly by posterior corpuscular cells , and to a lesser extent by transitional cells ; only the few most anteriorly positioned columnar cells do not normally produce any of the glue secretion (Lane et al. 1972; von Gaudecker 1972; Berendes and Ashburner 1978; Farkaš and Šuťáková 1999). However, even these most anterior columnar cells will produce such granules if the growth of the gland is allowed to continue e.g. by its transplantation into an adult host (Berendes and Holt 1965). Towards the end of the last larval instar, the steroid hormone ecdysone is released into circulation and induces a complex response that leads to the initiation of metamorphosis. In the salivary glands, this is accompanied by a series of polytene chromosome puffs that reflect a cascade of transcriptional regulation, and the secretion of the glue by exocytosis (Boyd and Ashburner 1977; Berendes and Ashburner 1978).
Laser confocal microscope image of paraformaldehyde-fixed SG. The 1st (a), 2nd (b), early 3rd (c) and late 3rd (d) instar larva showing the antibody-detected distribution of two cytosolic and cytoskeletal proteins: p127l(2)gl tumor suppressor (green) and non-muscle myosin II heavy chain (red) in (a), (b) and (d). In the early 3rd (c) instar the expression of two ecdysone-regulated proteins is shown: ecdysone receptor (green) and Broad-Complex (red). Blue indicates filamentous actin which concentrates predominantly on the apical membrane of the lumen . In corpuscular and transitional cells (d), the p127l(2)gl and myosin II proteins form a reticular network with numerous black vacuoles that correspond to secretory granules . The cytoplasm of the columnar cells show smooth and evenly distributed pattern for the proteins. Magnification of all confocal images 400×
(a) Light micrograph of a semithick section (0.5 μm) of a salivary gland. The organ was Araldite-embedded and glutaraldehyde-osmium fixed and a segment of adhering fat body (FB) from late 3rd instar larva, stained with toluidine blue-O in borax. The cytoplasm of corpuscular (C) and transitional (T) cells is filled with numerous (2500–3000 per cell) secretory granules of variable size (0.2–4 μm). The most anterior columnar cells (H – from German Halszellen) are devoid of any secretory vesicles . Magnification 250×. (b) Transmission electron micrograph of a glutaraldehyde-osmium fixed salivary gland from late 3rd instar larva at puff stage PS2 to PS4 showing numerous Golgi-derived electron-dense secretory granules (SG) filling the cytoplasm of corpuscular cells . L = indicates empty lumen of the gland. Magnification 2500×
1.1 Drosophila Salivary Glands as a Model Tissue
In the history of genetics, the Drosophila salivary glands are famous for their polytene chromosomes (Painter 1933). Their analyses has led to many conceptual advances, including establishing the first highly detailed cytogenetical maps (Bridges 1935, 1938, 1942; Bridges and Bridges 1939; Lindsley and Grell 1968; Lefevre 1976; Sorsa 1989; Lindsley and Zimm 1992), the elaboration of the elegant chromomere theory (Painter 1934; Pelling 1966; Beermann 1972), and correlating specific reversible changes in chromosomal structure (puffs) with the transcriptional activity of genes (Beermann 1952; Ashburner 1970; Pelling 1970).
Furthermore, studies using the Drosophila salivary glands have been at the forefront of research on the genetic and physiological responses to heat shock and stress (Ritossa 1962, 1963; Ashburner and Bonner 1979; Pardue et al. 1989) and glue gene regulation (Korge 1975; Giangrande et al. 1987, 1989; Lehmann 1996; Biyasheva et al. 2001). Detailed studies of how puffing patterns change during sequential gene activation in polytene chromosomes (Becker 1959; Ashburner 1972; Ashburner et al. 1974; Ashburner and Berendes 1978) and the molecular characterization of the puff-forming genes established a paradigm for understanding the mechanisms underlying temporal and tissue-specific transcriptional control (Burtis et al. 1990; Segraves and Hogness 1990; Thummel 1996, 2002). Because the larval salivary glands become obsolete early in the hormonally triggered metamorphosis of the larvae to the adult, they are also widely used as a model system to study hormone-regulated programmed cell death (PCD) .
With regard to PCD, the Drosophila salivary glands undergo close-to-synchronous histolysis of the entire organ about 16 h after pupariation (APF) in response to an endogenous pulse of ecdysone occurring about 6 h earlier (Jiang et al. 1997, 2000; Farkaš and Šuťáková 1998; Farkaš and Mechler 2000; Baehrecke 2003). Only a few of the cellular events that occur in the prepupal salivary glands prior to PCD have been elucidated. Jochova et al. (1997) and later Martin and Baehrecke (2004) described changes in the clumping and redistribution of actin and tubulin cytoskeleton in the prepupal salivary glands, emphasizing that the larval and various prepupal stages show differences due to the exocytosis of glue granules in the late third instar larva. However, more comprehensive laser confocal microscopy clearly revealed that there are multiple, dynamic changes in the salivary glands during the prepupal period, including changes in the distribution of vacuoles, the arrangement of non-actin and non-tubulin cytoskeletons, and the occurrence of noncanonical protein extrusion (Farkaš and Mechler 2000).
Recently, two novel and unexpected processes were described in the prepupal salivary glands . Immediately after pupariation up to about 6/7 h APF, the Drosophila salivary glands undergo a very intense vacuolation that is associated with complex endosomal trafficking . This is followed by the recently discovered apocrine secretion about 8–10 h APF (Farkaš et al. 2014, 2015). To understand the function of this intense vacuolization and the apocrine secretion that follows it, and to gain additional insight into the overall metabolism of this tissue, SG respiration was measured during precisely staged late larval and prepupal and very early pupal animals. This revealed that changes to the animal’s basal metabolism are correlated with feeding, postfeeding larval activity, the period of pupariation, and increased anabolic demands during the massive endosomal recycling in the early-to-mid prepupal period. There is a slow and gradual decline in respiration as the animal approaches pupation. Salivary glands stop their respiratory activity abruptly by completing histolysis 16 h after pupariation (Farkaš and Sláma 2015).
The Drosophila salivary glands provide a superb model to consider issues relevant to the main topics of this volume. This chapter will be devoted to two major and now clearly documented excretory functions of Drosophila larval SGs: (i) the production and release of a small and unique group of so-called glue proteins (Sgs-proteins), which accumulate in numerous vesicles at the end of the larval stage and are secreted by classical exocytosis , and (ii) the remarkable and massive apocrine secretion of almost all types of cellular proteins in late prepupae . In both cases, the secretory products of SG cells are delivered to the external environment. However, the secretory products are released by two mutually exclusive mechanisms, and serve functional roles that are also quite different in principle.
2 Larval Exocytotic Activity of Drosophila Salivary Glands
2.1 Production, Composition and Secretion of Sgs Proteins
As indicated above by about the middle of the third larval instar globular and highly refractile granules appear in the cytoplasm of the gland’s posteriormost cells. These granules constitute the components of the salivary glue secretion (Sgs). The Sgs represents a highly special and unique extracellular composite glue matrix that has not been identified so far outside of Cyclorrhaphous Dipterans; the majority of information on the composition of the Sgs that we have today comes from studies of it in Drosophila melanogaster.
As early as in (1948) Kodani found that the glue can be conveniently isolated after its secretion into the lumen by first fixing the glands in ethanol or an acetic acid-ethanol mixture, and then dissecting and removing the gland cells from the solid plug of precipitated glue. It was anticipated that the Sgs secretion would consist of mucinous glycoproteins (Korge 1975, 1977a; Beckendorf and Kafatos 1976). These electrophoretically separate into six to eight bands. Besides the PAS-positive histochemical reaction seen in the granules, the presence of glycomoieties was deduced from noticing that radioactively labelled 14C-glucose was incorporated into some of these bands (Beckendorf and Kafatos 1976; Korge 1977b; Kress 1979; Engoher and Kress 1980). The genes corresponding to these proteins have been named Sgs-1 to Sgs-8 according to the mobility of the proteins (salivary gland secretion genes 1–8; Korge 1975, 1981). Interestingly, the electrophoretic mobility of the secreted proteins varies between different strains of D. melanogaster. This variation is not only due to differences in glycosylation but also to allelic variation, that was used to genetically map the genes (Korge 1975). Moreover, the activity of a small group of interecdysial chromosomal puffs at the time of Sgs synthesis independently provided a guide for linking these proteins to their corresponding genetic loci (Korge 1977a, b; Ashburner and Berendes 1978; Velissariou and Ashburner 1980, 1981). These studies were shortly followed by the cloning of genes associated with each set of puffs : Cytological band 68C encodes the Sgs-3, Sgs-7 and Sgs-8 genes (Meyerowitz and Hogness 1982; Crowley et al. 1983, 1984; Crowley and Meyerowitz 1984; Crosby and Meyerowitz 1986), 3C encodes Sgs-4 (Muskavitch and Hogness 1980, 1982; Chen et al. 1987), 95B encodes Sgs-5 (Guild 1984; Guild and Shore 1984), and 25B2-3 encodes Sgs-1 (Roth et al. 1999). The origin of Sgs-2 and Sgs-6 remains unclear. Some authors claim that Sgs-2 and Sgs-6 fractions might be a cell-debris contamination associated with the secretion isolated from salivary gland lumens (Lindsley and Zimm 1992); there are no additional Sgs-related genes in the Drosophila genome (Adams et al. 2000, 2003). Sequence analysis has revealed unique features of Sgs-encoded proteins not previously found among known proteins in databases. These features have been ascribed to their secretory and glue-forming nature. Although strong glycosylation was expected in most of the Sgs proteins even before their amino-acid sequence was known, only Sgs-3 initially showed motifs that conclusively supported the contention that it is heavily glycosylated (Garfinkel et al. 1983). Later, detailed sequence analysis of Sgs-4 and Sgs-1 supported the view that they too are glycosylated (Furia et al. 1992; Roth et al. 1999). At the time of maximum synthesis, these Sgs proteins comprise for 25–30 % of the total protein content of the salivary glands (Zhimulev and Kolesnikov 1975), with each salivary gland cell containing 2500–3000 individual secretory granules ranging from 0.2 to 2.5 μm in diameter (Farkaš and Šuťáková 1999). These characteristics make the larval SGs of Drosophila an ideal and easily accessible model system to study various aspects of regulated exocytosis in metazoans.
The Sgs genes are coordinately activated: all of the cloned Sgs genes are heavily transcribed during the second half of the third larval instar only in the salivary glands (reviewed in Lehmann 1996) leading to the formation of dramatic puffs at their genes. These regress when the titre of the steroid hormone ecdysone increases at the end of the third larval instar (Becker 1959; Ashburner 1972; Richards 1981). Thus, this group of genes has provided an excellent opportunity to analyze the mechanisms that control tissue-specific and temporarily restricted gene expression. Presumably, the expression of all Sgs genes is controlled by the same trans-acting factors, possibly by the ecdysone receptor and auxiliary proteins (Lehmann and Korge 1995).
2.2 The Fate of Sgs-Secretory Granules
The Sgs proteins synthesized inside the salivary gland cells tend to form Golgi-derived electron-dense secretory vesicles that then fuse into larger granules (Farkaš and Šuťáková 1998, 1999). Several authors have studied secretory granules in D. melanogaster (von Gaudecker 1972; Lane et al. 1972; Farkaš and Šuťáková 1998), D. pseudoobscura (Harrod and Kastritsis 1972a, b; Pasteur and Kastritsis 1973) and D. hydei (Berendes 1965; Poels et al. 1971) and have described several different infrastructural elements within the granules: a foamy component, a paracrystalline component, and a fine particulate or electron-opaque component (Fig. 15.3). It is reasonable to assume that these infrastructural elements represent different states of granule maturation and reflect a level of densification that may be due to the gradual dehydration of granule contents. Detailed ultrastructural analysis of the Sgs-secretory granules indicates that densification is a continuous and permanent process inherent to the granules or Sgs-proteins, because when two highly dense electron-opaque granules fuse, they form local patches of foamy or paracrystalline infrastructure at the fusion sites, often close to the vesicle membrane (von Gaudecker 1972; Lane et al. 1972; Farkaš and Šuťáková 1998). That these observations are possible over relatively long periods of time make the Drosophila SGs an excellent model to study the basis of regulated exocytosis . Its secretory products are first synthesized and then stored for a long period (16–20 h) prior to their singular release, and so this model provides enough time to observe and investigate the molecular regulation of the process underlying their gradual maturation and release.
(a) Transverse section through the interecdysially active salivary gland with a centrally located lumen (L). Note the large number of electron-dense Sgs-secretory granules (SG) at the apical pole displaying three different infrastructures . Magnification 3000×. (b) Detailed view of three different infrastructural elements (densities) inside of the granule: I. foamy component, II. paracrystalline component, and III. fine particulate or electron-opaque component. Magnification 18,000×. (Reprinted with the permission of the publisher)
Through precise counting of the number and size of granules in late 3rd instar larval SGs of Drosophila, Farkaš and Šuťáková (1999) obtained clear evidence that the growth and maturation of Sgs-granules occurs as a continuous process through the gradual fusion of smaller granules. Niemeyer and Schwarz (2000) found that SNAP-24, a t-SNARE (soluble NSF (N-ethylmaleimide-sensitive fusion protein)-attachment receptor) homologue is present on the membrane of Sgs-granules, and that it acts to mediate granule-granule fusion. It is putatively involved also in exocytosis , i.e., mediating contact between the granule and the apical cell membranes. Additional key molecules in this process have also been identified. During the formation of Sgs-granules at the trans-Golgi network (TGN), newly synthesized glue proteins colocalize with clathrin and the clathrin adaptor protein complex subunit γ (AP1γ) at the TGN membranes (Burgess et al. 2011). Indeed, mutations affecting AP1 or its localization have dramatic effects. Mutations in AP1γ lead to a profound block in secretory granule formation or maturation. The localization of the AP1γ subunit to the TGN in salivary glands seems to require Gartenzwerg (Garz), the Drosophila ortholog of mammalian guanine nucleotide exchange factor GBF1, which is essential for Golgi complex biogenesis and surface delivery of proteins involved in cell-cell and cell-matrix interactions. The loss of Garz function, in addition to collapsing the Sgs-secretory pathway, inhibits trafficking of two adhesion molecules, DE-cadherin with the associated α- and β-catenins and Flamingo, to the cell surface, and disrupts the localization of the tumor suppressor Discs large, involved in the determination of polarity via the formation of septate junctions. By these mechanisms, the loss of Garz function leads to a dramatic disorganization of the morphology of the salivary glands (Szul et al. 2011). Drosophila SGs mutant for garz also display dysfunctional Arf1-COPI machinery (Wang et al. 2012). Arf1 (ADP-ribosylation factor 1), through assembly of a COPI coating onto membranes, is likely to promote the formation and maturation of Golgi elements including secretory vesicles, and thus likely to regulate anterograde transport of the secretory granules that are targeted for exocytosis (Wu et al. 2004; Munro 2005).
The classical approach to monitoring the release of Sgs-glue is to view the lumen of in vitro cultured SGs in a drop of diluted Grace’s or Schneider’s medium under a stereomicroscope or using phase contrast imaging under low magnification (Boyd and Ashburner 1977). Alternatively, PAS-positive histochemical staining of the whole-mount SGs can be employed (Berendes 1965; Poels 1970; von Gaudecker and Schmale 1974; Kolesnikov and Zhimulev 1975). We have routinely also used semi-thick sections of acrylate-resin embedded SGs metachromatically stained with Toluidine Blue O which strongly binds Sgs-glue (Farkaš and Sláma 1999). Currently, an easy method to view the process of granule secretion is to monitor it using a transgenic strain expressing GFP - or dsRED- fused to the Sgs-3 protein (Biyasheva et al. 2001). In these strains, the strongly fluorescent granules inside a cell’s cytoplasm are gradually released into the SG lumen with a corresponding rapid loss of cell volume (Fig. 15.4). After the contents of the granules are released into the lumen by exocytosis , the lumen becomes amorphous and rehydrated. After the complete or near-complete exocytosis of Sgs-granules which takes place over a period of about 2 h (Boyd and Ashburner 1977; Farkaš and Šuťáková 1998; Biyasheva et al. 2001), the lumen grows in volume by taking up the solute, most probably by active water transport from the haemolymph (Farkaš et al. 2015), to support the dilution of the glue in order to facilitate its later expectoration via the larval mouth. Indeed, the freshly formed puparium will become quickly cemented to the surface of a substrate after the evaporation of the water from the glue.
Exocytosis of Sgs-glue proteins. They were monitored using the GFP -SgsΔ3 strain. This strain was constructed by inserting 1.8 kb of the Sgs3 regulatory region and the N-terminal portion of the Sgs3 protein fused to enhanced GFP, into the pCaSpeR-4 transformation vector (Biyasheva et al. 2001). (a) Laser confocal image of most posterior region of the late 3rd instar larval salivary gland (during puff stages PS2 to PS4 corresponding to 1–3 h prior to glue exocytosis) with cells filled with numerous Sgs-granules containing GFP-fused Sgs-3 protein. (b) During PS5, when exocytosis is initiated by the elevated titre of ecdysteroids, glue granules containing Sgs proteins are transported towards the lumen where they become docked to the apical cell membrane via the actin cytoskeleton, highlighted by AlexaFluor488-conjugated phalloidin (blue). (c) The same region of the salivary gland with GFP-glue (green) released into the lumen (PS7 to PS8). Previously large and hexagonal salivary gland cells shrink upon massive glue exocytosis into the thin rim (red) around the lumen filled with secreted glue. To show this, Rab11 was detected in the cytoplasm of the SG cells using an anti-Rab11 polyclonal antibody and a Cy3-conjugated goat anti-rabbit secondary antibody
2.3 Physico-Chemical Properties of Sgs Proteins
Despite the initial interest in Sgs proteins during 1970s and 1980s, due to their striking characteristics as puff-encoded products, we do not have much published information about their properties. For example, we do not know if they are present in an equimolar ratio inside of the secretory granules and inside the expectorated secretory glue , so we cannot evaluate how their relative molar ratios are related to their overall function. We do not know the role of each individual protein in granule formation and maturation/densification, or in the subsequent hydration of the glue after it is exocytosed into the lumen . Since all of the Sgs genes have been molecularly characterized, some information can be gleaned from extensive in silico analysis of amino acid sequences.
The Sgs-1 protein is cornifin-related and adhesin-like, and potentially a chitin-binding protein, with a predicted molecular weight of 134.9 kDa and an isoelectric point of 12.1. It has 1286 amino acids and consists 109 tandemly arranged TTTTPRS repeats forming the core of the polypeptide chain. Overall, it contains 600 threonines representing 46.7 mole% of its total amino acid composition (for more details see Table 15.1). Remarkably, over 70 % of these threonines are predicted by several different methods to be O-glycosylated. Such a dense level of glycosylation has not been described so far for any other protein. At the same time, the majority of the tandem repeats are predicted by both the Garnier-Osguthorpe-Robson and the Chou-Fasman methods as being highly hydrophilic (Fig. 15.5a) which may reflect the distribution of hydroxyl- or polar amino acids. Except for a signal peptide in the N-terminal region and two stretches of a few amino acid residues at the C-terminal end, the majority of the Sgs-1 protein is predicted to be strongly disordered, most probably forming a coil (Fig. 15.7a). Only the more ordered N- and C-terminal regions are consistently predicted to form α-helices or β-sheets (Fig. 15.6a). The Sgs-1 protein displays 57 predicted disulfide bonds, with 40 of them having a very high score (between 0.8 and 1.0, where 1.0 means the highest degree of confidence).
Predictions of hydrophobicity, hydrophilicity and secondary structure of individual Sgs proteins. The Bayesian type Chou-Fasman and Garnier-Osguthorpe-Robson (GOR) methods were used. These methods are based on probability parameters derived from empirical studies of known protein tertiary structures solved by X-ray crystallography. In addition, the GOR algorithm takes into account not only the propensities of individual amino acids to form particular secondary structures, but also the conditional probability of the amino acid to form a secondary structure given that its immediate neighbors have already formed that structure. (a) Sgs-1, (b) Sgs-3, (c) Sgs-4, (d) Sgs-5, (e) Sgs-7, and (f) Sgs-8
The Sgs-3 protein, with a predicted molecular weight of 34.8 kDa and an isoelectric point of 10.5, has 307 amino acids and consists of 20 tandemly arranged TTTKX repeats (where the most frequent X is a P) that form the core of the polypeptide chain. As for Sgs-1, threonine residues are the most abundant amino acid (130 residues, 46.7 mole%) and at least 45 % of them are predicted to be O-glycosylated. Also as seen in Sgs-1, the tandem repeats in the Sgs-3 protein are predicted by both the Garnier-Osguthorpe-Robson and the Chou-Fasman methods to be very hydrophilic (Fig. 15.5b), and by PONDR to be strongly disordered, forming a coil as predicted by PsiPred , Predator and other algorithms (Figs. 15.6b and 15.7b). The entire Sgs-3 protein is predicted to have 28 disulfide bonds with only two having a score under 0.8. Over 12 of the disulfide bonds, however, are predicted within the signal peptide, and therefore these cannot contribute to the protein’s secondary structure and function (for more details see Table 15.1).
The Sgs-4 protein, with a predicted molecular weight 32.3 kDa and an isoelectric point of 9.1, has 297 amino acids. Structural parallels with Sgs-1 and Sgs-3 are readily apparent: 24 tandemly arranged TEPP or TKPP repeats encompass more than 80 % of the length of the polypeptide chain. Again, threonine is the most abundant amino acid (n = 53; 17.8 mole%) with at least 73 % of the threonines predicted to be O-glycosylated. Interestingly, Sgs-4 has also three N-glycosylable asparagines. As for Sgs-1 and Sgs-3, the tandem repeats of the Sgs-4 protein are predicted by both the Garnier-Osguthorpe-Robson and the Chou-Fasman methods to be very hydrophilic (Fig. 15.5c). In contrast to those proteins, however, Sgs-4 is less intrinsically disordered, containing two centrally and two C-terminally located short ordered regions (Fig. 15.7c). Out of 22 cysteins, at least eight are predicted to form disulfide bonds. Except for the signal peptide, there are no predicted α-helices or β-sheets (Fig. 15.6c). For more details see Table 15.1.
In constrast to Sgs-1, -2, -3, and -4, the Sgs-5 protein, with 161 amino acids, a predicted molecular weight of 18.6 kDa and an isoelectric point of 7.8, lacks any clearly identifiable tandem repeats . Threonine is far less abundant than in the previously discussed Sgs proteins (3.1 mol%). By contrast, Sgs-5 is rich in glutamic acid, leucine and serine (for more details see Table 15.1). The Garnier-Osguthorpe-Robson and the Chou-Fasman algorithms predict only a few hydrophilic and a few hydrophobic regions (Fig. 15.5d) and there appears to be just single N-glycosylation and a single O-glycosylation sites. Except for two regions, between amino acids 30 and 45, and between amino acids 75 and 95, which are intrinsically disordered, the majority of the Sgs-5 protein is highly ordered, containing five α-helices and four β-sheets (Figs. 15.6d and 15.7d). The Sgs-5 protein displays 12 predicted disulfide bonds, with eight of them having a very high score (between 0.8 and 1.0).
Secondary structure of Sgs proteins predicted by PsiPred algorithm employing neural network and machine learning methods which use the information of the evolutionarily related proteins (McGuffin et al. 2000). (a) Sgs-1, (b) Sgs-3, (c) Sgs-4, (d) Sgs-5, (e) Sgs-7, and (f) Sgs-8. For clarity, in the case of Sgs-1 (a) only the N-terminal and C-terminal portions of the entire sequence are shown because the central core region contain 109 tandemly repeated stretches of TTTTPRS that fail to form either α-helices or β-sheets. Legend: magenta cylin der = α-helix , yellow arrow = β-sheet, black line = coil, blue oblonger bars above secondary structures represents confidence of prediction
The Sgs-7 protein, with 74 amino acids, a predicted molecular weight of 7.9 kDa and an isoelectric point of 7.9, is also unlike Sgs-1, -2, -3, and -4 nonglycosylated and lacks their typical tandem repeats . The most abundant amino acids are cysteine, isoleucine, leucine and glutamine (for more details see Table 15.1). The Garnier-Osguthorpe-Robson and the Chou-Fasman algorithms predict only a few hydrophilic sites inside the core region and a few hydrophobic sites, the majority of which overlap with the N-terminal signal peptide (Fig. 15.5e). It lacks expected glycosylation sites, and surprisingly no disulfide bonds are predicted despite the presence of numerous cysteines. However, the potential for an unusual pattern of disulfide bonding deserves more investigation. The entire Sgs-7 protein is predicted to be strongly ordered with two long α-helices flanking a single β-sheet (Figs. 15.6e and 15.7e).
The Sgs-8 protein is highly related to Sgs-7, with a predicted molecular weight of 7.8 kDa and an acidic isoelectric point of 4.8. It has 74 amino acids without any typical tandem repeats . The most abundant amino acids are cysteine, glycine, valine and leucine (for more details see Table 15.1). The Garnier-Osguthorpe-Robson and the Chou-Fasman algorithms predict only few hydrophilic sites inside the core region and a few hydrophobic sites, the majority of which overlap with the N-terminal signal peptide (Fig. 15.5f). There are no glycosylation sites. In contrast to Sgs-7, however, the Sgs-8 protein is predicted with high confidence to have eight disulfide forming cysteines. Similar to Sgs-7, the Sgs-8 protein is strongly ordered harboring two long α-helices (Figs. 15.6f and 15.7f).
Posttranslational modification of individual Sgs proteins may have substantial effects on their properties and function. Different levels of glycosylation can have a significant effect on the final molecular weight and pI of a protein, and thus, its electrophoretic mobility may be quite different from that predicted, and several variants may be displayed. This issue deserves to be explored experimentally in more detail in the future. It will provide substantial insight into how individual Sgs proteins function as well as how the entire complex achieves its sticky properties. Currently, we can hypothesize that the glycosylated amino acid residues are likely to serve in at least three different functions: [1] Glycomoieties aid in protein folding and maturation; the nascent unmodified Sgs polypeptide would otherwise prefer a strongly disordered structure [2] Glycomoieties serve in hydration; they can be more easily dehydrated during granule densification than residues on a native protein, and vice versa, they can be more easily rehydrated after exocytosis . [3] Glycomoieties serve functional roles subsequent to exocytosis; a high level of glycosylation will serve to lubricate proteins allowing for more efficient transport via the gland lumen and mouth and so facilitate expectoration , and perhaps also facilitate adhesion to both the surface of the chitinous puparium and the attached substrate.
Analysis of ordered/disordered regions in the Sgs proteins by the PONDR protocol which utilizes feedforward neural networks that use sequence information from windows of 21 amino acids (Romero et al. 1997, 2001). If a residue value exceeds a threshold of 0.5, the residue is considered disordered. (a) Sgs-1, (b) Sgs-3, (c) Sgs-4, (d) Sgs-5, (e) Sgs-7, and (f) Sgs-8
2.4 Speculation on the Role of Sgs Proteins
From the predictions of various aspects of their secondary structures it is clear that the smaller and inherently ordered Sgs proteins are either not glycosylated or considerably less glycosylated than the larger and structurally disordered Sgs proteins that are quite heavily glycosylated. This identifies a specific paradox concerning the Sgs-proteins: the degree of order and disorder in the polypeptide chain is related to how much it is glycosylated. In addition, it appears that the structurally disordered, larger Sgs proteins also have more cysteines predicted to form disulfide bonds. We hypothesize that the higher level of glycosylation and disulfide bonding aids in reducing the inherently disordered state of the larger Sgs proteins since they lack α-helices and β-sheets, and that this may be required for secretory granule maturation and potentially, for fulfilling their function as glue proteins. In this context it seems logical to propose that the small nonglycosylated Sgs proteins like Sgs-7 and Sgs-8 or Sgs-5 have a higher likelihood to stably maintain their secondary structures as they proceed through the different conditions associated with granule formation, fusion, maturation, exocytosis, rehydration, and finally, expectoration . If this is the case, the higher ratio of α-helices and β-sheets in each of the small Sgs proteins, when compared to the highly glycosylated Sgs-1, Sgs-3 and Sgs-4 proteins, might allow them to serve as pivotal initiators or promotors of the densification process. On the other hand, the larger and highly glycosylated Sgs proteins are more likely to facilitate rehydration after exocytosis and the maintenance of lubrication during the process of expectoration and the cementing of the prepupa to the substrate.
It is not a rule of thumb that intrinsically disordered regions are typically involved in regulation, signaling and control pathways in which interactions with multiple partners and high-specificity/low-affinity interactions are expected (Dyson and Wright 2005; Bardwell and Jakob 2012; Mittal et al. 2013). It should be noted, however, that intrinsically disordered proteins or regions exist as dynamic ensembles in which the atom positions and the backbone Ramachandran angles vary significantly over time with no specific equilibrium values and typically involve non-cooperative conformational changes (Gunasekaran et al. 2004; Uversky et al. 2008). Such disorder may serve to provide a functional advantage by enhancing binding plasticity or allosteric coupling. Several studies showed that the conformational entropy conferred by disordered regions decreases the propensity of proteins to self-aggregate (Dyson and Wright 2005; Japrung et al. 2013). Thus, mutual interactions that can be anticipated between the inherently ordered and disordered Sgs-proteins can serve to prevent an unwanted aggregation process within the densely packed secretory granules or even after exocytosis before the glue is programmed to solidify. Presumably, the mutual interactions between the two fundamentally different types of Sgs proteins may serve in regulating when the glue will set.
2.5 Evolution of Sgs Proteins
Applying both of the BLAST and FASTA algorithms to D. melanogaster Sgs protein sequences revealed that these proteins are not present in the genomes of all Drosophila species sequenced to date (Adams et al. 2000; Misra et al. 2002; Kaminker et al. 2002; Celniker and Rubin 2003; Stark et al. 2007; Pfeiffer et al. 2010). Moreover, there are also species-specific differences in the distribution or occurrence of individual Sgs proteins. The Sgs-1 protein which is responsible for interecdysial puff 25 AC in D. melanogaster was unambiguously found only in D. sechelia . The most widespread Sgs proteins found among other Drosophila species are proteins related to those produced by the Sgs-5 and Sgs-7 genes which were found in the same nine non-melanogaster species ( D. simulans , D. sechelia, D. yakuba , D. ananassae , D. persimilis , D. pseudoobscura , D. erecta , D. virilis , D. mojavensis ). The orthologues of Sgs-8 were found in eight non-melanogaster species (D. simulans, D. sechelia, D. yakuba, D. erecta, D. ananassae, D. virilis, D. persimilis, and D. pseudoobscura), the orthologues of Sgs-3 were found in six species (D. simulans, D. yakuba, D. ananassae, D. pseudoobscura , D. sechelia , D. erecta), and finally, the orthologues of Sgs-4 were found in five non-melanogaster species ( D. simulans , D. yakuba , D. erecta, D. mojavensis, and D. virilis). Thus, only D. sechelia has all six of the Sgs proteins found in D. melanogaster, while D. simulans has 5 of them (all except Sgs-1). Present in D. mojavensis are only Sgs-4, Sgs-5 and the nonglycosylated Sgs-7. In summary, only those species belonging to melanogaster subgroup (D. simulans, D. sechelia, D. yakuba, D. erecta , and D. melanogaster) have all six or at least five Sgs proteins, whereas D. ananassae (melanogaster group), D. pseudoobscura (obscura group), and D. virilis , (virilis group) have four Sgs proteins. Finally, D. persimilis (obscura group) and D. mojavensis (repleta group) have only three Sgs proteins; Sgs-1 and Sgs-3 are absent in both, while Sgs-4 and Sgs-8 are missing in D. persimilis and D. mojavensis, respectively
In other species, the sequence data is either missing, or if it is available, neither BLAST nor FASTA searches, unless done under low stringency, identify positive hits. The majority of false positives are clearly not orthologues but sequences having only partial similarity (under 40 %) or identity (under 30 %). Nonetheless, we have made some experimental observations on morphological features found in the late larval salivary glands in few additional species. From these unpublished data, it is clear that species such as D. willistoni , D. atripex , D. mauritiana and D. parabipectinata show a reticulate network of cytoskeletal components indicating the presence of Sgs-like granules in the cytoplasm of SGs of late 3rd instar feeding and wandering larvae (Farkaš et al. unpublished observations). This indicates the potential presence of Sgs orthologues in these additional species. Detection of Sgs-like secretory granules in some species, for example, D. willistoni and D. mauritiana is surprising because under obligatory or natural conditions, the larvae prefer to pupariate on the surface of or inside their food. It is notwithstanding that after small pieces of leporello or accordion-folded filter paper were inserted into the food, more than 70 % of the larvae choose to climb out of the food and pupariate on the filter paper. Thus, these species are able to display a shift from sitter to rover larval behaviour (Farkaš et al., unpublished observations). It may be that in these species the use of a Sgs-based glue is facultative and not obligatory, so that its production depends on the surrounding habitat or type of material provided inside the culture. Interestingly, also we observed a similar facultative pupariation behaviour in D. simulans , a very close relative of D. melanogaster, in which five out of six Sgs proteins were identified (all except Sgs-1). Thus, we were surprised that BLAST and FASTA searches failed to identify any orthologue in D. willistoni . It may be that the evolutionary distance of 35–37 MYA that separates between D. melanogaster and D. willistoni (Garfinkel et al. 1983; Meyerowitz and Martin 1984; Parsons 1978, 1981, 1994; Barker et al. 1990; Korol et al. 2006) is sufficient for the Sgs genes to acquire a level of sequence divergence which prevents the identification of orthologues .
Certainly, there are several alternative explanations for these data. Still, one conclusions that can currently be made is that evolutionary older species such as D. mojavensis and D. persimilis have fewer Sgs genes, primarily Sgs-5 and Sgs-7, while more recently-evolved species such as representatives of melanogaster subgroup ( D. simulans , D. sechelia , D. yakuba , D. erecta , and D. melanogaster) have five or six Sgs genes. Thus, those Sgs proteins that are more structurally ordered are either not or less glycosylated, such as Sgs-5, Sgs-7 and Sgs-8 appear to be older, while those genes encoding unordered but heavily glycosylated proteins like Sgs-1 or Sgs-3, were acquired during evolution more recently. Currently, it would be very speculative to state which of the genes are ancestral for the less ordered and heavily glycosylated Sgs proteins. The high number of short tandem repeats , however, indicates that their internal disordered structure could evolve relatively quickly by repeated duplication of a simple tandem motif. Although the information presented thus far does not let us draw a clear or unambiguous conclusion about the evolutionary or habitat-prone adaptation associated with the function and expression of each particular Sgs protein, it is evident that as a group of excretory products with a highly specified function (glue), they appear to be flexible in their adaptation to environmental and habitat factors. The evolution of the Sgs proteins will therefore serve as a very useful model to study the evolution of Drosophila species in the context of habitat adaptation. Further investigations along these lines in more species from more diverse habitats will be necessary to gain deeper insights about the role of environment and evolution in the composition of the Sgs-glue, as well as the expression and the sequence variability of individual Sgs proteins.
According to many classical papers, the Sgs-glue proteins were considered to be mucins (Korge 1975, 1977a, b; Beckendorf and Kafatos 1976; Kress 1979; Engoher and Kress 1980). According to currently accepted criteria, however, mucins are characterized by poorly conserved repeated sequences that are rich in prolines and potentially glycosylated threonines and serines (PTS). If among the Sgs proteins in D. melanogaster, only Sgs-1 and Sgs-3 meet these stipulations (Syed et al. 2008), thus only about a third of glue proteins are true mucins . In the light of this conclusion the Drosophila glue system provides a challenging opportunity to understand the coevolution of proteins having considerably different structural features that nonetheless interact to form components of the same extracellular matrix.
3 Apocrine Secretion by Drosophila SGs
Drosophila SGs have been a model organ for many genetic, cytological and developmental studies, including those mentioned above. However, for a long period of time, their only well-characterized and consequently the major function associated with them was the production of the Sgs glue during the second half of the last larval instar. When released by exocytosis during pupariation, this serves to affix the freshly formed puparia to a substrate (Fraenkel and Brookes 1953). Because of their large cell size and otherwise excellent suitability to study processes underlying programmed cell death (PCD) , the SGs have become also the tissue of choice for investigating developmentally-linked and hormonally-triggered PCD. Indeed, it was during a set of experiments on PCD in Drosophila in our laboratory that we discovered that the doomed larval salivary glands release additional proteins, distinct from and well after their secretion of Sgs-glue, by an unusual extrusion process during the late prepupal period (Farkaš and Mechler 2000). Later we showed that this hitherto neglected protein extrusion process, which takes place just 6–4 h prior to the execution of PCD , occurs via a typical apocrine mechanism (Farkaš et al. 2014). Not only it was the first description of apocrine secretion in Drosophila, but the rich array of methods and molecular-genetic tools available in the fruitfly offer an outstanding opportunity to dissect the mechanism of this process and identify the genes regulating it. Below, are summarized the complex light and electron microscopical evidence for the apocrine process in the prepupal salivary glands , with a description of its dynamics and characterization of the secreted proteins that provide a foundation on which to achieve this long-term goal.
The main significance of finding an apocrine process in the SGs, especially late in their life and after their glue has been secreted, lies in the fact that the only type of widespread and well-known secretory process is exocytosis . This intensely studied mechanism has identified many dozens of factors and their encoding genes (Jahn 2004; Südhof 2004; Chieregatti and Meldolesi 2005; Südhof and Rothman 2009; Blank 2011; Porat-Shliom et al. 2013). Exocytosis or merocrine secretion is the process regulating the specific membrane contact, priming and fusion events required for the selective release of compartmentalized compounds such as signaling molecules (antibodies, neurotransmitters, cytokines, morphogens, growth factors, chemokines, hormones, etc.). It became widely accepted that the initial phase of the exocytotic secretory pathway involves the formation of vesicles in the trans-Golgi, then targeted translocation of these vesicles to specific sites on the plasma membrane, the preparation of these docked vesicles for full fusion competence (priming), and the subsequent triggered fusion of these membranes, resulting in their coalescence and the release of vesicular contents to the extracellular space. A complex composed of the three major membrane proteins, NSF, SNAP, and SNARE, each representing a small protein family conserved from yeast to humans, has emerged as key player in exocytosis (Malsam et al. 2008; Saraste et al. 2009; Walter et al. 2010). The role of the hexameric ATPase NSF (N-ethylmaleimide-sensitive fusion protein) is to put energy into the system. Members of the SNAP (soluble NSF-attachment protein) family appear to function as adaptors between NSF and the third type of protein in the complex, the SNAREs (SNAP receptors). SNARE proteins are found on both the target membrane (t-SNAREs) and the secretory vesicle (v-SNAREs), and are therefore assumed to be the major “targeting” components of the process (Shen et al. 2007; Maximov et al. 2009; Kasai et al. 2012).
In addition to exocytosis , which takes place by targeted fusion of secretory vesicles with the plasma membrane, there exist two additional types of noncanonical secretion: apocrine and holocrine secretion. During these processes, entire portions of the cell are released and homotypic membrane fusion is not required. In the apocrine mechanism, a glandular cell loses a portion of its cytoplasm and is then completely or partially renewed. In the case of holocrine secretion, the material is released into the gland lumen upon cell death and the dissolution of the cellular structure. In contrast to exocytosis , no protein components, factors or genes affecting apocrine and/or holocrine secretion have yet been identified, and thus the mechanisms underlying these processes remain enigmatic. However, finding that apocrine secretion occurs in the Drosophila salivary glands, several hours after the exocytosis of Sgs glue is completed, provides a mean to reappraise our understanding of apocrine secretion. Insights made using of this wonderful molecular genetic model organism, provide a glimmer of hope for elucidating the mechanistic aspects of this fundamental, and so far, almost uncharacterized process.
3.1 Identification of Apocrine Secretion in the Prepupal SGs
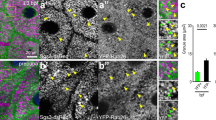
As we described more fully elsewhere (Farkaš et al. 2014, 2015), during the first hours after pupariation and glue expectoration , the salivary gland cells become vacuolized by enormous amounts of endocytosis (Fig. 15.8a). Within 6–7 h after puparium formation (APF), the vacuoles are consolidated by continued endosomal trafficking towards the ER and Golgi (Fig. 15.8b). Surprisingly however, many different types of proteins detectable using specific antibodies, are released into the centrally located gland lumen during the 8th hour of prepupal development, a process that continues for the next ~2 h (Fig. 15.8c). Using a panel of antibodies indicates that there is a differential release of different proteins over time, depending on the phase of the secretion and the type of protein secreted. For example, even though non-muscle myosin II and β-tubulin are being released into the lumen during the first hour of the secretory process, there is a strong accumulation of unsecreted filamentous actin at the apical membrane. Similarly, proteins such as cytoplasmic α-catenin and nuclear Smrter, the EcR-coupled transcriptional corepressor, are released almost completely during the first hour of secretion, but the transcription factor BR-C remains localized in nuclei during this time. When the lumen is at its widest during the more advanced phase of the protein extrusion (9th h APF), the lumen becomes filled with ecdysone-regulated transcription factor BR-C while cytosolic Rop is still retained in the cytoplasm. By this time, the nuclear histone deacetylase Rpd3 along with myosin II are both present in the lumen. During the tenth hour APF, any remaining nuclear receptor EcR and the ribosomal protein P21 as well as filamentous actin are all released into lumen . As a consequence of this massive extrusion by the end of the tenth hour APF, the signal of many intracellular proteins, as detected by antibodies, becomes weaker or undetectable. However, at 11 h APF some of the proteins once again can be detected, at least in modest amounts, at their original sites. This indicates either that the entire pool of cell proteinaceous components is not released, or alternatively, that at least some proteins are quickly replaced by new protein synthesis. In summary, this massive protein secretion corresponds with the relocation of measurable fluorescence signal from the salivary gland cells to the extracellular gland lumen .
The time course of the major developmental events in the prepupal salivary glands. They are illustrated by staining with antibodies to highlight appropriate structures. (a) About +2 h APF, the salivary gland cells become highly vacuolized by membrane recycling due to massive endocytosis , a consequence of exocytosis ; BR-C (red), p127l(2)gl (green) and filamentous actin (blue). (b) The process of vacuolization and membrane recycling is consolidated by +7 h APF, shortly prior to the next secretion; BR-C (green), p127l(2)gl (red) and filamentous actin (blue). (c) At +8 h APF, the salivary glands are showing an early phase of release of myosin II, p127l(2)gl and filamentous actin into the centrally located lumen . fb piece of adherent fat body. All confocal images 400×. (Reprinted with the permission of the publisher)
Since light microscopy found no indication for the involvement of secretory vesicles in this secretion, and there were no fluorescently-detectable increases in Golgi zone areas or other exocytosis-associated activity, transmission electron microscopy (TEM) was used to verify that this massive protein extrusion was not being achieved by exocytosis . Not only did the TEM images of the extrusion process in 8–10 h old prepupal glands confirm that proteins are not released by exocytosis during this period, but they also revealed typical attributes of apocrine secretion, which entails the loss of part of the cytoplasm. TEM images revealed also apical protrusions and cytoplasmic fragments inside the lumen of the glands. These cytoplasmic fragments contain various types of electron-dense material, including small pieces of membranes, free ribosomes, mitochondria, endoplasmic reticulum , and a plethora of amorphous structures (Fig. 15.9a–d). At the very earliest phase of apocrine secretion, during the eighth hour APF, the salivary gland cells show prominent and numerous microvilli and their lumen is filled with an “uncertain” whorling membranous-like (Fig. 15.9d) or electron-lucent filament-like material (Fig. 15.9e). Slightly later, while the apical surface of the cells still contains plenty of microvilli, the material inside the lumen becomes electron dense and almost evenly distributed, consisting of many small pieces of the cytoplasm (Fig. 15.9f). At the mid phase of apocrine secretion (9 h APF), microvilli are still present though less abundant, while larger pieces of more electron dense and compacted material start to appear in the lumen (Fig. 15.9g). At the later stages of secretion, the microvilli are almost absent and the luminal material becomes flocculent. It is electron-dense, irregularly scattered in the lumen in the form of larger pieces, some of which clearly contains structured cytoplasmic materials including ER, Golgi or mitochondria, etc. (Fig. 15.9h). Because the process of pinching-off, constriction and decapitation of the stalk of the apical protrusions was not clearly recognizable in 8–10 h prepupal salivary glands using TEM , we assessed this possibility using scanning electron microscopy (SEM) . The presence of numerous aposome-like structures on the apical membrane surface of the gland lumen was identified. Some of these aposome-like structures displayed constrictions and features consistent with the decapitation of the stalk of an aposome (Fig. 15.10a, b). Thus, a combination of light, TEM and SEM methods certify that the massive protein secretion in 8–10 h prepupal SGs of Drosophila occurs via an apocrine process.
Transmission electron microscopy of an apocrine process in 8–10 h old prepupal SGs . (a) Prima vista evidence of apocrine secretion is documented by apical protrusions (arrows) and numerous cytoplasmic fragments (arrowheads) inside the lumen of the salivary glands from a +9 h APF animal; 2700×. Higher magnification views (b and c) of the apocrine process showing details of electron-dense material (arrows) released from the apical surface (arrowheads) of 9-h old prepupal SG cells; 8000× and 10,000×, respectively. However, at the very early phases of apocrine secretion, +8 h APF, the SG cells show prominent and numerous microvilli (m) and the lumen is filled with “uncertain” whorled membraneous-like (arrows) (d) or electron-translucent filament-like material (e); both 2700×. Slightly later (+8.5 h APF), the apical surface of the cells still containsFig. 15.9 (continued) numerous microvilli (m), but the material inside the lumen becomes electron dense and almost evenly distributed (arrows), consisting of many small pieces (f); 4000×. At the mid-phase of apocrine secretion (+9 h APF), microvilli (m) are less abundant (arrows), and larger pieces and more electron dense material (arrowheads) start to appear in the lumen (g); 6700×. At later stages of apocrine secretion (+10 h APF), the microvilli are absent and the luminal material becomes flocculent; it stays electron-dense, and larger pieces of material (arrows) are irregularly scattered in the lumen. Some of these clearly contain structured material of the cytoplasm including ER, Golgi (G), mitochondria (M) or multivesiculated elements (MVE) (h, i, j); 2700×, 8000× and 14,000×, respectively. L in all images means lumen. (Reprinted with the permission of the publisher)
Scanning electron micrographs of the apocrine process in the 9 h old prepupal SG . The gland, dissected under the stereomicroscope and having a lumen evidently filled with material, was fixed and processed to critical point drying, after which it was broken up to expose the inferior portion that included the luminal surface, and then sputter coated. The image reveals (a) numerous aposome-like spheres (arrows) and various material-bearing structures on the surface of apical membrane (10,000× ). In addition, at higher magnification (b), some of these spheroid structures (arrows) displayed constrictions and show a decapitation of the aposome’s stalk (arrowheads) (20,000× ). (Reprinted with the permission of the publisher)
3.2 Further Characteristics of Apocrine Secretion in Drosophila SGs
Our initial observations indicated that prepupal salivary gland undergoing apocrine secretion release various kinds of proteins, and therefore, a fundamental question rose as to what kind or categories of proteins the glands release and whether the secreted material contains any specific proteins that could help shed light on the process’ physiological significance. To address this question, we used two different approaches to characterize the protein composition of the secretion: immunohistochemical detection at the light microscope level and top-down proteomic identification of components present in the secretion. For the former, a panel of antibodies available in our laboratory or antibodies that were readily available from colleagues was used. We also randomly selected several LacZ - and GFP-protein trap transgenic fly stocks available in Drosophila research community, known to be expressed either ubiquitously or strongly in the salivary glands, and assessed whether LacZ or GFP signal was present in the SG lumen of 8–10 h old prepupae . For the proteomic analysis, multiple samples each containing the secretion released into the lumen of prepupal glands from several hundreds gland pairs were collected. The pooled samples, whether separated by 1-dimensional electrophoresis or not, were reduced, alkylated, trypsin-digested, chromatographically separated and their proteins identified by MALDI-TOF/TOF or ESI-MS/MS mass spectrometry. The initial analysis by this approach identified 279 proteins (Farkaš et al. 2014). By pursuing this proteomic approach we found over 1000 proteins in the secretion from all sorts of categories including cytoskeletal proteins, cytoplasmic/cytosolic proteins, signaling molecules, membrane components, ER, mitochondria, Golgi and other organellar proteins, nuclear or chromosomal proteins including transcription factors and chromatin remodeling proteins, and even nucleolar proteins (Farkaš et al. 2014). Their ontological distribution shows that they include 41.2 % cytosolic proteins, 11.2 % ER chaperones + Golgi proteins, 6.9 % mitochondrial proteins, 15.9 % membrane proteins, and 11.6 % chromosomal, nucleolar and RNA/DNA binding/editing/modifying proteins (Fig. 15.11a). They also reflect a very wide range of biological processes: 11.7 % are transport and secretory proteins, 17 % are cytoskeletal proteins, 8.3 % are involved in signaling, 25.2 % are involved in basal metabolism, 7.3 % are nuclear proteins and transcription factors, 12.6 % are involved in protein synthesis and modification, 2.9 % are involved in storage, and 6.3 % have unknown functions (Fig. 15.11b). In addition, they also represent many cellular/molecular functions: e.g. enzymes 38 %, proteins associated with development 12 %, DNA and RNA binding proteins 10 %, cytoskeletal proteins 9 %, transport proteins 8 % etc. (Fig. 15.11c). Thus, the apocrine secretion is not selective for different protein categories.
Ontological classification of proteins released via apocrine secretion. The proteins were detected by a combination of immunohistochemistry, GFP -/EYFP-/RFP-fusions fluorescence, chromogenic staining of LacZ -insertions and mass spectrometry. The pies show (a) categories of proteins according to subcellular localization, (b) distribution by biological process, and (c) distribution by cellular/molecular function. (Reprinted with the permission of the publisher)
Nonetheless, our finding that some proteins in the Drosophila salivary glands are released by apocrine secretion earlier, while other proteins are released later, documents that this is not a random, but rather a highly regulated process. This also opens up a potentially new area for further research. We cannot unambiguously infer what categorical features of proteins determine their earlier versus later release. From an ultrastructural perspective, the early phases of secretion seem to be associated with the extrusion of more soluble proteins, whereas larger pieces of cytoplasm, which are harder to solubilize, are released at later stages. However, even at very early phases we documented release of larger pieces of the cytoplasm. Most likely, it is easier to detect the occurrence of such “less soluble” material at later stages because the released materials are being accumulated in the lumen over a secretory phase that lasts 2 h, which increases the chances for the detection of larger pieces (Farkaš et al. 2014). When we investigated the order of protein secretion during this two-hour time window using antibody staining, we found that it was highly reproducible in its regularity. To shed more light on the molecular mechanism that controls this gradual release of proteins, it will be helpful to identify the time-course of the secretion of individual proteins, using both microscopical as well as mass spectrometric approaches.
The temporal profile of the cytoplasmic accumulation of glue-containing granules, described above, demonstrates that the larval salivary glands are chiefly involved in the production and secretion of Sgs-glue. However, the typical exocytotic secretion that accomplishes this function is temporarily separated from the later apocrine secretion in the Drosophila salivary glands by a 14–16 h period. Although this interval may appear to be a relatively short time in the vertebrate world, it is a period of rapid and dramatic change in this insect. In response to a metamorphic pulse of ecdysterone, the relatively mobile and actively feeding larva stops feeding, enters a short wandering stage, become motionless, pupariates and then enters the early pupal stage where larval tissues initiate programmed histolysis and imaginal discs initiate metamorphosis. In this short interval, the larva undergoes dramatic morphogenetic changes that are associated with numerous and complex biochemical and cellular events. Therefore, the 14–16 h period between exocytosis and apocrine secretion can be considered as a substantial time interval. It is significant that the very same cells exercise these two apparently separate and independent processes. To answer the question of whether these two processes are truly separate and independent, the immense potential of Drosophila model system can be used for molecular genetic dissection of exocytosis vs. the apocrine secretion.
3.3 Postapocrine Fate of Drosophila SGs
As mentioned above, the apocrine secretion in the prepupal salivary glands takes place just a few hours prior to programmed cell death (PCD) . Therefore, it was logical to ask whether the material released from the cells 4–6 h prior to their histolysis is already degraded, as this would link apocrine secretion with their temporally close senescence. To address this issue, secretory material from 8–10 h old prepupal salivary glands was isolated, proteins extracted, and probed by western blotting with selected antibodies. The tested antigens (Rab11 membrane component, BR-C transcription factor, tumor suppressor protein p127, myosin II, Rop, β-tubulin, EcR, Scrib, Arm and several other proteins) remained as intact and undegraded in the prepupal secretion when compared to the total protein extracted from the late larval salivary glands when probed by western blotting (Farkaš et al. 2014). Thus, the proteins and associated complexes released by apocrine secretion are intact, and most probably also fully functional.
This raises yet another question with regard to the programmed cell death . Are salivary gland cells that are losing the majority of their cellular protein components able to retain basic vital functions? We experimentally documented that glands in the final phases of protein extrusion (+10 h APF), as well as glands several hours older (12–14 h APF) still incorporate radioactively labeled uridine ([14C]-uridine or [3H]-uridine) and amino acids ([35S]-methionine or [3H]-leucine) into newly synthesized RNA and proteins, respectively. Furthermore, the pattern of proteins synthesized is not static, but changes as the glands continue to age. These prepupal salivary glands also have viable cells as assessed by a dye exclusion test with trypan blue. Thus, even at time points past the massive, noncanonical apocrine secretion, these glands have cells that are fully alive and continue to maintain a pattern of transcriptional and protein synthetic activities (Farkaš et al. 2014). Indeed, this fits precisely with our understanding of the well-defined puffing pattern of salivary gland polytene chromosomes during this developmental period (Ashburner 1970, 1972; Richards 1976a, b; Ashburner and Berendes 1978). Therefore, this secretory cycle appears to be one of the vital and programmed functions of salivary gland prepupal development and appears to not be associated with PCD .
Interestingly, many of the proteins identified in our initial top-down proteomic analysis or immunodetected microscopically are encoded by genes recovered by Maybeck and Röper (2009) in their targeted gain-of-function screen for embryonic salivary gland morphogens. These include genes such as chic, egl, btsz, Arp87C, and others, and according to the modENCODE project and FlyAtlas tissue expression data (Chintapalli et al. 2007; Graveley et al. 2011; Robinson et al. 2013), such genes are known to be moderately to highly expressed in salivary glands. This indicates that these genes, which are important for the embryonic morphogenesis of this tissue remain active and are highly or increasingly expressed throughout the life of the gland, and so may be essential or vital for maintaining this organ’s identity, structure or function until the realization of cell death. On the other hand, several polypeptides detected by mass spectrometry, such as transferrin, and the larval serum proteins (yolk proteins) are almost surely not endogenous products of salivary glands, but exemplary representatives of haemolymph or fat body proteins. This strongly indicates that these are transsudated , similar to previously observed transsudated proteins e.g. albumin in mammalian tears (Ng et al. 2000; Grus et al. 2005; Zhou et al. 2009; Versura et al. 2010). And indeed, we recently described that there is tremendous endocytosis and vacuolation in the early-to-mid prepupal salivary glands of Drosophila. This appears to be associated with complex endosomal trafficking that is able to bring in various cargoes from the circulating haemolymph. After consolidation of the numerous vacuoles with the salivary gland’s ER and Golgi systems, these would easily be recruited into the apocrine pathway few hours later (Farkaš et al. 2015).
In this context, it should be also emphasized that we were unable to detect any low-molecular weight degradation products, even on overexposed X-ray films from western blots. As we detected only undegraded proteins in the released material by western blotting as well as morphologically “perfect” pieces of cellular structures in the lumen by electron microscopy, it implies that the apocrine secretion process is a real secretory activity with a different functional significance (Farkaš et al. 2014). Therefore, it can be concluded that apocrine secretion is a selective process; only undegraded proteins are released whereas those targeted for proteasomal degradation are retained in cells. This is a novel and important attribute of Drosophila apocrine secretion.
All the observations showed that only proteins, and not nuclear DNA, are released during apocrine secretion. To verify this result for all cells in the entire gland, which is composed of columnar, transitional and corpuscular cells , we detected DNA with Hoechst 33258 and various proteins with antibodies at 8, 9 and 10 h after pupariation. These experiments confirmed that during all of the three time points when various proteins are unambiguously secreted, nuclear DNA remains intact in all of the cells of the gland. This appears as one of the major hallmarks of the apocrine process (Farkaš et al. 2014; Farkaš 2015). The major outstanding question is what physiological purpose is served by the apocrine secretion in the prepupal glands, and also, whether it occurs in other Drosophila species or cyclorrhaphous dipterans.
3.4 Major Conclusions from Apocrine Secretion in the Drosophila SGs and Their Relevance to Vertebrates
Its identification in the prepupal SGs of Drosophila suggests that we should consider this type of a cell externalization mechanism in a wider context. Though it is a rarely investigated process, studying apocrine secretion has a very long history. The first identified paper on an apocrine secretory organ is that of Harder (1694) who described a special lachrymal gland in rodents. Then Purkyně (also known as Purkinje) (1833a, b) discovered the human sweat gland, currently the most intensely studied apocrine organ, which was then described in detail by his pupil Wendt (1833, 1834). Almost simultaneously, their findings were confirmed and extended by Breschet and Roussel de Vouzeme (1834), and by Gurlt (1835). The axillary armpit glands, which contain the highest known concentration of apocrine sweat glands in the human skin, were first recognized by Horner (1846). Independently, Velpeau (1839) and later Verneuil (1854) described a chronic acneiform infection of the cutaneous sweat (apocrine) glands that later was named hidradenitis suppurativa (HS) (Richter 1932; Brunsting 1939; Lasko et al. 2008; Blok et al. 2013). This identifies a specific medical problem closely associated with the apocrine process. Talke (1903) then described the presence of two types of glandular cells, clear cells and dark cells, in the human sweat apocrine gland. Mislawsky (1909) suggested that these are transitional to each other and are fundamentally of the same cell type. Today investigators studying sweat glands believe that the glandular secretory cells of the apocrine sweat glands are only of one type, being different from those of the eccrine sweat glands.
Ranvier (1879) was the first to distinguish “holocrine” secretion in the sebaceous gland from “eccrine/merocrine” secretion in the sweat glands. But it was not until (1917) and (1921) when Schiefferdecker, based on Ranvier’s observations, suggested that the sweat gland cells be classified functionally according to how they secreted their contents, by an eccrine/merocrine, apocrine or holocrine mechanism. This contribution provided a conceptual breakthrough. It established a clear functional definition of three substantially different categories of secretion based on the mechanism underlying the externalization of cellular materials (Fig. 15.12). Since that time, apocrine secretion has been confirmed and studied by many authors (Herzenberg 1927; Richter and Schmidt 1934; Kuno 1938; Kato and Minamitani 1941; Iwashige 1951; Hibbs 1962; Schaumburg-Lever and Lever 1975).
Schematic illustration of the three major secretory mechanisms. (a) exocytosis (merocrine secretion) involves homotypic membrane fusion between vesicles and the cell membrane, thus allowing for only the externalization of the cargo present inside the secretory granules . (b) Apocrine secretion occurs by discharging a portion of the cell when intracellular components are freed into a lumen through the shedding of whole pieces of the cytoplasm. After release, the cytoplasm is reconstituted and a new cycle of secretion may occur. (c) Holocrine secretion means “complete secretion” of the cell’s entire contents and occurs when the cell becomes completely dissolved. All of its compartments, including the nucleus, can serve as secretory products. The cell never recuperates. (Reprinted with the permission of the publisher)
In spite of the literature that accumulated over time, the mechanism and purpose of the apocrine secretion remains enigmatic. So, our puzzlement of its function in the dying SGs highlights a general lack of understanding. One of the reasons is the lack of suitable model organism or model tissue, as well as not having a set of clearly defined characteristics of the process, which currently stems from many controversial and incomplete observations made in various animals and human samples. The careful analysis of the apocrine process in Drosophila SGs has opened the door to comprehensive and synthetic comparison of many previous studies. Unfortunately, these often have misleading conclusions when compared to the current data that helped us to draw an elementary outline of the apocrine secretion process. First of all, observations in Drosophila clearly establish that apocrine secretion is a non-canonical and non-vesicular transport and secretory mechanism. It has been observed or unambiguously described in sweat glands, mammary glands, lacrimal tear glands as well as in many other tissues including the cerumenal glands, epididymis, and many others (Kawabata and Kurosumi 1976; Kurosumi and Kawabata 1977; Agnew et al. 1980; Gudeman et al. 1989; Morales and Cavicchia 1991; Paulsen 2003; Farkaš 2015). Though some of the glands (notably the pituitary and parathyroid glands) (Ream and Principato 1981; Schwarz et al. 1988) are typical endocrine glands, all of the other secretory organs are exocrine glands, many of which serve as barrier epithelia, just as do the Drosophila SGs. Therefore, in the majority of cases, apocrine secretion may serve a means to interface communication with the external environment.
It is very interesting that the Drosophila salivary gland cells have the capability to secrete jellyfish GFP - or bacterial lacZ-fusion proteins as well as lacZ -nonfusion reporters. This indicates that heterologous proteins can be recruited to the apocrine secretory machinery/pathway (Farkaš et al. 2014). This evokes the question of what mechanism is used to label and/or recruit proteins into the apocrine trafficking pathway? We can speculate about what potential complex posttranslational modification(s) would be able to recruit such a massive and diverse amount of varied proteins. However, there is also one additional and unexpected possibility we raise as a question: if the apocrine machinery is capable of recruiting such divergent categories of proteins from all cellular compartments and organelles , and such an enormous population of these proteins can be released, would it not be wise for a cell to coopt or invent an energetically less expensive summoning system? Using such a system would enable cells to devote much less effort towards labeling and modification if they marked the relatively fewer number of proteins that should be retained, and not mark those to be recruited for apocrine secretion. If this is the case, it opens research vistas towards a novel selection mechanism.
In contrast to previously published views, apocrine secretion is characterized by a massive protein release, rather than just devoted to the secretion of oily substances. Our current, albeit preliminary proteomic analyses of the apocrine secretory material, revealed that it is a very complex mixture consisting of hundreds or even thousands proteins from different subcellular locations with highly variable functions. The ontological distribution of these protein categories strongly resembles that of various organellar proteomes (Farkaš et al. 2014). Making use of recent advances in mass spectrometry and improved peptidome prefractionation, in-depth proteomic analyses of human body fluids, although without any special attention to apocrine secretion, were documented in the last 3–4 years. Interestingly and fortunately, some of them have provided insights into the proteomic composition of milk, sweat, tears, cerumen, saliva, etc. (Bandhakavi et al. 2009; Gao et al. 2012; Raiszadeh et al. 2012; Zhou et al. 2012; Feig et al. 2013), all of which are produced by apocrine organs. In accordance with the expectations and conclusions on apocrine secretion from the larval Drosophila salivary glands, these fluids are also remarkably complex. Though none of the currently available proteomes can be considered definitive, they can be organized into mutually comparable ontological categories. This comparison revealed that the distribution of categories between various proteomes is quite similar (Fig. 15.13). Among these human fluid proteomes, all of the same categories seen in the apocrine secretion from Drosophila SGs seem to be represented. This suggests that there must be a common purpose and function of apocrine secretion even among evolutionary distant metazoan groups, and that this could evolve from a common, fundamentally advantageous, ancestral trait. Comparison of five most complex proteomes identified to date (sweat, tears, cerumen, saliva and milk) reveal more than 300 shared entries. Put simply, between 30 and 65 % of apocrine fluids contain identical proteins regardless of their anatomical origin (Farkaš 2015).
Mass spectrometry analysis of the apocrine secretions. It contains a strikingly similar overall distribution of major ontological categories of proteins (A = subcellular localization, B = biological process). Secretions isolated from (a) Drosophila salivary glands; (b) human tears; (c) human sweat; (d) human cerumens; (e) human milk; (f) human bronchoalveolar fluid. (Reprinted with the permission of the publisher)
Merocrine exocytosis appears to be common to all eukaryotic cells from microbial yeasts to humans (Alberts et al. 2007; Lodish et al. 2012), while apocrine secretion is observed only in cells organized into tissues or organs; it is not found in individual cells. As such, apocrine secretion represents one of the few mechanisms that demarcate an evolutionary signature which uniquely characterizes metazoan eukaryotes . As already mentioned, as a very important feature of metazoan eukaryotes, apocrine secretion should have evolved quite early during or just after the Cambrian radiation, probably not later than during the Devonian period. With a period of 470–500 MYA (Hedges and Kumar 2009), the identification of genes that control apocrine secretion would significantly contribute to our understanding of molecular determinants that comprise a metazoan signature.
Apocrine secretion serves a fundamentally different purpose than other types of secretion. Furthermore, exocytosis can release only soluble proteins, solubilized inside of vesicle and bound on a cargo receptor (Machado et al. 2010). In contrast, apocrine secretion can release any kind of protein including polypeptides that would be insoluble in canonical vesicles. From an energetic perspective, it is a quite efficient mechanism. It saves the energy that would have been required to pack individual proteins into a vesicle and make them soluble for vesicular release. In fact, during apocrine discharge, the majority of secreted proteins inside gland’s lumen appear to be in their in situ location within their original subcellular compartments, which maintains them as both soluble and functional.
3.5 Distinguishing Molecular, Cellular and Evolutionary Attributes of Apocrine Secretion
The finding and comprehensive analysis of apocrine secretion in the Drosophila salivary glands (Farkaš et al. 2014) combined with a careful comparison of many historical and recent data in vertebrate models (Farkaš 2015), has made it clear that apocrine secretion has several specific characteristics and features that distinguish it from canonical exocytosis . (1) Apocrine secretion is non-canonical and non-vesicular trafficking and secretory pathway present exclusively in multicellular organisms. (2) It represents the en masse secretion of cellular components. (3) Apocrine secretion releases a secretory material that is a highly complex proteinaceous mixture with organellar components from all cellular compartments including the nucleus and nucleolus - the nuclear DNA, however, itself remains intact. (4) This secretory process is tightly regulated and selective, as some proteins or components are always released earlier than others. (5) Phylogenetic comparison reveals that anatomically divergent apocrine glands secrete very similar components with a conserved ontological distribution, with different secretions sharing 30–65 % of their proteins in common. (6) The apocrine secretion is an attribute restricted to metazoan, organized and polarized epithelial tissues or glands and is not observed in unicellular organisms or individual cells. (7) Even after a massive apocrine secretion, the released cellular components are renewed in the secretory cell by continued transcription and protein synthesis.
4 Conclusions
The Drosophila larval SGs have two major excretory functions that have been unambiguously documented. The first is the production and secretion of Sgs glue proteins at the very end of larval development and their expectoration during pupariation. The second is associated with the production and release of a highly complex proteinaceous mixture at the end of prepupal period. This is accomplished by apocrine secretion. Thus, the first large secretory activity is associated with the widely-known and well-described classical ER-Golgi-linked vesicular secretory machinery and results in the exocytosis of targeted proteins, whereas the second secretory activity is associated with a much less understood non-canonical and non-vesicular apocrine secretion. A comparison of the secretory material and its properties between exocytotic Sgs proteins and apocrine secretion reveals unexpected capabilities of this organ – it can reprogram its function to achieve two distinctly different roles. In contrast to exocytosis which releases and delivers a single product or a very small group of proteins or peptides, apocrine secretion serves to deliver hundreds or thousands of membranous, cytoskeletal, cytosolic, microsomal, mitochondrial, ribosomal, Golgi, and even nuclear as well as nucleolar proteins across the interface with the external environment. Moreover, an in-depth proteomic analysis of apocrine secretion from the Drosophila SGs and comparison with secretory materials from human apocrine glands has shown that all major ontological groups of proteins and their mutual distribution, either categorized by their subcellular location or biological/molecular function, remains highly conserved among evolutionary distant apocrine glands.
References
Abrams EW, Vining MS, Andrew DJ (2003) Constructing an organ: the Drosophila salivary gland as a model for tube formation. Trends Cell Biol 13:247–254
Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Sidén-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Worley KC, Woodage T, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC (2000) The genome sequence of Drosophila melanogaster. Science 287(5461):2185–2195
Adams MD, Sutton GG, Smith HO, Myers EW, Venter JC (2003) The independence of our genome assemblies. Proc Natl Acad Sci U S A 100:3025–3026
Agnew WF, Yuen TG, Achtyl TR (1980) Ultrastructural observations suggesting apocrine secretion in the choroid plexus: a comparative study. Neurol Res 1:313–332
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2007) Molecular biology of the cell, 5th edn. New York, Garland Science
Andrew DJ, Horner MA, Petitt MG, Smolik SM, Scott MP (1994) Setting limits on homeotic gene function: restraint of Sex combs reduced activity by teashirt and other homeotic genes. EMBO J 13:1132–1144
Armbruster K, Luschnig S (2012) The Drosophila Sec7 domain guanine nucleotide exchange factor protein Gartenzwerg localizes at the cis-Golgi and is essential for epithelial tube expansion. J Cell Sci 125:1318–1328
Ashburner M (1970) Function and structure of polytene chromosomes during insect development. Adv Insect Physiol 7:1–95
Ashburner M (1972) Puffing patterns in Drosophila melanogaster and related species. In: Beerman W (ed) Developmental studies on giant chromosomes. Springer, Berlin, pp 101–151
Ashburner M, Berendes HD (1978) Puffing of polytene chromosomes. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila, vol 2b. Academic, London, pp 315–395
Ashburner M, Bonner JJ (1979) The induction of gene activity in Drosophila by heat shock. Cell 17:241–254
Ashburner M, Chihara C, Meltzer P, Richards G (1974) Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol 38:655–662
Ashburner M, Golic KG, Hawley RS (2005) Drosophila: a laboratory handbook. CSHL Press, New York
Baehrecke EH (2003) Autophagic programmed cell death in Drosophila. Cell Death Differ 10:940–945
Bandhakavi S, Stone MD, Onsongo G, Van Riper SK, Griffin TJ (2009) A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J Proteome Res 8:5590–5600
Bardwell JCA, Jakob U (2012) Conditional disorder in chaperone action. Trends Biochem Sci 37:517–525
Barker JSF, Starmer WT, MacIntyre RJ (1990) Ecological and evolutionary genetics of Drosophila. Springer, New York
Beckendorf SK, Kafatos FC (1976) Differentiation in the salivary glands of Drosophila melanogaster: characterization of the glue proteins and their developmental appearance. Cell 9:365–373
Becker HJ (1959) Die Puffs der Speicheldrüssenchromosomen von Drosophila melanogaster. I. Beobachtungen zum Verhalten des Puffmuster im Normalstamm und bei zwei Mutanten, giant und lethal-giant-larvae. Chromosoma 10:654–678
Beermann W (1952) Chromomerkonstantz und spezifische Modifikation der Chromosomenstruktur in der Entwicklung und Organdifferenzierung von Chironomus tentans. Chromosoma 5:139–198
Beermann W (1972) Chromomeres and genes. In: Beermann W (ed) Developmental studies on giant chromosomes. Springer, Berlin, pp 1–53
Berendes HD (1965) Salivary gland function and chromosomal puffing patterns in Drosophila hydei. Chromosoma 17:35–77
Berendes HD, Ashburner M (1978) The salivary glands. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila, vol 2b. Academic Press, London, pp 453–498
Berendes HD, Holt TKH (1965) Differentiation of transplanted larval salivary glands of Drosophila hydei in adults of the same species. J Exp Zool 160:299–317
Biyasheva A, Do TV, Lu Y, Vaskova M, Andres AJ (2001) Glue secretion in the Drosophila salivary gland: a model for steroid-regulated exocytosis. Dev Biol 231:234–251
Blank U (2011) The mechanisms of exocytosis in mast cells. Adv Exp Med Biol 716:107–122
Blok JL, van Hattem S, Jonkman MF, Horváth B (2013) Systemic therapy with immunosuppressive agents and retinoids in hidradenitis suppurativa: a systematic review. Br J Dermatol 168:243–252
Bodenstein D (1950) The postembryonic development of Drosophila. In: Demerec K (ed) Biology of Drosophila. Wiley, New York, pp 1–35
Boyd M, Ashburner M (1977) The hormonal control of salivary gland secretion in Drosophila melanogaster: studies in vitro. J Insect Physiol 23:517–523
Bradley PL, Haberman AS, Andrew DJ (2001) Organ formation in Drosophila: specification and morphogenesis of the salivary gland. Bioessays 23:901–911
Breschet G, De Vauzeme R (1834) Recherches anatomiques et physiologiques sur les appareils tegumentaires des animaux. Ann des Sci Nat, 2e ser., Zool II: 167–238, 321–370
Bridges CB (1935) Salivary chromosome maps. J Hered 26:60–64
Bridges CB (1938) A revised map of the salivary gland X-chromosome of Drosophila melanogaster. J Hered 29:11–13
Bridges CB (1942) A new map of the salivary 2L chromosome of Drosophila melanogaster. J Hered 33:403–408
Bridges CB, Bridges PN (1939) A new map of the second chromosome: a revised map of the right limb of the second chromosome of Drosophila melanogaster. J Hered 30:475–476
Brunsting HA (1939) Hidradenitis suppurativa; abscess of apocrine sweat glands – a study of the clinical and pathologic features with a report of twenty-two cases and a review of the literature. Arch Dermatol Syphilol 39:108–120
Burgess J, Jauregui M, Tan J, Rollins J, Lallet S, Leventis PA, Boulianne GL, Chang HC, Le Borgne R, Krämer H, Brill JA (2011) AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Mol Biol Cell 22:2094–2105
Burgess J, Del Bel LM, Ma CI, Barylko B, Polevoy G, Rollins J, Albanesi JP, Krämer H, Brill JA (2012) Type II phosphatidylinositol 4-kinase regulates trafficking of secretory granule proteins in Drosophila. Development 139:3040–3050
Burtis KC, Thummel CS, Jones CW, Karim FD, Hogness DS (1990) The Drosophila 74EF early puff contains E74, a complex ecdysone-inducible gene that encodes two ets-related proteins. Cell 61:85–99
Campos-Ortega JA, Hartenstein V (1997) The embryonic development of Drosophila melanogaster, 2nd edn. Springer, Berlin
Celniker SE, Rubin GM (2003) The Drosophila melanogaster genome. Annu Rev Genomics Hum Genet 4:89–117
Chen CN, Malone T, Beckendorf SK, Davis RL (1987) At least two genes reside within a large intron of the dunce gene of Drosophila. Nature 329:721–724
Chieregatti E, Meldolesi J (2005) Regulated exocytosis: new organelles for non-secretory purposes. Nat Rev Mol Cell Biol 6:181–187
Chintapalli VR, Wang J, Dow JAT (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39:715–720
Crosby MA, Meyerowitz EM (1986) Drosophila glue gene Sgs3: sequences required for puffing and transcriptional regulation. Dev Biol 118:593–607
Crowley TE, Meyerowitz EM (1984) Steroid regulation of RNAs transcribed from the Drosophila 68C polytene chromosome puff. Dev Biol 102:110–121
Crowley TE, Bond MW, Meyerowitz EM (1983) The structural genes for three Drosophila glue proteins reside at a single polytene chromosome puff locus. Mol Cell Biol 3:623–634
Crowley TE, Mathers PH, Meyerowitz EM (1984) A trans-acting regulatory product necessary for expression of the Drosophila melanogaster 68C glue gene cluster. Cell 39:149–156
Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6:197–208
Engoher E, Kress H (1980) Glucosamine metabolism in Drosophila virilis salivary gland cells: ontogenic changes of enzyme activities and metabolic changes. Dev Biol 78:63–75
Farkaš R (2015) Apocrine secretion: new insights into an old phenomenon. Biochim Biophys Acta 1850:1740–1750
Farkaš R, Mechler BM (2000) The timing of Drosophila salivary gland apoptosis displays an l(2)gl-dose response. Cell Death Differ 7:89–101
Farkaš R, Sláma K (1999) Effect of bisacylhydrazine ecdysteroid mimics (RH-5849 and RH-5922) on chromosomal puffing, imaginal discs proliferation and pupariation in larvae of Drosophila melanogaster. Insect Biochem Mol Biol 29:1015–2027
Farkaš R, Sláma K (2015) Respiratory metabolism of salivary glands during the late larval and prepupal development of Drosophila melanogaster. J Insect Physiol 81:109–117
Farkaš R, Šuťáková G (1998) The ultrastructural changes of larval and prepupal salivary glands of Drosophila cultured in vitro with ecdysone. In Vitro Cell Dev Biol 34:813–823
Farkaš R, Šuťáková G (1999) Developmental regulation of granule size and numbers in larval salivary glands of Drosophila by steroid hormone ecdysone. Cell Biol Inl 23:671–676
Farkaš R, Ďatková Z, Mentelová L, Löw P, Beňová-Liszeková D, Beňo M, Sass M, Řehulka P, Řehulková H, Raška O, Kováčik L, Šmigová J, Raška I, Mechler BM (2014) Apocrine secretion in Drosophila salivary glands: subcellular origin, dynamics, and identification of secretory proteins. PLoS One 9:e94383
Farkaš R, Beňová-Liszeková D, Mentelová L, Mahmood S, Ďatková Z, Beňo M, Pečeňová L, Raška O, Šmigová J, Chase BA, Raška I, Mechler BM (2015) Vacuole dynamics in the salivary glands of Drosophila melanogaster during prepupal development. Dev Growth Differ 57:74–96
Feig MA, Hammer E, Völker U, Jehmlich N (2013) In-depth proteomic analysis of the human cerumen-a potential novel diagnostically relevant biofluid. J Proteomics 83:119–129
Fraenkel G (1952) A function of the salivary glands of the larvae of Drosophila and other flies. Biol Bull 103:285–286
Fraenkel G, Brookes VJ (1953) The process by which the puparia of many species of flies become fixed to a substrate. Biol Bull Mar Lab Woods Hole 105:442–449
Furia M, D’Avino PP, Digilio FA, Crispi S, Giordano E, Polito LC (1992) Effect of ecd 1 mutation on the expression of genes mapped at the Drosophila melanogaster 3C11-12 intermoult puff. Genet Res 59:19–26
Gao X, McMahon RJ, Woo JG, Davidson BS, Morrow AL, Zhang Q (2012) Temporal changes in milk proteomes reveal developing milk functions. J Proteome Res 11:3897–3907
Garfinkel MD, Pruitt RE, Meyerowitz EM (1983) DNA sequences, gene regulation and modular protein evolution in the Drosophila 68C glue gene cluster. J Mol Biol 168:765–789
Giangrande A, Mettling C, Richards GP (1987) Sgs-3 transcript levels are determined by multiple remote sequence elements. EMBO J 6:3079–3084
Giangrande A, Mettling C, Martin M, Ruiz C, Richards GP (1989) Drosophila Sgs3 TATA: effects of point mutations on expression in vivo and protein binding in vitro with staged nuclear extracts. EMBO J 8:3459–3466
Gloor RD (1962) Untersuchungen uber die wirkung der letalfaktoren l 52 und l 8 von Drosophila melanogaster. Revue Suisse Zool 69:409–463
Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, Brown JB, Cherbas L, Davis CA, Dobin A, Li R, Lin W, Malone JH, Mattiuzzo NR, Miller D, Sturgill D, Tuch BB, Zaleski C, Zhang D, Blanchette M, Dudoit S, Eads B, Green RE, Hammonds A, Jiang L, Kapranov P, Langton L, Perrimon N, Sandler JE, Wan KH, Willingham A, Zhang Y, Zou Y, Andrews J, Bickel PJ, Brenner SE, Brent MR, Cherbas P, Gingeras TR, Hoskins RA, Kaufman TC, Oliver B, Celniker SE (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471(7339):473–479
Gregg TG, McCrate A, Reveal G, Hall S, Rypstra AL (1990) Insectivory and social digestion in Drosophila. Biochem Genet 28:197–207
Grob H (1952) Entwicklungsphysiologische Untersuchungen an den Speicheldrusen dem Darmtraktus und den Imaginalscheiben einer Letalrasse (lgl) von Drosophila melanogaster. Z Indukt Abstamm- u VererbLehre 84:320–360
Grus FH, Podust VN, Bruns K, Lackner K, Fu S, Dalmasso EA, Wirthlin A, Pfeiffer N (2005) SELDI-TOF-MS ProteinChip array profiling of tears from patients with dry eye. Invest Ophthalmol Vis Sci 46:863–876
Gudeman DM, Brightman MW, Merisko EM, Merril CR (1989) Release from live choroids plexus of apical fragments and electrophoretic characterization of their synthetic products. J Neurosci Res 24:184–191
Guild GM (1984) Molecular analysis of a developmentally regulated gene which is expressed in the larval salivary gland of Drosophila. Dev Biol 102:462–470
Guild GM, Shore EM (1984) Larval salivary gland secretion proteins in Drososphila. Identification and characterization of the Sgs-5 structural gene. J Mol Biol 179:289–314
Gunasekaran K, Tsai CJ, Nussinov R (2004) Analysis of ordered and disordered protein complexes reveals structural features discriminating between stable and unstable monomers. J Mol Biol 341:1327–1341
Gurlt EF (1835) Vergleichende Untersuchungen über die Haut des Menschen und der Haus-Säugethiere, besonders in Beziehung auf die Absonderungs-Organe des Haut-Talges und desSchweisses. Archiv für Anatomie, Physiologie und wissenschaftliche Medicin, pp 399–418
Hadorn E, Faulhaber I (1962) Range of variability in cell number of larval salivaries. Drosoph Inf Serv 36:71
Harder JJ (1694) Glandula nova lachrymalis una cum ductu excretorio in cervis et damis. Acta Eruditorum Lipsiae 43:49–52
Harrod MJE, Kastritsis CD (1972a) Developmental studies in Drosophila. II. Ultrastructural analysis of the salivary glands of Drosophila pseudoobscura during some stages of development. J Ultrastruct Res 38:482–499
Harrod MJE, Kastritsis CD (1972b) Developmental studies in Drosophila. VI. Ultrastructural analysis of the salivary glands of Drosophila pseudoobscura during the late larval period. J Ultrastruct Res 40:292–312
Hedges SB, Kumar S (2009) The timetree of life. Oxford University Press, Oxford
Henderson KD, Andrew DJ (2000) Regulation and function of Scr, exd, and hth in the Drosophila salivary gland. Dev Biol 217:362–374
Herzenberg H (1927) Neue Beiträge zur Lehre von den apokrinen Schweissdrüsen. Virchows Arch Path Anat 266:422–455
Hibbs RG (1962) Electron microscopy of human apocrine sweat glands. J Invest Dermatol 38:77–84
Horner WE (1846) Special anatomy and histology, vol 1, 7th edn. Lea and Blanchard Press, Philadelphia
Hsu WS (1948) The Golgi material and mitochondria in the salivary glands of the larva of Drosophila melanogaster. Q J Microsc Sci 89:401–414
Iwashige K (1951) Beiträge zur Kenntnis der Eisenreaktion bei den apokrinen Schweissdrüsen der Achselhaut von Japaneren. Arch Histol Jap 2:367–374
Jahn R (2004) Principles of exocytosis and membrane fusion. Ann N Y Acad Sci 1014:170–178
Japrung D, Dogan J, Freedman KJ, Nadzeyka A, Bauerdick S, Albrecht T, Kim MJ, Jemth P, Edel JB (2013) Single-molecule studies of intrinsically disordered proteins using solid-state nanopores. Anal Chem 85:2449–2456
Jayaram SA, Senti KA, Tiklová K, Tsarouhas V, Hemphälä J, Samakovlis C (2008) COPI vesicle transport is a common requirement for tube expansion in Drosophila. PLoS One 3:e1964
Jiang C, Baehrecke EH, Thummel CS (1997) Steroid regulated programmed cell death during Drosophila metamorphosis. Development 124:4673–4683
Jiang C, Lamblin A-FJ, Steller H, Thummel CS (2000) A steroid-triggered transcriptional hierarchy controls salivary gland cell death during Drosophila metamorphosis. Mol Cell 5:445–455
Jochova J, Zakeri Z, Lockshin RA (1997) Rearrangement of the tubulin and actin cytoskeleton during programmed cell death in Drosophila salivary glands. Cell Death Differ 4:140–149
Kaminker JS, Bergman CM, Kronmiller B, Carlson J, Svirskas R, Patel S, Frise E, Wheeler DA, Lewis SE, Rubin GM, Ashburner M, Celniker SE (2002) The transposable elements of the Drosophila melanogaster euchromatin: a genomics perspective. Genome Biol 3:0084
Kasai H, Takahashi N, Tokumaru H (2012) Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev 92:1915–1964
Kato S, Minamitani K (1941) Kurze Mitteilung über die apokrinen Schweissdrüsen in der Aussenhaut des Nasenflügels bei den Chinesen. Okajimas Folia Anat Jap Bd 20:71–80
Kawabata I, Kurosumi K (1976) Transmission and scanning electron microscopy of the human ceruminous apocrine gland. II. Myoepithelial cells. Arch Histol Jpn 39:231–255
Kerman BE, Cheshire AM, Andrew DJ (2006) From fate to function: the Drosophila trachea and salivary gland as models for tubulogenesis. Differentiation 74:326–348
Kodani M (1948) The protein of the salivary gland secretion in Drosophila. Proc Natl Acad Sci U S A 34:131–135
Kolesnikov NN, Zhimulev IF (1975) Synthesis of mucoprotein secretory substance in salivary glands of the Drosophila melanogaster 3rd instar larvae. Ontogenez 6:171–182 (in Russian with English summary)
Korge G (1975) Chromosome puff activity and protein synthesis in larval salivary glands of Drosophila melanogaster. Proc Natl Acad Sci U S A 72:4550–4554
Korge G (1977a) Larval saliva in Drosophila melanogaster: production, composition, and relationship to chromosome puffs. Dev Biol 58:339–355
Korge G (1977b) Direct correlation between a chromosome puff and the synthesis of a larval saliva protein in Drosophila melanogaster. Chromosoma 62:155–174
Korge G (1981) Genetic analysis of the larval secretion gene Sgs-4 and its regulatory chromosome sites in Drosophila melanogaster. Chromosoma 84:373–390
Korol A, Rashkovetsky E, Iliadi K, Nevo E (2006) Drosophila flies in “Evolution Canyon” as a model for incipient sympatric speciation. Proc Natl Acad Sci U S A 103:18184–18189
Kress H (1979) Ecdysone-induced changes in glycoprotein synthesis and puff activities in Drosophila virilis salivary glands. Chromosoma 72:53–66
Kuno Y (1938) Variations in secretory activity of human sweat glands. Lancet 231:299–303
Kurosumi K, Kawabata I (1977) Transmission and scanning electron microscopy of the human ceruminous apocrine gland. Arch Histol Jpn 40:203–244
Lane NJ, Carter YR, Ashburner M (1972) Puffs and salivary gland function: the fine structure of the larval and prepupal salivary glands of Drosophila melanogaster. Wilhelm Roux Arch Entwickl Mechanik Org 169:216–238
Lasko LA, Post C, Kathju S (2008) Hidradenitis suppurativa: a disease of apocrine gland physiology. JAAPA 21:23–25
Lefevre G (1976) A photographic representation and interpretation of the polytene chromosomes of Drosophila melanogaster salivary glands. In: Ashburner M, Novitski E (eds) The genetics and biology of Drosophila, vol 1a. Academic, London, pp 31–66
Lehmann M (1996) Drosophila Sgs genes: stage and tissue specificity of hormone responsivness. BioEssays 18:47–54
Lehmann M, Korge G (1995) Ecdysone regulation of the Drosophila Sgs-4 gene is mediated by the synergistic action of ecdysone receptor and SEBP 3. EMBO J 14:716–726
Lesher S (1951a) Studies on the larval salivary gland of Drosophila. I. The nucleic acids. Exp Cell Res 2:577–585
Lesher S (1951b) Studies on the larval salivary gland of Drosophila. II. Changes in nuclear and nucleolar volumes and their possible significance. Exp Cell Res 2:586–588
Lesher S (1952) Studies on the larval salivary gland of Drosophila. III. The histochemical localization and possible significance of ribonucleic acid, alkaline phosphatase and polysaccharide. Anat Rec 114:633–652
Lindsley DL, Grell EH (1968) Genetic variations of Drosophila melanogaster, Carnegie Institution publication, no. 627. Carnegie Institution, Washington, DC
Lindsley DL, Zimm GG (1992) The genome of Drosophila melanogaster. Academic, San Diego
Lodish H, Berk A, Kaiser CA, Krieger M, Bretscher A, Ploegh H, Amon A, Scott MP (2012) Molecular cell biology, 7th edn. W. H. Freeman Publishers, San Francisco
Machado JD, Díaz-Vera J, Domínguez N, Alvarez CM, Pardo MR, Borges R (2010) Chromogranins A and B as regulators of vesicle cargo and exocytosis. Cell Mol Neurobiol 30:1181–1187
Makino S (1938) A morphological study of the nucleus in various kinds of somatic cell of Drosophila virilis. Cytologia 9:272–282
Malsam J, Kreye S, Söllner TH (2008) Membrane fusion: SNAREs and regulation. Cell Mol Life Sci 65:2814–2832
Martin DN, Baehrecke EH (2004) Caspases function in autophagic programmed cell death in Drosophila. Development 131:275–284
Maximov A, Tang J, Yang X, Pang ZP, Südhof TC (2009) Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323:516–521
Maybeck V, Röper K (2009) A targeted gain-of-function screen identifies genes affecting salivary gland morphogenesis/tubulogenesis in Drosophila. Genetics 181:543–565
McGuffin LJ, Bryson K, Jones DT (2000) The PSIPRED protein structure prediction server. Bioinformatics 16:404–405
Meyerowitz EM, Hogness DS (1982) Molecular organization of a Drosophila puff site that responds to ecdysone. Cell 28:165–176
Meyerowitz EM, Martin CH (1984) Adjacent chromosomal regions can evolve at very different rates: evolution of the Drosophila 68C glue gene cluster. J Mol Evol 20:251–264
Mislawsky AN (1909) Zur Lehre von der sogenannten blasenformigen Sekretion. Arch Mikrosk Anat 73:681–698
Misra S, Crosby MA, Mungall CJ, Matthews BB, Campbell KS, Hradecky P, Huang Y, Kaminker JS, Millburn GH, Prochnik SE, Smith CD, Tupy JL, Whitfied EJ, Bayraktaroglu L, Berman BP, Bettencourt BR, Celniker SE, de Grey AD, Drysdale RA, Harris NL, Richter J, Russo S, Schroeder AJ, Shu SQ, Stapleton M, Yamada C, Ashburner M, Gelbart WM, Rubin GM, Lewis SE (2002) Annotation of the Drosophila melanogaster euchromatic genome: a systematic review. Genome Biol 3: RESEARCH0083
Mittal J, Yoo T, Georgiou G, Truskett T (2013) Structural ensemble of an intrinsically disordered polypeptide. J Phys Chem B 117:118–124
Morales A, Cavicchia JC (1991) Release of cytoplasmic apical protrusions from principal cells of the cat epididymis, an electron microscopic study. Tissue Cell 23:505–513
Munro S (2005) The Arf-like GTPase Arl1 and its role in membrane traffic. Biochem Soc Trans 33:601–605
Muskavitch MA, Hogness DS (1980) Molecular analysis of a gene in a developmentally regulated puff of Drosophila melanogaster. Proc Natl Acad Sci U S A 77:7362–7366
Muskavitch MA, Hogness DS (1982) An expandable gene that encodes a Drosophila glue protein is not expressed in variants lacking remote upstream sequences. Cell 29:1041–1051
Myat MM (2005) Making tubes in the Drosophila embryo. Dev Dyn 232:617–632
Myat MM, Andrew DJ (2002) Epithelial tube morphology is determined by the polarized growth and delivery of apical membrane. Cell 111:879–891
Ng V, Cho P, To CH (2000) Tear proteins of normal young Hong Kong Chinese. Graefe’s Arch Clin Exp Ophthalmo 238:738–745
Niemeyer BA, Schwarz TL (2000) SNAP-24, a Drosophila SNAP-25 homologue on granule membranes, is a putative mediator of secretion and granule-granule fusion in salivary glands. J Cell Sci 113:4055–4064
Painter TS (1933) A new method for the study of chromosome rearrangements and the plotting of chromosome maps. Science 178:585–586
Painter TS (1934) Salivary chromosomes and the attack on the gene. J Hered 25:464–476
Painter TS (1945) Nuclear phenomena associated with secretion in certain gland cells with especial reference to the origin of cytoplasmic nucleic acid. J Exp Zool 100:523–544
Pardue ML, Feramisco J, Lindquist S (1989) Stress induced proteins. In: UCLA symposia on molecular and cellular biology, vol 96. Alan R. Liss, New York
Parsons PA (1978) Habitat selection and evolutionary strategies in Drosophila: an invited address. Behav Genet 8:511–526
Parsons PA (1981) Habitat selection and speciation in Drosophila. In: Atchley WR, Woodruff DS (eds) Evolution and speciation. Essays in honor of MJD White. Cambridge University Press, London, pp 219–240
Parsons PA (1994) Habitats, stress, and evolutionary rates. J Evol Biol 7:387–397
Pasteur N, Kastritsis CD (1973) Developmental studies in Drosophila. V. Alkaline phosphatases, dehydrogenases, and oxidases in organs and whole fly homogenates during development of D. pseudoobscura. Wilhelm Roux Arch Entwickl Mech Org 173:346–354
Paulsen F (2003) The human nasolacrimal ducts. Adv Anat Embryol Cell Biol 170:1–106
Pelling C (1966) A replicative and synthetic chromosomal unit – the modern concept of the chromomere. Proc R Soc B 164:279–289
Pelling C (1970) Puff-RNA in polytene chromosomes. Cold Spring Harb Symp Quant Biol 35:521–531
Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM (2010) Refinement of tools for targeted gene expression in Drosophila. Genetics 186:735–755
Poels CLM (1970) Time sequence in the expression of various developmental characters induced by ecdysterone in Drosophila hydei. Dev Biol 23:210–225
Poels CLM, De Loof A, Berendes H (1971) Functional and structural changes in Drosophila salivary gland cells triggered by ecdysterone. J Insect Physiol 17:1717–1729
Porat-Shliom N, Milberg O, Masedunskas A, Weigert R (2013) Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol Life Sci 70:2099–2121
Poulson DF (1937) Chromosomal deficiencies and the embryonic development of Drosophila melanogaster. Proc Natl Acad Sci U S A 23:133–137
Poulson DF (1950) Histogenesis, organogenesis and differentiation in the embryo of Drosophila melanogaster Meigen. In: Demerec M (ed) Biology of Drosophila. Wiley, New York, pp 168–274
Purkinje JE (1833a) Beobachtungen der spiralen Schweisscanäle der menschlichen Epidermis. Amtl Bericht über die Vers deutcher Naturf u Arzte zu Breslau 11:59
Purkinjie JE (1833b) Beobachtungen der spiralen Schweisscanäle der menschlichen Epidermis. Notizen aus dem Gebiete der Natur- und Heilkunde (Froriep) Weimar 38:152
Raiszadeh MM, Ross MM, Russo PS, Schaepper MA, Zhou W, Deng J, Ng D, Dickson A, Dickson C, Strom M, Osorio C, Soeprono T, Wulfkuhle JD, Petricoin EF, Liotta LA, Kirsch WM (2012) Proteomic analysis of eccrine sweat: implications for the discovery of schizophrenia biomarker proteins. J Proteome Res 11:2127–2139
Ranvier L (1879) Sur la structure des glandes sudoripares. Compt Rend Acad Séanc Acad Sci (Paris) 89:1120–1123
Ream LJ, Principato R (1981) Ultrastructural observations on the mechanism of secretion in the rat parathyroid after fluoride ingestion. Cell Tissue Res 214:569–573
Richards GP (1976a) The control of prepupal puffing patterns in vitro: implications for prepupal ecdysone titres in Drosophila melanogaster. Dev Biol 48:191–195
Richards GP (1976b) Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. IV. The mid prepupal period. Dev Biol 54:256–263
Richards G (1981) The radioimmune assay of ecdysteroid titres in Drosophila melanogaster. Mol Cell Endocrinol 21:181–197
Richter W (1932) Beiträge zur normalen und pathologischen Anatomie der apokrinen Hautdrüsen des Menschen mit besonderer Berücksichtigung des Achselhöhlenorgans. Virchows Arch Pathol Anat Physiol Klin Med 287:277–296
Richter W, Schmidt W (1934) Über das Vorkommen apokriner Drüsen in der Haut des Nasenflügels. Z mikr anat Forsch 35:529–532
Ritossa FM (1962) A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 18:571–573
Ritossa FM (1963) New puffs induced by temperature shock, DNP and salicylate in salivary chromosomes of D. melanogaster. Drosoph Inf Serv 37:122–123
Robinson SW, Herzyk P, Dow JAT, Leader DP (2013) FlyAtlas: database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Res 41(Database issue):D744–D750
Romero P, Obradovic Z, Dunker AK (1997) Sequence data analysis for long disordered regions prediction in the calcineurin family. Genome Inform 8:110–124
Romero P, Obradovic Z, LiX GE, Brown C, Dunker AK (2001) Sequence complexity of disordered protein. Proteins 42:38–48
Ross EB (1939) The postembryonic development of the salivary glands of Drosophila melanogaster. J Morphol 65:471–496
Roth GE, Wattler S, Bornschein H, Lehmann M, Korge G (1999) Structure and regulation of the salivary gland secretion protein gene Sgs-1 of Drosophila melanogaster. Genetics 153:753–762
Saraste J, Dale HA, Bazzocco S, Marie M (2009) Emerging new roles of the pre-Golgi intermediate compartment in biosynthetic-secretory trafficking. FEBS Lett 583:3804–3810
Schaumburg-Lever G, Lever WF (1975) Secretion from human apocrine glands: an electron microscopic study. J Invest Dermatol 64:38–41
Schiefferdecker P (1917) Die Hautdrüsen des Menschen und der Säugetiere. Biol Zentralbl 37:11–12
Schiefferdecker P (1921) Über morphologische Sekretionserscheinungen in den ekkrinen Hautdrüsen des Menschen. Arch Dermatol Syph 132:130–132
Schnitter M (1961) Zur Genetic und Entwicklungsphysiologie des Faktors ‘letal scheiben defekt’ (lsd) bei Drosophila melanogaster. Revue suisse Zool 68:345–418
Schwarz R, MR F e-B, Peukert-Adam I (1988) The mode of secretion in the anterior pituitary gland of the cow and the ewe. Anat Anz 167:183–189
Segraves WA, Hogness DS (1990) The E75 ecdysone-inducible gene responsible for the 75B early puff in Drosophila encodes two new members of the steroid receptor superfamily. Genes Dev 4:204–219
Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ (2007) Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128:183–195
Skaer H (1993) The alimentary canal. In: Bate M, Martinez Arias A (eds) The development of Drosophila melanogaster. Cold Spring Harbor Press, New York, pp 941–1012
Sonnenblick BP (1940) The salivary glands in the embryo of Drosophila melanogaster. Genetics 25:137
Sonnenblick BP (1950) The early embryology of Drosophila melanogaster. In: Demerec M (ed) Biology of Drosophila. Wiley, New York, pp 62–167
Sorsa V (1989) Chromosome maps of Drosophila melanogaster. CRC Press, Boca Raton
Stark A, Lin MF, Kheradpour P, Pedersen JS, Parts L, Carlson JW, Crosby MA, Rasmussen MD, Roy S, Deoras AN, Ruby JG, Brennecke J, Harvard FlyBase curators, Berkeley Drosophila Genome Project, Hodges E, Hinrichs AS, Caspi A, Paten B, Park SW, Han MV, Maeder ML, Polansky BJ, Robson BE, Aerts S, van Helden J, Hassan B, Gilbert DG, Eastman DA, Rice M, Weir M, Hahn MW, Park Y, Dewey CN, Pachter L, Kent WJ, Haussler D, Lai EC, Bartel DP, Hannon GJ, Kaufman TC, Eisen MB, Clark AG, Smith D, Celniker SE, Gelbart WM, Kellis M (2007) Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures. Nature 450:219–232
Südhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547
Südhof TC, Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323:474–477
Syed ZA, Härd T, Uv A, van Dijk-Härd IF (2008) A potential role for Drosophila mucins in development and physiology. PLoS One 3:e3041
Szul T, Burgess J, Jeon M, Zinn K, Marques G, Brill JA, Sztul E (2011) The Garz Sec7 domain guanine nucleotide exchange factor for Arf regulates salivary gland development in Drosophila. Cell Logist 1:69–76
Talke L (1903) Über die grossen Drüsen der Achselhöhenhaut des Menschen. Arch Mikrosk Anat 61:537–555
Thummel CS (1996) Flies on steroids – Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet 12:306–310
Thummel CS (2002) Ecdysone-regulated puff genes 2000. Insect Biochem Mol Biol 32:113–120
Tsarouhas V, Senti KA, Jayaram SA, Tiklová K, Hemphälä J, Adler J, Samakovlis C (2007) Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell 13:214–225
Uversky VN, Oldfield CJ, Dunker AK (2008) Intrinsically disordered proteins in human diseases: introducing the D2Concept. Annu Rev Biophys 37:215–246
Velissariou V, Ashburner M (1980) The secretory proteins of the larval salivary gland of Drosophila melanogaster: cytogenetic correlation of a protein and a puff. Chromosoma 77:13–27
Velissariou V, Ashburner M (1981) Cytogenetic and genetic mapping of a salivary gland secretion protein in Drosophila melanogaster. Chromosoma 84:173–185
Velpeau A (1839) Aiselle. In: Dictionnaire de Médecine, un Répertoire Général des Sciences Médicales sous la Rapport Théorique et Practique tome. 2, 2nd edn. Bechet Jeune Paris, pp 86–109
Verneuil AS (1854) Études sur les tumeurs de la peau et quelques maladies de glandes sudoripares. Arch Gén Méd 4:447–468
Versura P, Nanni P, Bavelloni A, Blalock WL, Piazzi M, Roda A, Campos EC (2010) Tear proteomics in evaporative dry eye disease. Eye 24:1396–1402
Vining MS, Bradley PL, Comeaux CA, Andrew DJ (2005) Organ positioning in Drosophila requires complex tissue-tissue interactions. Dev Biol 287:19–34
von Gaudecker B (1972) Der Strukturwandel der larvalen Speicheldrüse von Drosophila melanogaster. Ein Beitrag zur Frage nach der steuernden Wirkung aktiver Gene auf das Cytoplasma. Z Zellforsch Mikrosk Anat 127:50–86
von Gaudecker B, Schmale EM (1974) Substrate-histochemical investigations and ultrahistochemical demonstrations of acid phosphatase in larval and prepupal salivary glands of Drosophila melanogaster. Cell Tissue Res 155:75–89
Walter AM, Wiederhold K, Bruns D, Fasshauer D, Sørensen JB (2010) Synaptobrevin N-terminally bound to syntaxin-SNAP-25 defines the primed vesicle state in regulated exocytosis. J Cell Biol 188:401–413
Wang S, Tsarouhas V, Xylourgidis N, Sabri N, Tiklová K, Nautiyal N, Gallio M, Samakovlis C (2009) The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat Cell Biol 11:890–895
Wang S, Meyer H, Ochoa-Espinosa A, Buchwald U, Onel S, Altenhein B, Heinisch JJ, Affolter M, Paululat A (2012) GBF1 (Gartenzwerg)-dependent secretion is required for Drosophila tubulogenesis. J Cell Sci 125:461–472
Wendt A (1833) De epidermide humana. Dissertatio inauguralis anatomica 4. pag. VI + 36. Accedit tabula aenea. Universitas Vratislaviae
Wendt A (1834) Über die menschliche Epidermis. Archiv für Anatomie, Physiologie und wissenschaftliche Medicin. pp 278–291
Wu M, Lu L, Hong W, Song H (2004) Structural basis for recruitment of GRIP domain golgin-245 by small GTPase Arl1. Nat Struct Mol Biol 11:86–94
Zhimulev IF, Kolesnikov NN (1975) Synthesis and secretion of mucoprotein glue in the salivary gland of Drosophila melanogaster. Wilhelm Rouxs Arch Dev Biol 178:15–28
Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, Tong L, Liu S, Stern ME, Tan D (2009) Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res 8:4889–4905
Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW (2012) In-depth analysis of the human tear proteome. J Proteomics 75:3877–3885
Acknowledgements
I appreciate the comments, critical reading of the manuscript and many helpful suggestions by Bruce A. Chase. The author also would like to thank Denisa Beňová-Liszeková, Milan Beňo, Ludmila Pečeňová, Lucia Mentelová, Magda Bardačová and other members of the lab and close colleagues for help and continuous support during preparation of this manuscript. Drosophila Bloomington Stock Center, Indiana University and the Developmental Studies Hybridoma Bank, University of Iowa are acknowledged for making fly stocks and antibodies available to us during many previous years. This work was supported by an APVT-51-027402, a VEGA 2/0170/10, 2/0109/13 grants, a MVTS-32060600/EC-Instruct-FP7-211252 grant, and EEA-Norwegian FM SK-0086 grant to R.F.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Farkaš, R. (2016). The Complex Secretions of the Salivary Glands of Drosophila melanogaster, A Model System. In: Cohen, E., Moussian, B. (eds) Extracellular Composite Matrices in Arthropods. Springer, Cham. https://doi.org/10.1007/978-3-319-40740-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-40740-1_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40738-8
Online ISBN: 978-3-319-40740-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)