Abstract
Multiple myeloma (MM) is a disease of the elderly, with a median age at diagnosis of approximately 70 years old, and more than 30 % of patients aged >75 years. This latter and very elderly population is going to significantly rise in the near future given the increase in life expectancy in Western countries, and, most importantly, global health status of elderly patients is improving, justifying appropriate treatments. Changes in treatment paradigm from the old melphalan-prednisone regimen used since the 1970s to its use as a backbone in a nontransplant setting since the late 1990s have highlighted different subgroups in elderly MM. Some “elderly” patients could be treated like transplant eligible patients, more likely those aged between 65 and the early 70; while a second group would rather be referred to current approved treatment regimens for the non-transplant setting. A dose-intensity approach seems reasonable for this group, aiming for the best response, eventually the complete response (CR) or even minimal residual disease (MRD). The advent of novel agents such as thalidomide, bortezomib, and most recently lenalidomide have allowed a major improvement in outcome as compared to historical combinations, and soon the novel class of monoclonal antibodies should help to further improve these patients’ survival. Nonetheless, elderly patients are more susceptible to side effects and are often unable to tolerate full drug doses, and thus require lower dose intensity regimens, or novel drugs or combinations with more favourable safety profile. Recent developments in MM have focused on identifying these vulnerable patients through geriatric assessment and novel myeloma scoring system, including the notions of frailty, disability and comorbidities. Eventually, we have reached an era in which we should be able to provide individualized treatment strategies and drug doses—“tailored therapy”—to improve tolerability and optimize efficacy and ultimately survival for most elderly MM patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Multiple myeloma (MM) is a malignant neoplasia characterized by clonal plasma cell proliferation, driven by intrinsic genomic abnormalities and extrinsic bone marrow stromal cell support, associated with a monoclonal protein present in the blood and/or urine [1]. In Western countries, MM represents 1.5 % of all malignant diseases, with an annual age-adjusted incidence of 5.6 cases per 100,000 people [2].
MM is a disease of the elderly: median age at diagnosis is close to 70 years, with about two-third aged ≥65 years—including 34.8 % of patients diagnosed after 75 years, and 9.6 % after 85 years [2]. The number of elderly MM patients is expected to increase over time, thanks to the increased life expectancy of the general population, but also to the improved survival enabled by the increase use of potent novel agents.
However, MM remains a fatal disease and its prognosis remains poor in elderly patients, with a median overall survival of 24 months in patients aged over 75 years at diagnosis in the US [2], and a 5-year overall survival of 26 % for the 70–79 years old, and 14 % for the 80–99 year old in the UK [3]. There still is an unmet medical need in this population, as early as the first relapse setting for most of them, and even at diagnosis for the very elderly and frail; progress is therefore needed for these patients. Still, despite the efforts in drug development and progress in understanding the physiopathology of MM, management of elderly patients with MM will remain challenging, because of specific clinical and biological features but essentially because of frailty, comorbidities, financial, and psychosocial factors.
We will review current treatments, discuss various improvements in global appreciation of the health status, and display future perspectives.
2 Geriatric Assessment
Frailty. A precise clinical assessment is essential when treating elderly patients, as age alone is obviously very insufficient, knowing this population is characterized by an important heterogeneity. Several studies have showed that the “in the ballpark” geriatric assessment drove to a certain failure in many elderly patients. The notion of frailty has therefore been introduced to help qualify these patients characterized by a certain risk of significant side effects during treatment—and shorter survival due to these safety issues. It is now a consensual term, but no single sign of symptom is sufficient to define it [4]. Indexes of frailty have been developed according to several factors such as weakness, poor endurance, weight loss, low physical activity, and slow gait speed. At least three factors should be present in order to define a “clinically frail elderly patient”, and the presence of this “frailty” has been identified as an independent pejorative factor in elderly adults [5]. The different degrees of frailty are summarized in Table 1.
Comorbidities also have to be taken into account, formally defined as the concurrent presence of at least two diseases diagnosed in the same person [4]. The frequency of individual chronic conditions, along with the incidence of comorbid conditions, rises with age. Comorbidity is associated with polymedication and increased risk of drug interactions. Many prognostic indices for the elderly incorporating comorbidity are available [6–8], but these scores are often complicated.
Disability. Disability is an important notion in geriatric assessment, and can include both physical and mental impairments. It is defined as the difficulty or dependency in carrying out activities essential to independent living, including both essential personal care and household tasks, and activities that are important to maintain a person’s quality of life [9]. Disability, independent of its causes, is associated with a higher risk of mortality; disabled adults are more likely to become hospitalized [10]. In patients with MM, disability can be caused by orthopaedic problems and pain; otherwise, the main causes of physical disability in the elderly are chronic diseases such as cardiovascular disease, stroke or arthritis [10].
Scoring system. It should therefore be mandatory to perform a geriatric assessment to all elderly patients with MM, at least over the age of 70, and/or suffering from any kind of frailty, comorbidities or disability. One might consider that these patients should be seen and assessed by geriatricians which expertise is indisputable, but unfortunately this ideal assessment is rarely feasible due to the lack of geriatricians and the increased number of elderly MM patients.
Very recently, a frailty score that combines age, comorbidities and functional status (disability) has been proposed for elderly patients with MM [11]. In addition to age, three tools were used: the Katz Activity of Daily Living (ADL), the Lawton Instrumental Activity of Daily Living (IADL), and the Charlson Comorbidity Index (CCI). In a multivariate analysis, adjusted for ISS, chromosome abnormalities and type of therapy, a higher risk of death was observed for patients aged 75–80 years (score −1), and over 80 years (score = 2), and for those with an ADL score ≤4 (score = 1), an IADL ≤5 (score = 1) or a CCI ≥2 (score = 1). By combining the risk scores (range, 0–5) for these variables, patients were stratified into three distinctive risk groups for overall survival: fit (score = 0), intermediate fitness (score = 1) and frail (score ≥2). This frailty score could predict survival and toxicity, as the “frail” group displayed an increased risk of death, progression, non-hematologic adverse events and treatment discontinuation, regardless of ISS stage, chromosome abnormalities and type of treatment [11]. The authors even proposed an association of this frailty score with the ISS score.
Several questions remain unanswered; for instance, whether all patients should benefit from this evaluation or only patients selected according to their age and comorbidities. In routine practice, geriatric assessment is performed especially for patients aged over 70–75 years and identified with comorbidities. However, if geriatric assessment can help to better understand the precise geriatric risk that fits each elderly patient, it could thus also be useful to identify elderly patients (65–70 years, or even over 70) that could benefit from a “young” patient-based therapy, if they are deemed fit enough.
3 Biologic and Cytogenetic Features
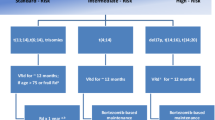
Biologic and cytogenetic features in MM are quite similar amongst the young and the elderly. Most of the cytogenetic data collected in the past few years came from younger, transplant-eligible newly diagnosed patients. Recently, the Intergroupe Francophone du Myélome (IFM) group reported on a series of 1890 elderly patients (>65 years) [12]. Patients were classified in two groups: 66–75 years (n = 1,239), and >75 years (n = 651), and incidence and clinical impact of three chromosomal aberrations [del(13), t(4;14), or del(17p)] were analyzed. Interestingly, they found a lower incidence of t(4; 14) and del(13) in the oldest patients, whereas incidence of del(17p) was remarkably stable. Regardless of treatment, both t(4; 14) and del(17p) were associated with a worse clinical outcome in this cohort of elderly patients with MM, highlighting the importance of cytogenetic analysis at diagnosis in all MM patients.
However, even if some data seem to suggest that VMP (melphalan-prednisone-bortezomib) may overcome the adverse prognosis associated with certain high-risk cytogenetic abnormalities [13], there is no certainty yet about the optimal management of these patients.
4 Response to Therapy as Primary Goal in Elderly Patients
The primary goal in MM has always been to improve survival across all age categories, as MM remains lethal for the vast majority of patients in a median of 5–7 years. In elderly patients, things are not always as simple: even if prolongation of disease-free survival and overall survival remains the ultimate goal, achieving prolonged treatment-free intervals and good quality of life have indeed also become important aims, along with avoiding complications—especially bone disease and thromboembolic events.
A surrogate marker to survival has long been to obtain at least VGPR (very good partial response). More recently, deeper responses such as CR (complete response) or even MRD (minimal residual disease) have become the optimal short-term endpoint, highly correlated to prolonged survival, including in elderly patients. The role of CR has indeed been evaluated in a retrospective analysis of 1175 elderly patients with newly diagnosed MM treated with novel agents and MP [14]. In this study, achieving CR was associated with improved progression-free survival (PFS) and overall survival (OS). Moreover, upon using more sensitive parameters such as serum free light-chain and multiparameter flow cytometry to define the depth of response, the Spanish group’s prospective analysis of elderly patients receiving novel agents showed that achieving an immunophenotypic response translated into better PFS compared with conventional CR or stringent CR [15].
However, in older patients, settling for a lower degree of response may be reasonable from case to case as treatment-related toxicities could outshine any benefit derived from the achievement of a CR. Despite improvement in overall survival, novel agents are indeed associated with adverse events that may impair quality of life (QOL) [16], which tempers down the benefit in improvement of MM-related symptoms such as skeletal-related events. This impairment in QOL can, however, be transient—as seen in the VISTA trial where Bortezomib was associated with a deterioration of the QOL indices for the first four cycles only [17]. In the absence of difference in treatment efficacy, the choice of initial treatment should thus be based on QOL indicators in elderly patients.
5 Supportive Care
Besides specific therapies, supportive care is essential in MM and especially in elderly patients. These patients need special attention in terms of management of anemia, pain (with a special focus on painkillers’ adverse effects), hypercalcemia, bone disease (especially use of intravenous bisphosphonates), infections prophylaxis (crucial in elderly patients) and nutrition.
Occurrence of adverse events during treatment should also be carefully taken into account to adjust doses and schedule.
6 Review of Current Approved First-Line Therapy
Melphalan-prednisone (MP) remained the gold standard for many years since its first description by Alexanian in [18]. Combining MP with conventional agents such as anthracyclines and vincristine did not improve outcome [19]; but combinations to novel agents such as Thalidomide and Bortezomib finally led to an improvement in overall survival. The current standards of care upfront in elderly MM patients ineligible for autologous stem cells transplantation are thus MPT (melphalan-prednisone-thalidomide) and VMP (bortezomib-melphalan-prednisone), with derivatives in the alkylating agent-based backbone, with either cyclophosphamide (CTD: cyclophosphamide-thalidomide-dexamethasone) [20], and bendamustine.
6.1 Thalidomide-Based Therapy
Thalidomide is particularly appealing in the elderly because of its lack of myelosuppression and its simple use in case of renal insufficiency, but will probably become more and more outshined by the advent of novel generation drugs. Ludwig et al. first showed the superiority of thalidomide-dexamethasone compared with MP in elderly patients, and especially in the over 75 subgroup [21]. Hulin et al. in the IFM 01/01 then reported the superiority of MPT (melphalan-prednisone-thalidomide) over MP-placebo in patients older than 75 years with newly diagnosed MM [22]. A significant benefit in progression-free survival and overall survival was indeed observed, and toxicity was acceptable with however more grade 2–4 neuropathy and grade 3–4 neutropenia in the MPT arm. A meta-analysis of published data from six randomized trials confirmed the improvement in progression-free survival (PFS) and overall survival (OS) with MPT (melphalan-prednisone-thalidomide) compared with MP [23]. The longer the treatment was continued, the better the outcome was. The reported median PFS and OS with MPT were 20.3 and 39.3 months, respectively. Toxicity, nevertheless, was always higher in the MPT arm [22, 24, 25], and this regimen is likely to be dethroned by less-toxic associations.
The combination of cyclophosphamide, thalidomide and dexamethasone (CTD) also improved response rates compared with MP. Evidence from the Myeloma IX trial suggested a survival benefit in CTD-treated patients with favourable cytogenetics, although early deaths from infections related to high-dose dexamethasone were significant [26, 27].
6.2 Bortezomib-Based Therapy
Bortezomib has excellent activity in MM at any stage of the disease and is synergistic with other agents, which led to several combination strategies.
First developed in the VISTA trial [17, 28], the addition of twice-weekly intravenous bortezomib to MP (VMP) is now a well-established regimen. VMP was proven superior to MP in response rate, CR rate, median TTP (time to progression) and OS, even over all cytogenetic and renal failure subgroups [29]. This superiority was sustained after a median follow-up of 60 months, in terms of median time to second-line antimyeloma therapy (31 months with VMP versus 20.5 months with MP) and median OS (56 months versus 43 months, respectively).
Neuropathy was the major side effect of this regimen. Changes in schedule and administration have then been made in order to reduce toxicity: the twice-weekly schedule was replaced by a weekly schedule in 2010 based on new clinical evidence [30–33] and from intravenous to subcutaneous administration in 2012 [28, 34]. Once weekly regimens are better tolerated especially in the elderly, and are associated with reduced toxicity such as neuropathy, diarrhea, constipation and thrombocytopenia [35]. Two schedules can however be discussed: a once weekly regimen from the start [31], or a twice weekly regimen for the first cycle (“VISTA” regimen) followed by a once weekly regimen for the remaining cycles [33]. It has been shown that a higher cumulative bortezomib dose, resulting from an increased dose/intensity or a prolonged treatment duration, is associated with improved OS [36]. The authors propose that dose/schedule modifications—and for instance beginning with a twice-weekly schedule, continuing therapy in responding patients, proactive management of adverse events, and subcutaneous administration of bortezomib, could help to achieve higher cumulative doses and maximize treatment duration and outcomes. The subcutaneous administration of bortezomib is indeed associated with a reduced toxicity (especially neuropathy) and similar activity [34].
Given its known efficacy and its improved safety profile, plus its easiness and in dose adaptation, VMP has now become the most prescribed regimen worldwide upfront for elderly MM.
6.3 Bendamustine Upfront in Elderly MM
The data on bendamustine are scarcer, but this drug is approved upfront with prednisone in elderly patients that could not benefit from MPT or MPV because of peripheral neuropathy. The rationale for this approval was based on a randomized trial in which bendamustine-prednisone has been proven superior to MP [37], with respect to CR rate (32 % vs. 13 %, p = 0.007), and with a benefit in terms of time-to-treatment failure (14 months vs. 10 months; p = 0.020), but without any benefit on overall survival. Bendamustine-prednisone is now an interesting option for patients ineligibile for autologous stem cell transplantation, and ineligible for VMP or MPT regimens.
Bendamustine plus prednisone in combination with bortezomib is currently being evaluated in several pilot clinical trials.
6.4 Autologous Stem Cell Transplantation (ASCT)
Although age does not affect the outcome of ASCT [38], the 65-year-old cut-off was commonly used to determine ASCT eligibility in patients with MM for safety reasons. However, the feasibility of ASCT is now well-established in fit patients up to the age of 70, although it should remain a “case-per-case” decision [39]. Even if evidence from the IFM 99-06 trial did not suggest any benefit of ASCT in this population [24], early ASCT may nevertheless be appropriate in selected fit patients between 65 and 70 years of age. Intermediate-dose melphalan (140 mg/m2) should be preferred to high-dose melphalan (200 mg/m2) in this population, as retrospective data suggests a better safety profile and a similar efficacy [40]. Lower doses (100–140 mg/m2) can be used for older patients.
Tandem ASCT with melphalan 100 mg/m2 (MEL100) is another option: Palumbo et al. indeed showed that tandem MEL100 ASCT was superior to conventional MP therapy, especially in patients aged 65–70 [41]. They then reported another valuable option including tandem MEL100 ASCT for elderly patients with MM, especially for those aged <70: 4 cycles of bortezomib-pegylated liposomal doxorubicin-dexamethasone, tandem MEL100 ASCT, 4 cycles of lenalidomide-prednisone consolidation, and lenalidomide maintenance until disease progression. After a median follow-up of 66 months, this sequential approach resulted in a median time-to-progression of 55 months, a median PFS of 48 months, a median OS not reached and 5-year OS of 63 % [42].
ASCT in elderly patients with significantly compromised renal function should however be avoided.
6.5 A New Standard of Care, Lenalidomide-Based
Recently, lenalidomide plus low-dose dexamethasone (Rd) has emerged as a promising new option especially in relapsed MM, or upfront in elderly patients. It is an attractive option for elderly patients because of its excellent tolerability, convenience and efficacy: amongst the patients 70 and older from the ECOG study, the 3-year OS rate was indeed 70 % [43].
The IFM2007-01/MM020/FIRST study [44] compared lenalidomide-low dose dexamethasone upfront in elderly MM patients, to the standard of care MPT. This phase 3 multicenter trial randomized 1623 newly diagnosed elderly MM patients aged 65 years or older and ineligible for ASCT, between three treatment arms: melphalan-prednisone-thalidomide (MPT) administered for 12 cycles so 18 months, versus lenalidomide-dexamethasone given either for 18 cycles so 18 months (Rd18) or until progression or intolerance (continuous Rd). Lenalidomide was given at 25 mg/day for 21 days out of 28, and dexamethasone at 20 or 40 mg per week. Approximately, 35 % of patients included were aged over 75 years, 47–50 % had a creatinine clearance <60 mL/min and 8–10 % <30 mL/min.
Compared with MPT, continuous Rd significantly improved PFS, and even showed an OS benefit at the interim analysis. With a median follow-up of 37 months, median PFS was 25.5 months for Rd, compared with 20.7 months for Rd 18 and 21.2 months for MPT. Improvement in OS was significant when comparing continuous Rd with MPT (estimated 4-year OS, 59.4 % vs. 51.4 %, p = 0.0168), but not when comparing continuous Rd with Rd18 (4-year OS, 59.4 % vs. 55.7 %, p = 0.307). In addition, Rd was superior to MPT across all other efficacy endpoints, including response rate, TTP, time to treatment failure, time to second-line antimyeloma therapy and duration of response.
Moreover, median PFS and OS achieved with MPT in the FIRST study compare favourably with those reported in published data: median PFS of 21.2 months versus 20.3 months in the meta-analysis, and median OS of about 46 months versus 39.3 months, respectively [23]. Rd was thus superior to MPT intrinsically, and not because MPT was less efficient than expected in this study.
It is worth noting that evaluation of PFS2 (PFS on second-line therapy), which is now adopted as a surrogate marker for OS and was a secondary endpoint in the FIRST study, also showed improvement in favour of continuous Rd as compared with MPT (HR = 0.78, p = 0.0051).
Bahlis et al. recently reported that duration of response was remarkably longer in patients treated with continuous Rd (35 months) versus Rd18 (22.18 months, p < 0.01) or MPT (22.3 months, p < 0.01) regardless of the depth of response, but the benefits of continuous Rd were even more pronounced in patients who achieved a greater depth of response. When comparing continuous Rd versus Rd18 and MPT, median PFS was indeed not reached versus 31 and 34.7 months, respectively, for patients in VGPR, and median PFS not reached versus 45.2 and 44.6 months, respectively, for patients in CR [45].
Concerning the safety profile, Rd was also generally better tolerated than MPT [44]. Interestingly, most of the adverse events—and especially infections—were mainly imputable to dexamethasone, more than to lenalidomide itself. The incidence of thromboembolic events was slightly higher in the continuous Rd arm: 8 %, versus 6 % in Rd18, and 5 % in the MPT arm. Second primary malignancies were higher with MPT (5 %) than with continuous Rd (3 %), which is consistent with reports suggesting that the increased risk of a second primary cancer among patients treated with lenalidomide may be related to prior or concurrent melphalan use. Quality of life was also assessed, and was improved in all three arms of treatment.
Lenalidomide plus low-dose dexamethasone is thus becoming a new standard of care upfront for MM patients ineligible for ASCT, and has been recently approved by the EMA in this indication.
This FIRST study has pushed the boundaries of MM treatment at least twice, defining not one but 2 new changes in treatment paradigm in elderly MM patients upfront: for the first time an alkylator-free option is suitable for first-line therapy, and a doublet-based regimen, supposedly safer, could prove more effective than a triplet-based regimen. One could foresee that some patients might never be exposed to alkylators throughout their MM disease history in the near future. On the other hand, one would also find of interest to compare Rd to VMP to validate the superiority of Rd not only over MPT but over all MP-triplet-based regimens. Indeed, the FIRST results should be interpreted with caution, as the benefit was mainly observed in the continuous Rd arm once the continuous phase started (while no treatment was then proposed to the other arms), and especially for responding patients. Available data are insufficient for now to firmly recommend continuous Rd over Rd18.
7 Continuous Treatment or Maintenance Therapy
Several studies have recently evaluated the role of continuous therapy in the form of maintenance or continuous treatment for elderly MM patients upfront. These approaches included:
7.1 Bortezomib-Based Treatments
-
Bortezomib-thalidomide (VT) maintenance, following VMPT induction [32, 33],
-
VT or VP (bortezomib-prednisone) maintenance, following VMP or VTP (bortezomib-thalidomide-prednisone) induction [30].
7.2 Lenalidomide-Based Treatments
-
Lenalidomide maintenance after MPR (melphalan-prednisone-lenalidomide) in MM015 study [46, 47],
-
Continuous Rd in the FIRST study [44].
Taken together, these studies support the role of continuous/maintenance therapy in elderly MM patients, at least in terms of PFS and time to second-line anti-myeloma therapy. These survival end points indeed are almost systematically prolonged by more than one year for patients exposed to maintenance versus no treatment.
-
With a bortezomib-based maintenance, median PFS varies from 31 to 39 months, versus 27 months without maintenance. No significant OS benefit has been proven for now. Amongst patients achieving CR (38–42 % of patients), results are impressive, with a median PFS of 54 months and a 5-years OS of 78 % [30].
-
With a lenalidomide-based maintenance, median PFS varies from 25.5 to 31 months, versus 13–21.2 months without maintenance, and 3-years OS is estimated to be 70 % versus 62–66 % without maintenance.
Maintenance therapy did not manage to overcome the adverse prognosis of cytogenetic abnormalities in these studies, but no increased toxicity was seen as compared to standard therapy.
For now, lenalidomide maintenance is the only regimen that has proven safe enough for long-term use, bortezomib having been studied only intravenously for now. The role of subcutaneous bortezomib, novel generation proteasome inhibitors particularly of oral form, or monoclonal antibodies in this setting is currently under study and should pave the way for novel strategies.
Whether all elderly patients should receive a maintenance therapy, what type (for instance monotherapy or combination), and for how long, remains an important question that future studies should address.
7.3 Sequential Versus Alternating Therapy, Two Keywords in One Trial: Continuous and Switch
-
If VMP and Rd are now considered the two most effective regimens in the first-line treatment of elderly MM patients, one way to further improve outcome might be to find a way to combine all these drugs. However, this would probably result toxic if used simultaneously. Mateos et al. recently reported preliminary results for the GEM2010MAS65 trial, which compared a sequential arm consisting of 9 cycles of VMP followed by 9 cycles of Rd, to an alternating arm consisting on one cycle of VMP alternating with one Rd, up to 18 cycles, in elderly MM patients with newly diagnosed MM [48]. These two approaches were both very effective, and no difference was seen between the two arms: median PFS 30 months, median OS not reached, and 3-years OS was 67 and 68 %. The safety profile was acceptable, although in a much lesser extent above 75 years old.
This study provided the best results ever reported in elderly patients upfront compared to any other treatment approach in elderly MM; and depict what may very much look like the introduction of continuous treatment in elderly MM upfront. One may foresee either VMP followed by R(d) or Rd ±X followed by R(X)(d) or R(X) or X as the very likely next most used regimens for countries with access to all drugs and able to prescribe continuous treatment. Nonetheless, so many questions lay upon us, still.
8 Future Perspectives
Future perspectives in the treatment of elderly patients with MM include improvement in treatment decision with geriatric assessment and optimization of tailored therapy, favouring all-oral regimens with progress in safety profile, and new families of drugs such as monoclonal antibodies.
8.1 Tailored Therapy
Tailored therapy in elderly patients should begin by a geriatric assessment; for instance using the new frailty score recently published which includes evaluation of age, disability and comorbidities [11]. The therapy decision based on frailty should help us propose the optimal therapy—the safer and most efficient regimen—for each category of patients with the help of specific end-points, dose adjustments and toxicity management recommendations.
Consensual options for first-line therapy now include VMP and the newcomer Rd, whereas the use of MPT should decline in the near future. It is not yet possible to officially recommend one regimen over another, although several patient- and disease-related characteristics may suggest one approach over the other. For instance, VMP does not lead to a risk of thrombosis but instead favours neuropathy. Rd is an all-oral regimen, compared to VMP that needs an hospital stay for the subcutaneous administration of bortezomib.
An important concern is also to try and improve the survival of the poor risk elderly MM patients who currently have a very short survival, and ideally overcome their adverse risk profile. Indeed, while we have a clear understanding of the adverse events of each therapy and thus know which patients we should avoid exposure to a particular treatment, little is known about efficient tailored therapy based on the risk profile, either good or poor. In the same vein, we also need to propose appropriate treatments options to patients with a very good risk MM, who could benefit from an intensive treatment and tend to a prolonged survival similar to that of matched age-related normal individuals.
8.2 Lenalidomide, a New Platform onto Which New Regimens Are Developed, Especially in the Elderly MM
Since the FIRST study reported the impressive results obtained with lenalidomide-low dose dexamethasone, a two-drug based regimen, one wondered about the efficacy of a three-drug regimen using Rd as a platform. This aspect has actually already been anticipated, and we should soon start to contemplate the results of the first phase 3 trials with Rd used as a platform for the studied arm, mostly in the context of triplet-based regimens, in the upfront setting.
Ongoing studies developed in this setting include:
-
Rd + proteasome inhibitor bortezomib: SWOG-SO777, versus Rd
-
Rd + proteasome inhibitor carfilzomib: ECOG E1A11, versus Bortezomib +Rd
-
Rd + novel generation proteasome inhibitor: Tourmaline MM2: Ixazomib, versus Rd
-
Rd + novel class of monoclonal antibodies, elotuzumab: Eloquent 1, versus Rd
-
Rd + novel class of monoclonal antibodies, daratumumab: MAIA, versus Rd.
Other lenalidomide-based combinations have already been studied, such as lenalidomide-melphalan-prednisone (MPR), which despite a clear efficacy was proven too toxic in elderly patients [46]. Dose-adjusted cyclophosphamide-lenalidomide-dexamethasone (CRDa) is also under investigation by the UK group (MRC-XI), with promising results in terms of early response and toxicity [49].
If one of these Rd-based regimens is proven effective and is approved in first-line therapy for elderly MM patients, the choice of upfront therapy between Rd + X compared to VMP will still have to be clarified.
8.3 Monoclonal Antibodies
Monoclonal antibodies finally arrived in the therapeutic arsenal of MM, even if none was approved in MM so far. The recent very positive results with at least two of them represent a major step forward in the management of MM. Two targets are particularly promising: anti-CD38 (daratumumab and more recently SAR650984) and anti-CS1/SLAMF7 (elotuzumab).
Great hopes are based on these antibodies, in terms of their expected ability to strengthen the efficacy of current regimens and combinations, and also because they are known for their very good safety profiles in the short and long term. Interestingly, it is not expected for tumour cells to develop mechanisms of resistance to these agents, which makes them even more attractive. Finally, monoclonal antibodies will almost naturally combine to IMiDs (including thalidomide, lenalidomide and the last in line pomalidomide), the second most effective class of agents in MM, whom immunomodulatory effect should reinforce significantly the action of monoclonal antibodies towards tumour cells.
-
CD38 is a transmembrane glycoprotein which plays a role in adhesion, signalling and intracellular calcium mobilization via enzymatic activity. It is overexpressed on the surface of malignant plasma cells in MM, making it an ideal therapeutic target. Daratumumab is a promising anti-CD38 monoclonal antibody which effectively mediates destruction of CD38-expressing malignant plasma cells. It was first tested as single agent in the GEN501 trial with remarkable tolerance but rather modest efficacy, with an overall response rate of 35 % a median PFS of 23 weeks [50]. In the GEN503 trial, daratumumab was tested in combination with lenalidomide and dexamethasone in relapsed or refractory MM (RRMM). Tolerance was excellent and efficacy was outstanding, as 75 % of patients obtained at least a very good partial response [51]. Daratumumab was also tested in combination with various platforms (VD, VMP, VTP, POM-D), which led to an overall response rate of 100 % for newly diagnosed MM patients, and 50 % in relapsed MM [52]. Moreover, the addition of Daratumumab was well tolerated in all evaluable patients and did not result in significant additional toxicity.
-
SAR650984 is another anti-CD38 antibody whose association with lenalidomide and dexamethasone allowed an overall response rate of 64.5 % in heavily treated patients with RRMM, and a median PFS of 6.2 months [53]. This combination was well tolerated with impressive durable responses and warrants further evaluation.
-
Elotuzumab is an anti-CS1/SLAMF7 antibody. The exact function of CS1 (also called SLAMF7) in MM cells is not completely understood; however, previous reports suggest that CS1 may be involved in cell adhesion (MM cells and bone marrow stromal cells), cell cycle regulation and other growth and survival pathways [54]. Targeting of SLAMF7 by elotuzumab on NK cells activate NK cells. As a single agent, Elotuzumab did not show any activity in MM despite plasma cell target saturation at the higher elotuzumab doses studied [55]. Encouraging response rates have been observed in combination with lenalidomide [56] in phase 1/2 trials, and in a much lesser extent with bortezomib [57]. Impressive response rate (92 %) and median PFS (not reached at a median follow-up of 20.8 months) have been described with elotuzumab at 10 mg/kg in combination with lenalidomide and low-dose dexamethasone in RRMM patients in a phase II trial [58]. Ongoing phase 3 trials are testing elotuzumab in combination with lenalidomide and low-dose dexamethasone both in the relapse (Eloquent 2) and the frontline setting (Eloquent 1 and 2).
Monoclonal antibodies thus seem very promising agents in MM therapy, and their very favourable safety profile makes them ideal candidates for elderly patients. Their exact place however remains to be determined, whether they should be used in addition to known regimens, and/or in consolidation or maintenance setting, as an add-on or even a backbone onto which to build upon.
8.4 Other Drugs
Other proteasome inhibitors (such as carfilzomib or ixazomib), IMIDs (pomalidomide), and novel families of drugs like HDAC inhibitors (panobinostat, vorinostat) or kinesin spindle inhibitors (Filanesib) are currently under investigation in the relapse setting, and for some of them in the upfront setting as well already, and could become valuable options in MM management in the future.
9 Conclusion
Management of elderly patients with MM remains challenging. The availability of novel agents such as thalidomide, lenalidomide and bortezomib has improved the treatment options and outcome of these patients, but has also taught us about the frailty of some of these elderly patients. For the first time achievement of CR was not necessarily followed by a prolonged survival, if the treatment was stopped in relation to drug toxicity profile. Out of the “battlefield” that drug development looks like in MM, the standards of care in first-line therapy of elderly MM patients are VMP, and Rd for the very near future.
Other combinations, including the second and third generation of novel classes and monoclonal antibodies, are under clinical development. Maintenance treatment with novel agents is emerging as a new strategy to sustain disease control and delay disease progression; however, the optimal maintenance regimen or molecule has yet to be determined, and longer follow-up is needed to assess the optimal duration and the OS benefit.
The optimal treatment strategy should allow a good efficacy but also a favourable safety profile, and quality of life needs to be taken into account especially in elderly patients. No data are available that assess screening for vulnerability before choosing and starting therapy for MM, but geriatric assessment should help to develop tailored therapies for these patients in the future.
10 Disclosures of Potential Conflicts of Interest
XL and TF have received honorarium from Janssen, Celgene, Takeda, Amgen, LeoPharma, Novartis, BMS, TEVA, Pierre Fabre
AP has received honorarium from Janssen, Celgene, Takeda, Amgen, Novartis, BMS,
SB has received honorarium from Janssen, Celgene, Mundifarma; AL has received honorarium from Janssen, Celgene, FG has received honorarium from Janssen, Celgene, Sanofi, Mundifarma
References
Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, Morgan G, Van Ness B, Chesi M, Minvielle S, Neri A, Barlogie B, Kuehl WM, Liebisch P, Davies F, Chen-Kiang S, Durie BG, Carrasco R, Sezer O, Reiman T, Pilarski L, Avet-Loiseau H, International Myeloma Working G (2009) International myeloma working group molecular classification of multiple myeloma: spotlight review. Leukemia: official journal of the Leukemia Society of America. Leuk Res Fund 23(12):2210–2221. doi:10.1038/leu.2009.174
SEER data,. US Population data (1969–2012) SEER Datasets [Internet]. [cited 2015 Jan 4]. http://seer.cancer.gov/data/citation.html
ONS. Office for National Statistics (ONS) [Internet]. Office for National Statistics (2010) [cited 2015 Jan 4]. http://www.ons.gov.uk/ons/index.html
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G (2004) Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 59(3):255–263
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research G (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146–156
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251
Walter LC, Brand RJ, Counsell SR, Palmer RM, Landefeld CS, Fortinsky RH, Covinsky KE (2001) Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA 285(23):2987–2994
Lee SJ, Lindquist K, Segal MR, Covinsky KE (2006) Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 295(7):801–808. doi:10.1001/jama.295.7.801
Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Blade J, Mateos MV, Rosinol L, Boccadoro M, Cavo M, Lokhorst H, Zweegman S, Terpos E, Davies F, Driessen C, Gimsing P, Gramatzki M, Hajek R, Johnsen HE, Leal Da Costa F, Sezer O, Spencer A, Beksac M, Morgan G, Einsele H, San Miguel JF, Sonneveld P (2011) Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood 118(17):4519–4529. doi:10.1182/blood-2011-06-358812
Fried LP, Guralnik JM (1997) Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc 45(1):92–100
Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, Offidani M, McCarthy P, Evangelista A, Lonial S, Zweegman S, Musto P, Terpos E, Belch A, Hajek R, Ludwig H, Stewart AK, Moreau P, Anderson K, Einsele H, Durie BG, Dimopoulos MA, Landgren O, San Miguel JF, Richardson P, Sonneveld P, Rajkumar SV (2015) Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 125(13):2068–2074. doi:10.1182/blood-2014-12-615187
Avet-Loiseau H, Hulin C, Campion L, Rodon P, Marit G, Attal M, Royer B, Dib M, Voillat L, Bouscary D, Caillot D, Wetterwald M, Pegourie B, Lepeu G, Corront B, Karlin L, Stoppa AM, Fuzibet JG, Delbrel X, Guilhot F, Kolb B, Decaux O, Lamy T, Garderet L, Allangba O, Lifermann F, Anglaret B, Moreau P, Harousseau JL, Facon T (2013) Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myelome experience. J Clin Oncol Official J Am Soc Clin Oncol 31(22):2806–2809. doi:10.1200/jco.2012.46.2598
Hideshima T, Richardson PG, Anderson KC (2011) Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther 10(11):2034–2042. doi:10.1158/1535-7163.mct-11-0433
Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R, Genuardi M, Romano A, Liberati AM, Siniscalchi A, Petrucci MT, Nozzoli C, Patriarca F, Offidani M, Ria R, Omede P, Bruno B, Passera R, Musto P, Boccadoro M, Sonneveld P, Palumbo A (2011) Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood 117(11):3025–3031. doi:10.1182/blood-2010-09-307645
Paiva B, Martinez-Lopez J, Vidriales MB, Mateos MV, Montalban MA, Fernandez-Redondo E, Alonso L, Oriol A, Teruel AI, de Paz R, Larana JG, Bengoechea E, Martin A, Mediavilla JD, Palomera L, de Arriba F, Blade J, Orfao A, Lahuerta JJ, San Miguel JF (2011) Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol Official J Am Soc Clin Oncol 29(12):1627–1633. doi:10.1200/jco.2010.33.1967
Sonneveld P, Verelst SG, Lewis P, Gray-Schopfer V, Hutchings A, Nixon A, Petrucci MT (2013) Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia: official journal of the Leukemia Society of America. Leuk Res Fund 27(10):1959–1969. doi:10.1038/leu.2013.185
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG, Investigators VT (2008) Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. New Engl J Med 359(9):906–917. doi:10.1056/NEJMoa0801479
Alexanian R, Bergsagel DE, Migliore PJ, Vaughn WK, Howe CD (1968) Melphalan therapy for plasma cell myeloma. Blood 31(1):1–10
Cavo M, Benni M, Ronconi S, Fiacchini M, Gozzetti A, Zamagni E, Cellini C, Tosi P, Baccarani M, Tura S, Writing Committee of the “Bologna 90” Clinical T (2002) Melphalan-prednisone versus alternating combination VAD/MP or VND/MP as primary therapy for multiple myeloma: final analysis of a randomized clinical study. Haematologica 87(9):934–942
Ludwig H, Miguel JS, Dimopoulos MA, Palumbo A, Garcia Sanz R, Powles R, Lentzsch S, Ming Chen W, Hou J, Jurczyszyn A, Romeril K, Hajek R, Terpos E, Shimizu K, Joshua D, Hungria V, Rodriguez Morales A, Ben-Yehuda D, Sondergeld P, Zamagni E, Durie B (2014) International Myeloma Working Group recommendations for global myeloma care. Leukemia: official journal of the Leukemia Society of America. Leuk Res Fund 28(5):981–992. doi:10.1038/leu.2013.293
Ludwig H, Hajek R, Tothova E, Drach J, Adam Z, Labar B, Egyed M, Spicka I, Gisslinger H, Greil R, Kuhn I, Zojer N, Hinke A (2009) Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood 113(15):3435–3442. doi:10.1182/blood-2008-07-169565
Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, Dib M, Guillerm G, Salles B, Eschard JP, Lenain P, Casassus P, Azais I, Decaux O, Garderet L, Mathiot C, Fontan J, Lafon I, Virion JM, Moreau P (2009) Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol Official J Am Soc Clin Oncol 27(22):3664–3670. doi:10.1200/jco.2008.21.0948
Fayers PM, Palumbo A, Hulin C, Waage A, Wijermans P, Beksac M, Bringhen S, Mary JY, Gimsing P, Termorshuizen F, Haznedar R, Caravita T, Moreau P, Turesson I, Musto P, Benboubker L, Schaafsma M, Sonneveld P, Facon T, Nordic Myeloma Study G, Italian Multiple Myeloma N, Turkish Myeloma Study G, Hemato-Oncologie voor Volwassenen N, Intergroupe Francophone du M, European Myeloma N (2011) Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood 118(5):1239–1247. doi:10.1182/blood-2011-03-341669
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix C, Dib M, Voillat L, Maisonneuve H, Troncy J, Dorvaux V, Monconduit M, Martin C, Casassus P, Jaubert J, Jardel H, Doyen C, Kolb B, Anglaret B, Grosbois B, Yakoub-Agha I, Mathiot C, Avet-Loiseau H (2007) Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 370(9594):1209–1218. doi:S0140-6736(07)61537-2 [pii] 10.1016/S0140-6736(07)61537-2
Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, Montanaro M, Ria R, Capaldi A, Zambello R, Benevolo G, Derudas D, Dore F, Cavallo F, Gay F, Falco P, Ciccone G, Musto P, Cavo M, Boccadoro M (2008) Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood 112(8):3107–3114. doi:10.1182/blood-2008-04-149427
Morgan GJ, Davies FE, Gregory WM, Russell NH, Bell SE, Szubert AJ, Navarro Coy N, Cook G, Feyler S, Byrne JL, Roddie H, Rudin C, Drayson MT, Owen RG, Ross FM, Jackson GH, Child JA, Group NHOS (2011) Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood 118(5):1231–1238. doi:10.1182/blood-2011-02-338665
Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Cook G, Drayson MT, Owen RG, Ross FM, Jackson GH, Child JA (2013) Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res Official J Am Associa Cancer Res 19(21):6030–6038. doi:10.1158/1078-0432.ccr-12-3211
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Delforge M, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Deraedt W, Cakana A, van de Velde H, Richardson PG (2013) Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol Official J Am Soc Clin Oncol 31(4):448–455. doi:10.1200/jco.2012.41.6180
San-Miguel JF, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, Blade J, Boccadoro M, Cavenagh JD, Neuwirth R, Boral AL, Esseltine DL, Anderson KC (2008) Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia: official journal of the Leukemia Society of America. Leuk Res Fund 22(4):842–849. doi:10.1038/sj.leu.2405087
Mateos MV, Oriol A, Martinez-Lopez J, Gutierrez N, Teruel AI, Lopez de la Guia A, Lopez J, Bengoechea E, Perez M, Polo M, Palomera L, de Arriba F, Gonzalez Y, Hernandez JM, Granell M, Bello JL, Bargay J, Penalver FJ, Ribera JM, Martin-Mateos ML, Garcia-Sanz R, Lahuerta JJ, Blade J, San-Miguel JF (2012) Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. Blood 120(13):2581–2588. doi:10.1182/blood-2012-05-427815
Palumbo A, Bringhen S, Larocca A, Rossi D, Di Raimondo F, Magarotto V, Patriarca F, Levi A, Benevolo G, Vincelli ID, Grasso M, Franceschini L, Gottardi D, Zambello R, Montefusco V, Falcone AP, Omede P, Marasca R, Morabito F, Mina R, Guglielmelli T, Nozzoli C, Passera R, Gaidano G, Offidani M, Ria R, Petrucci MT, Musto P, Boccadoro M, Cavo M (2014) Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol Official J Am Soc Clin Oncol 32(7):634–640. doi:10.1200/jco.2013.52.0023
Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, Offidani M, Patriarca F, Nozzoli C, Guglielmelli T, Benevolo G, Callea V, Baldini L, Morabito F, Grasso M, Leonardi G, Rizzo M, Falcone AP, Gottardi D, Montefusco V, Musto P, Petrucci MT, Ciccone G, Boccadoro M (2010) Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol Official J Am Soc Clin Oncol 28(34):5101–5109. doi:10.1200/jco.2010.29.8216
Mateos MV, Oriol A, Martinez-Lopez J, Gutierrez N, Teruel AI, de Paz R, Garcia-Larana J, Bengoechea E, Martin A, Mediavilla JD, Palomera L, de Arriba F, Gonzalez Y, Hernandez JM, Sureda A, Bello JL, Bargay J, Penalver FJ, Ribera JM, Martin-Mateos ML, Garcia-Sanz R, Cibeira MT, Ramos ML, Vidriales MB, Paiva B, Montalban MA, Lahuerta JJ, Blade J, Miguel JF (2010) Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol 11(10):934–941. doi:10.1016/s1470-2045(10)70187-x
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine DL, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau JL (2011) Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 12(5):431–440. doi:10.1016/s1470-2045(11)70081-x
Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, Miguel JS, Blade J, Boccadoro M, Cavenagh J, Alsina M, Rajkumar SV, Lacy M, Jakubowiak A, Dalton W, Boral A, Esseltine DL, Schenkein D, Anderson KC (2007) Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood 110(10):3557–3560. doi:10.1182/blood-2006-08-036947
Mateos MV, Richardson PG, Dimopoulos MA, Palumbo A, Anderson KC, Shi H, Elliott J, Dow E, van de Velde H, Niculescu L, San Miguel JF (2015) Effect of cumulative bortezomib dose on survival in multiple myeloma patients receiving bortezomib-melphalan-prednisone in the phase III VISTA study. Am J Hematol 90(4):314–319. doi:10.1002/ajh.23933
Ponisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G, Dachselt K, Richter P, Schirmer V, Schulze A, Subert R, Harksel B, Grobe N, Stelzer E, Schulze M, Bittrich A, Freund M, Pasold R, Friedrich T, Helbig W, Niederwieser D, East German Study Group of H, Oncology (2006) Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone–a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO). J Cancer Res Clin Oncol 132(4):205–212. doi:10.1007/s00432-005-0074-4
Siegel DS, Desikan KR, Mehta J, Singhal S, Fassas A, Munshi N, Anaissie E, Naucke S, Ayers D, Spoon D, Vesole D, Tricot G, Barlogie B (1999) Age is not a prognostic variable with autotransplants for multiple myeloma. Blood 93(1):51–54
Gertz MA, Dingli D (2014) How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood 124(6):882–890. doi:10.1182/blood-2014-03-544759
Badros A, Barlogie B, Siegel E, Morris C, Desikan R, Zangari M, Fassas A, Anaissie E, Munshi N, Tricot G (2001) Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol 114(3):600–607
Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, Lauta VM, Bergonzi C, Barbui A, Caravita T, Capaldi A, Pregno P, Guglielmelli T, Grasso M, Callea V, Bertola A, Cavallo F, Falco P, Rus C, Massaia M, Mandelli F, Carella AM, Pogliani E, Liberati AM, Dammacco F, Ciccone G, Boccadoro M (2004) Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood 104(10):3052–3057. doi:10.1182/blood-2004-02-0408
Gay F, Magarotto V, Crippa C, Pescosta N, Guglielmelli T, Cavallo F, Pezzatti S, Ferrari S, Liberati AM, Oliva S, Patriarca F, Offidani M, Omede P, Montefusco V, Petrucci MT, Giuliani N, Passera R, Pietrantuono G, Boccadoro M, Corradini P, Palumbo A (2013) Bortezomib induction, reduced-intensity transplantation, and lenalidomide consolidation-maintenance for myeloma: updated results. Blood 122(8):1376–1383. doi:10.1182/blood-2013-02-483073
Jacobus S, Callander NS, Siegel D, Abonour R, David P, Fonseca R et al (2010) Outcome of elderly patients 70 years and older with newly diagnosed myeloma in the ECOG randomized trial of lenalidomide/high-dose dexamethasone (RD versus lenalidomide/low-dose dexamethasone (RD). (abs.0370). Haematologica 95(s2):149
Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, Pinto A, Weisel K, Ludwig H, Bahlis N, Banos A, Tiab M, Delforge M, Cavenagh J, Geraldes C, Lee JJ, Chen C, Oriol A, de la Rubia J, Qiu L, White DJ, Binder D, Anderson K, Fermand JP, Moreau P, Attal M, Knight R, Chen G, Van Oostendorp J, Jacques C, Ervin-Haynes A, Avet-Loiseau H, Hulin C, Facon T, Team FT (2014) Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. New Engl J Med 371(10):906–917. doi:10.1056/NEJMoa1402551
Bahlis NJ, Corso A, Mugge L, Shen Z, Desjardins P, Stoppa A, Decaux O, De Revel T, Granell M, Marit G, Nahi H, Demuynck H, Huang S, Basu S, Guthrie TH, Ervin-Haynes A, Leupin N, Marek J, Chen G, Facon T (2014) Impact of response quality on survival outcomes in transplant-ineligible newly diagnosed multiple myeloma (NDMM) patients (Pts): results from the first trial. Blood (ASH Annual Meeting Abstract) Abstract 3458
Falco P, Cavallo F, Larocca A, Rossi D, Guglielmelli T, Rocci A, Grasso M, Siez ML, De Paoli L, Oliva S, Molica S, Mina R, Gay F, Benevolo G, Musto P, Omede P, Freilone R, Bringhen S, Carella AM, Gaidano G, Boccadoro M, Palumbo A (2013) Lenalidomide-prednisone induction followed by lenalidomide-melphalan-prednisone consolidation and lenalidomide-prednisone maintenance in newly diagnosed elderly unfit myeloma patients. Leukemia: official journal of the Leukemia Society of America. Leuk Res Fund 27(3):695–701. doi:10.1038/leu.2012.271
Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, Gisslinger H, Wiktor-Jedrzejczak W, Zodelava M, Weisel K, Cascavilla N, Iosava G, Cavo M, Kloczko J, Blade J, Beksac M, Spicka I, Plesner T, Radke J, Langer C, Ben Yehuda D, Corso A, Herbein L, Yu Z, Mei J, Jacques C, Dimopoulos MA, Investigators MM (2012) Continuous lenalidomide treatment for newly diagnosed multiple myeloma. New Engl J Med 366(19):1759–1769. doi:10.1056/NEJMoa1112704
Mateos M, Martinez-Lopez J, Hernandez M, Martinez R, Rosiñol L, Ocio EM, Echeveste MA, Pérez de Oteyza J, Oriol A, Bargay J, Gironella M, Martín J, Cabrera C, De La Rubia J, Gutiérrez NC, Martin M, Paiva B, Montalbán MA, Bladé J, Lahuerta JJ, San Miguel JF (2014) Comparison of sequential vs alternating administration of bortezomib, melphalan, prednisone (VMP) and lenalidomide plus dexamethasone (Rd) in elderly pts with newly diagnosed multiple myeloma (MM) patients: GEM2010MAS65 trial. Blood (ASH Annual Meeting Abstract) Abstract 178
Pawlyn C, Brioli A, Gregory W, Hinsley S, Lindsay J, Cook G, Milligan D, Chapman C, Owen RG, Drayson MT, Russell N, Jackson GH, Davies FE, Morgan GJ (2014) Lenalidomide combined with cyclophosphamide and dexamethasone is effective and well tolerated induction treatment for newly diagnosed myeloma patients of all ages. Blood (ASH Annual Meeting Abstracts) Abstract 540
Lokhorst HM, Plesner T, Gimsing P, Nahi H, Lisby S, Richardson P (2013) Daratumumab, a CD38 monoclonal antibody study in advanced multiple myeloma—An open-label, dose escalation followed by open-label extension in a single-arm Phase I/II study. Proc EHA 2013 Abstract S576
Plesner T, Arkenau H, Lokhorst HM, Gimsing P, Krejcik J, Lemech C, Minnema MC, Lassen U, Laubach JP, Ahmadi T, Yeh H, Guckert ME, Feng H, Brun NC, Lisby S, Basse L, Palumbo A, Richardson PG (2014) Safety and Efficacy of Daratumumab with Lenalidomide and Dexamethasone in relapsed or relapsed, refractory multiple myeloma. Blood (ASH Annual Meeting Abstract) Abstract 84
Moreau P, Mateos M, Bladé J, Benboubker L, de la Rubia J, Facon T, Comenzo RL, Fay JW, Qin X, Masterson T, Schecter J, Ahmadi T, San Miguel J (2014) An open-label, multicenter, phase 1b study of daratumumab in combination with backbone regimens in patients with multiple myeloma. Blood (ASH Annual Meeting Abstract) Abstract 176
Martin TG, Baz R, Benson Jr. DM, Lendvai N, Campana F, Charpentier E, Vij R (2014) A phase Ib dose escalation trial of SAR650984 (Anti-CD-38 mAb) in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma. Blood (ASH Annual Meeting Abstracts) Abstract 83
Moreau P, Touzeau C (2014) Elotuzumab for the treatment of multiple myeloma. Future Oncol (London, England) 10(6):949–956. doi:10.2217/fon.14.56
Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, Fry J, Afar DE, Singhal AK (2012) A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 120(3):552–559. doi:10.1182/blood-2011-06-360552
Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, Kaufman JL, Leleu X, Tsao LC, Westland C, Singhal AK, Jagannath S (2012) Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol Official J Am Soc Clin Oncol 30(16):1953–1959. doi:10.1200/jco.2011.37.2649
Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, Richardson PG, Afar DE, Singhal AK, Anderson KC (2012) Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol Official J Am Soc Clin Oncol 30(16):1960–1965. doi:10.1200/jco.2011.37.7069
Lonial S, Jagannath S, Moreau P, Jakubowiak AJ, Raab MS, Facon T, Vij R, Bleickardt E, Reece DE, Benboubker L, Zonder JA, Deng W, Singhal AK, Richardson PG (2013) Phase (Ph) I/II study of elotuzumab (Elo) plus lenalidomide/dexamethasone (Len/dex) in relapsed/refractory multiple myeloma (RRMM), updated Ph II results and Ph I/II long-term safety. J Clin Oncol 31(Suppl.), Abstract 8542 (2013)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Fouquet, G. et al. (2016). Treatment of Newly Diagnosed Elderly Multiple Myeloma. In: Roccaro, A., Ghobrial, I. (eds) Plasma Cell Dyscrasias. Cancer Treatment and Research, vol 169. Springer, Cham. https://doi.org/10.1007/978-3-319-40320-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-40320-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40318-2
Online ISBN: 978-3-319-40320-5
eBook Packages: MedicineMedicine (R0)