Abstract
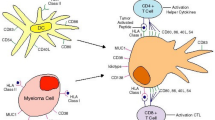
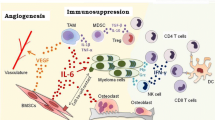
Multiple myeloma (MM) is a hematologic cancer derived from malignant plasma cells within the bone marrow. Unlike most solid tumors, which originate from epithelial cells, the myeloma tumor is a plasma cell derived from the lymphoid cell lineage originating from a post-germinal B-cell. As such, the MM plasma cell represents an integral component of the immune system in terms of both antibody production and antigen presentation, albeit not efficiently. This fundamental difference has significant implications when one considers the implications of immunotherapy. In the case of lymphoid malignancies such as myeloma, immune-based strategies must take into consideration this important difference, potentially necessitating immunotherapy targeted toward MM to be altered from that targeted at solid tumors. Typically, the immune system “surveys” cells within our body and is able to recognize and attack cancerous cells that may arise. However, some cancer cells are able to evade immune surveillance and continue to flourish, causing disease. The major mechanism leading to an effective tumor-specific response is one that enables effective antigen processing and presentation with subsequent T-cell activation, expansion, and effective trafficking to the tumor site. Plasma cells employ several mechanisms to escape immune surveillance which include altered interactions with T-cells, DCs, bone marrow stromal cells (BMSC’s), and natural killer cells (NK Cells) that can be mediated by immunosuppressive cells such as and myeloid-derived suppressor cells (MDSC’s) and cytokines such as IL-10, TGFβ, and IL-6 as well as down-regulation of the antigen processing machinery. Many therapies have been developed to reestablish a functional immune system in MM patients. These include adoptive T-cell therapies to deliver more tumor-specific T-cells, vaccines to increase the tumor-specific precursor frequency of the endogenous T-cell population, immunomodulatory agents (IMiDs) such as thalidomide and lenalidomide to enhance global endogenous immunity, immunostimulatory cytokines, and antibodies to specifically target tumor-specific cell-surface proteins or cytokines. This review will dissect these various approaches currently being explored in MM as well as highlight some future directions for myeloma-specific immune-based strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keyword
1 Interactions with Bone Marrow Stromal Cells (BMSC)
As with most tumors, interactions with the surrounding microenvironment are critical to their survival [1–3]. BMSC interactions with plasma cells are a key factor for the survival of MM tumor cells. Adhesion of MM cells to BMSC’s stimulates the production of various anti-apoptotic and cell cycle activating proteins [4, 5]. A major factor contributing to plasma cell survival through BMSC interaction is IL-6, which has a wide range of functions that serve to increase MM proliferation and survival [4] as well as IL-1β which is largely responsible for the production of IL-6 in BMSC’s.
IL-6 induces MM proliferation by activating the Ras/Raf/MEK/ERK pathway as well as by blocking p21 and p27, which inhibit CDK. IL-6 also initiates a pro-survival cascade in MM cells though the MAPK/JAK/STAT3 pathway by activating Mcl1, Bcl-xL, and cMyc as well as down-regulating Bim [4].
The Wnt pathway has been found to be aberrantly expressed in myeloma and is responsible for cell proliferation, differentiation, and apoptosis. Dickkopf 1 (Dkk1) inhibits Wnt signaling and plays a role in osteolytic bone disease. Dkk1 inhibition with a neutralizing antibody has been shown to increase osteoblast activity, reduce osteoclast activation, and restore bone mineral density. Additionally, it reduces IL-6 levels and adhesion of MM cells to BMSC’s [6].
CCL25 and its receptor CCR9 can increase MM survival in a stroma-dependent interaction [5]. CCL25 is secreted by MM tumor cells and is proposed to be a chemo-attractant for mesenchymal stem cells (MSCs), promoting the survival MM tumor. Detectable CCL25 expression was found in 5 of 6 MM cell lines and 11 of 14 primary MM tumor cells by Western blot. When mice were co-injected with MM and mesenchymal stem cells (MSC’s), more than twice as many MM cells were observed in the femur compared to injecting MM cells alone. Additionally, an in vitro transwell system showed that MSCs had elevated levels of IL-6, VEGF, IL-10, IGF-1, and DKK1 after 48 h of coculture with MM cells, and MM cells showed both increased levels of pAKT, pERK, Cyclin D2, CDK4, and Bcl-xL as well as decreased levels of Caspase-3 and PARP after 24 h of coculture with MSC’s.
1.1 CD4/CD8 T-Cells
Extensive evidence exists to suggest that disease progression in MM is associated with a loss of tumor-specific immunity [7]. suggesting that immune surveillance may play a role in the prevention of MM disease progression. In MM patients demonstrating long-term survival (MM-LTS), clonally expanded T-cell populations have a much higher proliferative capacity than in non-LTS-MM patients. Interestingly however, no significant differences in IFNγ production were observed between T-cells from LTS versus non-LTS patients. Additionally, MM patients have fewer TH17 cells than LTS-MM patients by both absolute number and percentage of total T-cells. The Treg/Th17 ratio was also higher in MM patients compared to MM-LTS patients, with the ratio in MM-LTS rivaling that of healthy donors [8].
1.2 Th17
Th17 cells are pro-inflammatory T-cells that may aid in mounting T-cell responses against tumor. Conversely, Tregs have been shown to suppress immune responses, including those mounted by Th17 cells. In MM, the balance between immunosuppressive Tregs and pro-inflammatory Th17 cells is thought to play an important role in mediating an effective immune response. Patients with MM show increased levels of Th17 cells in the peripheral blood (PB) compared to healthy donors. Additionally higher PB Th17 levels (3.7 % vs. 5.14 %; p = 0.019) correlate with more advanced clinical disease (Stage I/II vs. stage III, respectively) [9].
When the ratio of Treg:Th17 in PB was examined, patients with a Treg/Th17 ratio greater than 2SD above the mean had significantly reduced OS (p = 0.025) [10]. Of note in this study, patients with high Treg/Th17 ratios all had normal Treg levels, indicating that a decrease in the number of Th17 cells in PB is the driving force in increased disease burden, contradicting the previously discussed data.
While these data look at Th17 cells in the peripheral blood, MM is a disease that originates in the bone marrow (BM) which provides the rationale for examining immune responses in that compartment. IL-6 plays a role in both Th17 and Treg cells promoting Th17 differentiation while suppressing Treg production [11, 12]. Furthermore, cytokines such as IL-6, TGFβ and IL-1b that skew CD4 cells toward a TH17 phenotype are largely found in the BM of MM patients, but not in the PB or in BM or healthy donors [13].
1.3 Regulatory T-Cells
Regulatory T-cells (Tregs) are CD4+ T-cells that express high levels of CD25 as well as FoxP3, CTLA-4, and GITR. Tregs secrete the inhibitory cytokines, IL-10 and TGFβ, suppress immune function, and prevent autoimmunity by killing T-cells via granzyme and perforin. IL-10 is required for the suppressive function of Tregs in vivo [14] as well as for transplant immunity [15]. TGFβ is both secreted and expressed on the surface of Tregs. Transmembrane TGFβ is important for inhibition of NK activation via NKG2D [16], while secreted TGFβ is necessary for T-cell suppression [17–19]. Tregs suppress the immune system but the full effect this has on an immune-derived malignancy such as MM remains to be fully elucidated.
Peripheral blood (PB) Tregs are elevated in MM patients compared to healthy volunteers [20, 21]. Additionally, it was observed that both naïve and activated Tregs were elevated (p = 0.015 and p = 0.036, respectively). However, no differences were seen in PB Tregs between healthy volunteers and MGUS, SMM, or patients in remission. BM Tregs were elevated in relapse patients compared to healthy volunteers (p = 0.035), however, no differences were seen between any other groups of patients. Furthermore, Treg function was similar in MM and healthy patients [20].
In contrast to the above-mentioned data, patients with MGUS and MM have significantly reduced numbers of Tregs compared to healthy donors [22]. This shows that Tregs in MGUS and MM patients are significantly less functional and ineffectively suppress T-cells, which could ultimately result in greater numbers of hyperactivated T-cells.
When CD4+/CD25+ cells from the PB are compared to that of the BM, MM patients were found to have significantly more FoxP3+ cells in the PB compared to BM (52.2 % versus 2.2 %). Additionally, these FoxP3+/CD4+/CD25+ cells from the PB had the ability to suppress T-cell proliferation by greater than 90 %, whereas those from the BM demonstrated no suppressive capabilities. These data indicate that FoxP3+/CD4+/CD25+ cells in the PB and BM of MM patients are functionally distinct cell types [13].
CD8+ Tregs have been identified as well [23, 24] and while they also express FoxP3, CTLA4, and GITR, their mechanism of action does not involve TGFβ or IL-10, but rather TNFα and CCL4, causing cells to become cytostatic. CD8+ Tregs in both PB and BM are elevated in patients with MM compared to healthy donors. Multiple studies have demonstrated that tumor-induced CD4+ iTregs are created in a contact-dependent manner by MM tumor cells and express higher levels of FoxP3, PD-1 and GITR than nTregs [23, 25]. Additionally, proliferation of MM induced CD4+ iTregs is mediated by ICOS/ICOS-L interactions and not by the standard IL-10 or TGFβ interactions [25].
Tregs have multifaceted interactions and functions with T-cells in the myeloma setting. Tregs cannot simply be grouped together as having a single mechanism of action or effect on T-cells or the myeloma environment. There are clear differences in both phenotype and function of Tregs, which must be taken into consideration and studied, to better understand their role in Myeloma.
2 NK Cells
Natural killer (NK) cells are cytotoxic lymphocytes that do not express CD3, defined by the expression of CD56 in humans and function as cytotoxic cells in a non-HLA-restricted manner. Immunophenotyping was done on MM patients following HSCT. They found that at 1 month post-transplant, NK levels were predictive of PFS. Patients with NK counts below 100 cells/uL had an average PFS of 2.2 months compared to 11.6 months for patients with NK counts above 100 cells/uL. However, this was not predictive of OS or time to next treatment [26].
NK cells from healthy donors do not express PD-1, however, NK cells from MM patients do. This phenotypic difference indicates that there is potentially a functional change occurring in NK cells in response to the MM tumor helping to create an immunosuppressive environment for the tumor to thrive [27].
NK cells also express a variety of surface receptors, including NKG2D, that can be used to recognize tumor cells, or cells that are in a state of stress expressing ligands such as MICA, MICB, and ULBP1-6. The NKG2D receptor, also expressed on CD4 and CD8 cells, has been shown to be critical for the recognition and lysis of MM cells [28]. Additionally, MICA expression on plasma cells and shedding of MICA (sMICA) correlates with MM progression. MGUS patients express high levels of MICA on their plasma cells compared to normal donors, and MM patients express intermediate MICA levels but high sMICA levels. MGUS patients also have high levels of anti-MICA antibodies whereas MM patients do not [29]. The presence of anti-MICA antibodies ameliorates the suppressive effects of MICA on NKG2D, whereas the loss of these antibodies contributes to MM disease progression. Therefore, the use of anti-MICA antibodies may prove to be a viable therapeutic in MM.
3 DC’s
Dendritic cells (DCs) are one of the most effective antigen presenting cells (APCs) for stimulating naïve T-cells. However, MM patients have not only fewer DC cells and precursors compared to healthy donors [30, 31], but also have impaired DC function. Peripheral blood DCs (PBDCs) from MM patients have decreased expression of HLA-DR, CD40, and CD80 and are nonfunctional. This effect is mediated by IL-6. Conversely, CD14+ derived DCs (Mo-DCs) are fully functional in both normal in MM patients [31]. This observation brings into question the use of PBDCs for therapeutic use in MM, discussed later.
4 MDSC’s
Myeloid-derived suppressor cells (MDSC’s) are immunosuppressive cells of the myeloid lineage. They suppress the function of T-cells mainly via up-regulation of iNOS, ROS, and Arg-1. Two subsets of MDSC’s have been described, polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs). PMN-MDSCs are Ly6Clo/Ly6G+ and use ROS to mediate T-cell suppression, while M-MDSC are Ly6Chi/Ly6G− and have increased levels of NO, but not ROS [32]. Significant MDSC accumulation is observed in BM and PB of MM patients compared to healthy donors [32, 33], and the majority of MDSC’s in the BM are PMN-MDSC’s. Additionally, the frequency of MDSCs increases with disease progression (newly diagnosed < relapsed < relapsed/refractory) with significantly different (p < 0.025) frequencies between healthy donors and relapsed/refractory patients [33].
In a murine model in which MDSCs are unable to accumulate in tumor-bearing mice (S100A9 model), reduced tumor growth is observed, accompanied by an accumulation of antigen-specific CD8+ T-cells in the spleen and BM. Either adoptive transfer of MDSCs or anti-CD8 mAb negate this effect. Interestingly, there is no correlation between disease extent and MDSC levels in this murine model [32].
PDE5 inhibition is an approach to inhibiting MDSC function. PDE5 inhibition increases proliferation and infiltration of T-cells in the myeloma tumor environment that can render adoptive T-cell therapy more effective [34]. In fact, in one case study, PDE5 inhibition regenerated tumor-specific T-cell function in a patient with end-stage MM [35].
5 Therapies
5.1 Autologous Stem Cell Transplant
Autologous stem cell transplantation (ASCT) is largely considered frontline therapy for myeloma patients under the age of 65. The primary goal of ASCT is a platform to deliver high dose chemotherapy with stem cell rescue. However, there is an increasing appreciation of the role myeloablative chemotherapy can play as a platform for immunotherapy. Specifically, through its myeloablation, it can: (1) provide effective lymphodepletion to enhance homeostatic lymphocytic proliferation following adoptive T-cell transfer; (2) abolish the intrinsic tolerogenic mechanisms that usually serve to impair the generation of effective antitumor immunity; and (3) potentially augment the efficacy of tumor-specific vaccines by enabling such vaccine to effectively skew the developing T-cell repertoire toward greater tumor recognition [36, 37].
5.2 Allogeneic Bone Marrow Transplant (BMT)
Allogeneic SCT (allo-SCT) using HLA-identical (or haploidentical) donor cells has also been used to treat MM. However, graft versus host disease (GVHD) remains a sizeable obstacle with a significant morbidity and mortality. Patients who receive allo-SCT upfront as opposed to upon relapse or progressive disease show an increase in 2-year progression free survival (PFS) (63 % vs. 25 %) and overall survival (OS) (81 % vs. 52 %) [38]. However, myeloablative allo-SCT has historically been associated with an unacceptably high transplant-related mortality of >40 % [39–41]. In conclusion, the high graft v myeloma effect of allogeneic transplants must be balanced with the significant mortality. An approach to reduce such toxicity includes the use of post-transplant cyclophosphamide which has dramatically reduced the incidence of GVHD, minimized the use of immunosuppression in HLA-identical transplants and opened this modality as a therapeutic option even to HLA-mismatched donors thus dramatically increasing donor availability [42].
6 Adoptive T-Cell Therapy
Adoptive T-cell therapy activates and expands tumor-specific T-cells ex vivo to reverse T-cell tolerance and increase their numbers. One advantage of adoptive T-cell therapy is the quantity of T-cells that can be generated and infused into patients. Evidence from several studies has shown the extent to which T-cells can rapidly and efficiently eradicate rather large tumor burdens. A limitation of this approach has been the expense and manufacturing needs required to implement such an approach.
7 Marrow-Infiltrating Lymphocytes
Marrow-infiltrating lymphocytes (MILs) are bone marrow-derived T-cells expanded ex vivo using CD3/CD28 beads in the presence of all cells from the bone marrow, including the tumor cells. The rationale for the development of this approach was to utilize an endogenous T-cell population obtained from the tumor microenvironment. However, in addition to it being the site of disease in hematologic malignancies such as myeloma, the bone marrow also has several unique immunologic properties. It is also a reservoir for central memory T-cells, which are the most efficient at maintaining long-term immunity [43, 44]. Furthermore, MILs possess many other important and unique properties essential to effective adoptive T-cell therapy: they are present in all patients; they can traffic to the bone marrow upon reinfusion; and can kill tumor and persist over time [45]. This has led to the development of clinical trials showing the ability of MILs to impart a measurable myeloma-specific response that correlates with clinical outcomes in patients.
8 CARs
Chimeric antigen receptors (CARs) are T-cells with genetically modified transmembrane proteins that confer target specificity. CARs posses an antigen-specific extracellular domain as well as an intracellular domain that transduces an activation signal. While first generation CARs targeted CD19 and were stimulated via a CD3ζ, second generation CARs have co-stimulatory molecules, such as CD28 or 41BB in addition to CD3ζ and third generation CARs have multiple intracellular signaling domains. A group at Ohio State has developed a second generation CAR using NK cells that target CS-1/SLAM7, a surface protein highly expressed in MM cells. These SLAM7 CAR NK cells demonstrate increased cytotoxicity via cell lysis of tumor cell lines, and increased specificity for myeloma tumor compared to mock-transduced or empty vector NK cells. Promising results were seen in both in vitro models as well as in mouse models [46]. Mice with IM9 MM tumor showed a significant reduction in tumor burden and increased survival when treated with SLAM7 CAR NK cells as compared to mock-transduced NK cells and untreated controls (p < 0.05 and <0.01, respectively) [46].
Another CAR NK cell (NK-92MI) has been developed targeting CD138 with encouraging preclinical activity. These CD138 CARs show increased cytotoxicity and antitumor activity in both in vitro and xenograft studies [47]. Both IFNγ and granzymeB were significantly increased in the NK-92MI cells compared to mock transfected cells in response to CD138+ tumor cell lines as well as primary patient tumor cells, demonstrating tumor-specific cytotoxicity. Furthermore, the cytotoxicity of the NK-92MI cells was maintained even after irradiation with 10 Gy. This finding has important implications in ameliorating the risk of unrestrained proliferation after transplant of the transplanted cells.
Non-antigen-specific CARs may also prove to be effective in MM. NKG2D is a receptor expressed on some CD8+ T-Cells, γδT-cells, and NK cells. It facilitates cell lysis in a non-MHC restricted and TCR-independent manner and has multiple ligands, several of which are selectively expressed in MM tumor cells. Enriching for NGK2D+CD3+CD8+ T-cells increases autologous myeloma cell lysis and demonstrates a role for NKG2D in killing autologous myeloma tumor [28, 48]. Therefore, creating a CAR targeting NKG2D or other non-antigen-specific molecules may prove to be an effective means of targeting the MM tumor as well.
9 Vaccines
Cancer vaccines aim to prime an endogenous T-cell response to tumor-specific antigens. Approaches used are highly variable but all seek to achieve this goal.
10 DC Vaccines
Dendritic cell (DCs) vaccines aim to elicit antitumor responses by overcoming the immunosuppressive environment created by the tumor. Several approaches have been applied to DC vaccines to ultimately generate measurable tumor-specific T-cell responses. One historical approach used idiotype-pulsed (Id-pulsed) DCs [49–53]. However, a potential drawback in using Id as a tumor target in myeloma is that it is largely a secreted protein potentially more likely to induce tolerance but incapable of effectively mounting a tumor-specific immune response that could result in tumor cell killing.
Tumor lysate-pulsed DCs is another approach that has been used in MM. In the 5TGM1 myeloma mouse model, DCs pulsed with tumor lysate have a much more potent antitumor response than Id-pulsed DCs [54]. This data suggests that tumor lysates serve as better tumor antigens than Id. Considering that the whole tumor cell likely possesses multiple tumor antigens that include, but are not limited to Id, these findings are not unexpected. A potential risk in using whole tumor lysates, however, is that antigens expressed on normal cells may also be expressed in the tumor, presented by the DC and recognized by T-cells as a foreign antigen, mounting a non-tumor-specific response.
Creating DC/tumor fusions is another means of creating at DC-based vaccine. This approach attempts to combine the entire antigenic repertoire of autologous tumors with efficient antigen presentation of a DC to maximize the immune response that can be generated by such an approach. In a clinical trial with patients who had undergone ASCT and had a minimum of 20 % plasma cells in the BM, 36 patients received a DC/tumor fusion vaccine and 12 of these patients also received GM-CSF. 78 % of patients achieved a CR or VGPR (47 % and 31 %, respectively) and at a median follow-up time of 45.6 months, the 2-year PFS was 57 % [55].
10.1 Tumor Associated Antigen Vaccines
MM lacks many defined tumor-specific antigens making it difficult to specifically target the tumor using an antigen-specific approach. One study used the RHAMM-R3 peptide (CD168) as a vaccine to treat MM. RHAMM is expressed in 100 % of MM tumor cells, but is not expressed in PBMCs or healthy donor CD34+ BMSCs. Following treatment with R3, 2 of 4 (50 %) of patients had reduced plasma cells and β2-microglobulin in the BM as well as a reduction of free light chains in the serum and/or urine [56]. Additionally, in 4/9 (44 %) of patients, a CD8+ RHAMM-R3-specific T-cell response was observed. Of these 4 patients showing an immunological response, one had decreased blasts in the BM and another had a reduction of free light chains. However, the other two patients showed no clinical changes [57].
Wilms tumor gene (WT-1) is a universal tumor antigen that is processed by MM cells and expressed in the context of HLA class 1. WT-1 is recognized by cytotoxic CD8+ T-cells, that are able to efficiently kill MM tumor cells via perforin-mediated cytotoxicity and WT-1-specific CTLs are found in MM patients [58]. Based on this preclinical data, a patient was given WT-1 vaccine. In this case report, the percentage of MM cells in the BM decreased from 85 to 25 % and the M-protein levels decreased from 3.6 to 0.6 g/day [59] making a strong case for further studies using a WT-1 vaccine.
While vaccines against tumor antigens is a promising means of eliciting a tumor-specific immune response, finding MM-specific antigens has proven to be a major obstacle.
10.2 Whole Cell Vaccines
Allogeneic vaccines can be used to target MM via shared tumor antigens between the MM tumor and the cells used in the vaccine. In one study, a combination of MM tumor cell lines (H929 and U266) and a GM-CSF-producing cell line (K562/GM) are irradiated and combined to form a vaccine. Patients received 3 monthly vaccinations and a booster vaccination at 6 months. All patients were previously on a Len-containing regimen, which was continued as a single agent in addition to the vaccine. Additionally, these patients needed to be in one of three categories to receive vaccine: (1) in a stable nCR for at least 4 months, (2) converting from IFE negative to positive, or (3) show signs of early relapse from nCR to an M-spike <0.3 g/dL. Twelve patients were vaccinated and 16 were observed without vaccination. PFS was significantly improved for patients who received the vaccine compared to those who did not and the overall CR rate for patients receiving vaccine was 64 %. Of the 4 patients receiving vaccine in category 1, 3 achieved a true CR and one achieved an nCR. All 3 patients in category 2 achieved true CRs. In category 3, 1 patient achieved a CR, 1 SD and 3 PD. Additionally, patients achieving a CR had more central memory CD8 T-cells at baseline in the BM and blood, more phenotypically active CD8 in the BM and had more tumor-specific IFNγ production. This study demonstrates that in the MRD setting, the combination of an allogenic vaccine with Len has significant clinical benefit compared to Len alone [60].
Allogeneic vaccines are an off-the-shelf product. Since it is made using cell lines and not primary cells, there is a theoretically unlimited supply available for treating patients.
11 Antibodies
Using monoclonal antibodies (mAbs) to specifically target proteins expressed on tumor cells is a desirable treatment approach for many cancers, including MM, since it is a universally applicable, highly specific form of therapy available to all patients. Several mAbs have been developed to target the MM tumor based on cell-surface expression of proteins as well as secreted survival factors. Rituximab, targets CD20 and it’s primary use is in non-Hodgkin lymphomas, but has been used in myeloma as well. Rituximab targets CD20, which is differentially expressed on MM plasma cells. Less than one third of MM tumor cells express CD20, as mature plasma cells tend to lose CD20 expression. However, patients who do have CD20-expressing MM tend to have more aggressive disease and lower overall survival [61]. Furthermore, there are data suggesting that CD20 is expressed on what may be precursor myeloma cells [62], providing suitable rationales for targeting CD20 in myeloma. In one study, out of 19 MM patients treated, 6 (32 %) had a clinical response to Rituximab therapy (1 PR, 5 SD), and all 6 of these patients had CD20+ BMPCs (Bone Marrow Plasma Cells) [63]. Another study in relapsed/refractory MM showed that no patients achieved an objective response. 22 % of patients (2 of 9) had SD at 6 months while the rest (88 %) had progressive disease [64]. A subsequent study selected only for patients with CD20+ MM and showed that 7 % of patients (1 of 14) had a minor response while 36 % (5 of 14) had stable disease yielding a clinical response rate of 43 % [65].
CD38 is expressed in many heme malignancies including MM and may have a pro-survival role [66]. CD38 ligation to CD31 activates NFκB, leading to cytokine secretion and proliferation of T-cells. Several CD38 mAbs have been created, including daratumumab (DARA) and SAR650984. DARA is an IgG1 k antibody that has therapeutic efficacy in MM patients, especially in combination with IMiDs and/or proteasome inhibitors [67]. Several mechanisms have been proposed for DARA, including Ab-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) [68], Fc receptor-mediated crosslinking [69] and antibody-dependent cellular phagocytosis (ADCP) [70]. Promising preliminary clinical results have been seen with DARA. When treated with 4, 8, or 16 mg/kg DARA, patients showed 80–100 % reduction of bone marrow plasma cells [71]. Additionally, in combination with lenalidomide and dexamethasone, 8/11 patients achieved a PR or better while the remaining 3 patients achieved MR (2) and SD (1) [72].
SAR650984 is a newer IgG1 antiCD38 mAb that also has ADCC, CDC, and ADCP activity against MM tumor cell lines and cytotoxicity of both MM cell lines and primary patient tumor. Additionally, SAR650984 drastically decreased tumor volume in xenograft studies of MOLP-8 and H929 cells compared to bortezomib [73]. However, SAR650984 has not yet entered the clinic.
CD56, also known as neural cell adhesion molecule (N-CAM) is not expressed by benign plasma cells, but is highly expressed in MM cells [74]. Lorvotuzumab mertansine (LM) is an antibody-drug conjugate that targets MM cells via high affinity CD56 mAb and is conjugated to the drug Maytansine, which inhibits tubulin. As a single agent in heavily pretreated patients with CD56+ MM, 16.2 % had objective responses (OR) and 40.5 % had SD for greater than 3 months, including those with ORs [75]. LM in combination with Lenalidomide and Dexamethasone shows greater clinical benefit with a 56.4 % OR and 66.1 % CR rate in relapsed/refractory MM [76].
CS1 (CRACC, SLAMF7) is a glycoprotein highly expressed on the surface of plasma cells. It is a member of the CD2 receptor family and contains an immunoreceptor tyrosine-based switch motif (ITSM) that is involved in lymphocyte activation [77]. Elotuzumab targets CS1 and is currently entering phase III clinical trials. Data from phase I trials show that elotuzumab alone yielded no objective responses (≥PR), while 26.5 % of patients had stable disease after about two months and the rest had progressive disease [78]. When combined with bortezomib, 48 % of patients had an objective responses and a median time to progression (TTP) of 9.5 months [79]. When elotuzumab is combined with lenalidomide and low-dose dexamethasone, objective responses were seen in 82 % of patients with a median TTP not reached after 16.4 months [80]. Preliminary data from a phase II study with elotuzumab combined with lenalidomide and dexamethasone show an overall ORR of 84 %, with a 92 % ORR in the cohort receiving 10 mg/kg elotuzumab and 76 % in those receiving 20 mg/kg. Patients receiving 10 mg/kg had not reached PFS at 20.8 months while PFS was 18.6 months for those receiving 20 mg/kg. While elotuzumab may not work well as a single agent, in combination with lenalidomide and dexamethasone elotuzumab appears to be a promising new therapeutic for MM.
CD40 is a member of the TNF-receptor family. It is required for B-cell activation and differentiation and is more highly expressed in patients with B-cell malignancies, such as MM. Dacetuzumab, a CD40 mAb, has not shown promising results in a phase 1 trial as a single agent; no patients achieved an OR and 20 % achieved SD [81]. Lucatumumab, another CD40 mAb, has completed a phase 1 study in which 33 patients were evaluated. One patient achieved a PR while 12 (43 %) had SD as a best response [82]. Neither Lucatumumab nor Dacetuzumab show promising clinical results as a single agent, however, like many therapeutics they warrant further investigation in combination with other therapies, but no data has been published to date.
IL-6 is not expressed on the surface of MM cells, however, it is an important factor in their proliferation. Siltuximab is an anti-IL-6 mAb that so far has shown minimal clinical benefit. In a Phase I trial, 13 MM patients were treated and 2 (15 %) had a CR [83]. In a Phase II trial and RRMM, initially 14 patients received siltuximab alone, 10 whom later received dexamethasone. 39 additional patients were then treated with a combination of siltuximab and dexamethasone with a response rate of 23 % (17 % CR and 6 % PR). No responses were seen with siltuximab alone [84]. Another phase II trial evaluated bortezomib, melphalan and prednisone therapy with or without siltuximab (VMP versus S + VMP) in newly diagnosed transplant eligible patients. ORR was only 8 % higher in S + VMP than VMP alone (88 % versus 80 %, respectively) whereas ≥VGPR was 20 % higher in S + VMP than VMP (71 % vs. 51 %). Finally, median PFS as well as 1 year OS were equivalent in both groups showing definitively that siltuximab has no additional benefit to VMP therapy in MM [85].
12 Checkpoint Blockade
The role of PD-1 is to maintain T-cell homeostasis by limiting the proliferation and activation of T-cells. However, many tumors usurp this mechanism and over-express PD-L1, engaging PD-1 on T-cells and generating tumor-specific T-cell tolerance [86–89]. Therapy targeted at blocking programmed death-1 (PD-1) and its ligand (PD-L1) transformed the field of immunotherapy in immunogenic solid tumors, such as renal cell carcinoma and melanoma [90, 91] and more recently Hodgkin’s lymphoma [92]. More recently, inhibition of PD-1 and PD-L1 has also been shown to work in non-immunogenic solid tumors such as lung, ovarian, and breast cancers [91, 93]. These findings have opened the door to studying PD-1 and PD-L1 in a wide variety of tumor types. There are four PD-1 mAbs (Nivolumab, Pidilizumab, Pembrolizumab, and MK-3475) and four PD-L1 mAbs (BMS-936559, MSB0010718C, MEDI4736, and MDPL3280A) currently in clinical trials. Despite the significant efficacy in certain malignancies, the overall efficacy of single agent PD-1 blockade in relapsed MM is negligible [94].
Syngeneic mice lacking PD-1 completely suppress MM tumor (J558L) formation whereas mice expressing PD-1 form rapidly growing tumor demonstrating a clear role of PD-1 and its interaction with PD-L1 in MM [89]. More recently, data shows that treatment with a PD-L1 mAb in combination with lymphodepletion with irradiation eliminate MM in a murine 5T33 model, again highlighting the importance of the PD-1/PD-L1 axis in MM [95].
CTLA4 is an inhibitory molecule expressed on T-cells that binds to CD80 and competes with CD28, a co-stimulatory molecule that also binds CD80, but with lower affinity. CTLA4 has been shown to maintain control to T-cell proliferation [96] as well as being critical for proper function of Tregs [97, 98]. 70 % of MM patients have increased CTLA4 expression and may be associated with an accumulation of immunosuppressive Tregs in the bone marrow microenvironment [99]. Blocking CTLA4 has proven to be an effective therapeutic strategy in patients with metastatic melanoma [100], opening the doors for its use in other malignancies, including MM.
13 IMiDs
Immunomodulatory drugs (IMiDs), such as thalidomide, lenalidomide, and pomalidomide, are often used as both frontline and maintenance therapy for MM. Beneficial clinical outcomes are seen with the use of IMiDs, and while many studies have given insights into how these drugs work, their exact mechanism of action is only recently being understood.
IMiDs have been shown to increase the proliferation and function of T-cells. Greater lytic capacity as well as higher percentages of polyfunctional T-cells (T-cells secreting multiple cytokines) was observed in MM patients receiving IMiDs following ASCT, due at least in part to decreased Tregs and increased DC function [101]. Furthermore, the immunomodulatory properties of lenalidomide (Len) was confirmed by demonstration about its ability to enhance both infectious vaccines [102] as well as possibly tumor vaccines [60]. The induction of Rho GTPases with pomalidomide (Pom) may contribute to improving immune synapses in T-cells [103]. IMiDs effects on DCs could also play a role in the enhanced T-cell functionality. DCs treated with lenalidomide or pomalidomide have enhanced endocytic ability as well as an increased levels of CD86, MHC I, and MHC II on the DC cell surface [104]. Additionally, DCs that have been pretreated with Len or Pom show significantly enhanced cross-priming of CD8+ T-cells and priming of CD4+ T-cells. Additionally, these CD8+ T-cells are more cytotoxic, exhibiting increased levels of both IFNγ and perforin.
However, other studies suggest that IMiDs may prove to be harmful to immune surveillance long-term for myeloma patients. Clave et al., observed that 6 months post ASCT, terminally differentiated CD8 T-cells are reduced in the presence of Len. Pro-inflammatory cytokines such as TNFα were also reduced in the blood of patients receiving Len after ASCT. Furthermore, Len also significantly increased the NK cell activity as well as the percentage of Treg cells 18 months post ASCT [105, 106].
14 Summary
There are many promising immunotherapeutic treatments available for the Multiple myeloma, however, we have not yet been able to cure this disease by enabling the immune system to fight off the cancer completely. The immune system is an intricate network of cells and signaling molecules with multifaceted microenvironments creating a complex system for targeted therapies. However, as we learn more about the immune system and how it interacts with the tumor, we can continue to create more potent and targeted therapies.
References
Alifano M et al (2014) Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS ONE 9:e106914–11
Song G et al (2014) Effects of tumor microenvironment heterogeneity on nanoparticle disposition and efficacy in breast cancer tumor models. Clin Cancer Res 20:6083–6095
Kumar V, Gabrilovich DI (2014) Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 143:512–519
Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM (2012) Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol 2012:1–5
Xu S et al (2012) Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells 30:266–279
Fulciniti M et al (2009) Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114:371–379
Spisek R et al (2007) Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med 204:831–840
Bryant C et al (2013) Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J 3:e148
Shen CJ, Yuan ZH, Liu YX, Hu GY (2012) Increased numbers of T helper 17 cells and the correlation with clinicopathological characteristics in multiple myeloma. J Int Med Res 40:556–564
Favaloro J et al (2014) Myeloid derived suppressor cells are numerically, functionally and phenotypically different in patients with multiple myeloma. Leuk Lymphoma 55:2893–2900
Zhou L et al (2007) IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8:967–974
Lichtenstein A, Berenson J, Norman D, Chang M-P, Charlie A (1989) Production of cytokines by bone marrow cells obtained from patients with multiple myeloma. Blood 74:1266–1273
Noonan K et al (2010) A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood 116:3554–3563
Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F (1999) An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190:995–1004
Kingsley CI, Karim M, Bushell AR, Wood KJ (2002) CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol 168:1080–1086
Ghiringhelli F (2005) CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-dependent manner. J Exp Med 202:1075–1085
Strauss L et al (2007) A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res 13:4345–4354
Fahlen L (2005) T cells that cannot respond to TGF- escape control by CD4+CD25+ regulatory T cells. J Exp Med 201:737–746
Shull MM et al (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359:693–699
Muthu Raja KR et al (2012) Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS ONE 7:e47077–11
Giannopoulos K, Kaminska W, Hus I, Dmoszynska A (2012) The frequency of T regulatory cells modulates the survival of multiple myeloma patients: detailed characterisation of immune status in multiple myeloma. Br J Cancer 106:546–552
Prabhala RH (2006) Dysfunctional T regulatory cells in multiple myeloma. Blood 107:301–304
Muthu Raja KR et al (2012) Functionally suppressive CD8 T regulatory cells are increased in patients with multiple myeloma: a cause for immune impairment. PLoS ONE 7:e49446–10
Ablamunits V, Bisikirska B, Herold KC (2010) Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF. Eur J Immunol 40:2891–2901
Feyler S et al (2012) Tumour cell generation of inducible regulatory T-cells in multiple myeloma is contact-dependent and antigen-presenting cell-independent. PLoS ONE 7:e35981–10
Rueff J, Medinger M, Heim D, Passweg J, Stern M (2014) Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol Blood Marrow Transplant 20:896–899
Benson DM et al (2010) The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116:2286–2294
Talebian L et al (2014) The natural killer-activating receptor, NKG2D, on CD3+CD8+ T cells plays a critical role in identifying and killing autologous myeloma cells. Transfusion 54:1515–1521
Jinushi M et al (2008) MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci 105:1285–1290
Pasiarski M, Grywalska E, Kosmaczewska A, Gozdz S, Rolinski J (2013) The frequency of myeloid and lymphoid dendritic cells in multiple myeloma patients is inversely correlated with disease progression. Potepy Hig Med Dosw 67:1–7
Ratta M et al (2002) Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood 100:230–237
Ramachandran IR et al (2013) Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J Immunol 190:3815–3823
Görgün GT et al (2013) Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 121:2975–2987
Serafini P et al (2006) Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 203:2691–2702
Noonan KA, Ghosh N, Rudraraju L, Bui M, Borrello I (2014) Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol Res 2:725–731
Borrello I et al (2000) Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing tumor vaccines. Blood 95:3011–3019
Gorin NC (2000) New developments in the therapy of acute myelocytic leukemia. Hematology 2000:69–89
Gerull S et al (2013) Allo-SCT for multiple myeloma in the era of novel agents: a retrospective study on behalf of Swiss Blood SCT. Bone Marrow Transplant 48:408–413
Bjorkstrand BB et al (1996) Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood 88:4711–4718
Gahrton G et al (1999) Syngeneic transplantation in multiple myeloma—a case-matched comparison with autologous and allogeneic transplantation. Bone Marrow Transplant 24:741–745
Barlogie B (2006) Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US intergroup trial S9321. J Clin Oncol 24:929–936
Kanakry CG et al (2014) Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 124:3817–3827
Mazo IB et al (2005) Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity 22:259–270
Di Rosa F, Pabst R (2005) The bone marrow: a nest for migratory memory T cells. Trends Immunol 26:360–366
Noonan K et al (2005) Activated marrow-infiltrating lymphocytes effectively target plasma cells and their clonogenic precursors. Cancer Res 65:2026–2034
Chu J et al (2014) CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 28:917–927
Jiang H et al (2014) Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol 8:297–310
Meehan KR et al (2013) Adoptive cellular therapy using cells enriched for NKG2D+CD3+CD8+ T cells after autologous transplantation for myeloma. Biol Blood Marrow Transplant 19:129–137
Curti A et al (2007) Phase I/II clinical trial of sequential subcutaneous and intravenous delivery of dendritic cell vaccination for refractory multiple myeloma using patient-specific tumour idiotype protein or idiotype (VDJ)-derived class I-restricted peptides. Br J Haematol 139:415–424
Reichardt VL et al (1999) Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma—a feasibility study. Blood 93:2411–2419
Lim SH, Bailey-Wood R (1999) Idiotypic protein-pulsed dendritic cell vaccination in multiple myeloma. Int J Cancer 83:215–222
Yi Q, Desikan R, Barlogie B, Munshi N (2002) Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol 117:297–305
Rollig C et al (2011) Induction of cellular immune responses in patients with stage-I multiple myeloma after vaccination with autologous idiotype-pulsed dendritic cells. J Immunother 34:100–106
Hong S et al (2012) Optimizing dendritic cell vaccine for immunotherapy in multiple myeloma: tumour lysates are more potent tumour antigens than idiotype protein to promote anti-tumour immunity. Clin Exp Immunol 170:167–177
Rosenblatt J et al (2013) Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res 19:3640–3648
Schmitt M et al (2007) RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood 111:1357–1365
Greiner J et al (2010) High-dose RHAMM-R3 peptide vaccination for patients with acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma. Haematologica 95:1191–1197
Azuma T (2004) Myeloma cells are highly sensitive to the granule exocytosis pathway mediated by WT1-specific cytotoxic T lymphocytes. Clin Cancer Res 10:7402–7412
Tsuboi A et al (2007) Wilms tumor gene WT1 peptide-based immunotherapy induced a minimal response in a patient with advanced therapy-resistant multiple myeloma. Int J Hematol 86:414–417
Noonan K et al (2014) Lenalidomide immunomodulation with an allogeneic myeloma GVAX in a near complete remission induces durable clinical remissions. Blood 124:2137
Miguel JFS et al (1990) Immunophenotypic heterogeneity of multiple myeloma: influence on the biology and clinical course of the disease. Br J Haematol 77:185–190
Matsui W et al (2004) Characterization of clonogenic multiple myeloma cells. Blood 103:2332–2336
Treon SP et al (2002) CD20-directed serotherapy in patients with multiple myeloma: biologic considerations and therapeutic applications. J Immunother 25:72–81
Zojer N, Kirchbacher K, Vesely M, Hübl W, Ludwig H (2006) Rituximab treatment provides no clinical benefit in patients with pretreated advanced multiple myeloma. Leuk Lymphoma 47:1103–1109
Moreau P et al (2007) Rituximab in CD20 positive multiple myeloma. Leukemia 1–2. doi:10.1038/sj.leu.2404558
Deaglio S (2006) In-tandem insight from basic science combined with clinical research: CD38 as both marker and key component of the pathogenetic network underlying chronic lymphocytic leukemia. Blood 108:1135–1144
van der Veer MS et al (2011) The therapeutic human CD38 antibody daratumumab improves the anti-myeloma effect of newly emerging multi-drug therapies. Blood Cancer J 1:e41–e43
de Weers M et al (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840–1848
Jansen JHM et al (2012) Daratumumab, a human CD38 antibody induces apoptosis of myeloma tumor cells via Fc receptor-mediated crosslinking. Blood 120:2974
Overdijk MB et al (2012) Phagocytosis is a mechanism of action for daratumumab. Blood 120:4054
Plesner T et al (2012) Daratumumab, a CD38 monoclonal antibody in patients with multiple myeloma—data from a dose-escalation phase I/II study. Blood 120:73
Plesner T et al (2013) Preliminary safety and efficacy data of daratumumab in combination with lenalidomide and dexamethasone in relapsed or refractory multiple myeloma. Blood 122:1986
Deckert J et al (2014) SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res 20:4574–4583
Harada H et al (1993) Phenotypic difference of normal plasma cells from mature myeloma cells. Blood 81:2658–2663
Chanan-Khan A et al (2010) Efficacy analysis from phase I study of Lorvotuzumab mertansine(IMGN901), used as monotherapy, in patients with heavily pre-treated CD56-positive multiple myeloma—a preliminary efficacy analysis. Blood 116:1962
Berdeja JG et al (2012) Phase i study of Lorvotuzumab mertansine (LM, IMGN901) in combination with Lenalidomide (Len) and Dexamethasone (Dex) in patients with CD56-positive relapsed or relapsed/refractory multiple myeloma (MM). Blood 120:4048
Kumaresan PR, Lai WC, Chuang SS, Bennett M, Mathew PA (2002) CS1, a novel member of the CD2 family, is homophilic and regulates NK cell function. Mol Immunol 39:1–8
Zonder JA et al (2012) A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 120:552–559
Jakubowiak AJ et al (2012) Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol 30:1960–1965
Lonial S et al (2012) Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 30:1953–1959
Hussein M et al (2010) A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica 95:845–848
Bensinger W et al (2012) A phase 1 study of lucatumumab, a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br J Haematol 159:58–66
Kurzrock R et al (2013) A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res 19:3659–3670
Voorhees PM et al (2013) A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol 161:357–366
San-Miguel J et al (2014) Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood 123:4136–4142
Chapon M et al (2011) Progressive Upregulation of PD-1 in Primary and Metastatic Melanomas Associated with Blunted TCR Signaling in Infiltrating T Lymphocytes. Journal of Investigative Dermatology 131:1300–1307
Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212
Yao S, Chen L (2013) Adaptive resistance: a tumor strategy to evade immune attack. Eur J Immunol 43:576–579
Iwai Y et al (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci 99:12293–12297
Topalian SL et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving Nivolumab. J Clin Oncol 32:1020–1030
Brahmer JR et al (2012) Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Ansell SM et al (2015) PD-1 blockade with Nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372:311–319
Creelan BC (2014) Update on immune checkpoint inhibitors in lung cancer. Cancer Control 21:1–10
Lesokhin AM et al (2014) Preliminary results of a phase I study of Nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies. Blood 124:291–291
Kearl TJ, Jing W, Gershan JA, Johnson BD (2013) Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol 190:5620–5628
Schneider H et al (2006) Reversal of the TCR stop Signal by CTLA-4. Sci 313(5795):1972–5
Schmidt EM et al (2008) CTLA-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol 182:274–282
Friedline RH et al (2009) CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med 206:421–434
Braga WMT, Vettore AL, Carvalho AC, Atanackovic D, Colleoni GWB (2011) Overexpression of CTLA-4 in the bone marrow of patients with multiple myeloma as a sign of local accumulation of immunosuppressive Tregs—perspectives for novel treatment strategies. Blood 118:1829
Hodi FS et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
De Keersmaecker B et al (2014) Immunomodulatory drugs improve the immune environment for dendritic cell-based immunotherapy in multiple myeloma patients after autologous stem cell transplantation. Cancer Immunol Immunother 63:1023–1036
Noonan K et al (2012) Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res 18:1426–1434
Xu Y et al (2009) Immunomodulatory drugs reorganize cytoskeleton by modulating Rho GTPases. Blood 114:338–345
Henry JY et al (2013) Enhanced cross-priming of naive CD8+ T cells by dendritic cells treated by the IMiDs ®immunomodulatory compounds lenalidomide and pomalidomide. Immunology 139:377–385
Clave E et al (2014) Lenalidomide consolidation and maintenance therapy after autologous stem cell transplant for multiple myeloma induces persistent changes in T-cell homeostasis. Leuk Lymphoma 55:1788–1795
Hayashi T et al (2005) Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol 128:192–203
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lee, S.J., Borrello, I. (2016). Role of the Immune Response in Disease Progression and Therapy in Multiple Myeloma. In: Roccaro, A., Ghobrial, I. (eds) Plasma Cell Dyscrasias. Cancer Treatment and Research, vol 169. Springer, Cham. https://doi.org/10.1007/978-3-319-40320-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-40320-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40318-2
Online ISBN: 978-3-319-40320-5
eBook Packages: MedicineMedicine (R0)