Abstract
Induced hypothermia or targeted temperature management has been studied as a potential therapy for improving outcome following traumatic brain injury (TBI) and to a lesser extent, spinal cord injury (SCI). Hypothermia has been clearly demonstrated to improve clinical outcome following cardiac arrest. Preclinical TBI models have shown efficacy. Unfortunately, to date, this is not true for clinical moderate to severe TBI in humans. The same is true for spinal cord injury (SCI). As a result, there is insufficient evidence to support the routine use of induced hypothermia for improving outcome following TBI or SCI. The one clinical condition for which induced hypothermia may be useful is reducing elevated intracranial pressure (ICP) that is recalcitrant to standard medical therapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Traumatic brain injury (TBI)
- Spinal cord injury (SCI)
- Intracranial pressure (ICP)
- Cardiac arrest
- Targeted temperature management
- Hypothermia
- Neuroinflammation
- Decompression
- Pediatric
Introduction
In clinical practice today, there is no therapy that will cure traumatic brain injury (TBI) or spinal cord injury (SCI) . Furthermore, there are no clinically available neuro rescue or neuroprotective therapies. Management of patients suffering from either TBI or SCI is based on optimizing general physiology and avoiding exacerbating conditions, such as seizure, hypotension, or hypoxia. Induced hypothermia or targeted temperature management is a promising potential therapy for TBI and SCI. Preclinical animal models, especially rats and mice, of both TBI and SCI provide provocative evidence that induced hypothermia is highly beneficial for improving both neurological outcome and survival. Unfortunately, similar evidence in humans is lacking. There have been a number of clinical trials conducted but the outcomes have not supported widespread clinical adoption. Nevertheless, the ease of application, relatively low toxicity, dramatic benefit in preclinical models, and lack of any other effective therapeutic options make hypothermia still worthy of consideration.
Background
The central nervous system (CNS) is comprised of the brain and spinal cord. Both have gray matter and white matter. Gray matter is primarily neurons whereas white matter is axons, glia, and astrocytes. The CNS is highly dynamic with a consequently high metabolic demand. To meet this demand, the CNS receives 15 % of cardiac output and accounts for 20–25 % of total body oxygen and 25 % of glucose consumption [1]. Jain and colleagues, employing a noninvasive technique that uses magnetic resonance susceptometry-based oximetry and venous oxygen saturation demonstrate that in humans, global cerebral metabolic rate (CMRO2) is about 130 mol per 100 g per min [2]. Others report cerebral oxygen consumption rate in adults as 3.5 ml per 100 g per min [3]. The gray matter uses about 94 % of CNS oxygen consumption whereas the white matter uses approximately 6 % [3]. Almost 80 % of gray matter oxygen consumption is devoted to glutamate-mediated neurotransmission [1]. Under normal conditions, the blood flow to the CNS is autoregulated to about 50 ml per 100 g tissue per minute. Autoregulation is the process whereby cerebral blood flow is maintained at this constant rate over a wide range of systemic blood pressures.
When injured, the CNS becomes pressure passive. Autoregulatory function is compromised so the CNS is dependent on the systemic blood pressure for adequate perfusion. So, when the CNS is injured, systemic blood pressure rises. The injured tissue is able to receive the perfusion it requires whereas the uninjured tissue is able to autoregulate so as not to be over perfused [4].
Injury has 2 phases—primary and secondary [4]. Primary injury refers to tissue destruction resulting directly from the inciting event. This occurs virtually instantaneously and is complete very soon after injury. Secondary injury is the cascade of events that include inflammation, free radical production, and release of excitatory mediators such as calcium and glutamate. This develops shortly after injury and develops over time in hours to days. The best approach for mitigating primary injury is prevention. Pretreatment may be an option analogous to aspirin for primary prevention of sudden coronary syndrome. However, a clinically effective TBI or SCI pretreatment therapy has not yet been identified. Secondary injury is an opportunity to treat. The period of development is a window in which an effective treatment can be ameliorative.
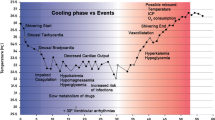
Hypothermia is believed to reduce neuroinflammatory processes, cause a reduction in CMRO2, and improve the efficiency of glucose and energy metabolism [5]. Hibernating animals have been shown to tolerate very low perfusion states for prolonged periods. This became a basis for investigating induced hypothermia as a potential treatment for TBI and SCI. Many basic science investigators have and are exploring this field. In 1994, Dietrich and colleagues showed in a TBI rat model that reduction of core body temperature to 30 °C resulted in significantly less neuron necrosis and brain contusion volume [6]. Since then, a number of investigators have confirmed these findings in rats and other animal subject species.
In 2002, two landmark studies were published demonstrating human clinical efficacy for induced hypothermia or targeted temperature management in patients who suffer out-of-hospital cardiac arrest and remain unconscious. One study was performed by Bernard and colleagues in which 77 patients were randomized to either hypothermia to 33 oC or normothermia [7]. Hypothermia was induced within 2 h of return-to-spontaneous circulation and maintained for 12 h. They found that 49 % of the hypothermia patients were able to leave the hospital to either home or a rehabilitation facility versus only 26 % of the normothermia patients. The other study enrolled 275 patients and also randomized them to either induced hypothermia to 32–34 oC [8]. In this group, hypothermia was induced within 4 h of return-to-spontaneous circulation and maintained for 24 h. They were rewarmed over 8 h. The hypothermia group did much better than the normothermia. About 41 % of hypothermia patients died as compared to 55 % of normothermia and, of those who survived, 55 % had favorable neurological recovery versus 39 %, respectively.
A Cochrane database systematic review was conducted in 2012 by Arrich et al. [9]. They confirmed the efficacy of induced mild hypothermia for improving outcome after cardiac arrest . Since its efficacy has been revealed, this therapy has become part of clinical practice guidelines for managing adult cardiac arrest [10].
Unfortunately, a recent 2015 study by Moler and co-workers did not demonstrate the same efficacy when induced hypothermia was used for pediatric patients who suffered cardiac arrest [11].
Thus, for adult patients with impaired consciousness after cardiac arrest , targeted temperature management , or induced hypothermia provides clear benefit.
Traumatic Brain Injury
In 1997, Marion and colleagues demonstrated the first conclusive evidence showing that mild hypothermia had a benefit in improving clinical outcome in patients who suffered TBI [12]. Unfortunately, it was temporary. There were better outcomes with induced hypothermia at 3 and 6 months after injury. However, this benefit was not sustained so that by 12 months after injury, there was no difference between the hypothermia group versus the normothermia group. Importantly, very severely impaired patients, i.e., those with admission GCS scores of 3–4, did not have any benefit at any time with hypothermia.
An interesting finding of the 1997 Marion et al. study was that both glutamate and IL-1 levels were significantly decreased in the hypothermia group [12]. Glutamate is an excitatory amino acid implicated in secondary neuro-injury. IL-1 is an important proinflammatory cytokine. This suggests that hypothermia did reduce excitatory amino acid release and neuroinflammation as previously hypothesized. However, the study was not designed to determine if lower hypothermia levels would have led to even more glutamate and IL-1 suppression or if even that would have had greater clinical impact.
In 2001, Clifton and colleagues studied whether an earlier induction and longer period of hypothermia would be beneficial [13]. They achieved hypothermia within 8 h of injury and maintained it for 24 h. Unfortunately, outcome and mortality were not significantly different between the 2 groups.
In 2002, post hoc analysis of the 2001 Clifton et al. study revealed that patients who were hypothermic on admission and then subsequently maintained in a hypothermic state had better clinical outcomes [14]. This suggested that the induction of hypothermia very quickly after injury with subsequent maintained cooling could be beneficial. This seemed rational as preclinical animal studies induced hypothermia within minutes after injury and outcomes were significantly better.
In 2010, Clifton and co-workers conducted a trial to test the hypothesis that very early and even longer period of induced hypothermia would provide clinical benefit [15]. Patients were cooled to 33 °C within 2.5 h of injury and maintained for 48 h. Unfortunately, in spite of this achievement, after 232 patients were enrolled, interim analysis did not reveal any clinical benefit. The study was terminated due to futility. Thus, it appears in humans, even very early induction of hypothermia is not beneficial.
In 2015, Andrews and colleagues of the Eurotherm3225 consortium studied the impact of adding induced hypothermia to controlling increased intracranial pressure (ICP) in the setting of TBI [16]. Hypothermia added to standard of care treatments, such as mannitol, was able to control ICP better than without temperature management. Fewer patients needed third tier intervention such as decompressive hemicraniectomy for ICP control. However, an overall benefit could not be demonstrated for adding hypothermia. Instead, there was a worse outcome as more patients had a favorable outcome in the control group as opposed to hypothermia.
For pediatric TBI victims, induced hypothermia also has not been shown to improve outcome. In 2008, Hutchison et al. reported a study of pediatric TBI patients where induced hypothermia was associated with increased toxicity but without benefit [17]. In fact, there was a concerning trend toward more patients with poor outcomes and death in the hypothermia group. This study was followed by a study in 2013 by Adelson and associates [18]. Unfortunately, this study was terminated early for lack of efficacy. Most recently, in 2015, Beca and co-investigators showed no clinical benefit following hypothermia. Thus, hypothermia is not used for improving TBI outcome in the pediatric TBI population.
However, there may be hope for hypothermia as a treatment for TBI. A number of systematic literature reviews have concluded that although there is insufficient evidence presently to endorse the routine use of induced hypothermia for improving TBI clinical outcome, there may be benefit from hypothermia in specific TBI populations [19–21]. The most effective depth of temperature reduction, duration of hypothermia, and other goal directed strategies are needed. They all conclude that additional well controlled randomized studies are warranted.
Spinal Cord Injury
Very few human clinical studies have been conducted evaluating the efficacy of induced hypothermia or targeted temperature management for ameliorating SCI. There is only one prospective study of induced hypothermia as a treatment for acute SCI [22]. The remaining literature is limited to case reports, case series, and retrospective analysis.
In 2008, Kwon et al. conducted a systematic review of the literature of induced hypothermia for SCI. They noted that preclinical animal studies revealed conflicting results. Furthermore, over the prior 2 decades, there were no published peer reviewed human clinical studies using local induced hypothermia for SCI and none ever for systemic induced hypothermia [22].
In 2009, Levi et al. conducted a small retrospective SCI case series that showed that systemic hypothermia with surgery may be beneficial. At 12-months post-injury, 6 patients demonstrated improvement of at least one ASIA grade; 3 patients moved to grade B, 2 to grade C, and 1 to grade D. Any improvement that did occur happened within the first 3-months. The extent that surgical decompression accounted for this improvement could not be determined.
In 2013 Dididze et al. published a case controlled prospective study of induced systemic hypothermia [23]. To date, this is the largest such trial. Most of the hypothermia patients also underwent early surgical decompression . The patients that improved did so within 3 months. As in the Levi study, the relative role of surgical decompression could not be ascertained. Notably, the thromboembolic rate in the prospective cohort group remained significant despite prophylaxis.
In 2014, a small prospective case-series of local epidural cooling was described by Hansebout and Hansebout [24]. All of these SCI ASIA A patients underwent surgical decompression and also received dexamethasone. Most of them (80 %) demonstrated some degree of sensory and motor recovery over the next 5 years. This is important as most patients with complete SCI ASIA A rarely recover any function below the level of injury. Of the enrolled patients, 65 % exhibited some improvement; 30 % improved to ASIA grade B, 25 % to ASIA grade C, and 10 % to ASIA grade D. Two patients even regained the ability to walk. Of note, even though patients uncommonly recover from ASIA grade A to ASIA grade C, when they do, it typically takes 3–5 years. As some patients had a very prolonged recovery, there remains the possibility that some of these may have recovered without hypothermia treatment.
For SCI, there is suggestive evidence that induced hypothermia, either systemic or local, may be clinically beneficial. However, the lack of any randomized well controlled prospective human clinical trials prevents endorsement of either approach as standard of care practice.
Managing Intracranial Hypertension
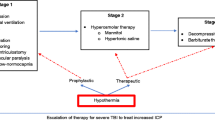
Induced hypothermia may have utility in reducing elevated ICP. In particular, the use of temperature management may reduce the need for more invasive ICP interventions such as decompressive hemicranitectomy.
An important hallmark of TBI management is keeping ICP below 20 mmHg. A retrospective review by Sadaka and associates shows that there is clinical evidence supporting the use of induced hypothermia to manage elevated ICP [25]. Reducing systemic temperature to between 32°–35 °C can decrease ICP by 5 mmHg [16, 26]. However, this may be accompanied by a reduction in cerebral blood flow. For patients suffering from severe TBI (GCS <5), Tokutomi et al. advocate cooling to 35 °C instead of lower temperatures to obtain ICP levels <20 mmHg [27]. Work by Andrews et al. have shown that adding induced hypothermia to clinical management options for elevated ICP recalcitrant to medical therapy can reduce the need for neurosurgical interventions [16]. A cautionary note is that even with ICP control, induced hypothermia has not been shown to improve outcome.
Conclusions
An effective neuroprotection or neurorescue therapy is desperately needed for both TBI and SCI. There simply is no cure for these dreaded conditions. Sadly, at this time, there is insufficient evidence to support the routine use of induced hypothermia or targeted temperature management for improving outcome from TBI or SCI. However, as the investigators all noted, there are still reasons to continue exploring the potential of this therapy.
Disclaimer
The opinions expressed herein belong solely to those of the authors. They do not and should not be interpreted as belonging to or being endorsed by the Uniformed Services University of the Health Sciences, the Dept of Defense or any other element of the US federal government.
References
Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–38.
Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab. 2010;30(9):1598–607.
Clinic C. Human Nerophysiology 2007. Available from: http://www.humanneurophysiology.com/cbfo2consumption.htm.
Ling G. Traumatic brain injury and spinal cord injury. In: Goldman L, Schafer A, editors. Cecil’s Textbook of Medicine, 25th Edition: Elsevier; 2015.
Broessner G, Fischer M, Shubert G, Metzler B, Schumtzhard E. Therapeutic hypothermia in traumatic brain injury. Crit Care. 2012;16(Suppl 2):A1–42.
Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994;87(3):250–8.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128.
Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–21.
Moler FW, Holubkov R, Dean JM. Therapeutic Hypothermia in Children. N Engl J Med. 2015;373(10):980.
Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336(8):540–6.
Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR Jr, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556–63.
Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR Jr, et al. Hypothermia on admission in patients with severe brain injury. J Neurotrauma. 2002;19(3):293–301.
Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, et al. Very early hypothermia induction in patients with severe brain injury (the national acute brain injury study: hypothermia II): a randomised trial. Lancet Neurol. 2011;10(2):131–9.
Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, et al. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015;373(25):2403–12.
Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–56.
Adelson PD, Wisniewski SR, Beca J, Brown SD, Bell M, Muizelaar JP, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol. 2013;12(6):546–53.
Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev. 2009(2):CD001048.
Fox JL, Vu EN, Doyle-Waters M, Brubacher JR, Abu-Laban R, Hu Z. Prophylactic hypothermia for traumatic brain injury: a quantitative systematic review. Cjem. 2010;12(4):355–64.
Crossley S, Reid J, McLatchie R, Hayton J, Clark C, MacDougall M, et al. A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care. 2014;18(2):R75.
Kwon BK, Mann C, Sohn HM, Hilibrand AS, Phillips FM, Wang JC, et al. Hypothermia for spinal cord injury. Spine J. 2008;8(6):859–74.
Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395–400.
Hansebout RR, Hansebout CR. Local cooling for traumatic spinal cord injury: outcomes in 20 patients and review of the literature. J Neurosurg Spine. 2014;20(5):550–61.
Sadaka F, Veremakis C, Lakshmanan R, Palagiri A. Therapeutic hypothermia in traumatic brain injury. Therapeutic Hypothermia in Brain Injury: InTech, Chapters; 2013.
Andrews RJ, Bringas JR, Alonzo G. Cerebrospinal fluid pH and PCO2 rapidly follow arterial blood pH and PCO2 with changes in ventilation. Neurosurgery. 1994;34(3):466–70; discussion 70.
Tokutomi T, Miyagi T, Takeuchi Y, Karukaya T, Katsuki H, Shigemori M. Effect of 35 degrees C hypothermia on intracranial pressure and clinical outcome in patients with severe traumatic brain injury. J Trauma. 2009;66(1):166–73.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Haji, S., Ling, G.S.F. (2017). Therapeutic Hypothermia for Traumatic Brain Injury and Spinal Cord Injury. In: Ecklund, J., Moores, L. (eds) Neurotrauma Management for the Severely Injured Polytrauma Patient. Springer, Cham. https://doi.org/10.1007/978-3-319-40208-6_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-40208-6_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40206-2
Online ISBN: 978-3-319-40208-6
eBook Packages: MedicineMedicine (R0)