Abstract
A number of studies have reported benefits associated with the application of hyperbaric oxygen treatment (HBO) delivered immediately prior to radiation therapy. While these studies provide evidence that pre-treatment with HBO may be beneficial, no measurements of intratumoral pO2 were carried out and they do not directly link the apparent benefits to decreased hypoxic fractions at the time of radiation therapy. While there is empirical evidence and some theoretical basis for HBO to enhance radiation therapy, without direct and repeated measurements of its effects on pO2, it is unlikely that the use of HBO can be understood and optimized for clinical applications. In vivo EPR oximetry is a technique uniquely capable of providing repeated direct measurements of pO2 through a non-invasive procedure in both animal models and human patients. In order to evaluate the ability of pretreatment with HBO to elevate tumor pO2, a novel small animal hyperbaric chamber system was constructed that allows simultaneous in vivo EPR oximetry. This chamber can be placed within the EPR magnet and is equipped with a variety of ports for multiplace gas delivery, thermoregulation, delivery of anesthesia, physiologic monitoring, and EPR detection. Initial measurements were performed in a subcutaneous RIF-1 tumor model in C3H/HeJ mice. The mean baseline pO2 value was 6.0 ± 1.2 mmHg (N = 7) and responses to two atmospheres absolute pressure HBO varied considerably across subjects, within tumors, and over time. When an increase in pO2 was observed, the effect was transient in all but one case, with durations lasting from 5 min to over 20 min, and returned to baseline levels during HBO administration. These results indicate that without direct measurements of pO2 in the tissue of interest, it is likely to be difficult to know the effects of HBO on actual tissue pO2.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
It has been recognized for over 60 years that hypoxia has a profound effect on the radiosensitivity of tissue and that hypoxic cells are approximately three times less sensitive to radiation than well oxygenated cells. A practical consequence of this fact is that tumors with significant hypoxic fractions that are treated with radiation therapy (RT) have decreased probabilities for complete tumor control and reduced patient survival rates. Despite the fundamental importance of tumor oxygenation during RT, measurements of pO2 are not routinely performed in the clinical setting due, at least in part, to the lack of an available quantitative non-invasive measurement technique. EPR oximetry is a technique that is capable of providing direct measurements of pO2 through a repeatable and non-invasive procedure, following one-time implantation of a paramagnetic oxygen reporter, such as India ink. Oxygen-dependent broadening of the EPR signal from the implanted India ink reports the pO2 of the surrounding has been used successfully in a wide array of animal studies [1–3] and in human subjects [4–6].
A number of studies have reported benefits associated with the application of HBO immediately preceding RT [7–12]. The hypothesis behind these studies is that increased tumor oxygenation due to HBO treatment is maintained for a significant period following decompression and a return to room pressure, and that this provides a window where the hypoxic fraction of the tumor is decreased and RT can be applied precisely using the most modern technology. While the above studies provide evidence that pre-treatment with HBO can be beneficial, no measurements of intratumoral pO2 were carried out in these studies, and they do not directly link the apparent benefits to decreased hypoxic fractions at the time of RT nor do they provide insights as to the mechanism by which HBO affects the tissue pO2. The effects of HBO on physiological parameters such as perfusion and cell metabolism make it difficult to conclude that an effect on outcomes has occurred simply due to increased pO2 in the circulation. Data describing normal tissue and tumor oxygen dynamics following HBO are limited. The oxygenation of normal rat cerebral tissues following exposure to four atmospheres absolute pressure (ATA) HBO were measured by Jamieson and van den Brenk using polarographic electrodes and found to be elevated for over 30 min [13]. Kinoshita et al. [12] monitored tumor oxygenation in subcutaneous SCCVII tumors of C3H/He mice following HBO (2 ATA) using semi-quantitative T1-weighted MRI and found that the oxygen-dependent signal intensity remained elevated for more than 60 min. They also reported that the signal intensity in normal muscle tissue declined much more rapidly. Becker et al. [14] measured pO2 in squamous cell cancers of the head and neck in seven patients using polarographic electrodes during and following HBO at 2.4 ATA and found that significantly elevated pO2 values were maintained in all patients for 5–25 min after leaving the hyperbaric chamber. They also report that maximum pO2 values during HBO were achieved after 10–33 min of treatment, with a mean of 17 min. Beppu et al. [9] measured pO2 in clinical glioblastoma tumors and peritumoral tissue (n = 18) using Clark-type electrodes at 5-min intervals following the application of HBO (60-min at 2.8 ATA, w/ 20-min (de)compression periods). The intratumoral pO2 remained significantly higher than the baseline level for 30 min following decompression. These measurements indicate that pO2 in various tissues remains elevated following HBO, but there is variability across tissues and individual tumors. Under these conditions, especially in a clinical setting, confirmation of reduced hypoxia and optimal application of RT would require direct pO2 measurements during and following HBO .
In summary, while there is some good empirical evidence and some theoretical basis for HBO to enhance radiation therapy , without direct and repeated measurements of the effects on pO2 it is unlikely that the use of HBO can be understood and optimized for clinical applications. To date, no studies have combined RT and HBO pre-treatment with direct measurement of the tumor pO2. Without such measurement of pO2, especially in the face of variability across subjects, it is not possible to determine if RT was performed at a time of increased oxygenation as expected. As investigation of this promising treatment protocol continues, such confusion could lead to inaccurate assessment of the effects of the combined therapy. Furthermore, optimization of the application of HBO and the timing of RT following HBO will benefit from knowledge of the pO2 values in the tumor tissue and the rates at which pO2 declines once HBO application ceases.
2 Methods
2.1 Animal and Tumor Model
Developments and studies were performed using the subcutaneous RIF-1 tumor model in C3H/HeJ mice (6–8 weeks, The Jackson Laboratory) [1, 2]. This tumor model was chosen because it has been widely studied within the radiobiology community and it has a significant hypoxic fraction of 1–11 % [15]. Established procedures for cell culture and implantation were followed [2]. Approximately 12 days after subcutaneous inoculation with tumor cells within the hind leg, when the tumors are 100–200 mm3, aggregates of the EPR oxygen probe lithium phthalocyanine (LiPc) were introduced into the tumor. These paramagnetic crystals are biologically inert and have EPR spectra which broaden linearly with increasing pO2. LiPc was introduced at 2 sites within each tumor to sample pO2 within the tumor volume. Each group of LiPc crystals encompassed a volume of approximately 0.5 × 0.5 × 1.5 mm3 with a separation between sites of 4 mm.
EPR oximetry measurements were acquired at least 24 h after insertion of the LiPc aggregates to allow for resolution of the minimal acute trauma of the procedure [16]. Mice were anesthetized using an intraperitoneal bolus injection of ketamine (90 mg/kg) and xylazine (9 mg/kg). Following anesthetization, mice were intubated according to procedures described by Hallowell EMC (Pittsfield, MA, USA) and using an otoscope and supplies within the Hallowell mouse intubation package. Mice were then positioned and secured on the animal tray and mechanical ventilation was initiated. Prior to insertion of the mouse into the HBO chamber and EPR system, a boost of anesthesia (0.5× initial dose) was applied; this combination of bolus and boost was sufficient to typically provide the 1.5 h of anesthesia necessary for the measurement protocol. A rectal thermometer was used to monitor the core temperature of the mice, which was maintained at 37 ± 1 °C throughout the experiments through use of an IR lamp prior to installation in the chamber and the radiant heat supply surrounding the mouse within the chamber.
2.2 HBO Application Protocol
Hyperbaric oxygen was applied using a customized small animal hyperbaric chamber (Model B11, Reimers Systems Inc, Springfield VA, USA) in operation at the Dartmouth EPR Center (Fig. 48.1). This chamber is capable of delivering 100 % O2 at pressures of up to 3.72 ATA. It is equipped with a variety of ports for drug delivery, physiologic monitoring, and EPR detection. An additional set of ports allow the use of an existing external small animal ventilator (Model SAR-830, CWE Inc.), which is rated for use with pure oxygen and at hyperbaric pressures. In addition to supporting the respiratory function, the ventilator allows the chamber to be operated as a multiplace hyperbaric chamber where the chamber is filled with an inert gas (e.g. N2) and O2 is supplied directly to the animal. This increases safety tolerances and alleviates the need for time consuming O2 flushing of the chamber. The chamber can be installed inside the permanent magnet of the clinical EPR spectrometer and it is constructed entirely of non-magnetic materials. A customized EPR resonator and animal warming bed have been constructed for use in the chamber. For all experiments in this initial study, mice were exposed to HBO at 2 ATA, though the duration of HBO exposure varied. Compression and decompression of the chamber were performed over 2-min periods, resulting in biologically acceptable rates of ~15 (feet of seawater)/min.
2.3 In Vivo EPR Oximetry
Mice were positioned with tumors centrally located with respect to the magnet and directly under the surface loop detector [17]. Multi-site EPR spectroscopy was used to simultaneously measure the EPR signals from each site, characterize their spectral shapes, and estimate pO2 at their locations. Quality assurance spectra were acquired during the study to verify proper calibration of the instrumental settings that could affect the observed linewidth and derived pO2 estimates. Instrumental parameters, such as the Zeeman modulation amplitude and RF power, were set adaptively based on the measured spectra to optimize the precision and accuracy of the pO2 measurements. Throughout periods of baseline (normobaric air breathing), HBO (2ATA with 100 % O2), and recovery periods with normobaric air, the pO2 was measured continuously using spectra collected with periods of 10 seconds. EPR spectra were analyzed using least-squares fitting to estimate the oxygen-dependent linewidth and linewidths were converted to pO2 through an established oxygen calibration curve.
3 Results
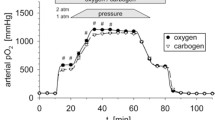
Following series of normobaric trials and studies to assess the role of the ketamine/xylazine anesthesia, pO2 measurements were accomplished in seven mice during the application of HBO. The mean baseline pO2 value was 6.0 ± 1.2 mmHg, which is consistent with prior measurements in this mouse tumor model [16]. Responses to the application of HBO varied considerably across subjects and for individual subjects at the 2 measurement sites. In 6/7 mice a change in pO2 of at least 2 mmHg was observed for at least one of the measurement sites. In one mouse there was no response to HBO. When an increase in pO2 was observed, the effect was transient in all but one case, with durations lasting from 5 min to over 20 min. A typical response is shown in Fig. 48.2. HBO was initiated at the 21-min time point and ceased 30 min later. In one of the measurement sites (Site #1) a ~4 mmHg increase in pO2 was observed within the initial 10 min of HBO, after which the pO2 returned to the baseline values of ~6 mmHg. The second measurement site (Site #2) remained near its baseline value of 9 mmHg throughout the measurement and did not exhibit any increase in pO2 when HBO was applied.
Within this set of measurements, and consistent with all other measurements, there was a very large degree of variability in the response to HBO. This highlights the need for such measurements of tumor pO2 if the radiosensitizing ability of HBO is to be assessed and investigated effectively.
4 Discussion and Conclusions
We have developed the instrumentation and procedures necessary to perform tumor pO2 measurements during and following the application of HBO treatment. The developments of the instrumentation and procedures have enabled initial measurement of tumor pO2 in the mouse tumor model prior to, during, and after HBO. We observed a large degree of inter- and intra-subject variability in the response of tumor tissue to the application of HBO . Variability in the existence, timing, and level of response were observed. The methods were designed to enable adequate physiologic controls to minimize the impact of likely confounders, including ventilation and temperature. The role of anesthesia as a potential confounder must be further considered. Additional systematic studies are warranted to provide more definitive characterization of the effects of HBOT in pertinent tumor model systems and normal tissues, and eventually within clinical trials. Currently the use of HBO for enhancing therapy has been limited by a lack of knowledge of the optimum conditions for enhancing therapy in regard to both the HBO parameters to be used to maximize radiation sensitization and the effects of radiation on the effectiveness of repeated HBO applications to change tumor pO2. In the face of variability such as that observed in our study, especially without knowledge of the individual pO2 dynamics, effective use of HBO to radiosensitize tumors prior to treatment would likely not be possible. Our research is ongoing to better understand the sources and patterns of the observed variations, including both physiologic and experimental considerations, with the aim of enhancing the effectiveness of radiation therapy. Recently, clinical EPR systems have been developed [4] which will allow measurement of human tumor pO2 following HBO treatment and, with the use of multiplace HBO chambers, could provide data during HBO as well.
References
Goda F, O’Hara JA, Rhodes ES, Liu KJ, Dunn JF, Bacic G, Swartz HM (1995) Changes of oxygen tension in experimental tumors after a single dose of X-ray irradiation. Cancer Res 55(11):2249–2252
O’Hara JA, Goda F, Demidenko E, Swartz HM (1998) Effect on regrowth delay in a murine tumor of scheduling split-dose irradiation based on direct pO2 measurements by electron paramagnetic resonance oximetry. Radiat Res 150(5):549–556
O’Hara JA, Goda F, Liu KJ, Bacic G, Hoopes PJ, Swartz HM (1995) The pO2 in a murine tumor after irradiation: an in vivo electron paramagnetic resonance oximetry study. Radiat Res 144(2):222–229
Swartz HM, Williams BB, Zaki BI, Hartford AC, Jarvis LA, Chen EY, Comi RJ, Ernstoff MS, Hou H, Khan N and Swarts SG (2014) Clinical EPR: unique opportunities and some challenges. Acad Radiol 21(2):197–206
Khan N, Williams BB, Swartz HM (2006) Clinical applications of in vivo EPR: rationale and initial results. Appl Magn Reson 30:185–199
Swartz HM, Khan N, Buckey J, Comi R, Gould L, Grinberg O, Hartford A, Hopf H, Hou H, Hug E, Iwasaki A, Lesniewski P, Salikhov I, Walczak T (2004) Clinical applications of EPR: overview and perspectives. NMR Biomed 17(5):335–351
Kunugita N, Kohshi K, Kinoshita Y, Katoh T, Abe H, Tosaki T, Kawamoto T, Norimura T (2001) Radiotherapy after hyperbaric oxygenation improves radioresponse in experimental tumor models. Cancer Lett 164(2):149–154
Kohshi K, Kinoshita Y, Terashima H, Konda N, Yokota A, Soejima T (1996) Radiotherapy after hyperbaric oxygenation for malignant gliomas: a pilot study. J Cancer Res Clin Oncol 122(11):676–678
Kohshi K, Kinoshita Y, Imada H, Kunugita N, Abe H, Terashima H, Tokui N, Uemura S (1999) Effects of radiotherapy after hyperbaric oxygenation on malignant gliomas. Br J Cancer 80(1–2):236–241
Kohshi K, Yamamoto H, Nakahara A, Katoh T, Takagi M (2007) Fractionated stereotactic radiotherapy using gamma unit after hyperbaric oxygenation on recurrent high-grade gliomas. J Neurooncol 82(3):297–303
Jamieson D, Van Den Brenk HAS (1963) Measurement of oxygen tensions in cerebral tissues of rats exposed to high pressures of oxygen. J Appl Physiol 18(5):869–876
Kinoshita Y, Kohshi K, Kunugita N, Tosaki T, Yokota A (2000) Preservation of tumour oxygen after hyperbaric oxygenation monitored by magnetic resonance imaging. Br J Cancer 82(1):88–92
Becker A, Kuhnt T, Liedtke H, Krivokuca A, Bloching M, Dunst J (2002) Oxygenation measurements in head and neck cancers during hyperbaric oxygenation. Strahlenther Onkol 178(2):105–108
Beppu T, Kamada K, Yoshida Y, Arai H, Ogasawara K, Ogawa A (2002) Change of oxygen pressure in glioblastoma tissue under various conditions. J Neurooncol 58(1):47–52
Clifton K, Briggs R, Stone HB (1966) Quantitative radiosensitivity studies of solid carcinomas in vivo: methodology and effect of anoxia. J Natl Cancer Inst 36:965–974
Hou H, Lariviere JP, Demidenko E, Gladstone DJ, Swartz HM, Khan N (2009) Repeated tumor pO2 measurements by multi-site EPR oximetry as a prognostic marker for enhanced therapeutic efficacy of fractionated radiotherapy. Radiother Oncol 91(1):126–131
Salikhov I, Walczak T, Lesniewski P, Khan N, Iwasaki A, Comi R, Buckey J, Swartz HM (2005) EPR spectrometer for clinical applications. Magn Reson Med 54(5):1317–1320
Acknowledgments
This research was supported by a grant from the GEMI Fund, Lidingö, Sweden and American Cancer Society Research Grant #IRG-82-003-22.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Williams, B.B., Hou, H., Coombs, R., Swartz, H.M. (2016). EPR Oximetry for Investigation of Hyperbaric O2 Pre-treatment for Tumor Radiosensitization. In: Luo, Q., Li, L., Harrison, D., Shi, H., Bruley, D. (eds) Oxygen Transport to Tissue XXXVIII. Advances in Experimental Medicine and Biology, vol 923. Springer, Cham. https://doi.org/10.1007/978-3-319-38810-6_48
Download citation
DOI: https://doi.org/10.1007/978-3-319-38810-6_48
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-38808-3
Online ISBN: 978-3-319-38810-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)