Abstract
Obstructive sleep apnea syndrome (OSAS) is associated with an increased prevalence of hypertension (HTN) and is currently recognized as a cause of secondary HTN [1], with the severity of OSAS being directly correlated with the degree of blood pressure (BP) elevation, with its resistance to antihypertensive treatment and with the presence of alterations in day-to-night BP changes [2–4]. The adverse cardiovascular (CV) prognosis associated with these alterations underlines the importance of OSAS-related hypertension and the need of implementing specific treatment strategies (that is, continuous positive airway pressure (CPAP) in order to promote BP control and optimize CV protection. The present chapter will review the evidence supporting the association of OSAS with often resistant arterial HTN and the proposed mechanisms for this association. It will also address the role of ambulatory blood pressure monitoring (ABPM) in the confirmation of HTN in subjects with OSAS and whether the proper identification and management of OSAS in subjects with resistant HTN will improve BP control. Particular emphasis will be put on the role of CPAP ventilation for the treatment of OSAS, which is known to be effective in reducing the sympathetic nervous system overdrive, a major contributing mechanism for OSAS-related HTN.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Resistant hypertension
- Obstructive sleep apnea
- Cardiovascular risk
- Antihypertensive treatment
- Continuous positive airway pressure treatment
1 Introduction
Obstructive sleep apnea syndrome (OSAS) is associated with an increased prevalence of hypertension (HTN) and is currently recognized as a cause of secondary HTN [1], with the severity of OSAS being directly correlated with the degree of blood pressure (BP) elevation, with its resistance to antihypertensive treatment and with the presence of alterations in day-to-night BP changes [2–4]. The adverse cardiovascular (CV) prognosis associated with these alterations underlines the importance of OSAS-related hypertension and the need of implementing specific treatment strategies (that is, continuous positive airway pressure (CPAP)) in order to promote BP control and optimize CV protection. The present chapter will review the evidence supporting the association of OSAS with often resistant arterial HTN and the proposed mechanisms for this association. It will also address the role of ambulatory blood pressure monitoring (ABPM) in the confirmation of HTN in subjects with OSAS and whether the proper identification and management of OSAS in subjects with resistant HTN will improve BP control. Particular emphasis will be put on the role of CPAP ventilation for the treatment of OSAS, which is known to be effective in reducing the sympathetic nervous system overdrive, a major contributing mechanism for OSAS-related HTN.
2 OSAS and BP Levels
OSAS, combining nighttime occurring intermittent obstruction of upper airways with daytime somnolence, is not only a recognized cause of secondary HTN [1] but is associated with a high prevalence of severe and resistant BP elevation [2–4]. OSAS is defined as the presence of recurrent obstructive breathing events generated by complete upper airway obstruction during sleep or of sleep hypoventilation syndrome, accompanied by daytime symptoms [5]. Alterations in breathing patterns in OSAS may importantly influence many regulatory mechanisms involved in BP control. OSA events occurring during night (i.e., obstructive apnea and hyperventilation episodes alternating during sleep) have been shown to be accompanied by acute changes in autonomic CV control and in hemodynamic regulation, which in turn induce marked increases in BP levels during nighttime [6]. Indeed, HTN related to OSAS is predominantly nocturnal in its early stages and frequently accompanied by a non-dipper profile of BP (i.e., nocturnal BP fall <10 % compared to daytime BP levels) [7, 8]. Nonetheless, the increase in BP levels in OSAS subjects is not limited to the nighttime hours, during which OSA episodes occur, but is often sustained also during the daytime. Indeed, case–control studies using 24-h ABPM have provided evidence that, compared to matched control subjects, OSAS patients show significantly higher ambulatory BP levels not only during the nighttime sleep but also during daytime wakefulness [9–11].
3 Epidemiological Evidence of the Association Between OSAS and Hypertension
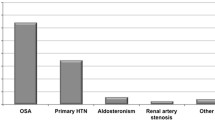
The association between OSAS and HTN has been extensively confirmed across a number of studies of different natures either in the general population or in cohorts of OSAS patients [2, 12–15]. Overall, these studies have indicated a variable frequency of HTN in subjects with OSAS that may range from 35 to 80 % [7, 16]. Conversely, when properly investigated, OSAS has been shown to be present in up to 40 % of hypertensive subjects [17]. Whether the association between OSAS and HTN is explained by the presence of other CV risk factors other than OSAS itself, this is still a matter of debate. For instance, OSAS is often associated with obesity, which in turn is considered to explain about 65–75 % of cases of essential HTN [18, 19]. Indeed, some studies have indicated that much of the relationship between OSAS and HTN may be explained by the associated obesity [20]. Conversely, other reports have indicated that the association between obesity and HTN may be explained by the presence of OSAS in a substantial number of subjects. In addition to body mass index (BMI), other factors such as sex and age have been shown to significantly influence the relationship between OSAS and elevated BP levels [21]. Evidence for this has been provided both from cross-sectional [12] and longitudinal studies [20, 22] showing that OSAS is more strongly associated with resistant HTN in young to middle-aged adults (<50 years of age) [21]. This association is more frequently observed in men than in women [23]. It is thus clear that disentangling the independent contribution of OSAS, obesity, and other CV risk factors to elevation in BP levels is rather difficult. Despite this difficulty, several longitudinal studies have supported the association between OSAS and HTN independently of other potential contributing factors such as BMI, also indicating that OSAS is not only associated with an increased risk of prevalent HTN but may also be an independent and significant predictor of future development of HTN in particular if not properly treated [3, 15, 20, 24] (Fig. 5.1).
Predicted increase in systolic blood pressure (SBP) and in diastolic blood pressure (DBP) associated with sleep-disordered breathing at three body mass index (BMI) categories in the Wisconsin Sleep Cohort Study (Modified from young et al. [15] by permission)
In particular, in the Wisconsin Sleep Cohort Study, a dose–response relationship between sleep-disordered breathing at baseline and the development of HTN after 4 years of follow-up was reported independently of baseline BP levels, BMI, neck and waist circumference, age, sex, and other potential confounders, suggesting that sleep-disordered breathing is likely to be an independent risk factor for HTN and resultant CV morbidity in the general population [3].
However, in spite of such suggestion, there is still some controversy regarding the specific role of OSAS in the development of HTN. This is because of the variable role played by several confounders, the large heterogeneity of the available studies populations in terms of ethnicity, age, BMI and metabolic risk factors and the variable methodologies used to ascertain the presence of OSAS (i.e., in-laboratory polysomnography, in-home polysomnography, in-home polygraphy), combined with variable follow-up periods and different definitions of hypertension.
Most available longitudinal studies suffer from an inherent weakness, i.e., the lack of relevant history on subjects’ sleep breathing patterns prior to the start of the study. Thus, it is possible that subjects who had HTN at baseline and were thus excluded from the study were those rapidly developing HTN because of previous exposure to sleep apnea, while normotensive subjects who had obstructive sleep apnea (OSA) at baseline were less responsive to the apneic events and therefore less likely to develop HTN at follow-up. The percentage of subjects who had HTN at baseline in these studies is indeed of substantial size.
Change in body weight over time, another important confounder for incident HTN, was not taken into account in most of available prospective studies, and majority of participants in these studies had mild OSA, with less than 13 % of patients having moderate-to-severe OSA.
A relatively short follow-up period of OSA cohorts predominantly comprising patients with mild OSA may thus importantly contribute to explain discrepant findings reported so far.
More recently, the results of a prospective cohort study of 1889 participants without HTN at baseline more convincingly showed a risk of incident hypertension to be directly related to the severity of OSA [25]. In addition, this study showed that OSA treatment with CPAP therapy was associated with a lower risk of hypertension (Fig. 5.2).
Risk of incident hypertension and severity of OSA. OSA indicates obstructive sleep apnea. Severity of OSA was defined by the apnea–hypopnea index (AHI) as mild OSA (AHI, 5.0–14.9), moderate OSA (AHI, 15.0–29.9), and severe OSA (AHI, ≥30.0). P value reflects an overall log-rank χ 2 test, providing an overall survival difference among the four study groups (Taken from Marin et al. [25] by permission)
Several studies have identified OSAS as an important risk factor for resistant HTN also showing a dose–response relationship between OSAS severity and the degree of BP elevation [2, 26, 27]. It has also been shown that HTN occurring in individuals with OSAS is more likely to be severe, resistant to treatment and associated with alterations in day-to-night BP changes (i.e., nocturnal HTN and non-dipping profile of BP on 24-h ABPM) [2, 26, 27]. Conversely, an extremely high prevalence of OSA of about 80 % has been reported among adult patients with drug-resistant HTN [23]. It has also been shown that rates of BP control decrease as the severity of sleep-related breathing disorder increases [2]. Although all the above evidence supports a potential role of OSAS in the pathogenesis of HTN and drug-resistant HTN, there is still only partial understanding of the pathophysiological mechanisms by which OSAS promotes arterial HTN.
4 Proposed Mechanisms for OSAS-Related Hypertension
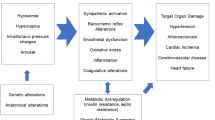
Evidence from experimental and clinical studies has indicated that the pathogenesis of OSAS-related HTN is likely to be multifactorial, involving alterations in several regulatory systems: activation of the sympathetic nervous system in the frame of complex alterations in autonomic CV modulation involving both arterial baro- and chemoreflexes, activation of renin–angiotensin–aldosterone system, endothelial dysfunction, systemic and vascular inflammation, oxidative stress, metabolic abnormalities, increased arterial stiffness and alterations in cardiac function and structure.
Sympathetic Nervous System Activation
Activation of the sympathetic nervous system is considered a major pathophysiological mechanism underlying the alterations in BP regulation reported in OSAS (Fig. 5.3). This has been consistently demonstrated by several studies implementing direct techniques for assessment of sympathetic nervous system activity (i.e., recording of efferent postganglionic muscle sympathetic nerve activity via microneurography (MSNA) and assessment of norepinephrine plasma levels) in which an increase in central sympathetic drive was positively correlated with increases in BP levels independently of other contributing factors. The sympathetic activation in OSAS is largely explained by stimulation of the peripheral and central chemoreflexes, triggered by the reductions in arterial oxygen content and by hypercapnia, respectively. Moreover, sleep fragmentation, related to repeated arousals after each apnea/hypopnea event, might play an additional role in this context. The resultant increases in sympathetic drive to the heart and peripheral vasculature lead to important increases in heart rate and vascular tone which in turn are responsible for the marked increases in BP levels during resumption of ventilation after each apnoeic episode [28] (Fig. 5.4a).
(a) Recordings of sympathetic nerve activity (SNA), respiration (RESP), and blood pressure (BP) during 3 min of stage II sleep, showing incessant oscillations in BP and SNA in response to the repetitive OSAs. These oscillations occurred continuously during sleep, throughout all sleep stages. (b) Recordings of SNA during wakefulness in patients with OSAS and matched controls showing high levels of SNA in patients with OSA (Taken from reference Somers et al. [28] by permission)
This increase in central sympathetic drive has also been shown to be associated with alterations in circadian BP variation (i.e., absence of nocturnal BP fall or increase in BP at night), and nocturnal HTN is frequently observed in OSAS patients [29]. In addition, several studies using MSNA recordings have indicated that the sympathetic activation in OSAS subjects is not only limited to nighttime but may persist even after resuming normal breathing pattern during daytime wakefulness, despite normal arterial oxygen saturation and carbon dioxide levels [28, 30] (Fig. 5.4b). Remarkably, in several studies long-term implementation of CPAP resulted in marked reductions in sympathetic nerve traffic [28] and BP levels [31] both during nighttime and daytime wakefulness [32], further supporting the pathogenetic role of the sympathetic activation in explaining BP elevation in OSAS.
Alterations in Integrated Autonomic CV Modulation
In normal physiological conditions, control of BP levels is achieved through a complex combination between central and reflex neural influences, leading to a continuous modulation of efferent sympathetic and parasympathetic nerve activity and the associated activity of neurohormonal systems primarily regulated by the hypothalamus. In OSAS, the sustained chemoreflex activation, the related adrenergic overactivity, and the resulting HTN may blunt and/or reset arterial and cardiopulmonary reflexes which in turn may lead to chemoreflex potentiation [33, 34]. In addition, dysfunction of neural reflexogenic areas (i.e., baroreflex impairment) may lead to a reduced sympathoinhibition and to impaired cardiac parasympathetic modulation [35, 36] further contributing to adrenergic overdrive and rise in BP levels (Fig. 5.3).In particular, the observation of a reduced spontaneous cardiac baroreflex sensitivity (as assessed by the sequence method), and the absence of 24-h baroreflex modulation (i.e., blunted increase in baroreflex sensitivity during sleep compared with its values during wakefulness) in OSAS patients [35], has provided indirect support to the concept that baroreflex dysfunction and not only chemoreceptor stimulation by hypoxia may contribute to the acute and long-term sympathetic activation in OSAS patients (Fig. 5.5). The depressed cardiac baroreflex sensitivity during sleep may thus in turn contribute to the pathophysiology of HTN in OSAS patients.
Relationship between spontaneous baroreflex sensitivity (BRS) and the severity of obstructive sleep apnea syndrome, as quantified by the apnea–hypopnea index (AHI). Data are shown as individual values in 11 patients separately for a period of wakefulness (W), a period of non-rapid eye movement (NREM) sleep, and a period of rapid eye movement (REM) sleep (Taken from Parati et al. [35] by permission)
This concept has been further supported by the results of interventional studies in OSAS patients showing a significant improvement in baroreflex sensitivity after long-term implementation of CPAP treatment [37–39]. Other independent studies, applying spectral analysis to estimate variability of MSNA, BP, and heart rate, have provided additional evidence on the impaired autonomic CV modulation in OSA based on the demonstration of significant increases in heart rate and sympathetic drive, but also of a reduced heart rate variability and a marked increase in BP variability (more than double the variance in healthy controls) [40] (Fig. 5.6).
RR interval and systolic blood pressure (SBP) mean values and their variances and normalized low-frequency (LF) and high-frequency (HF) spectral components of RR interval in control subjects, patients with mild OSA, and patients with moderate-to-severe OSA *P < 0.05 versus control subjects. †P < 0.05 versus mild OSA. Data are mean ± SEM (Modified from Ref. [40] by permission)
Further evidence that sleep-related breathing disorders may induce alterations in autonomic CV modulation has been provided by a study in untreated subjects with OSA of different severities indicating that excessive daytime sleepiness is accompanied by lower baroreflex sensitivity and significantly higher low-to-high-frequency power ratio of heart rate variability (which is believed to be a marker of sympathetic activity) during the different stages of nocturnal sleep as compared not only to control subjects but also to OSA patients without daytime somnolence [41] (Fig. 5.7).
Trends of baroreflex sensitivity (BRS), and of the ratio between low- and high-frequency powers of RRI (LF⁄HF), in healthy controls without sleep-related breathing disorders (SRBD, square symbols), in patients with OSA and excessive daytime sleepiness (EDS, open circles), and in patients with OSA not affected by EDS (nEDS, solid circles) (Taken from Lombardi et al. [41] by permission)
Activation of Renin–Angiotensin–Aldosterone System (Increase in Aldosterone Levels)
The frequent association of OSAS with hyperaldosteronism reported in patients with resistant HTN has led to suggest that both these factors may interact on a pathophysiological basis contributing to BP elevation [42–44]. Although evidence is still needed to determine the causality of this association, it has been hypothesized that OSAS may contribute to the pathogenesis of resistant HTN by stimulating aldosterone secretion [45] (Fig. 5.3). Evidence supporting this concept has been provided by several studies showing positive and significant correlations between plasma aldosterone concentrations and OSAS severity in patients with resistant HTN, but not in normotensive subjects nor in treated hypertensives with controlled BP [46, 596]. It is likely that aldosterone excess by promoting fluid accumulation in the neck, and thus increasing upper airway resistance, may increase the severity of OSA and the related increase in BP levels [17, 47]. Indirect evidence favoring this concept has been provided by interventional studies in subjects with OSAS and resistant HTN where addition of spironolactone to current antihypertensive treatment resulted in significant reductions in the severity of OSA (i.e., reductions in apnea–hypopnea index and the number of central and obstructive events) on top of its BP-lowering effects [48]. Additional evidence is still needed, however, to consistently determine a causal association between aldosterone excess in OSAS and resistant hypertension.
Endothelial Dysfunction
The intermittent hypoxia, the associated neural and humoral alterations, and repeated BP surges during OSA episodes may contribute to impairment in endothelial function. In turn, the inhibition of nitric oxide (NO) production, decreased vasodilatation, and increased vasoconstriction associated with endothelial dysfunction may substantially contribute to BP elevation (Fig. 5.3). Several studies assessing brachial artery endothelium-dependent flow-mediated dilation (FMD, an indirect marker of endothelial NO-mediated reactivity) and forearm blood flow responses to different stimuli (i.e., infusion of acetylcholine, sodium nitroprusside, nitroglycerin) have shown that compared to healthy controls, patients with OSAS often exhibit an impairment of resistance-vessel endothelium-dependent vasodilation [49, 50]. Even when accounting for important confounding factors such as body weight, brachial artery FMD has been shown to be significantly lower in normal-weight OSAS patients than in OSAS-free controls [51]. Additional evidence that OSAS may importantly influence both indices of macrovascular and microvascular endothelial function was provided by a recent study showing abnormal myocardial perfusion, attenuated brachial artery reactivity, and reduced cutaneous perfusion response in OSAS patients compared to healthy controls [52]. Remarkably, several interventional studies have shown substantial improvements in different indices of endothelial function following implementation of regular CPAP use in subjects with hypertension and OSAS [50–52] which indirectly supports a role for endothelial dysfunction in the pathogenesis of arterial hypertension in OSAS.
Vascular Inflammation and Oxidative Stress
Repetitive episodes of hypoxia/reoxygenation during transient cessation of breathing in OSA may also reduce NO availability, promoting vascular endothelial inflammation and elevated oxidative stress [49, 50, 53–55] (Fig. 5.3). When compared to OSAS-free controls and regardless of the presence of obesity, OSAS patients have been shown to present a reduced expression of endothelial NO synthase (eNOS) and phosphorylated eNOS (proteins that regulate basal NO production and activity) as well as an increased expression of nitrotyrosine (a marker of oxidative stress) and of nuclear factor-K B (NFkB) (a marker of inflammation) [51]. Most importantly, after 1 month of regular treatment with CPAP, flow-mediated dilation, expression of eNOS, and phosphorylated eNOS were significantly increased, whereas expression of nitrotyrosine and nuclear factor-K B was decreased [51]. It has also been proposed that intermittent hypoxia/hypercapnia associated with OSAS may contribute to the pathogenesis of hypertension by increasing endothelin-1 production. This has been supported by experimental studies in rats showing significant increases in plasma levels of endothelin-1 (a potent vasoconstrictor) and higher BP levels in rats exposed to intermittent hypoxia (i.e., cycles of hypoxia/hypercapnia of 8 h a day during 11 days) compared to those breathing normoxic air [56].
Data from several studies have indicated that selective activation of inflammatory pathways may be an additional important molecular mechanism for the pathogenesis of arterial HTN in OSAS. This has been supported by translational studies showing a selective activation of the pro-inflammatory transcription factor NFkB in HeLa cells of OSAS patients exposed to intermittent hypoxia/reoxygenation cycles [57]. In addition, compared to healthy controls, subjects with OSAS showed significantly higher levels of circulating pro-inflammatory cytokines (i.e., tumor necrosis factor-alpha (TNF-alpha) and the adaptive factor erythropoietin) as well as higher levels of circulating neutrophils. Interestingly, levels of TNF-alpha were normalized after 6 weeks of continuous treatment with CPAP [57]. Other studies have shown that compared to healthy controls, serum levels of inflammatory markers (i.e., C-reactive protein, CRP) are significantly higher in OSAS patients and independently associated with OSAS severity [58]. Interestingly, interventional studies have shown significant reductions in serum levels of CRP and interleukin-6 following implementation of regular CPAP treatment [59]. Finally, evidence has also been provided that OSAS may induce activation of adhesion molecules participating in inflammation. This has been supported by case–control studies showing significantly higher levels of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and L-selectin in OSA patients compared to healthy controls [60].
Arterial Stiffness
An increased arterial stiffness is a recognized risk factor contributing to the pathogenesis of arterial HTN [61–63]. A recent systematic review of relevant studies has indicated an independent effect of OSAS on arterial stiffness, which in turn may contribute to elevation in BP levels and to resistant HTN [64] (Fig. 5.3). A number of studies have consistently reported significantly higher values of carotid–femoral pulse wave velocity (cfPWV) (which is considered the “gold standard” measure of aortic stiffness), in patients with OSAS compared to healthy controls [64, 65]. Of note, the increase in cfPWV has been shown to be directly related to the severity of OSAS and to be even higher in subjects with OSAS and associated HTN or in the presence of other CV risk factors [66]. In Asian populations, several studies implementing brachial–ankle PWV (baPWV) have also reported significant associations between OSAS and increased arterial stiffness [67]. Even when comparisons have been performed between individuals with or without OSAS entirely free from other CV risk factors, an independent effect of OSAS on arterial stiffening has been reported [68]. Remarkably, in randomized interventional studies, effective treatment of OSAS with CPAP has been associated with significant decreases in arterial stiffness [69, 70]. In one of such studies, CPAP was also associated with significant reductions in sympathetic nerve activity and in ambulatory BP and with significant improvements in arterial baroreflex sensitivity [69].
Metabolic Factors
In addition to the hemodynamic changes, OSAS has been frequently associated with metabolic alterations (i.e., alterations in glucose metabolism, insulin resistance, and leptin resistance) which in turn may contribute to the pathogenesis of arterial hypertension (Fig. 5.3). Although alterations in glucose metabolism are thought to be the consequence of other conditions associated with OSAS (i.e., an increased BMI, metabolic syndrome, and/or type 2 diabetes) rather than being OSAS outcomes, evidence has been provided that OSAS, independently of the presence of other confounding factors, is associated with alterations in glucose metabolism which may indeed favor development of type 2 diabetes [71]. In addition, interventional studies have shown the efficacy of regular CPAP treatment in improving the abnormalities in glucose metabolism in OSAS patients [71]. Compared to healthy controls, OSAS patients have also been shown to have a higher degree of insulin and leptin resistance [72–74] even after accounting for body fat content [75]. Although the above-mentioned metabolic alterations should theoretically contribute to the pathogenesis of HTN in OSAS, their relative contribution to BP elevation independent of other concomitant factors still needs to be further explored.
5 Prognostic Relevance of OSAS-Related Hypertension
Evidence from several studies has supported an independent association between OSAS and CV disease [76]. OSAS, particularly if severe, has been linked to fatal and nonfatal CV events [77–81], to development and progression of congestive heart failure [79], and with all-cause mortality [82, 83]. However, because the link between OSAS and CV disease may be related to age, obesity, and visceral adiposity, in some of these studies, the associations have lost strength when adjusting for these factors. When it comes to subclinical organ damage, evidence has also been provided that OSAS is independently associated with cardiac (i.e., LV hypertrophy and dysfunction) [70, 84, 85], vascular (i.e., increased carotid intima-media thickness, increased arterial stiffness) [64], renal organ damage (i.e., increased urinary albumin excretion) [86, 87], and endothelial dysfunction (i.e., blunted endothelium-dependent dilatation) [64]. Evidence has also been provided that resistant HTN which is more frequent among OSAS patients considerably increases the risk for CV complications including myocardial infarction, stroke, congestive heart failure, and chronic kidney disease [88–90]. In consideration of the increased CV risk associated with OSAS and resistant HTN, current guidelines for the management of arterial HTN include OSAS among the modifiable causes to be considered in the diagnostic approach to resistant HTN, in order to properly manage both of these conditions [1, 91, 92]. It should be mentioned, however, that no studies have specifically addressed how and to which extent the addition of HTN to OSAS may increase the risk of CV disease independent of other CV risk factors that are often clustered in the context of OSAS. Although OSAS and resistant hypertension have been shown to be independent predictors of CV prognosis, evidence is still needed to determine the actual prognostic relevance of their interaction independently of other concomitant CV risk factors.
Not only the presence of resistant HTN but also the higher frequency of alterations in day-to-night BP profiles and nocturnal HTN contributed to the elevated CV risk of OSAS patients. As mentioned above, nocturnal sympathetic activation during OSAS episodes importantly contributes to increases in BP during sleep, thus attenuating the physiologic nocturnal dipping of BP (i.e., on average by 10–20 % of daytime BP values) or even increasing nocturnal BP levels (rising pattern of nighttime BP). It is thus not surprising the high frequency of non-dipping profile of BP (i.e., nocturnal BP fall <10 % compared to daytime BP levels) reported in OSA patients independently of the presence of HTN [93]. Remarkably, the degree of impairment in nocturnal BP fall has been found to be related to the severity of OSAS [94]. On the other hand, an increased prevalence of alterations in day-to-night BP profiles and nocturnal HTN has been reported in subjects with resistant HTN regardless of the presence of OSAS [90, 95, 96]. It is thus expected that alterations in day-to-night BP changes might be even more pronounced in subjects with OSAS and resistant HTN. From a prognostic point of view, identification of nocturnal HTN and alterations in day-to-night BP changes in subjects with OSAS-related HTN is of utmost relevance on the background of the evidence showing the superior prognostic value of nocturnal BP compared to awake or 24-h BP means in predicting CV morbidity and mortality [97–102], the development of CV events [97, 98, 103–105] as well as overall mortality [97–99, 104, 106, 107]. Identification of “non-dipping” pattern of BP in OSAS patients is also important if we consider that subjects in whom nocturnal decrease in BP is blunted have been reported to have a higher prevalence of subclinical organ damage [108, 109] and an increased risk of CV events [110] and mortality [102], which is even higher in patients in whom BP increases rather than decreases at night (so-called risers or inverted dippers). Despite the very high prevalence of nocturnal HTN and alterations in day-to-night BP changes in OSAS patients, these are often undiagnosed (thus representing a form of so-called masked resistant hypertension), mainly because BP measurements are prevalently measured during daytime at the moment of the clinical visit. Given their relevant prognostic value, alterations in circadian BP should be properly investigated in patients with OSAS-resistant HTN through the use of 24-h ABPM in order to guide antihypertensive treatment toward their normalization and optimization of CV protection.
6 Diagnostic Approach to OSAS-Related Resistant Hypertension
Confirming the diagnosis of OSAS in subjects with HTN and in particular in those with resistant HTN is relevant in order to implement specific treatment strategies (i.e., CPAP, weight reduction). This might allow achievement of BP control reducing the elevated CV risk of these subjects. Polysomnography is currently considered the standard technique for diagnosis of OSAS and requires simultaneous monitoring of several CV and respiratory variables during night sleep (i.e., sleep, air flow, respiratory effort, oxygen saturation, and brain activity through electroencephalogram). Based on the number of apneas and hypopneas lasting >10 s during each hour of recording, the severity of the disease is graded using the apnea–hypopnea index (AHI) [111]. Whether polysomnography should be employed systematically in individuals with resistant HTN is still a matter of debate in the absence of cost-effectiveness studies supporting this suggestion. According to a recent position paper of the European Respiratory Society (ERS)/European Society of Hypertension (ESH) [112], polysomnography should be performed in all subjects with a high pretest probability of OSA based on structured questionnaires (e.g., Epworth and Berlin questionnaires).
Considering the extremely high frequency of alterations in ambulatory BP profiles during nighttime in subjects with resistant HTN and OSAS, the task force of the ERS/ESH also recommends performing ABPM in order to identify alterations in day-to-night BP changes in subjects with resistant HTN in order to guide the decision to perform polysomnography in subjects with otherwise a low probability of OSA based on questionnaires. Indeed, in subjects with a low pretest probability of OSAS, polysomnography is only recommended in those who present alterations in day-to-night BP changes (i.e., non-dipping pattern of BP). See Fig. 5.8.
Proposed algorithm for the diagnostic management of patients with hypertension associated with obstructive sleep apnea (OSA). BP blood pressure, SBP systolic BP, DBP diastolic BP, ABPM ambulatory blood pressure monitoring, PSG polysomnography. # according to clinical evaluation and questionnaires, e.g., Epworth and Berlin; ¶ hypertension guidelines recommend the use of home BP monitoring in most hypertensive patients (Reproduced by permission from Parati et al. [112])
It is worth mentioning that before starting the instrumental tests to discard OSAS, a first step in the diagnostic approach of the patient with suspected OSAS-related hypertension consists in confirming whether resistance to antihypertensive treatment is true, or corresponds to false resistance. Current guidelines for the management of arterial hypertension define resistant hypertension as the persistence of BP values above the BP goal (i.e., ≥140/90 mmHg for office systolic/diastolic BP) despite the concomitant use of three optimally dosed antihypertensive medications from different classes at near-maximal doses, one of which should ideally be a diuretic [91, 92]. However, this definition is based on office BP measurements which have acknowledged limitations in assessing BP control including the inherent inaccuracy of the technique, the observer’s bias and digit preference, a variable interference by the “white-coat effect,” and the inability of this approach to collect information on BP during subjects’ usual activities and over a long period of time [113]. Thus, for confirmation of true resistant HTN, out-of-office BP-measuring techniques such as ambulatory and/or home BP monitoring (which are not affected by the limitations of office BP) should be performed in addition to office BP measurements. Based on the measures obtained with these methods, a substantial and sometimes larger than expected number of subjects initially diagnosed with resistant hypertension or with BP control based on OBP may actually correspond to false resistant HTN or white-coat resistant HTN (i.e., elevated OBP but normal out-of-office BP values) or to masked HTN (i.e., normal OBP but elevated out-of-office BP values) [90, 114, 115].
From a prognostic point of view, identification of OSAS patients with true resistant HTN as well as of those with masked resistant HTN (treated patients with normal OBP and elevated ABP or HBP) [116, 117] is of the highest relevance on the background of the evidence showing these conditions to be associated with a higher prevalence of target organ damage [118, 119], as well as with a higher risk of future CV and renal events when compared to those with true BP control [105, 120, 121] which ultimately translates in greater healthcare costs [27, 122, 123]. The most recent ESH/ESC arterial HTN guidelines have included OSAS among the causes responsible for true resistant HTN [92].
7 Effects of Different Therapeutic Strategies on OSAS-Related Resistant Hypertension
7.1 Effects of Lifestyle Changes and Weight Loss on OSAS-Related Hypertension
Obesity is the single most important cause of OSAS and elevation in BP levels. It is thus expected that weight loss might reduce the severity of OSAS and BP levels. Indeed, in subjects who achieve significant reductions in body weight either through dietary [124], pharmacological [125], or surgical [126] measures, considerable reductions of various indices of OSA severity (i.e., AHI) and in BP levels have been reported. In particular, bariatric surgery has been shown to be a highly effective measure to achieve OSAS improvement and BP control as supported by a large meta-analysis of 136 randomized controlled trials [127]. It has to be emphasized that BP was normalized in 61.7 % of patients and normalized or better controlled in 78.5 %. OSA was cured in 85.7 % of patients and was cured or improved in 83.6 % of patients [127]. However, despite its efficacy, bariatric surgery is reserved for selected patient groups, i.e., type 2 diabetes mellitus, patients with severe obesity (BMI >35 kg/m2), and moderately obese patients (BMI 30–35 kg/m2) who are inadequately controlled by conventional medical and behavioral therapies to reduce body weight.
7.2 Effects of CPAP Treatment on OSAS-Related Hypertension
Nasal CPAP is currently considered the optimal treatment for OSA [128]. When properly implemented, CPAP not only provides relative instant relief of clinical symptoms [129] and reduction in the severity of OSA (i.e., AHI) but also improves many of the acute and chronic pathophysiological alterations induced by OSAS, such as arterial baroreflex impairment and sympathetic activation, systemic inflammation, endothelial dysfunction, RAAS activation, arterial stiffness, and metabolic alterations (insulin resistance).
Of note, CPAP use has been shown to induce marked and acute reductions in MSNA not only during nighttime sleep but also during daytime wakefulness if maintained in the long term [28] (Fig. 5.9). As mentioned above, several studies have indeed also shown the effectiveness of CPAP in improving baroreflex impairment [69], systemic inflammation [51, 57, 59], endothelial dysfunction [50–52], RAAS activation [130], arterial stiffness [69, 70], and metabolic alterations [71].
Elimination of apneas by continuous positive airway pressure (CPAP) reduces muscle sympathetic nerve activity (SNA) and prevents blood pressure (BP) surge during rapid eye movement (REM) sleep (Taken from Somers et al. [28] by permission)
Although improvements in these pathophysiological alterations should theoretically translate into substantial BP reductions, most interventional trials in OSAS and subsequent meta-analyses have indicated that although CPAP has a significant effect on BP levels, the overall effect on 24-h, daytime, and nighttime systolic and diastolic ambulatory BP levels is rather small (in the order of 1–3 mmHg only) [131–133]. In spite of this, the effects of CPAP on BP levels have been shown to be variable as a function of patients’ compliance with nocturnal CPAP, of the number of CPAP hours during nighttime, and of the implementation of ambulatory BP monitoring to assess its effects. In some subgroups of patients, in particular those with more severe OSAS [134], or with resistant HTN [135], substantial effects of CPAP on BP levels have been reported. Indeed, effective CPAP treatment in patients with moderate-to-severe OSAS has been shown to induce important reductions both in day- and nighttime BP levels [134]. This has also been the case of subjects with resistant HTN in whom regular CPAP implementation has resulted in marked reductions in ambulatory BP levels not only during nighttime but also during daytime wakefulness [135]. In a study addressing the effects of 1-year treatment with CPAP, whereas no effects on BP levels were observed in patients with BP controlled at baseline, marked and significant reductions in BP levels were observed in subjects with resistant HTN [136].
A critical aspect when assessing the clinical effects of CPAP is to guarantee patients’ adherence to therapy. Given the mechanical nature of CPAP (i.e., facial interface mask and the pressure required to prevent airway collapse), this therapeutic intervention is not always well accepted by patients specially those free of OSA-related symptoms. Indeed, compliance with CPAP has been shown to be directly related to the severity of OSAS [137]. On the other hand, several studies have indicated that in order to observe an effect of CPAP on BP, CPAP treatment should be implemented for enough time and for a sufficient number of hours per night, and its effects on BP levels ideally assessed by means of ABPM. Proof of this has been provided by several studies in OSAS in which the benefits of CPAP have been evident only in subjects with confirmed resistant HTN (i.e., persistent elevation of both in-office and out-of-office BP levels), in whom CPAP has been implemented for at least 3 months and for more than 5.8 h per night [138]. A positive effect of CPAP has also been reported in non-sleepy hypertensive patients with OSA, among whom the most significant reductions in BP have been observed in those patients using CPAP for more than 5.6 h per night [137]. Further studies are still needed, however, focusing on early start of CPAP treatment before HTN organ damage develops and makes HTN control more difficult, in order to better determine whether CPAP implementation in OSAS patients with HTN is indeed associated with better BP control rates and/or with reduction in the number of antihypertensive medications needed in order to achieve BP control.
A recent meta-analysis of a randomized control trial (RCT) [139] addressing the effect of CPAP on BP in patients with OSA and HTN evaluated seven randomized controlled trials reporting 24-h ambulatory BP data. Overall, CPAP was associated with significant reductions in 24-h ambulatory systolic BP (−2.32 mmHg; 95 % confidence interval [CI], −3.65 to −1.00) and diastolic BP (−1.98 mmHg; 95 % CI, −2.82 to −1.14). CPAP led to more significant improvement in nocturnal systolic BP than that in daytime systolic BP. Subgroup analysis showed that patients with resistant HTN or receiving antihypertensive drugs benefited most from CPAP. Meta-regression indicated that CPAP compliance, age, and baseline systolic BP were positively correlated with decrease in 24-h diastolic BP, but not with reduction in 24-h systolic BP.
A recent study addressing the effect of CPAP treatment on BP in patients with OSA and resistant HTN reported that CPAP treatment for 12 weeks compared with untreated OSA patients as controls resulted in a significant decrease in 24-h mean BP (3.1 mmHg [95 % CI, 0.6–5.6]; P = 0.02) and 24-h DBP (3.2 mmHg [95 % CI, 1.0–5.4]; P = 0.005), but not in 24-h systolic BP (3.1 mmHg [95 % CI, −0.6–6.7]; P = 0.10). Moreover, the percentage of patients displaying a nocturnal BP dipping pattern at the 12-week follow-up was greater in the CPAP group than in the control group (35.9 vs. 21.6 %; adjusted odds ratio [OR], 2.4 [95 % CI, 1.2–5.1]; P = 0.02) [140].
Another study evaluated the effect of CPAP on BP in patients with resistant HTN and OSA in the frame of a randomized controlled clinical trial with blinded assessment of outcomes in 117 patients with moderate/severe OSA, defined by apnea–hypopnea index ≥15 apneic events per hour. Subjects were randomized to 6-month CPAP treatment (57 patients) or no therapy (60 patients), while maintaining antihypertensive treatment. Clinic and 24-h ambulatory BPs were obtained before and after 6-month treatment. Primary outcomes were changes in clinic and ambulatory BPs and in nocturnal BP fall patterns. On intention-to-treat analysis, there was no significant difference in any BP change, neither in nocturnal BP fall, between CPAP and control groups. The best effect of CPAP was on nighttime systolic BP in per-protocol analysis, with a tendentially although nonsignificantly greater reduction of 4.7 mmHg (95 % confidence interval, −11.3 to +3.1 mmHg; P = 0.24) and an increase in nocturnal BP fall of 2.2 % (95 % confidence interval, −1.6 to +5.8 %; P = 0.25), in comparison with control group. The conclusion of this study is that CPAP treatment had no significant effect on clinic and ambulatory BPs in patients with resistant HTN and moderate/severe OSA, although a beneficial effect on nighttime systolic BP and on nocturnal BP fall might exist in patients with uncontrolled ambulatory BP levels [141].
Overall, also in the light of these recent trials, the reported poor efficacy of CPAP in reducing BP levels in OSA patients with HTN may depend on a combination of different factors, including poor patients’ compliance with nocturnal CPAP use, too short treatment duration, inaccurate CPAP calibration, failure to use 24-h ABPM to evaluate CPAP effects on BP, and, most importantly, delayed use of CPAP in the clinical history of OSA patients when HTN may have become more resistant to treatment due to appearance of organ damage.
7.3 Effects of Renal Sympathetic Denervation in OSAS-Related Resistant Hypertension
Sympathetic activation in OSAS determines an increase in sympathetic drive to the heart, the peripheral vasculature and the kidneys. In relation to the latter, the sympathetic nerves arriving to the renal district have been identified as a major contributing factor to the pathophysiology of HTN both in experimental models and in human studies [142]. This has been the basis for the development of interventional strategies aimed at modulating renal sympathetic nerve activity through radiofrequency catheter-based renal sympathetic denervation (RDN) [143]. In subjects with uncontrolled HTN, RDN has been shown to induce significant reductions in renal sympathetic efferent nerve activity, in whole-body sympathetic nerve activity and norepinephrine spillover, as well as substantial and sustained reductions in BP levels [144]. Small interventional studies in OSAS patients who were refractory to lifestyle modifications, weight loss, pharmacological treatment, and CPAP have also suggested that RDN may represent an effective strategy for the management of resistant HTN associated with OSA, inducing significant and sustained changes in BP levels at 3 and 6 months of follow-up [145]. Remarkably, the changes in BP levels reported in this study have also been accompanied by improvements in OSAS severity as indicated by the significant reductions in AHI at 3 and 6 months after denervation [145]. Renal sympathetic denervation might thus represent a potentially useful option for the management of resistant HTN in OSAS patients, who are refractory to lifestyle modifications, weight loss, pharmacological treatment and CPAP. Nonetheless, given the very small sample size of this paper, adequately powered longitudinal studies are needed to confirm these anecdotal findings and to assess the long-term impact of RDN on HTN control, as well as its benefits in terms of organ damage and incidence of CV morbid-mortality in subjects with OSAS.
8 Do Different Antihypertensive Drug Classes Have Different Effects on OSAS-Related Hypertension?
Different antihypertensive drug classes might have a differential effect on the pathophysiological mechanisms involved in the pathogenesis of OSAS-related HTN. However, the few studies that have comparatively assessed the BP-lowering effects of different drug classes in OSAS have been of small size, and their statistical power was limited to derive consistent conclusions. In a randomized study assessing the effects of different classes of antihypertensive drugs (i.e., beta-blockers, calcium antagonists, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and thiazide diuretics) on office and ambulatory BP levels in patients with HTN and OSAS, no significant differences between drug classes were observed in their ability to reduce office and daytime ambulatory BP levels. However, treatment with β-blockers was more effective in reducing nighttime ambulatory BP than administration of other compounds, probably through their effects on sympathetic activation. In general, however, no consistent evidence has been provided supporting a superior antihypertensive efficacy of any antihypertensive drug in OSA patients [146]. Long-term effects of treatment with different antihypertensive agents on hypertension severity in OSAS have not been systematically addressed in clinical trials, however. Evidence is therefore still needed in order to identify preferred compounds for an adequate BP control in this group of high-risk patients.
Recent studies in resistant HTN have suggested that spironolactone should be considered in all patients with uncontrolled HTN on three or more antihypertensive agents [147]. In some studies, addition of spironolactone in doses of 25–50 mg a day to the current antihypertensive treatment in resistant hypertensive patients was shown to reduce the severity of OSAS on top of its BP-lowering effects [48]. This is in line with the concept that aldosterone-mediated chronic fluid retention may influence severity of OSA.
9 Conclusions
Consistent evidence has supported the association between OSAS and HTN [2, 4, 12–15] showing a dose–response relationship between OSAS severity and the degree of BP elevation [2, 26, 27]. It has also been shown that HTN occurring in individuals with OSAS is more likely to be severe, resistant to treatment, and associated with alterations in day-to-night BP changes [2, 26, 27]. The pathogenesis of OSAS-related HTN is likely to be multifactorial, involving alterations in several regulatory systems. However, the mechanisms by which OSAS promotes arterial HTN still need to be better understood. Although OSAS and drug-resistant HTN are independent predictors of CV morbid-mortality, evidence from longitudinal studies is still needed to determine the actual prognostic relevance of OSAS-related HTN. In a subject with resistant HTN and suspected OSAS, ABPM should be performed whenever possible for confirmation of resistant HTN, for identification of alterations in day-to-night BP changes and in order to define the need of performing additional diagnostic procedures (i.e., polysomnography) and/or implementing more aggressive pharmacological or interventional strategies for the management of resistant HTN. In turn, identification of OSAS and proper implementation of specific treatment strategies for its treatment (i.e., CPAP) in subjects with resistant HTN might favor achievement of BP control optimizing CV protection. Evidence from additional longitudinal interventional studies in OSAS controlling for potential confounders (i.e., visceral obesity, increased BMI) is still needed, however, not only to determine the prognostic relevance of the interaction between OSAS and HTN but also for determining whether treating OSAS in resistant HTN confers significant benefits in terms of CV protection.
Abbreviations
- ABPM:
-
Ambulatory blood pressure monitoring
- AHI:
-
Apnea–hypopnea index
- baPWV:
-
Brachial–ankle pulse wave velocity
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- cfPWV:
-
Carotid–femoral pulse wave velocity
- CPAP:
-
Continuous positive air pressure
- CRP:
-
C-reactive protein
- CV:
-
Cardiovascular
- eNOS:
-
Endothelial nitric oxide synthase
- ERS:
-
European Respiratory Society
- ESH:
-
European Society of Hypertension
- FMD:
-
Flow-mediated dilation
- HTN:
-
Hypertension
- ICAM-1:
-
Intercellular adhesion molecule-1
- MSNA:
-
Muscle sympathetic nerve activity
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- OSA:
-
Obstructive sleep apnea
- OSAS:
-
Obstructive sleep apnea syndrome
- TNF-alpha:
-
Tumor necrosis factor-alpha
- VCAM-1:
-
Vascular cell adhesion molecule-1
References
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G et al (2007) 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 25(6):1105–1187. Epub 2007/06/15
Grote L, Hedner J, Peter JH (2000) Sleep-related breathing disorder is an independent risk factor for uncontrolled hypertension. J Hypertens 18(6):679–685. Epub 2000/06/29
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342(19):1378–1384. Epub 2000/05/11
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S et al (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283(14):1829–1836. Epub 2000/04/19
Medicine AAoS (2005) International classification of sleep disorders: diagnostic and coding manual, 2nd edn. American Academy of Sleep Medicine, Westchester
Coccagna G, Mantovani M, Brignani F, Parchi C, Lugaresi E (1972) Continuous recording of the pulmonary and systemic arterial pressure during sleep in syndromes of hypersomnia with periodic breathing. Bull Physiopathol Respir 8(5):1159–1172. Epub 1972/09/01
Baguet JP, Hammer L, Levy P, Pierre H, Rossini E, Mouret S et al (2005) Night-time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J Hypertens 23(3):521–527. Epub 2005/02/18
Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M (2008) Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep 31(6):795–800. Epub 2008/06/14
Lavie P, Herer P, Hoffstein V (2000) Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 320(7233):479–482. Epub 2000/03/04
Davies CW, Crosby JH, Mullins RL, Barbour C, Davies RJ, Stradling JR (2000) Case–control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax 55(9):736–740. Epub 2000/08/19
Pankow W, Nabe B, Lies A, Becker H, Kohler U, Kohl FV et al (1997) Influence of sleep apnea on 24-hour blood pressure. Chest 112(5):1253–1258. Epub 1997/11/21
Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A et al (2000) Association of hypertension and sleep-disordered breathing. Arch Intern Med 160(15):2289–2295. Epub 2000/08/06
Duran J, Esnaola S, Rubio R, Iztueta A (2001) Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 163(3 Pt 1):685–689. Epub 2001/03/20
Tanigawa T, Tachibana N, Yamagishi K, Muraki I, Kudo M, Ohira T et al (2004) Relationship between sleep-disordered breathing and blood pressure levels in community-based samples of Japanese men. Hypertens Res: Off J Jap Soc Hypertens 27(7):479–484. Epub 2004/08/11
Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B et al (1997) Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med 157(15):1746–1752. Epub 1997/08/11
Kiely JL, McNicholas WT (2000) Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. Eur Res J Off J Eur Soc Clini Res Physiol 16(1):128–133. Epub 2000/08/10
Calhoun DA (2010) Obstructive sleep apnea and hypertension. Curr Hypertens Rep 12(3):189–195. Epub 2010/04/29
Garrison RJ, Kannel WB, Stokes J 3rd, Castelli WP (1987) Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med 16(2):235–251. Epub 1987/03/01
Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H et al (2004) Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens 17(10):904–910. Epub 2004/10/16
O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S et al (2009) Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med 179(12):1159–1164. Epub 2009/03/07
Kapa S, Sert Kuniyoshi FH, Somers VK (2008) Sleep apnea and hypertension: interactions and implications for management. Hypertension 51(3):605–608. Epub 2008/01/30
Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA et al (2005) Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation 111(5):614–621. Epub 2005/02/09
Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M et al (2001) High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 19(12):2271–2277. Epub 2001/11/29
Cano-Pumarega I, Duran-Cantolla J, Aizpuru F, Miranda-Serrano E, Rubio R, Martinez-Null C et al (2011) Obstructive sleep apnea and systemic hypertension: longitudinal study in the general population: the Vitoria Sleep Cohort. Am J Respir Crit Care Med 184(11):1299–1304. Epub 2011/08/27
Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ et al (2012) Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 307(20):2169–2176. Epub 2012/05/24
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A et al (2008) Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118(10):1080–1111. Epub 2008/08/30
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD (2008) Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51(6):1403–1419. Epub 2008/04/09
Somers VK, Dyken ME, Clary MP, Abboud FM (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96(4):1897–1904. Epub 1995/10/01
Portaluppi F, Provini F, Cortelli P, Plazzi G, Bertozzi N, Manfredini R et al (1997) Undiagnosed sleep-disordered breathing among male nondippers with essential hypertension. J Hypertens 15(11):1227–1233. Epub 1997/12/31
Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK (1998) Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 98(8):772–776. Epub 1998/09/04
Ali NJ, Davies RJ, Fleetham JA, Stradling JR (1992) The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest 101(6):1526–1532. Epub 1992/06/01
Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK (1999) Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 100(23):2332–2335. Epub 1999/12/11
Hedner JA, Wilcox I, Laks L, Grunstein RR, Sullivan CE (1992) A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am Rev Respir Dis 146(5 Pt 1):1240–1245. Epub 1992/11/01
Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK (1999) Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99(9):1183–1189. Epub 1999/03/09
Parati G, Di Rienzo M, Bonsignore MR, Insalaco G, Marrone O, Castiglioni P et al (1997) Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleep. J Hypertens 15(12 Pt 2):1621–1626. Epub 1998/03/06
Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK (1998) Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension 32(6):1039–1043. Epub 1998/12/18
Noda A, Nakata S, Koike Y, Miyata S, Kitaichi K, Nishizawa T et al (2007) Continuous positive airway pressure improves daytime baroreflex sensitivity and nitric oxide production in patients with moderate to severe obstructive sleep apnea syndrome. Hypertens Res: Off J Jap Soc Hypertens 30(8):669–676. Epub 2007/10/06
Ryan S, Ward S, Heneghan C, McNicholas WT (2007) Predictors of decreased spontaneous baroreflex sensitivity in obstructive sleep apnea syndrome. Chest 131(4):1100–1107. Epub 2007/04/12
Bonsignore MR, Parati G, Insalaco G, Marrone O, Castiglioni P, Romano S et al (2002) Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med 166(3):279–286. Epub 2002/08/03
Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK (1998) Altered cardiovascular variability in obstructive sleep apnea. Circulation 98(11):1071–1077. Epub 1998/09/16
Lombardi C, Parati G, Cortelli P, Provini F, Vetrugno R, Plazzi G et al (2008) Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res 17(3):263–270. Epub 2008/05/28
Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM et al (2010) Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clini Sleep Med JCSM Official Pub Am Ac Sleep Med 6(4):363–368. Epub 2010/08/24
Pimenta E, Calhoun DA, Oparil S (2009) Sleep apnea, aldosterone, and resistant hypertension. Prog Cardiovasc Dis 51(5):371–380. Epub 2009/03/03
Goodfriend TL, Calhoun DA (2004) Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension 43(3):518–524. Epub 2004/01/21
Calhoun DA, Nishizaka MK, Zaman MA, Harding SM (2004) Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest 125(1):112–117. Epub 2004/01/14
Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA (2007) Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 131(2):453–459. Epub 2007/02/14
Dudenbostel T, Calhoun DA (2012) Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 26(5):281–287. Epub 2011/06/10
Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM et al (2010) Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens 24(8):532–537. Epub 2009/12/18
Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V et al (2000) Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 102(21):2607–2610. Epub 2000/11/22
Ip MS, Tse HF, Lam B, Tsang KW, Lam WK (2004) Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 169(3):348–353. Epub 2003/10/11
Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH et al (2010) Vascular inflammation in obesity and sleep apnea. Circulation 121(8):1014–1021. Epub 2010/02/18
Butt M, Khair OA, Dwivedi G, Shantsila A, Shantsila E, Lip GY (2011) Myocardial perfusion by myocardial contrast echocardiography and endothelial dysfunction in obstructive sleep apnea. Hypertension 58(3):417–424. Epub 2011/07/13
Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D et al (2008) Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 117(17):2270–2278. Epub 2008/04/17
Dyugovskaya L, Lavie P, Lavie L (2002) Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 165(7):934–939. Epub 2002/04/06
Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N et al (2005) Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 172(5):625–630. Epub 2005/08/27
Kanagy NL, Walker BR, Nelin LD (2001) Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension 37(2 Part 2):511–515. Epub 2001/03/07
Ryan S, Taylor CT, McNicholas WT (2005) Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112(17):2660–2667. Epub 2005/10/26
Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V et al (2002) Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 105(21):2462–2464. Epub 2002/05/30
Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G et al (2003) Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107(8):1129–1134. Epub 2003/03/05
Ohga E, Nagase T, Tomita T, Teramoto S, Matsuse T, Katayama H et al (1999) Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol 87(1):10–14. Epub 1999/07/20
O’Rourke MF, Nichols WW (2005) Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 45(4):652–658. Epub 2005/02/09
Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL Jr, Neutel J, Kerwin LJ et al (2003) Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation 108(13):1592–1598. Epub 2003/09/17
Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P et al (2012) Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 60(2):369–377. Epub 2012/07/04
Doonan RJ, Scheffler P, Lalli M, Kimoff RJ, Petridou ET, Daskalopoulos ME et al (2011) Increased arterial stiffness in obstructive sleep apnea: a systematic review. Hypertens Res: Off J Jap Soc Hypertens 34(1):23–32. Epub 2010/10/22
Tsioufis C, Thomopoulos K, Dimitriadis K, Amfilochiou A, Tousoulis D, Alchanatis M et al (2007) The incremental effect of obstructive sleep apnoea syndrome on arterial stiffness in newly diagnosed essential hypertensive subjects. J Hypertens 25(1):141–146. Epub 2006/12/05
Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G (2007) Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest 131(5):1379–1386. Epub 2007/05/15
Shiina K, Tomiyama H, Takata Y, Usui Y, Asano K, Hirayama Y et al (2006) Concurrent presence of metabolic syndrome in obstructive sleep apnea syndrome exacerbates the cardiovascular risk: a sleep clinic cohort study. Hypertens Res: Off J Jap Soc Hypertens 29(6):433–441. Epub 2006/08/31
Nagahama H, Soejima M, Uenomachi H, Higashi Y, Yotsumoto K, Samukawa T et al (2004) Pulse wave velocity as an indicator of atherosclerosis in obstructive sleep apnea syndrome patients. Intern Med 43(3):184–188. Epub 2004/04/22
Kohler M, Pepperell JC, Casadei B, Craig S, Crosthwaite N, Stradling JR et al (2008) CPAP and measures of cardiovascular risk in males with OSAS. Eur Res J Off J Eur Soc Clini Res Physiol 32(6):1488–1496. Epub 2008/07/26
Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF (2007) Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 176(7):706–712. Epub 2007/06/09
Rasche K, Keller T, Tautz B, Hader C, Hergenc G, Antosiewicz J et al (2010) Obstructive sleep apnea and type 2 diabetes. Eur J Med Res 15(Suppl 2):152–156. Epub 2010/12/22
Basoglu OK, Sarac F, Sarac S, Uluer H, Yilmaz C (2011) Metabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and C-reactive protein in obese patients with obstructive sleep apnea syndrome. Ann Thor Med 6(3):120–125. Epub 2011/07/16
Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA (2011) Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit Inter Med J Exper Clini Res 17(3):CR159–CR164. Epub 2011/03/02
Bonsignore MR, Esquinas C, Barcelo A, Sanchez-de-la-Torre M, Paterno A, Duran-Cantolla J et al (2012) Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. Eur Res J Off J Eur Soc Clini Res Physiol 39(5):1136–1143. Epub 2011/11/15
Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK (2000) Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol 279(1):H234–H237. Epub 2000/07/19
McNicholas WT, Bonsigore MR (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Res J Off J Eur Soc Clini Res Physiol 29(1):156–178. Epub 2007/01/02
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353(19):2034–2041. Epub 2005/11/12
Selim B, Won C, Yaggi HK (2010) Cardiovascular consequences of sleep apnea. Clin Chest Med 31(2):203–220. Epub 2010/05/22
Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF et al (2010) Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 122(4):352–360. Epub 2010/07/14
Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE et al (2010) Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 182(2):269–277. Epub 2010/03/27
Capampangan DJ, Wellik KE, Parish JM, Aguilar MI, Snyder CR, Wingerchuk D et al (2010) Is obstructive sleep apnea an independent risk factor for stroke? A critically appraised topic. Neurologist 16(4):269–273. Epub 2010/07/02
Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR (2008) Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 31(8):1079–1085. Epub 2008/08/22
Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ et al (2008) Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31(8):1071–1078. Epub 2008/08/22
Otto ME, Belohlavek M, Romero-Corral A, Gami AS, Gilman G, Svatikova A et al (2007) Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol 99(9):1298–1302. Epub 2007/05/05
Tavil Y, Kanbay A, Sen N, Ciftci TU, Abaci A, Yalcin MR et al (2007) Comparison of right ventricular functions by tissue Doppler imaging in patients with obstructive sleep apnea syndrome with or without hypertension. Int J Cardiovasc Imaging 23(4):469–477. Epub 2006/10/21
Agrawal V, Vanhecke TE, Rai B, Franklin BA, Sangal RB, McCullough PA (2009) Albuminuria and renal function in obese adults evaluated for obstructive sleep apnea. Nephron Clin Pract 113(3):c140–c147. Epub 2009/08/13
Sim JJ, Rasgon SA, Derose SF (2010) Review article: managing sleep apnoea in kidney diseases. Nephrology (Carlton) 15(2):146–152. Epub 2010/05/18
Persell SD (2011) Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 57(6):1076–1080. Epub 2011/04/20
Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC (2011) Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation 124(9):1046–1058. Epub 2011/08/10
de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P et al (2011) Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension 57(5):898–902. Epub 2011/03/30
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J et al (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311(5):507–520. Epub 2013/12/20
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M et al (2013) 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31(7):1281–1357. Epub 2013/07/03
Wolf J, Hering D, Narkiewicz K (2010) Non-dipping pattern of hypertension and obstructive sleep apnea syndrome. Hypertens Res: Off J Jap Soc Hypertens 33(9):867–871. Epub 2010/09/08
Lavie-Nevo K, Pillar G (2006) Evening-morning differences in blood pressure in sleep apnea syndrome: effect of gender. Am J Hypertens 19(10):1064–1069. Epub 2006/10/10
Muxfeldt ES, Cardoso CR, Salles GF (2009) Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch Intern Med 169(9):874–880. Epub 2009/05/13
Muxfeldt ES, Bloch KV, Nogueira AR, Salles GF (2003) Twenty-four hour ambulatory blood pressure monitoring pattern of resistant hypertension. Blood Press Monit 8(5):181–185. Epub 2003/11/19
Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW et al (1999) Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 282(6):539–546. Epub 1999/08/18
Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G et al (2005) Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 111(14):1777–1783. Epub 2005/04/06
Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S et al (2005) Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension 45(2):240–245. Epub 2004/12/15
Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML et al (2008) Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 51(1):55–61. Epub 2007/11/28
Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K et al (2007) Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 370(9594):1219–1229. Epub 2007/10/09
Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA (2011) Predictive role of the nighttime blood pressure. Hypertension 57(1):3–10. Epub 2010/11/17
Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH et al (2003) Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 348(24):2407–2415. Epub 2003/06/13
Fagard RH, Van Den Broeke C, De Cort P (2005) Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens 19(10):801–807. Epub 2005/06/17
Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM (1998) Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension 31(2):712–718. Epub 1998/02/14
Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S et al (2005) Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 46(1):156–161. Epub 2005/06/09
Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C (2005) Ambulatory blood pressure and mortality: a population-based study. Hypertension 45(4):499–504. Epub 2005/03/09
Sander D, Kukla C, Klingelhofer J, Winbeck K, Conrad B (2000) Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3-year follow-up study. Circulation 102(13):1536–1541. Epub 2000/09/27
Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V et al (2002) Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 347(11):797–805. Epub 2002/09/13
Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J et al (2006) Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension 47(2):149–154. Epub 2005/12/29
Parati G, Lombardi C, Hedner J, Bonsignore MR, Grote L, Tkacova R et al (2012) Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens 30(4):633–646. Epub 2012/03/13
Parati G, Lombardi C, Hedner J, Bonsignore M, Grote L, Tkacova R et al (2013) Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Res J Off J Eur Soc Clini Res Physiol 41(3):523–538
O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G et al (2003) European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 21(5):821–848. Epub 2003/04/26
Oikawa T, Obara T, Ohkubo T, Kikuya M, Asayama K, Metoki H et al (2006) Characteristics of resistant hypertension determined by self-measured blood pressure at home and office blood pressure measurements: the J-HOME study. J Hypertens 24(9):1737–1743. Epub 2006/08/18
de la Sierra A, Banegas JR, Oliveras A, Gorostidi M, Segura J, de la Cruz JJ et al (2012) Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J Hypertens 30(6):1211–1216. Epub 2012/04/25
Pickering TG, Davidson K, Gerin W, Schwartz JE (2002) Masked hypertension. Hypertension 40(6):795–796. Epub 2002/12/07
Parati G, Ulian L, Santucciu C, Omboni S, Mancia G (1998) Difference between clinic and daytime blood pressure is not a measure of the white coat effect. Hypertension 31(5):1185–1189. Epub 1998/05/12
Muxfeldt ES, Bloch KV, Nogueira Ada R, Salles GF (2005) True resistant hypertension: is it possible to be recognized in the office? Am J Hypertens 18(12 Pt 1):1534–1540. Epub 2005/12/21
Oliveras A, Armario P, Hernandez-Del Rey R, Arroyo JA, Poch E, Larrousse M et al (2010) Urinary albumin excretion is associated with true resistant hypertension. J Hum Hypertens 24(1):27–33. Epub 2009/05/08
Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM et al (2005) Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens 18(11):1422–1428. Epub 2005/11/11
Salles GF, Cardoso CR, Muxfeldt ES (2008) Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med 168(21):2340–2346. Epub 2008/11/26
Cuspidi C, Macca G, Sampieri L, Michev I, Salerno M, Fusi V et al (2001) High prevalence of cardiac and extracardiac target organ damage in refractory hypertension. J Hypertens 19(11):2063–2070. Epub 2001/10/26
Moser M, Setaro JF (2006) Clinical practice. Resistant or difficult-to-control hypertension. N Engl J Med 355(4):385–392. Epub 2006/07/28
Johansson K, Hemmingsson E, Harlid R, Trolle Lagerros Y, Granath F, Rossner S et al (2011) Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ 342:d3017. Epub 2011/06/03
Yee BJ, Phillips CL, Banerjee D, Caterson I, Hedner JA, Grunstein RR (2007) The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int J Obes (Lond) 31(1):161–168. Epub 2006/05/03
Pannain S, Mokhlesi B (2010) Bariatric surgery and its impact on sleep architecture, sleep-disordered breathing, and metabolism. Best Pract Res Clin Endocrinol Metab 24(5):745–761. Epub 2010/11/30
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292(14):1724–1737. Epub 2004/10/14
Leech JA, Onal E, Lopata M (1992) Nasal CPAP continues to improve sleep-disordered breathing and daytime oxygenation over long-term follow-up of occlusive sleep apnea syndrome. Chest 102(6):1651–1655. Epub 1992/12/01
Kribbs NB, Pack AI, Kline LR, Getsy JE, Schuett JS, Henry JN et al (1993) Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis 147(5):1162–1168. Epub1993/05/01
Bradley TD, Floras JS (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373(9657):82–93. Epub2008/12/23
Alajmi M, Mulgrew AT, Fox J, Davidson W, Schulzer M, Mak E et al (2007) Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung 185(2):67–72. Epub2007/03/30
Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A et al (2007) The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med 167(8):757–764. Epub2007/04/25
Bazzano LA, Khan Z, Reynolds K, He J (2007) Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 50(2):417–423. Epub2007/06/06
Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE et al (2003) Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 107(1):68–73. Epub2003/01/08
Logan AG, Tkacova R, Perlikowski SM, Leung RS, Tisler A, Floras JS et al (2003) Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Res J Off J Eur Soc Clini Res Physiol 21(2):241–247. Epub2003/03/01
Dernaika TA, Kinasewitz GT, Tawk MM (2009) Effects of nocturnal continuous positive airway pressure therapy in patients with resistant hypertension and obstructive sleep apnea. J Clini Sleep Med JCSM Official Pub Am Ac Sleep Med 5(2):103–107. Epub2009/12/09
Barbe F, Duran-Cantolla J, Capote F, de la Pena M, Chiner E, Masa JF et al (2010) Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med 181(7):718–726. Epub2009/12/17
Lozano L, Tovar JL, Sampol G, Romero O, Jurado MJ, Segarra A et al (2010) Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens 28(10):2161–2168. Epub2010/06/26
Hu X, Fan J, Chen S, Yin Y, Zrenner B (2015) The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 17(3):215–222. Epub2015/01/15
Martinez-Garcia MA, Capote F, Campos-Rodriguez F, Lloberes P, Diaz de Atauri MJ, Somoza M et al (2013) Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 310(22):2407–2415. Epub2013/12/12
Muxfeldt ES, Margallo V, Costa LM, Guimaraes G, Cavalcante AH, Azevedo JC et al (2015) Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension 65(4):736–742. Epub2015/01/21
DiBona GF, Kopp UC (1997) Neural control of renal function. Physiol Rev 77(1):75–197. Epub1997/01/01
Doumas M, Faselis C, Papademetriou V (2011) Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 20(6):647–653. Epub2011/09/03
Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD (2009) Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361(9):932–934. Epub2009/08/28
Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P et al (2011) Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 58(4):559–565. Epub 2011/08/17
Ziegler MG, Milic M, Sun P (2011) Antihypertensive therapy for patients with obstructive sleep apnea. Curr Opin Nephrol Hypertens 20(1):50–55. Epub 2011/02/18
Pimenta E, Calhoun DA (2010) Treatment of resistant hypertension. J Hypertens 28(11):2194–2195. Epub 2010/10/16
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Parati, G., Lombardi, C., Ochoa, J.E. (2016). Sleep Apnea. In: Tsioufis, C., Schmieder, R., Mancia, G. (eds) Interventional Therapies for Secondary and Essential Hypertension. Updates in Hypertension and Cardiovascular Protection. Springer, Cham. https://doi.org/10.1007/978-3-319-34141-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-34141-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-34140-8
Online ISBN: 978-3-319-34141-5
eBook Packages: MedicineMedicine (R0)