Abstract
Transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS) are noninvasive neuromodulatory techniques that deliver low-intensity currents facilitating or inhibiting spontaneous neuronal activity. These techniques have a number of advantages that have been applied in clinical settings; in particular, tDCS/tACS dose in principle is easily customized by varying electrode number, position, size, shape, and current. However, the ability to leverage this customization depends on how tDCS/tACS dose modulate the underling brain current flow. This relationship is not simple and can benefit from the use of computational models of current flow, personalized to individual subjects and cases. Tools for modeling range from Finite Element Method models to stand-alone GUI based software for clinicians. Many software packages can load individual’s MRI scans, allowing individualized therapy design. However, the challenge remains to design and interpret these models while remaining aware of their limitations. Current flow models alone cannot “make dose decisions,” but rather inform the rational design of electrotherapy. This is evidenced in exemplary studies combining computer modeling and clinical data, several examples of which are outlined in this chapter. Though modeling software is now widely available, newer generations of algorithms promise more precision and flexibility, and thus it is predicted that with increased validation, dissemination, simplification and dissemination of modeling tools, computational forward models of neuromodulation will become useful tools to guide the optimization of clinical electrotherapy. Essential for this adoption and refinement is an appreciation by clinicians of the uses and limitations of computational models, and conversely understanding by engineers and programmers of what software functions are relevant to clinical practice.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Transcranial direct current stimulation
- Computational models
- Finite element method
- Magnetic resonance imaging
- Computer-based modeling
Overview of Computational Models of Noninvasive Neuromodulation
This chapter introduces the rationale and approach behind modeling tDCS/tACS as well as the technical development and limitations of models currently in use. This chapter is intended to provide a broad introduction for both clinical researchers and engineers interested in translational work to develop and apply computational models of customized tDCS/tACS. A central premise of this chapter is that models cannot “make decisions” about tDCS/tACS, but rather are tools that inform how protocols should be interpreted and optimized. As such, it is incumbent on clinical researchers to appreciate the function and limitations of models, and conversely for programmers to consider the goals of the end user (investigator) when deciding what functionality is relevant for their modeling software.
Conventionally, stimulation techniques can be grouped into two categories: protocols that induce activity of neurons (supra-threshold), and protocols that exert modulatory effects on ongoing neuronal activity and excitability (sub-threshold). For a complete historical context of terminology see ref. [1]. The first group includes high-intensity short-pulse transcranial electrical stimulation (TES), transcranial magnetic stimulation (TMS), electroconvulsive therapy (ECT), and paired associative stimulation (PAS). The second group, includes forms of low-intensity sustained tES including transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcranial pulsed current stimulation (tPCS), and transcranial random noise stimulation (tRNS). The electric field intensities produced in the brain by supra-threshold techniques are two orders of magnitude above sub-threshold techniques [2–10] which allows for action potentials to be triggered [11]. However, it is important to recognize that supra-threshold techniques ultimately affect behavior by modulating endogenous networks while sub-threshold techniques can influence firing in the active system [12]. Based on the growing evidence that current delivered to specific brain regions can promote desirable plastic changes, stimulation techniques are emerging as promising tool in symptom management [13–15]. However, stimulation should be applied in a manner that is within safe and well-tolerated parameters. Complimentary to other brain stimulation approaches (Fig. 4.1), tDCS and tACS have been gaining considerable interest because they are well tolerated, can be used as add-on therapies, and have low maintenance costs [16]. This review focuses on low-intensity approaches and specifically tDCS and tACS (as they are most commonly used clinically); however, many of the conclusions of this chapter can be generalized.
Comparable stimulation techniques: deep brain stimulation, motor cortex stimulation, transcranial magnetic stimulation, and spinal cord stimulation (top row); classic transcranial direct current stimulation (tDCS) via sponge pads, optimized high definition-tDCS (HD-tDCS), and 4 × 1 HD-tDCS (bottom row). Transcranial direct current stimulation is an increasingly popular investigational form of brain stimulation, in part, due to its low cost, portability, usability, and safety. However, there are still many of unanswered questions. The number of potential stimulation doses is practically limitless. Stimulation can be varied by simply changing the electric current waveform and electrode shape, size, and position. These variations can thus be analyzed through computational modeling studies that have resulted in montages such as HD-tDCS and 4 × 1 HD-tDCS
In contrast to pharmacotherapy, noninvasive electrotherapy offers the potential for both anatomically specific brain activation and temporal control. Anatomical targeting can be achieved through the rational selection of electrode number, shape, and position. In training applications such as rehabilitation, neuromodulatory techniques such as tDCS/tACS can combine focal stimulation with specific training to reinforce a particular region of activation [17] including with “functional targeting” [18, 19]. Temporal control is possible due to the instantaneous delivery of electricity to the brain through the scalp. There is no electrical “residue” since the generated brain current disappears as soon as stimulation is paused. The tDCS/tACS dose can also be modeled for specific subjects and targeted in ways not possible with other interventions. Specifically, the “dose” of electrotherapy (see ref. [5] for definition) is readily adjustable by determining the location of electrodes (which determines spatial targeting) and selecting the stimulation waveform and intensity (which together determines the nature and timing of neuromodulation). Thus, a single programmable electrotherapy device can be simply configured to provide a diversity of dosages. Though this flexibly underpins the utility of neuromodulation, the myriad of potential dosages (stimulator settings and combinations of electrode placements) makes the optimal choice difficult to readily ascertain. The essential issue in dose design is to relate each externally controlled dose with the associated brain regions targeted (and spared) by the resulting current flow—and hence the desired clinical outcome. Computational forward models aim to provide precisely these answers (Fig. 4.2), and thus need to be leveraged in the rational design, interpretation, and optimization of neuromodulation.
Role of computational models in rational electrotherapy: (left) Neuromodulation is a promising therapeutic modality as it affects the brain in a way not possible with other techniques with a high degree of individualized optimization. The goal of computational models is to assist clinicians in leveraging the power and flexibility of neuromodulation (right). Computational forward models are used to predict brain current flow during transcranial stimulation to guide clinical practice. As with pharmacotherapy, electrotherapy dose is controlled by the operator and leads a complex pattern of internal current flow that is described by the model. In this way, clinicians can apply computational models to determine which dose will activate (or avoid) brain regions of interest
The precise pattern of current flow through the brain is determined not only by the stimulation dose but also by the underlying anatomy and tissue properties. Thus, in predicting brain current flow using computational models, important to not only precisely model both the stimulation itself, but also the relevant anatomy upon which it is delivered on an individual basis. The latter issue remains an area of ongoing technical development and is critical to establishing the clinical utility of these models. For example, cerebral spinal fluid (CSF) is so highly conductive (a preferred “super highway” for current flow) that details of CSF architecture profoundly shape current flow through adjacent brain regions. Especially relevant for rehabilitative applications is the recognition that individual anatomical idiosyncrasies can result in significant distortions in current flow. This is especially apparent when skull defects and brain lesions occur.
Methods and Protocols in the Generation of Computational Forward Models of tDCS/tACS
This section outlines the technical steps and pit-falls of computational models for tDCS/tACS and so aimed primarily to the engineers and programmers developing these tools. However, clinicians and experimentalists interested in understanding the technical challenges and limitations of modeling will also benefit from these sections, consistent with our emphasis that these are tools to be used by experientialists and clinicians—and only by understanding the nature and limits of tools can they be applied meaningfully.
During tDCS/tACS, current is generated in the brain [20]. While there are intrinsic electric fields in the brain as recording during electroencephalogram (EEG), models of tDCS/tACS predict an induced electric field given a source (the stimulation electrodes). Solving for the induced fields from a known source and vice-versa is what technically differentiates stimulation models from source localization models used in EEG. These modeling methods are dubbed the “forward” and “inverse” models respectively.
Because different electrode montages result in distinct brain current flow, researchers and clinicians can, in principle, adjust the montage to target or avoid specific brain regions in an application specific manner. Though tDCS/tACS montage design often follows basic rules-of-thumb (e.g., “increased/decreased excitability” under the anode/cathode electrode for tDCS and “boost oscillating activity” under one electrode for tACS), computational forward models of brain current flow provide more accurate insight into detailed current flow patterns and in some cases, can even challenge simplified electrode-placement assumptions.
We note two common over-simplifications using rule-of thumb for tDCA/tACS dose design. For example, clinical tDCS studies are often designed by placing the anode electrode directly over the targeted region desired to be excited, while the cathode electrode is placed over a far removed region from the target to avoid unwanted reverse effects. This region could be the contralateral hemisphere or in some cases even extracephalic locations like the neck, shoulder or the arm. However, the cathode remains active and an extracephalic location means extensive deep and mid brain current flow. More generally, all regions between electrodes are stimulated. As another example, researchers have used smaller stimulation electrode sizes and bigger reference electrode sizes to offset the focal limitations of tDCS/tACS; while clinical neurophysiology has established that electrode size can “shape” the pattern of current flow [21], the dispersion caused before current reaches the brain limits the role of electrode size [22, 23].
With the increasingly recognized value of computational forward models in informing tDCS/tACS montage design and interpretation of results, there has been recent advances in modeling tools and proliferation of technical publications, e.g., [6, 7, 10, 23–36]. At this stage, the limitations of computational models seem to rest largely in the clinical and experimental applications, including the continuing validation and refinement of modeling parameters (e.g., conductivities) and results. Nevertheless, careful consideration of the development of modeling techniques can provide insight on how models can be leveraged.
The work done by Miranda and Lomarev [32] was among the earliest numerical modeling efforts that specifically examined tDCS montages and intensities in the context of a “spherical head.” Later, the focality of cortical electrical fields was compared across small electrode configurations proposed to achieve targeted modulation [29]. Wagner et al. (2006) was the first CAD (Computer Aided Design) rendered head model that analyzed current density distributions for various montages, including healthy versus cortical stroke conditions. The more recent modeling efforts have been mostly MRI derived. Oostendorp et al. [33] was the first to consider anisotropy in the skull and the white matter, specifically the conductivity of these tissues were a function of direction/fiber alignment. Datta et al. [27] built the first high-resolution head model with gyri/sulci specificity. Suh et al. [7] concluded that skull anisotropy causes a large shunting effect and may shift the stimulated areas. Sadleir et al. [35] compared modeling predictions of frontal tDCS montages to clinical outcomes. Datta et al. [28] studied the effect of tDCS montages on TBI and skull defects. Parazzini et al. [34] was the first to analyze current flow patterns across subcortical structures. Dmochowski et al. [37] showed how a multi-electrode stimulation can be optimized for focality and intensity at the target.
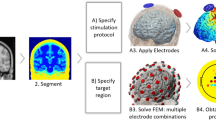
Recent efforts have focused to build patient-specific models and compare modeling predictions to experimental outcomes. In considering new electrode montages, especially in potentially vulnerable populations (e.g., skull damage, children), forward models are the main tool used to relate the externally controllable dose parameters (e.g., electrode number, position, size, shape, current) with resulting brain current flow. While the specific software applications can vary across groups, in general, the approach and workflow for model generation follow a similar pattern (Fig. 4.3).
Imaging and computational work-flow for the generation of high-resolution individualized models: Though the specific processes and software packages will vary across technical groups and applications, in each case high-resolution modeling initiated with precise anatomical scans that allow demarcation of key tissues. Tissues with distinct resistivity are used to form masks. These masks along with the representation of the physical electrodes are meshed to allow FEM calculations. The boundary conditions (generally simply reflecting how the electrodes are energized) and the governing equations (related to ohms law) are well established. The reproduction of the stimulation dose and the underlying anatomy thus allow for the prediction of resulting brain current. These current flow patterns are represented in false-color map and analyzed through various post-processing tools
The steps for generating high-resolution, anatomically specific, forward models of noninvasive neuromodulation are adapted from extensive prior work on computational modeling. These involve: (1) Demarcation of individual tissue types such as bone, cerebrospinal fluid, and brain from high-resolution anatomical data (e.g., magnetic resonance imaging slices obtained at 1 mm slice thickness) using a combination of automated and manual segmentation tools. Specifically, from the perspective of stimulating current flow, it is necessary to distinguish tissues by their resistivity; the majority of the effort that has gone into the development and implementation of models has involved this step (see also next section). The number and precision of the individual masks obtained is pivotal for the generation of accurate 3D models in order to capture critical anatomical details that may influence current flow. (2) Modeling of the exact physical properties of the electrodes (e.g., shape and size) and precise placement within the segmented image data (i.e., along the skin mask outer surface). (3) Generation of accurate meshes (with a high quality factor) from the tissue/electrode masks, whilst preserving resolution of subject anatomical data. The generation of meshes is a process where each mask is divided into small contiguous ‘elements’ which allow the current flow to then be numerically computed—hence the term “Finite Element Method” stimulations. In modern efforts, the number of elements in tDCS models can exceed tn million. (4) Resulting volumetric meshes are then imported into a commercial finite element (FE) solver. (5) At this step, resistivity is assigned to each mask (every element in each mask) and the boundary conditions are imposed, including the current applied to the electrodes. (6) The standard Laplacian equation is solved using the appropriate numerical solver and tolerance settings. In modern efforts the degrees of freedom can exceed 14 million. (7) Data is plotted as induced cortical electric field or current density maps (Fig. 4.3).
Though each of the above steps is required for high-resolution modeling, they rely on personnel technical expertise and hence result in variation in protocols across groups and publications [6, 7, 10, 23–36, 38, 39]. These variations are relevant to clinical practice only in the sense that they change predictions in current flow that meaningfully effect dose decisions. The sources and impact of these variations are addressed in the next section.
Initial models of transcranial current flow assumed simplified geometries such as concentric spheres that could be solved analytically as well as numerically [29, 32]. Such concentric sphere models are useful to address generic dose questions such as the global role of inter-electrode distance, electrode montage, or the relationship between electrode and brain current density, precisely because they exclude regional anatomical differences. More realistic models started to include explicit representation of human anatomy [36]. Datta et al. [27] published the first model of tDCS with gyri resolution, illustrating the importance of anatomical precision in determining complex brain current flow. Addition of diffusion tensor imaging (DTI) incorporates anisotropic properties in the skull and the white matter regions [7]. Fine resolution of gyri/sulci lead to current “hotspots” in the sulci, thereby reinforcing the need for high-resolution modeling [6]. An open-source head model comprising of several different tissue types was adapted to analyze current flow through cortical, subcortical, and brain stem structures [34]. Such models help determine whether current of sufficient magnitude reaches the deeper subcortical structures.
To this day, only a few studies have attempted to more directly link clinical outcomes and model predictions—and thus validate model utility. Clinical evaluation was combined with model predictions to investigate the effects of different montages on clinical disorders such as fibromyalgia [31]. Patient-specific models have been used to retrospectively analyze the therapeutic success of a given experimental stimulation montage [26] and compare model predictions with patterns of activation revealed by functional magnetic resonance imaging (fMRI) [30]. Postmortem “current flow imaging” has also used to validate general model prediction [40] and individualized tDCS models were validated with simultaneous scalp potential recordings [41]. In response to the anatomical localization problem of traditional tDCS, a more focal 4 × 1 high-definition tDCS was developed through computational models and then validated in a clinical neurophysiology trial [42]. The focal delivery of current using the 4 × 1 montage was further validated using supra-threshold TES) [43]; moreover, the models predicted individual variation in sensitivity to currents delivery among typical adults of >2×. These example applications open the door for potentially customizing tDCS on a subject-to-subject basis within the clinical setting [44].
In a subsequent section we describe avenues for clinicians to practically access computational modeling tools, but precisely because this is now a “standard” models approach, limitations of varied approaches need to be understood. If tDCS continues to emerge as an effective tool in clinical treatment and cognitive neuroscience, and concurrent modeling studies emphasize the need for rational (and in cases individualized) dose decisions, then it will become important for tDCS researchers to understand the applications (and limitations) of computational forward models [45].
Pitfalls and Challenges in the Application and Interpretation of Computational Model Predictions
Computational models of tDCS range in complexity from concentric sphere models, to biologically inspired synthetic shapes, to high-resolution models based on individuals MRI. The appropriate level of modeling detail depends on the clinical question being asked, as well as the available computational resources available. Whereas simple geometries (e.g., spheres) may be solved analytically [46], realistic geometries employ numerical solvers. Regardless of complexity, all forward models share the goal of correctly predicting brain current flow during transcranial stimulation to guide clinical therapeutic delivery. Special effort has recently been directed towards increasing the precision of tDCS models. However, it is important to note that increased model complexity does not necessarily equate with greater accuracy or clinical value.
To meaningfully guide clinical utility, attempts to enhance model precision must rationally balance detail (i.e., complexity) and accuracy. (1) Beginning with high-resolution anatomical scans, the entire model workflow should preserve precision. Any human head model is limited by the precision and accuracy of tissue segmentation (i.e., “masks) and of the assigned conductivity values. One hallmark of precision is that the cortical surface used in the final FEM solver should capture realistic sulci and gyri anatomy. Models incorporating gyri level resolution, starting with Datta [27], clearly show that current is “clustered” in local hot spots correlated with cortical folding. (2) Simultaneously, a priori knowledge of tissue anatomy and factors known to influence current flow should be applied to further refine segmentation. We believe that of critical importance are discontinuities not present in nature that result from limited scan resolution, notably both unnatural perforations in planar tissues (e.g., ventricular architecture, discontinuities in CSF where brain contacts skull, misrepresented skull fissures,) and microstructures (e.g., incomplete or voxelized vessels) can produce significant deviations in predicted current flow. Moreover, because of the sensitivity of current flow to any conductivity boundary, increasingly detailed segmentation (e.g., globe of the eye and related structures, glands, and deeper midbrain structures) without reliable reported human conductivity values in literature (especially at static frequency) may also lead to errors. It is worth noting that the respective contribution of the automated/manual interventions also depends on: (a) sophistication of the particular database or automated algorithm employed since they are usually not optimized for forward transcranial modeling [26, 47] and (b) the need for identification of anomalies in suspect populations like skull defects, lesions, and shunts. Thus, addition of complexity without proper parameterization can evidently decrease prediction accuracy. An improper balance between these factors can introduce distortions in predicted brain current flow.
Having mentioned the importance of balancing increased complexity with clinical access to modeling, it is fundamental to emphasize a difference between the “value” of adding precision (complexity) as it is evaluated in engineering papers versus clinical translation. Increasingly detailed computational approaches have been proposed in recent years of varying anatomical and physiological detail [33, 34, 48]. These include whole body models, additional tissues and layers with and without anisotropic properties, and image derived conductivity values using effective medium approximations [9, 49–51]. At the same time, computational models indicate subject specific variability in susceptibility to the same dose [44, 52–54], indicating the value of individualized modeling, or at least modeling across a set of archetypes. Real clinical translational utility must balance the value of increased sophistication with the cost associated with clinical scanning, computational time, and human resources/intervention (manual correction/pre- and post-processing etc.). Thus the question is not if different models will yield different predictions (as must be posed in an engineering paper) but rather does increased complexity change model predictions in a way that is clinically meaningful. While this is a complex and application specific question, a first step toward systematizing value across a myriad of groups and efforts is to develop a metric of change versus a simpler approach, and then applying a threshold based on perceived clinical value and added cost.
It is simplistically assumed that added detail/complexity will enhance model precision and, if done rationally, model accuracy [5, 55]. Though an engineering group can devote extended resources and time to a “case” modeling study, the number of potential electrode combinations and variations across normal heads [44] and pathological heads means that in clinical trial design the exact models will likely not be solved for all subjects (e.g., 4 × 1 over FP3 in a female head). However, while different models will yield different predictions; practical dose decision is based on study specific criterion making a meaningful clinical difference. Therefore, additional complexity and detail is only clinical meaningful if it results in a different clinical decision being made as far as dose individualization—otherwise, the additional detail is purely academic. Two clinical applications of modeling are considered (1) Deciding across montages—namely which montage is expected to achieves the optimal clinical outcomes (safety/efficacy) in a given subject or on average across subjects; (2) Deciding on dose variation across subjects—namely if and how to vary dose based on subject specific anatomy. These aspects of using computational models in clinical practice are addressed in the next sections.
Assuming accurate and precise representation of all tissue compartments (anatomy, resistivity, anisotropy) relevant to brain current flow, it is assumed that by using modern numerical solvers, the resulting prediction is independent of the numerical technique used. Our own experience across various commercial solvers confirms this implicit assumption when meshes are of sufficient detail. That is, a precise description in methods (use of publically available programs) and representation of resulting mesh density and quality (in figures or methods) as well as tests using various solvers provides explicit control for errors generated by the computation itself.
Literature regarding forward modeling, or more broadly the dissemination of modeling analysis to the clinical hands, introduces further issues in regard to (1) interpretability, reproducibility, and accuracy (tissue masks) and (2) graphical representation of regions of influence (degree of “activation). As there is no standard protocol for tissue imaging or segmentation, diversity in the resulting tissue masks will invariably influence predicted current flow. As such, it is valuable to illustrate each 3D tissue mask in a publication’s methods and/or classified serial sections. In regard to representation of relative activation, studies employ either maps of current density (unit of A/m2) or electric field (unit of V/m)., but because the two are related linearly by local tissue resistivity, when plotting activation in a region with uniform resistivity (for example the cortical surface), the spatial profile is identical. When plotting activation across tissues (e.g., coronal section), current density may be advantageous to illustrate overall brain current flow. However, the electric field in the brain is directly related to neuronal activation (e.g., for varied resistivity, the electric field, but not current density, provides sufficient information to predict activation). Despite best efforts, figure preparation invariably restricts tissue mask perspectives and comprehensive display of volumetric current flow, which can be supplemented with online data publication (http://www.neuralengr.com/bonsai).
When interpreting simulation predictions, it is important to recognize that the intensity of current flow in any specific brain region does not translate in any simple (linear) manner to the degree of brain activation or modulation, even when considering current direction. Moreover, recent neurophysiological studies indicate changes in” excitability “may not be monotonic with stimulation [4]. For example increasing stimulation amplitude or duration can invert the direction of modulation, as can the level of neuronal background activity [56]. However, to a first approximation, it seems reasonable to predict that regions with more current flow are more likely to be affected by stimulation while regions with little or no current flow will be spared the direct effects of stimulation. As a first step to understand the mechanism of action of tDCS, a relationship between model predicted regional current flow and changes in functional activation has been recently demonstrated [30]. The “quasi-uniform” assumption considers that if the electric field (or current density) is uniform on the scale of a region/neuron of interest, then “excitability” may be modulated with local electric field intensity [57] (see discussion in refs. [29, 58]). Though efforts to develop suitably detailed biophysical models that consider the myriad of neurons with distinct positions and morphologies or ‘continuum’ approximations [59] of modulation are pending, the current state-of-the-art requires (implicit) application of the “quasi-uniform” assumption.
Forward modeling studies and analysis are often published as case reports with predictions only evaluated on a single head [6, 10, 31, 34]. The suitability of single subject analysis reflects limited available resources and the clinical question being addressed. For a given electrode montage and stimulation dose, the sensitivity of global brain current to normal variation in anatomy (including across ages, gender) is unknown. However, high-resolution modeling suggests gyri-specific dispersion of current flow, which could potentially account for individual variability. More generally, gross differences in tissue dimensions, notably skull thickness and CSF architecture, are expected to influence current flow; in some cases, modeling efforts specifically address the role of individual anatomical pathology, such as skull defects [28] or brain lesions [26]. It is precisely because these studies have shown the importance of specific defect/lesion details, that findings cannot be arbitrarily generalized. This in turn stresses the importance of individualized modeling as illustrated in the next section.
Though this section focused on the technical features of modeling, there is a broader concern in promoting effective collaboration between engineers and clinicians. For analogy, clinicians are generally aware of the challenges and pitfalls in post-processing and feature selection of fMRI data—and indeed, are thus intimately involved in data analysis rather than blindly relying on a technician. For computational “forward” models of neuromodulation, where results may inform study design and patient treatment, it is as important to consider the uses and technical limitations of modeling approaches—and vigilance and skepticism on the part of clinicians will only enhance model rigor. Critically, for this reason, clinician/investigator experience and judgment supersedes all model predictions, even as these models form an important tool in dose design.
Use of Computational Models in Clinical Practice: Consideration for Efficacy
Before beginning our sections of consideration for clinical practice, we note that the ability of clinicians to leverage computational models is limited by access to modeling tools. For clinicians interested in using computational forward models to inform study design or interpretation, but who do not have the time and resources to establish an independent modeling program, several options are available. (1) A collaboration with a modeling group [10] or a company can allow for customized exploration of montage options; (2) referencing existing published reports or databases (www.neuralengr.com/bonsai); [60]) for comparable montages (with careful consideration of the role of individual variation and other caveats presented in the next section); (3) with some coding experience, using a novel process where a desired brain region can be selected and the optimized electrode montage is proposed within a single step has been developed [37]; (4) Graphical User Interface (GUI) based program to simulate arbitrary electrode montages in a spherical model is now available (www.neuralengr.com/spheres). GUI-based software using gyri-precise brain anatomy has now been developed as well [38, 39, 60]. This last solution illustrates an important trend: even as increasingly complex and resource expensive modeling tools are developed, parallel efforts to simplify and automate (high-throughput) model workflow are needed to facilitate clinical translation.
In regard to efficacy, it is typically the case that scientists and clinicians have identified one or more brain regions that they desire to modulate (e.g., based on fMRI and prior behavioral studies; [10, 61–64] and typically this modulation is expressed as a desire to enhance or inhibit function in the region. While this is a starting point for rational dose optimization using computational models, several additional parameters and constraints need to be specified.
A central issue relates to the concern, if any, about current flow through other brain regions. In one extreme, current flow through other regions outside of those targeted is considered unimportant for trial outcomes—and in such a case the optimization would be for intensity at the target while ignoring details of current flow through other brain regions. Conversely, it may be desired to minimize current flow through all other brain regions while maximizing current flow intensity in the targeted brain region—in such a case the optimization is for focality. The reason this distinction between optimization for intensity and optimization for focality is so critical is that produces highly divergent “best” dose solutions [37]. Optimization for intensity often produces a bipolar (one anode and one cathode) montage across the head, such montages typically produces broad current flow across both the target and other brain regions. Optimization for focality typically produces a “ring” montage (with one polarity surrounded by another, analogous to the HD-tDCS 4 × 1;[27]) that spares much of the brain regions outside of the target but also produces less relative current flow at the target then optimization for intensity. In practicality, though distinctions between optimization for intensity and optimization for focality must be made, the (iterative) process of dose optimization may be subtler. Certain brains regions outside of the target may be “neutral” as far as collateral stimulation, others may be “avoid” regions “and other may in fact be considered” beneficial “to the outcomes. A best montage therefore is highly dependent on both the trial design outcomes and the experimenter’s opinion on how distinct brain regions are implicated.
Another critical parameter to consider in trial design is the desired electric field intensity at the target (s). As emphasized throughout this review, optimization based on electric field at the target is expected produce more consistent outcomes then optimization by external current intensity. None-the-less, an experimenter may choose to select a current level (e.g., 1 mA, 2 mA) simply because of historical experience and trends. It is important to emphasize that at least for neurophysiological measures (such as TMS) and likely for behavioral and clinical outcomes, the relationship between current and outcomes is not linear and not necessarily monotonic [65, 66]—meaning reversing current direction (at the level of electrodes and the brain) may not reverse the direction of change, and increasing current intensity may not increase, and can even reverse, the direction of change. The effects of stimulation may vary with the brain region (e.g., prefrontal may not response as motor) or the state of that region, for example is there is ongoing activity (due to a concurrent task) or pathology (due to injury or disease; [67]), in ways that remain poorly understood. In general, more is thus not more with stimulation intensity and thus the decision of what current intensity is desired is a complex and critical one for outcomes. The same challenges applied to selecting a desired brain electric field where higher electric field at a target may not produce increased neuromodulation or more of the type of change desired—moreover increasing electric-field intensity at the target by increasing applied current will increase electric field intensity at every other brain region proportionally. Finally the orientation of the electric field at the target may be critical and depending on the orientation different montages may be considered.
Though the above paints an increasingly complex picture of dose optimization in tDCS it may be unwise to simply ignore these issues and use “historical” montages (e.g., whatever is popular in the literature) and not leverage computational models to the extent possible to optimize dose. In the face of complexity (and risk), experimenters may feel a desire to simply revert to using what has already been reported successful in the literature, but such an approach seems inconsistent with broader efforts to advance the field especially when these previous approach were not optimization (and indeed a very limited set of montages are used across highly disparate indications). None-the-less, given the complexity and unknowns, historical montages do represent a good starting point for dose optimization. Practically, we recommend the optimization process can begin by simulated previously used successful and unsuccessful montages to consider the brain current flow patterns generated in each case, it is against these standards montages that any optimized montage can be compared.
Use of Computational Models in Clinical Practice: Consideration for Safety
Computational models also provide a tool to support assessment of safety. tDCS is considered a well-tolerated technique [16] but vigilance is always warranted with an investigational tool; moreover, given that most montages produce current flow through many brain regions, combined with the desire to explore increasing intensities and durations/repetitions of treatment, as well as stimulation in susceptible subjects (e.g., children), computational models, though only predictions, provide quantitative methods to increase confidence and identify hazards.
We distinguish effects at the skin (which relate largely to electrode design/electrochemical issues and electrode current density) from effects at the brain (which relate to electric fields in the brain) [68]. Computational models predict current flow at both the skin and the brain. Often dose design simply avoids crossing (or even approaching) a threshold for intensity in any given region both inside and outside the target. This threshold is often based on historical approaches. Here the distinction between dose optimization based only on stimulation parameters (e.g., total current) verses brain electric field (with leverages computational models) is evident. Maintaining applied current (e.g., 1 mA) but changing electrode montage and/or subject inclusion (e.g., skull defects) may profoundly change current density/electric field in the skin and brain. Computational models are thus useful to relate new montages/approaches against historically safe ones. It is often the case that even when current density/electric field is predicted, the experimenter still applied the upper limited of applied current. Thus maximum current density/eclectic field and maximum current intensity become constraints in the efficacy optimization process.
Use of Computational Models in Clinical Practice: Consideration for Individual Dose Titration
There are two general uses for computational models in designing rational experiments and clinical trials. The first is the selection of the best generic dose as discussed above. The second “if” to consider is if and how to customize dose to individual subjects. Even across normal healthy adults there is a twofold difference in the electric field generated in the brain for a given applied current [43, 44, 49]. This variation is potentially profoundly significant when considering that twofold changes in applied current can invert the direction of change (see above). Therefore, anatomical differences, even across healthy adults may explain some of the know variation in existing tDCS studies and normalizing for brain electric field across subjects, by leveraging computational models, may in part correct for individual differences.
When considering extremes of age [52, 53] or body mass [9] or the presence of variable brain or skull injuries [28], the potential for individual differences to influence current flow increases [63]. While it is not unusual for tDCS montages to be changed based on individual disease etiology (e.g., stroke location) this is often done using basic rules of thumb (e.g., position the “active” electrode over the brain region) which may not always produce the desired brain current flow [26]. The need to normalize (wide) individual variations in response to tDCS is universally recognized (along with the desire to increase efficacy), and it is rational that normalizing brain electric field, should help reduce variability since brain electric field determines outcomes. Yet the use of computational models for individual optimization is rare and limited by accessibility to rapid modeling tools.
We note the value of individualization is evident in TMS studies when it is almost unheard of to apply the same intensity across subjects. It is no less important in tDCS, but as tDCS does not produce an overt physiological response such as TMS, computational models are valuable tool to individualize dose.
Example Results of Computational Analysis in Susceptible Populations
We conclude with some case studies to illustrate the application of computational models for informing clinical guidelines.
Case 1: Skull defects: There is interest in the application of tDCS during rehabilitation of patients with brain lesions or skull defects (i.e., with or without skull plates); for example subjects with traumatic brain injury (TBI) or patients undergoing neurosurgery. As some of the neurological sequelae are presumably consequences of disrupted cortical activity following the traumatic event, the use of tDCS to deliver current to both damaged and compensatory regions in such circumstances can be a useful tool to reactivate and restore activity in essential neural networks associated with cognitive or motor processing. In addition, because of the reported anti-seizure effects of tDCS [69], this technique might be useful for patients with refractory epilepsy who underwent surgery and have skull plates or decompressive craniectomy for trauma and cerebrovascular disease.
Despite rational incentives for investigation of tDCS in TBI or patients with other major neurological deficits and skull defects, one perceived limitation for the use of tDCS in these patients is the resulting modification of current flow by the skull defects and presence of surgical skull plates. Modeling studies can provide insight into how skull defects and skull plates would affect current flow through the brain and how to modify tDCS dose and/or electrode locations in such cases (Fig. 4.4, adapted from ref. [28]). For example, a skull defect (craniotomy) that is filled with relatively highly conductive fluid or tissue represents a “shunt” pathway for current entering the brain but in a manner highly dependent on defect position relative to electrode montage. In such cases, the underlying cortex would then be exposed to a higher intensity of focused current flow. This in turn might be either beneficial in targeting the underlying brain region or hazardous if the increased current levels resulted in undesired neurophysiologic or pathological changes. Our modeling results confirm the notion that skull defects and skull plates can change the distribution of the current flow induced in cortical areas by tDCS. However, the details of current modulation depend entirely on the combination of electrode configuration and nature of the defect/plate, thus indicating the importance of individual analysis. Based on model predictions, application of tDCS without accounting for skull defects can lead to suboptimal and undesired brain current.
Computational model of current flow in subjects with skull defects/plates. A defect in skull tissue which is the most resistive tissue in the head would hypothetically affect current flow in the underlying brain regions. Furthermore, the exact location of the defect (under/between the stimulation pads) in combination with the ‘material’ filling up the defect with the stimulation montage employed will influence induced current flow. Sample segmentation masks are shown on the left. A small defect under the anode pad (top right) leads to current flow in the cortex restricted to directly under the defect (avoiding the intermediate regions). A similar sized defect placed between the pads (bottom right) does not significantly alter current flow patterns in comparison with a healthy head with no defects (Adapted from ref. [28])
Case 2: Simulation of tDCS in subjects with idealized Deep Brain Stimulation (DBS) leads. Combination therapies incorporating tDCS are increasingly being investigated in drug-resistant instances of psychiatric disorders such as depression and schizophrenia [70, 71]. Subjects who have had DBS electrodes either as a comorbidity or due to an indication being investigated with tDCS or tACS do not necessarily have to be exclude from study. Computational models can the estimate the current flow artifact due to the presence of DBS implantation. At a minimum, safety can be inferred by comparing maximum current density or electric field in DBS subjects to known safe montages in healthy individuals. In Fig. 4.5, four montages were compared, once in a healthy-intact head and again in a head with a burr-hole defect resulting from the typical placement of subthalamic nucleus DBS. While a realistic DBS implantation would include insulation surrounding the lead and a protective cap in the skull opening, this model examined a worst case scenario in which only the burr hole from implantation is present. As seen in the cross-sectional current density images (dashed line), the fluid filled implantation defect draws a greater proportion of current than intact healthy tissue. While current density and in turn electric field distribution are affected by the presence of the defect, peak electric field has less than a twofold change in intensity, which is within the variations seen between individuals and common tDCS protocols (1–2 mA) [9, 44]. Stimulation amplitude could be lowered to 1 mA out of an abundance of caution. The use of HD-tDCS electrodes in the 4x1 configuration (bottom row) can also be used to restrict both maximum intensity and spread of current, especially to deep brain regions.
Simulation of tDCS in subjects with idealized Deep Brain Stimulation (DBS) leads. Finite element models of tDCS with and without burr-hole defects typical in subthalamic nucleus deep brain stimulation. Common sponge (conventional) and HD-tDCS montages for motor and cerebellar stimulation are compared. Fluid-filled burr holes draw a greater amount of current density than what would normally exist with healthy tissue (dashed images). However, peak current density and electric field are minimally affected (less than twofold). HD configurations have lower deep brain electric field intensities in general in addition to being more confined. (Adapted from Truong, Bikson et al. in preparation)
Case 3: Pediatric populations: There is increasing interest in the use of neuromodulation in pediatric populations for a range of indications including rehabilitation, cognitive performance, and epilepsy treatment [72–75]. However, a rational protocol/guideline for the use of tDCS on children, has not been formally established. Previous modeling studies have shown that current flow behavior is dependent on both the tDCS dose (montage and current intensity) and the underlying brain anatomy. Because of anatomical differences (skull thickness, CSF volume, and gray/white matter volume) between a growing child and an adult it is expected that the resulting brain current intensity in a child would be different as compared to that in an adult. Evidently, it would not be prudent to adjust stimulation dose for children through an arbitrary rule of thumb (e.g., reduce electrode size and current intensity by the ratio of head diameter). Again, computational forward models provide direct insight into the relation between external tDCS dose and resulting brain current and thus can inform dose design in children. Figure 4.6 shows an example of a model of tDCS in a 12-year-old compared to that of a standard adult model. Both the peak and spatial distribution of current in the brain is altered compared to the typical adult case. In fact, for this particular case, the peak electric fields, at a given intensity, were nearly double in the 12-year-old as compared to the adult. Though questions remain about the impact of gross anatomical differences (e.g., as a function of age or gender) in altering generated brain current flow during neuromodulation, computational “forward” models provide direct insight into this question, and may ultimately be used to rationally adjust stimulation dose.
Case 4: The wide range of uses for tDCS makes it applicable to a diverse population that can include obese subjects. Montages that have been evaluated for pain, depression, or appetite suppression have been modeled in average adults, but unique challenges exist in the obese model (Fig. 4.7, adapted from ref. [76]). The additional subcutaneous fat present in the obese model warranted an additional layer of complexity beyond the commonly used 5 tissue model (skin, skull, CSF, gray matter, white matter). Including fat in the model of a super obese subject led to an increase in cortical electric field magnitude of approximately 60 % compared to the model without fat (Fig. 4.7a.1–a.3). A shift was also seen in the spatial distribution of the cortical electric field, most noticeable on the orbitofrontal cortex.
Predicted cortical electric field during inferior prefrontal cortex stimulation via 5 × 7 pads. Two conditions, homogenous skin (a.1) and heterogeneous skin (a.2), are contrasted on the same scale (0.364 V/m per mA peak). The homogeneous skin condition is displayed (a.3) at a lowered scale (0.228 V/m per mA peak) to compare the spatial distribution to the heterogeneous condition (a.2). The effect due to a range of varying fat conductivities (b.1–b.8) is compared on a fixed scale (0.364 V/m per mA peak). The conductivity of fat (0.025 S/m) is within an optimum range of influence that causes an increase in peak cortical electric field when included (Adapted from ref. [76])
To gain an intuition for how subcutaneous fat influences cortical electric field and current density, additional models examined a range of conductivity values from the conductivity of skull (0.010 S/m, Fig. 4.7b.1) to the conductivity of skin (0.465 S/m, Fig. 4.7b.8). Coincidentally, the conductivity commonly used for fat (0.025 S/m, Fig. 4.7b.4) was in the range that causes a peak increase in cortical electric field magnitude. It was postulated that more current was blocked by subcutaneous fat at an extremely low conductivity (Fig. 4.7b.1), while more current was redirected at an extremely high conductivity. This, in effect, led to an “optimum” range of influence where the conductivity of fat is believed to reside.
Ultimately, the need to precisely parameterize models rests hand-in-hand with the intended use of the model. From an engineering perspective, the increased complexity of this model caused a noteworthy change within the subject modeled, but this change would not be clinically noteworthy if stimulation dose does not change from subject to subject. This clinical analysis requires an additional comparison between subjects and consideration of the wide variation already inherent in “typical” subjects [44]. What can be concluded, however, is that a comparison between models would require consistent parameterization of subcutaneous fat.
These cases demonstrate the potentially profound influence of lesions and skull defects on resulting current flow, as well as the need to customize tDCS montages to gross individual head dimensions. If tDCS continues to become a viable option for treatment in cases such as chronic stroke, the consideration of tDCS-induced current flow through the brain is of fundamental importance for the identification of candidates, optimization of electrotherapies for specific brain targets, and interpretation of patient-specific results. Thus, the ability and value of individualized tDCS therapy must be leveraged. Whereas, tDCS electrode montages are commonly designed using “gross” intuitive general rules (e.g., anode electrode positioned “over” the target region), the value of applying predictive modeling as one tool in the rational design of safe and effective electrotherapies is becoming increasingly recognized.
Electrode montage (i.e., the position and size of electrodes) determines the resulting brain current flow and, as a result, neurophysiological effects. The ability to customize tDCS treatment through electrode montage provides clinical flexibility and the potential to individualize therapies [24, 26, 31]. However, while numerous reports have been published in recent years demonstrating the effects of tDCS upon task performance, there remain fundamental questions about the optimal design of electrode configurations with computational “forward” models playing a pivotal role.
Conclusion
While numerous published reports have demonstrated the beneficial effects of tDCS upon task performance, fundamental questions remain regarding the optimal electrode configuration on the scalp. Moreover, it is expected that individual anatomical differences in the extreme case manifest as skull defects and lesioned brain tissue which consequently will influence current flow and should therefore be considered (and perhaps leveraged) in the optimization of neuromodulation therapies. Heterogeneity in clinical responses may result from many sources, but the role of altered brain current flow due to both normal and pathological is tractable using computational “forward” models, which can then be leveraged to individualize therapy. Increasing emphasis on high-resolution (subject specific) modeling provides motivation for individual analysis, leading to optimized and customized therapy.
References
Guleyupoglu B, Schestatsky P, Edwards D, Fregni F, Bikson M. Classification of methods in transcranial electrical stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J Neurosci Methods. 2013;219(2):297–311.
Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249:31–8.
Datta A, Dmochowski JP, Guleyupoglu B, Bikson M, Fregni F. Cranial electrotherapy stimulation and transcranial pulsed current stimulation: a computer based high-resolution modeling study. Neuroimage. 2013;65:280–7.
Lindenberg R, Zhu LL, Schlaug G. Combined central and peripheral stimulation to facilitate motor recovery after stroke: the effect of number of sessions on outcome. Neurorehabil Neural Repair. 2012;2012:18.
Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5:435–53.
Salvador R, Mekonnen A, Ruffini G, Miranda PC. Modeling the electric field induced in a high resolution head model during transcranial current stimulation. Conf Proc IEEE Eng Med Biol Soc. 2010;2010(2010):2073–6.
Suh HS, Kim SH, Lee WH, Kim TS. Realistic simulation of transcranial direct current stimulation via 3-d high resolution finite element analysis: effect of tissue anisotropy. Conf Proc IEEE Eng Med Biol Soc. 2009;2009(2009):638–41.
Suh HS, Lee WH, Cho YS, Kim JH, Kim TS. Reduced spatial focality of electrical field in tDCS with ring electrodes due to tissue anisotropy. Conf Proc IEEE Eng Med Biol Soc. 2010;1:2053–6.
Truong DQ, Magerowski G, Blackburn GL, Bikson M, Alonso-Alonso M. Computational modeling of transcranial direct current stimulation (tDCS) in obesity: impact of head fat and dose guidelines. Neuroimage Clin. 2013;2:759–66.
Turkeltaub PE, Benson J, Hamilton RH, Datta A, Bikson M, Coslett HB. Left lateralizing transcranial direct current stimulation improves reading efficiency. Brain Stimul. 2012;5(3):201–7.
Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2:215–28. 28 e1–3.
Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci. 2010;30:15067–79.
Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimultion of the human brain. J Physiol. 2005;568:653–63.
Zentner J. Noninvasive motor evoked potential monitoring during neurosurgical operations on the spinal cord. Neurosurgery. 1989;24:709–12.
Player MJ, Taylor JL, et al. Increase in PAS-induced neuroplasticity after a treatment course of transcranial direct current stimulation for depression. J Affect Disord. 2014;167:140–7.
Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5(3):175–95.
Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor Neurol Neurosci. 2009;27(3):199–207.
Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci. 2013;7:688.
Moreno-Duarte I, Morse LR, Alam M, Bikson M, Zafonte R, Fregni F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage. 2014;85(Pt 3):1003–13.
Ruffini G, Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, Salvador R, et al. Transcranial current brain stimulation (tCS): models and technologies. IEEE Trans Neural Syst Rehabil Eng. 2013;21(3):333–45.
Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97(4):3109–17.
Faria P, Hallett M, Miranda PC. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J Neural Eng. 2011;8(6):066017.
Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clin Neurophysiol. 2009;120(6):1183–7.
Bikson M, Datta A, Rahman A, Scaturro J. Electrode montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode’s position and size. Clin Neurophysiol. 2010;121(12):1976–8.
DaSilva AF, Mendonca ME, Zaghi S, Lopes M, DosSantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012;52(8):1283–95.
Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 2011;4(3):169–74.
Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–7.
Datta A, Bikson M, Fregni F. Transcranial direct current stimulation in patients with skull defects and skull plates: high-resolution computational FEM study of factors altering cortical current flow. Neuroimage. 2010;52(4):1268–78.
Datta A, Elwassif M, Battaglia F, Bikson M. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng. 2008;5(2):163–74.
Halko MA, Datta A, Plow EB, Scaturro J, Bikson M, Merabet LB. Neuroplastic changes following rehabilitative training correlate with regional electrical field induced with tDCS. Neuroimage. 2011;57(3):885–91.
Mendonca ME, Santana MB, Baptista AF, Datta A, Bikson M, Fregni F, et al. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. J Pain. 2011;12(5):610–7.
Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117(7):1623–9.
Oostendorp TF, Hengeveld YA, Wolters CH, Stinstra J, van Elswijk G, Stegeman DF. Modeling transcranial DC stimulation. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:4226–9.
Parazzini M, Fiocchi S, Rossi E, Paglialonga A, Ravazzani P. Transcranial direct current stimulation: estimation of the electric field and of the current density in an anatomical head model. IEEE Trans Biomed Eng. 2011;58(6):1773–80.
Sadleir RJ, Vannorsdall TD, Schretlen DJ, Gordon B. Transcranial direct current stimulation (tDCS) in a realistic head model. Neuroimage. 2010;51(4):1310–8.
Wagner T, Fregni F, et al. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007;35(3):1113–24.
Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8(4):046011.
Jung Y-J, Kim J-H, Im C-H. COMETS: a MATLAB toolbox for simulating local electric fields generated by transcranial direct current stimulation (tDCS). Biomed Eng Lett. 2013;3:39–46.
Thielscher A, Antunes A, et al. Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? 2015 37th annual international conference of the IEEE engineering in medicine and biology society (EMBC). 2015.
Antal A, Bikson M, Datta A, Lafon B, Dechent P, Parra LC, et al. Imaging artifacts induced by electrical stimulation during conventional fMRI of the brain. Neuroimage. 2014;85 Pt 3:1040–7.
Datta A, Zhou X, Su Y, Parra LC, Bikson M. Validation of finite element model of transcranial electrical stimulation using scalp potentials: implications for clinical dose. J Neural Eng. 2013;10:036018.
Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo M-F, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 2013;6(4):644–8.
Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–75.
Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front Psychiatry. 2012;3:91.
Borckardt J, Bikson M, Frohman H, Reeves S, Datta A, Bansal V, et al. A pilot study of the tolerability, safety and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 2012;13(2):112–20.
Rush S, Driscoll DA. Current distribution in the brain from surface electrodes. Anesth Analg. 1968;47(6):717–23.
Huang Y, Dmochowski JP, Su Y, Datta A, Rorden C, Parra LC. Automated MRI segmentation for individualized modeling of current flow in the human head. J Neural Eng. 2013;10:066004.
Parazzini M, Fiocchi S, Ravazzani P. Electric field and current density distribution in an anatomical head model during transcranial direct current stimulation for tinnitus treatment. Bioelectromagnetics. 2012;33(6):476–87.
Laakso I, Tanaka S, Koyama S, De Santis V, Hirata A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul. 2015;8(5):906–13.
Sadleir R, Argibay A. Modeling skull electrical properties. Ann Biomed Eng. 2007;35:1699–712.
Shahid S, Wen P, Ahfock T, editors. Effect of fat and muscle tissue conductivity on cortical currents – a tDCS study. 2011 IEEE/ICME International Conference on Complex Medical Engineering (CME), IEEE. 2011.
Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK, editors. Transcranial direct current stimulation in pediatric brain: a computational modeling study. 2012 Annual international conference of the IEEE engineering in medicine and biology society (EMBC). 2012.
Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK. Transcranial direct current stimulation in pediatric brain: a computational modeling study. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:859–62.
Shahid S, Wen P, Ahfock T. Numerical investigation of white matter anisotropic conductivity in defining current distribution under tDCS. Comput Methods Programs Biomed. 2013;109:48–64.
Bikson M, Datta A. Guidelines for precise and accurate computational models of tDCS. Brain Stimul. 2012;5(3):430–1.
Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–901.
Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–90.
Miranda PC, Correia L, Salvador R, Basser PJ. The role of tissue heterogeneity in neural stimulation by applied electric fields. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1715–8.
Joucla S, Yvert B. The “mirror” estimate: an intuitive predictor of membrane polarization during extracellular stimulation. Biophys J. 2009;96(9):3495–508.
Truong DQ, Hüber M, Xie X, Datta A, Rahman A, Parra LC, et al. Clinician accessible tools for GUI computational models of transcranial electrical stimulation: BONSAI and SPHERES. Brain Stimul. 2014;7(4):521–4.
Bikson M, Rahman A, Datta A, Fregni F, Merabet L. High-resolution modeling assisted design of customized and individualized transcranial direct current stimulation protocols. Neuromodulation. 2012;15(4):306–15.
Coffman BA, Trumbo MC, Clark VP. Enhancement of object detection with transcranial direct current stimulation is associated with increased attention. BMC Neurosci. 2012;13:108.
Dmochowski JP, Datta A, Huang Y, Richardson JD, Bikson M, Fridriksson J, et al. Targeted transcranial direct current stimulation for rehabilitation after stroke. Neuroimage. 2013;75:12–9.
Medina J, Beauvais J, Datta A, Bikson M, Coslett HB, Hamilton RH. Transcranial direct current stimulation accelerates allocentric target detection. Brain Stimul. 2013;6(3):433–9.
Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591(Pt 7):1987–2000.
Weiss M, Lavidor M. When less is more: evidence for a facilitative cathodal tDCS effect in attentional abilities. J Cogn Neurosci. 2012;24(9):1826–33.
Hasan A, Misewitsch K, Nitsche MA, Gruber O, Padberg F, Falkai P, et al. Impaired motor cortex responses in non-psychotic first-degree relatives of schizophrenia patients: a cathodal tDCS pilot study. Brain Stimul. 2013;6:821–9.
Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol. 2009;120(6):1033–4.
Fregni F, Thome-Souza S, Nitsche MA, Freedman SD, Valente KD, Pascual-Leone A. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epliepsia. 2006;47(2):335–42.
Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny M-F, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719–24.
Brunoni AR, Valiengo L, Baccaro A, Zanao TA, Oliveira AC, Goulart AC, et al. The sertraline versus electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–91.
Krause B, Cohen KR. Can transcranial electrical stimulation improve learning difficulties in atypical brain development? A future possibility for cognitive training. Dev Cogn Neurosci. 2013;6:176–94.
Mattai A, Miller R, Weisinger B, Greenstein D, Bakalar J, Tossell J, et al. Tolerability of transcranial direct current stimulation in childhood-onset schizophrenia. Brain Stimul. 2011;4(4):275–80.
Schneider HD, Hopp JP. The use of the Bilingual Aphasia Test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clin Linguist Phon. 2011;25(6–7):640–54.
Varga ET, Terney D, Atkins MD, Nikanorova M, Jeppesen DS, Uldall P, et al. Transcranial direct current stimulation in refractory continuous spikes and waves during slow sleep: a controlled study. Epilepsy Res. 2011;97(1–2):142–5.
Truong DQ, Magerowski G, Pascual-Leone A, Alonso-Alonso M, Bikson M, editors. Finite element study of skin and fat delineation in an obese subject for transcranial direct current stimulation. 34th Annual international conference of the IEEE engineering in medicine and biology society. 2012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Truong, D.Q., Adair, D., Bikson, M. (2016). Computer-Based Models of tDCS and tACS. In: Brunoni, A., Nitsche, M., Loo, C. (eds) Transcranial Direct Current Stimulation in Neuropsychiatric Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-33967-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-33967-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33965-8
Online ISBN: 978-3-319-33967-2
eBook Packages: MedicineMedicine (R0)