Abstract
Tendon functional competence and structural integrity rely on homeostasis of tendon cell metabolism and extracellular matrix macromolecules. The clear link between tendinopathies and increasing age suggests a slow change to tendon homeostasis, which increases susceptibility to damage. Despite this well evidenced association between increasing age and tendon damage, changes to tendon mechanical properties with ageing are not clear with different studies reporting conflicting results. More recent research suggests that age-related changes occur at specific sub-structure locations and may be overlooked by measuring properties of the whole tendon. In this chapter we review changes to tendon mechanical properties, structure and composition. Mechanisms speculated to contribute to tendon change with age such as cellular senescence, ageing stem cell population, reactive oxygen species and formation of advanced glycation end-product crosslinks are discussed. Understanding age-related changes to tendon homeostasis are key to understanding increased incidence of tendon injuries in the ageing population.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tendon
- Ageing
- Mechanical properties
- Extracellular matrix
- Collagen
- Gene expression
- Cellular senescence
- Stem cell
- Inflammageing
- Advanced glycation end-product crosslink
Ageing and Tendon Susceptibility to Injury
Chronic tendon/ligament disorders are highly debilitating and increasingly prevalent [1], accounting for one-third of all primary-care musculoskeletal consultations in the UK [2]. Injuries to ligaments, joint capsules and tendons account for approximately 50 % of the 23 million musculoskeletal injuries that occur in the USA annually [3]. They affect sporting and sedentary individuals [1] in addition to animals such as horses [4, 5]. However, there are no effective treatments or prevention strategies for these injuries [5, 6], as a result of limited understanding of the tissues and the aetiology of injury.
The prevalence of tendon injuries is thought to be increasing due to both increasing sports participation as well as an ageing population [1]. In particular, increasing age has been demonstrated to be a risk factor for a number of different tendinopathies. A recent systematic review of rotator cuff diseases has identified a prevalence of 9.7 % in patients 20 years or younger, rising to 62 % in patients 80 years or older [7]. A separate report identified that rotator cuff tears affected 40 % of individuals older than 60 years in the USA [8]. Achilles tendinopathy is most commonly observed in the fourth and fifth decade of life [9]. Another study identified Achilles, patellar and quadriceps tendon rupture as injuries of middle age, with rotator cuff tears and biceps tendon rupture occurring more frequently in old age [10]. In the horse, a species which frequently suffers from tendinopathy (Fig. 24.1), a number of studies have demonstrated an association between increasing age and risk of tendon injury [11–13].
Age-Related Changes to Mechanical Properties
The unequivocal evidence demonstrating increased susceptibility of tendon to injury with advancing age suggests that the ability of tendon to withstand mechanical forces declines. The strength of the tendon, the degree of elongation prior to failure and ease with which the tendon deforms (stiffness) are important properties for consideration. While it might be expected that the ultimate force and strain would show a negative correlation with age following maturity, studies have not been able to demonstrate a clear link. The ultimate tensile strength of the human patellar tendon was found to show a moderate 17 % decrease between age groups of 29–50 years and 64–93 years [14] but no difference between the ages of 17–54 years [15, 16] in in vitro mechanical tests. The Achilles tendon demonstrated a decrease in ultimate tensile strength for ages between 36 and 100 years in embalmed specimens [17] and a lower ultimate tensile stress in fresh samples over 35 years of age compared to less than 35 years [18]. Material stiffness (modulus) might be expected to increase with ageing however some studies show no effect in human tendon [14, 16, 19] or a slight decrease in human patellar [15] and Achilles tendon [18]. In horses, despite the clear association between horse age and incidence of injury, the ultimate stress, strain and modulus of the superficial digital flexor tendon (SDFT) do not appear to change with increasing horse age [20].
Measurement of mechanical properties in vivo is more problematic due to difficulties in measuring both force and elongation accurately. While it is not possible to measure the failure properties of tendons in vivo, some studies have quantified tendon stiffness using a combination of ultrasound or MRI to track tendon elongation and joint torque to calculate muscle force generation. Measurements of this type are possible for the Achilles and patellar tendon (Fig. 24.2). The forces that tendons are subjected to decrease with advancing age from skeletal maturity through into old age as a result of a decline in muscle mass and force generation [22–25]. Some studies suggest that tendon stiffness may compensate for the decrease in the ability of the associated muscle to generate force. The tendon-aponeurosis of the vastus lateralis muscle was found to decrease in stiffness in a group of women aged from 21 to 77 years [22] although tendon strain at maximum force also decreased. In another study, the patellar tendon in a group of aged men (60–69 years) had a lower stiffness than in a young group of men (21–32 years) although there was no difference in the stiffness of the Achilles tendon between groups and no difference in the maximum strain for either tendon between young and old groups [23]. A larger study including both men and women with a broader age range (18–80 years) found that Achilles tendon stiffness decreased in the older age group [25], a finding repeated in a later study with a smaller group of women [26]. The decrease in tendon stiffness cannot be accounted for purely by a decrease in tendon size. In the study by Csapo et al. [26] the Achilles tendon showed no significant difference in length and cross sectional area (CSA) between groups and in the study by Stenroth et al. [25] the Achilles tendon CSA increased significantly in the older group, hence both studies reported a significantly lower Young’s modulus in older tendons.
Ultrasound probe attached to human lower limb to visualize the muscle tendon junction (a) and images of the Achilles tendon junction with the lateral gastrocnemius (b) (Adapted from [21])
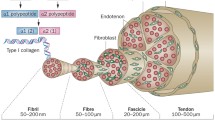
Given the complex hierarchical structure of tendon, studies investigating mechanical properties of the gross tendon structure in vitro and in vivo may miss important changes occurring at sub-structure level. The work of Screen and colleagues has investigated mechanical properties of tendon fascicles and fibres and changes related to ageing. The failure properties (stress and strain) and modulus of fascicles dissected from the equine SDFT, an energy-storing tendon, showed no significant differences between a young group of horses (3–8 years) and an old group of horses (15–20 years) [20]. The stiffness of the inter-fascicular matrix however, which binds the fascicles together, increased in stiffness significantly with increasing age (Fig. 24.3) [20]. This finding suggests that the load distribution within the tendon changes with ageing such that the fascicles are loaded earlier during tendon extension in older tendons. It is interesting to note that behavior of the subunits of tendon differ between tendon types; in the equine common digital extensor tendon (CDET), a positional tendon, the inter-fascicular matrix is much stiffer [27] and does not change significantly with increasing horse age (Fig. 24.3) [20].
Force extension curves for the fascicular interface in the SDFT (solid line) and CDET (dashed line) from a 3 year old horse (a) and a 20 year old horse (b) (Adapted from [20])
In addition, other mechanical properties less often considered such as hysteresis and fatigue properties are likely to be very relevant with regard to tendinopathies. These properties are particularly important in the human patellar and Achilles tendon and the equine SDFT as they are subjected to a high number of loading and unloading cycles and function as elastic energy stores. Although quantifying hysteresis in vivo is problematic [28], a study of the tendon-aponeurosis of the vastus lateralis showed an increase in hysteresis with ageing in a group of women between 21 and 77 years [22]. Hysteresis and post-loading recovery have been studied in fascicles from equine tendons in vitro and differences have been observed between tendon types and with ageing. Fascicles from the energy storing SDFT have lower hysteresis and a greater ability to recover after loading compared to those from the CDET. This difference can be explained by a difference in the extension mechanism; SDFT fascicles appear to extend by rotation of a helical structure while CDET fascicle extension is dominated by sliding of the component fibres [29]. In older horses, the ability of the fascicles from the SDFT to recover following loading is reduced and hysteresis increases [29]. Fatigue loading of SDFT fascicles in vitro results in changes similar to those seen in ageing; in fascicles from young horses rotation decreases and in fascicles from older horses where the helical structure is already compromised there is an increase in fibre sliding following fatigue loading [30]. The inter-fascicular matrix in the SDFT, in addition to an increase in stiffness with ageing, also shows less ability to resist repetitive loading [31]. These studies demonstrate that the mechanical behavior of tendons is complex, as are the changes associated with ageing.
Age Related Changes to Tendon Composition
Age related changes in the response of tendon to applied force stems from a difference in the matrix structure and composition. Information on age related changes to human tendon matrix composition is limited; the majority of research in this area has been conducted on animal tissue and often short-lived species such as rats and mice. The application of these findings to human tendon should be made with care. The horse however represents a good model to study tendon ageing as this species is relatively long-lived and shows an age related decline in tendon function. Morphometric study of the SDFT in horses aged from 2 to 23 years showed that fascicles decreased in size with increasing horse age [32], however this finding was not apparent in studies where fascicles were dissected free from the tendon and CSA measured [20]. In the study by Gillis et al., [32] tendon CSA did not decrease with advancing age thus fascicle numbers appeared to increase. This would suggest a greater proportion of inter-fascicular matrix in older tendon; an interesting finding given the role that the inter-fascicular matrix plays in tendon mechanical behavior. A decrease in unit size also appears to occur at the nano-scale with increasing age, as the average collagen fibril diameter reduces with advancing age in the equine SDFT [33]. The size of the collagen fibrils has also been linked to mechanical properties, where smaller diameter collagen fibrils have been suggested to provide a more creep resistant matrix [34]. Collagen fibrils in their longitudinal course show an abrupt change of direction giving rise to a ‘crimped’ structure [35]; in older horses the crimp becomes less pronounced in the central core of the SDFT (Fig. 24.4) [36, 37].
In terms of molecular composition, equine tendon water content and collagen content do not change significantly with ageing [38, 39]. In contrast, tissue biopsies from the human patellar tendon were found to have a lower collagen content and higher levels of the mature crosslinks hydroxylysylpyridinoline and lysylpyridinoline in an older group of men (67 ± 3 years) compared to a young group of men (27 ± 2 years) [40]. A measure of the total sulphated glycosaminoglycan (GAG) content gives an indication of proteoglycan levels in tendon. Changes in GAG levels with age appear to depend on tendon type, with the energy storing equine SDFT showing no significant change in levels [38, 39] whereas the positional CDET showed a significant decrease in GAG levels with increasing horse age [39]. In a study of human tendons from donors ranging in age from 11 to 95 years GAG levels decreased significantly with age in the supraspinatus tendon but not the common biceps tendon [41].
Using changes in total sulphated GAG levels as an indication of changes in proteoglycan content overlooks possible disparity between individual proteoglycans. For example, there is some evidence to suggest that lubricin, also known as superficial zone protein and proteoglycan 4 (PRG4), increases in rabbit ligament between the ages of 1 and 3 years [42]. Lubricin is a glycoprotein that acts as a lubricant enabling gliding of cartilage surfaces [43, 44] and gliding of tendons around joints and adjacent tendons [43]. More recently, lubricin has been found within the tendon structure where it is enriched in the interfascicular matrix [45, 46]. Interestingly, the rabbit ligaments where higher levels of lubricin mRNA expression were measured showed an increased ultimate strain and decreased elastic modulus [42]; a change that may be explained by increased ability of fascicles to glide relative to each other. However, studies investigating age related changes in human Achilles tendon found that lubricin was not differentially expressed between young (19 ± 5.8 years) and old (69.4 ± 7.3 years) tendons [47]. These studies indicate the need for detailed investigation with regard to specific proteins and glycoproteins as well as sub-structure location with a clear distinction made between maturation and ageing effects.
Contrary to what might be expected, DNA levels, which provide a measure of cellularity, do not seem to decrease in ageing tendon [38, 39].
Age Related Changes to Matrix Turnover Rate
In general, protein synthesis in tissues decreases with advancing age [48], and therefore it might be expected that extracellular matrix proteins in tendon are renewed less often. It is difficult to measure the turnover rate of long-lived proteins, however measuring the ratio of D to L isomers of amino acids allows an estimate of protein half-life in tendon to be made. All amino acids are incorporated into newly synthesized proteins in their L form however over time they can spontaneously convert to the D isomer form by a process known as racemization. This is a relatively slow process but happens more quickly in aspartic acid making this amino acid convenient to measure. A comparison of the rate of accumulation of D aspartic acid with the rate that would occur if the protein were not turned over at all allows a half-life to be calculated. Measurement of the ratio of D/L forms of aspartic acid in equine SDFT and CDET tissue from horses ranging in age from 4 to 30 years suggested an average protein half-life of 7.85 years for the SDFT and 8.02 years for the CDET and half-life increased significantly with increasing horse age for both tendon types [39]. Separation of the tissue into collagenous and non-collagenous proteins showed that the collagen component has a much longer half-life than the non-collagenous proteins and this was significantly longer in the SDFT (197.53 years) compared to the CDET (34.03 years). The half-life of the collagen component increased significantly with increasing age in the SDFT but not the CDET. Conversely, the non-collagenous proteins turned over more slowly in the CDET (average half-life 3.51 years) than the SDFT (average half-life 2.18 years) and half-life increased with increasing age in the CDET but not the SDFT [39]. These studies suggest that the majority of the collagen remains in the tendon for the life-time of the horse.
A similar study has been carried out in human tendon by taking advantage of the (14)C labeling of tissues as a result of the nuclear bomb tests in 1955–1963. Levels of (14)C were measured in Achilles tendon samples and compared to known atmospheric levels. The results suggested that after the cessation of growth at about 17 years of age the turnover of tendon tissue is essentially zero [49]. Other work however has suggested a much more rapid turnover of tendon matrix. Studies using incorporation of stable isotope labeled amino acids suggested that the half-life of collagen in human patellar tendon is about 2 months and close to that of skeletal muscle proteins and higher than muscle collagen [50, 51]. Studies using microdialysis catheters to extract metabolites from the peritendinous region of human Achilles tendon have also demonstrated active turnover of collagen by measuring the pro-peptide of type I collagen as a marker of synthesis and cross-linked carboxyterminal telopeptide of type I collagen (ICTP), as a marker of degradation [52, 53]. Thus it seems likely that a component of the tendon is degraded and renewed frequently while the bulk of the tendon is relatively inert.
A decline in matrix turnover with increasing age would be most easily explained by reduced gene expression of matrix and matrix degrading enzymes by tenocytes . Expression of a range of different matrix proteins including collagens and proteoglycans and matrix degrading enzymes has been quantified in equine SDFT tissue from horses ranging in age from 3 to 30 years [54]. This study showed no significant drop in expression levels of Col1a2, Col3a1, Col5a1, or Col12a1 or proteoglycans such as decorin, biglycan and fibromodulin with increasing horse age. Furthermore, despite previous studies suggesting a slowing of collagen turnover in the SDFT with ageing, no decrease in the levels of expression of the collagenases MMP1 and MMP13 were detected or the tendon-specific transcription factor scleraxis. A full transcriptome analysis using RNA-Seq of human Achilles tendon tissue from young (19 ± 5.8 years) and old (69.4 ± 7.3 years) donors found 191 transcripts were at higher levels in the older tendon and 134 were at lower levels in the older tendon [47]. The networks identified as associated with the differentially expressed genes were cellular function and maintenance, cellular growth and proliferation, cellular cycling, and cellular development, rather than networks relating to matrix proteins, as had been identified in a transcriptome analysis of ageing cartilage [55]. Interestingly, a proteomics study comparing young and old equine SDFT tissue also found cellular proteins featured strongly in the differential analysis; with the term ‘intermediate filament’ identified and several cyto-skeletal keratins and gap junction proteins higher in the old tendon group [56].
While data suggest that there is not a straightforward reduction in gene expression resulting in reduced matrix turnover, other age related processes may result in changes to cell behavior and response to growth factors, cytokines and mechanical signals.
Mechanisms for Age Related Decline in Matrix Turnover Rate
Cellular Senescence
Cellular senescence, the irreversibly arrest of cellular division is an intricate biological process causing alterations in the protein expression profile of the cell and resulting in replicative arrest, changes in metabolism, adhesion efficiency and secretory phenotype [57]. Several of these modifications produce beneficial tumour-suppressive effects as they diminish the proliferation capacity of mutated cells. However, senescent cells are characterised by an increase in the secretion of growth factors, inflammatory cytokines, and proteases; the ‘senescence-associated secretory phenotype’ (SASP), that can exert the opposite activity by creating a tumour-favoring milieu [57]. We can distinguish ageing from senescence by noting that the latter occurs at a cellular level [58].
Tendon fibroblasts from old mice exhibited low motility, a poorly organized actin cytoskeleton, and a different localization of key focal adhesion proteins as compared with young cells. Senescence associated β-galactosidase expression, a marker for senescence demonstrated that fibroblasts from old mice Achilles tendon were not senescent, but had a distinct phenotype [59] in contrast to ageing rat in which there was an increase in β-galactosidase in middle aged and old rats [60]. However, replicative senescence was demonstrated in mice Achilles tendon fibroblasts cultured for more than 50 passages [59]. Long term in vitro culture of cells (the Hayflick model of cellular senescence) has been used extensively to identify mechanisms of age related impairment of function [61]. This method demonstrates proliferation arrest after a number of population doublings and the associated biochemical and molecular changes. Telomere length is a further senescence marker as telomeres are known to shorten progressively during successive cell divisions [62]. In a recent study there was no decrease in cellularity or relative telomere length with increasing age in equine tendon [54].
There is a reduction in proliferation of tenocytes with age. The cellular senescence-inhibited gene (CSIG) is expressed abundantly in young tendon fibroblasts, but its expression declines during cellular senescence. In ageing tendon the reduction in proliferative capacity is associated with the down-regulation of CSIG and an increase in p27, a cell cycle inhibitor protein. CSIG modulates replicative senescence. A reduction in CSIG reduces cell growth and accelerates cellular senescence [60].
Ageing Stem Cell Population
Ageing in tissue such as muscle and brain is driven in part by an age-related reduction in regenerative potential of adult tissues related to a functional decline of their stem cell pool [63]. The number, stress resistance, and repair capacity of tissue-specific adult stem cells contributes to this [64]. The existence of tendon stem or progenitor cells (TSC) has been confirmed in a number of species [65–70].
A relationship between altered TSC properties and tissue ageing has been hypothesised. A study in human Achilles tendon demonstrated that ageing TSCs exhibited self-renewal and clonogenic insufficiencies and premature entry into senescence whilst retaining their multipotency. The group suggested that during tendon ageing the TSC pool size and functional capacity becomes exhausted [71]. It has been suggested that microRNA (miR) 135a has a role in TSC senescence through Rho-associated coiled-coil protein kinase 1 (ROCK1) whilst also promoting proliferation, migration and tenogenic differentiation [72]. Furthermore a loss of tenomodulin, a marker for the tenogenic lineage, may be a source of TSC senescence in ageing tendon. A tenomodulin knock-out mouse model demonstrated TSCs that had reduced self-renewal and demonstrated early entry into senescence [73]. A recently recognised regulator of TSC ageing is peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin 1) which has a role as a post phosphorylation control in protein function regulation and participates in cellular processes including cell cycle progression, cell survival, immune response and lineage commitment. It is involved in the regulation of adult stem cells and in TSCs it affects cellular senescence possibly through miR-140-5p [74].
In ageing rat [69] and human [75] TSCs there is a reduction in both the number of TSCs, their self-renewal and differentiation potential. In rat this has been associated with a concomitant decrease in tendon lineage markers [69]. However, TSCs seem to retain their pluripotency [69, 71, 75].
Interestingly, a recent study determined the mechanical properties of ageing stem cells and identified an increase in stiffness of ageing TSCs in rat that was attributed to a dense cytoskeleton resulting in an increase in size and irregular shape of older TSCs (see Chap. 6) [76].
Inflammageing
Inflammageing is considered the age-related increase in the systemic pro-inflammatory status. The process results in the breakdown of the multi-shell cytokine network as a consequence of remodeling of the innate and acquired immune system; leading to chronic inflammatory cytokine production. Genetic, environmental and age-related factors determine susceptibility to inflammageing. Thus there is a diminished ability to modulate inflammation [77]. Dakin et al. [78] described an age-associated reduction in FPR2/ALX (a g-coupled protein receptor which binds ligands including metabolites of arachidonic acid) along with increased PGE2 in tendon.. In addition interleukin 1β (IL1β) treated tendon explants from older horses had a reduced ability to express FPR2/ALX and tenocytes from older horses had a reduced response to IL1β induced PGE2. These results imply that inflammageing is present in ageing tendons and aged individuals exhibit a reduced capacity to resolve inflammation . Therefore ageing may contribute to deregulated tendon repair through these pathways.
In contrast, in an RNASeq study of ageing human Achilles tendon inflammatory pathways were not recognised following gene ontology of differentially expressed genes [47]. Furthermore, in a proteomics study of ageing tendon, inflammatory proteins were not differentially expressed [56].
Reactive Oxygen Species and the Free Radical Theory
One ageing theory is the ‘damage-accumulating theory/free radical theory’ in which there is a progressive accumulation of cell damage resulting in failure of repair and maintenance systems [79]. One of the causes of cell damage is reactive oxygen species (ROS) . A free radicle is any species capable of independent existence that contains one or more unpaired electrons. Trauma, environmental and physiological stimuli may enhance ROS production and ROS are continually produced during normal cell metabolism.
There are a lack of studies on the role of ROS in age-related tendinopathy. An increase in the expression of peroxiredoxin, a thioredoxin peroxidase with antioxidant properties, in tendon degeneration suggests that oxidative stress may be involved in the pathogenesis of tendon degeneration [80], as it is in the age-related disease osteoarthritis [81].
In a recent ageing tendon proteomic study there was a reduction in catalase, α-crystalline-β chain and a number of heat shock proteins, which have protective roles in oxidative and thermal stress respectively. This could point to ageing tendon being less able to respond to increases in ROS [56]. However there was no change in oxidative stress related genes in a tendon ageing transcriptomic study [47].
There is evidence for the involvement of age-related apoptosis in tendon pathology and ageing which may be a consequence of ROS. In degenerative joint disease of the knee, an age related condition, there is an association with increased susceptibility of periarticular tenocytes to Fas ligand induced apoptosis [82].
Glycation of Matrix Proteins
Although the majority of research concerning ageing has focused on mechanisms that are cell mediated [83], an alternative explanation is that the matrix proteins themselves undergo age related changes. The long-lived nature of tendon collagen renders it susceptible to attack by reactive carbonyl groups on sugars such as glucose, in a process known as ‘browning’ or glycation. A series of spontaneous chemical re-arrangements occurs and further reactions with neighboring peptides results in advanced glycation end-product (AGE) crosslinks such as pentosidine. Unlike the enzyme-mediated crosslinks, which are confined to the telopeptide regions of collagen molecules, AGE crosslinks can form throughout the length of the collagen molecule. Pentosidine is relatively easy to measure as it is resistant to the convenient method of acid hydrolysis to break proteins down into constituent amino acids and in addition, pentosidine is fluorescent, so easy to detect. A number of studies have quantified levels of pentosidine in tendon tissue and shown a positive correlation with donor age. For example pentosidine levels increase with age in human patellar tendon [40], posterior tibialis tendon [84], and equine SDFT and CDET (Fig. 24.5) [39]. The levels are however relatively low (1 crosslink per 70 collagen molecules) in the study by Thorpe et al. [39] and other AGE crosslinks that are present at much higher levels are likely to be more relevant to pathophysiology. One AGE crosslink of particular interest is glucosepane; this AGE crosslink was first identified in 2002 by Biemel and colleagues [85] and later shown to be present in human skin samples at levels equivalent to those for enzyme-mediated crosslinks (see Chap. 18) [86].
Accumulation of the AGE pentosidine in the SDFT as a function of horse age (inset shows the structure of pentosidine) [39]
Glucosepane is formed from lysine, arginine and glucose, is acid labile, non-fluorescent and present in several different stereoisomer forms making study in native tissues difficult. Initial work in our laboratory has however shown that glucosepane is present in human Achilles tendon tissue and levels increase with increasing donor age (Birch, unpublished data). The impact that these crosslinks will have on collagen properties are determined by the sites at which glucosepane forms. Computational studies using a fully atomistic model of an entire collagen molecule in a fibrillar environment have been used to identify, based on energetics, the residues responsible for forming intra-molecular glucosepane cross-links in type I collagen [87]. Using this approach 24 sites where lysine and arginine are in close enough proximity to form glucosepane were identified and six of these sites yielded an exothermic enthalpy change on formation of the glucosepane cross-link (Fig. 24.6). The six energetically favourable sites identified all occur within regions of the collagen molecule that are involved in interactions with other matrix and bioactive molecules. Other studies have investigated the effect of AGEs on mechanical properties of collagenous tissues by incubating the tissue with sugars or other reactive carbonyl metabolites [88–91]. Incubation of rat-tail tendon with methylglyoxal resulted in increased stiffness of collagen fascicles and this seemed to result from decreased sliding between collagen fibrils rather than increased stiffness of the fibril [88]. There remains much to be discovered about AGE formation as an ageing mechanism but this will undoubtedly be very important for understanding tendon homeostasis during ageing.
Distribution of identified intra-molecular cross-linking sites along the length of the collagen molecule (red areas show energetically unfavourable sites and green areas show energetically favourable sites) (Taken from [87])
Conclusion
In summary, although many characteristics of ageing tendon have been documented, there remain conflicting reports of age related effects and the cause of increased injury with age remains unresolved. We consider that age related decline in tendon function is likely to result from a combination of factors, rather than one single cause. Some of the studies discussed in this chapter suggest that a better understanding of normal tendon function and biology is required to allow the effects of ageing to be studied at more specific sub-structure levels. Understanding the factors, and their interactions, that contribute to age related changes in tendon would allow novel approaches to both prevention and better treatments for tendinopathies.
Abbreviations
- AGE:
-
Advanced glycation end-product
- CDET:
-
Common digital extensor tendon
- CSA:
-
Cross sectional area
- CSIG:
-
Cellular senescence-inhibited gene
- GAG:
-
Glycosaminoglycan
- ICTP:
-
Cross-linked carboxyterminal telopeptide of type I collagen
- IL1β:
-
Interleukin 1β
- MRI:
-
Magnetic resonance imaging
- PGE2 :
-
Prostaglandin E2
- PRG4:
-
Proteoglycan 4
- ROCK1:
-
Rho-associated coiled-coil protein kinase 1
- ROS:
-
Reactive oxygen species
- SASP:
-
Senescence-associated secretory phenotype
- SDFT:
-
Superficial digital flexor tendon
- TSC:
-
Tendon stem cell
References
Riley G (2008) Tendinopathy – from basic science to treatment. Nat Clin Pract Rheumatol 4(2):82–89
Bevan S, Passmore E, Mahdon M (2007) Fit for work? Musculoskeletal disorders and labour market participation. The Work Foundation, London. http://www.fitforworkeurope.eu/Downloads/Website-Documents/44_fit_for_work_small.pdf
Butler DL, Juncosa N, Dressler MR (2004) Functional efficacy of tendon repair processes. Annu Rev Biomed Eng 6:303–329
Thorpe CT, Clegg PD, Birch HL (2010) A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine Vet J 42(2):174–180
Clegg PD (2012) Musculoskeletal disease and injury, now and in the future. Part 2: Tendon and ligament injuries. Equine Vet J 44(3):371–375
Kingma JJ, de Knikker R, Wittink HM, Takken T (2007) Eccentric overload training in patients with chronic Achilles tendinopathy: a systematic review. Br J Sports Med 41(6):e3
Teunis T, Lubberts B, Reilly BT, Ring D (2014) A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J Shoulder Elb Surg 23(12):1913–1921
Jain NB, Higgins LD, Losina E, Collins J, Blazar PE, Katz JN (2014) Epidemiology of musculoskeletal upper extremity ambulatory surgery in the United States. BMC Musculoskelet Disord 15:4
Hess GW (2010) Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec 3(1):29–32
Clayton RA, Court-Brown CM (2008) The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 39(12):1338–1344
Kasashima Y, Takahashi T, Smith RK et al (2004) Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet J 36(4):346–350
Perkins NR, Reid SW, Morris RS (2005) Risk factors for injury to the superficial digital flexor tendon and suspensory apparatus in Thoroughbred racehorses in New Zealand. N Z Vet J 53(3):184–192
Reardon RJ, Boden LA, Mellor DJ et al (2012) Risk factors for superficial digital flexor tendinopathy in Thoroughbred racehorses in hurdle starts in the UK (2001–2009). Equine Vet J 44(5):564–569
Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL (1994) Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 12(6):796–803
Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC (1994) The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med 22(3):328–333
Flahiff CM, Brooks AT, Hollis JM, Vander Schilden JL, Nicholas RW (1995) Biomechanical analysis of patellar tendon allografts as a function of donor age. Am J Sports Med 23(3):354–358
Lewis G, Shaw KM (1997) Tensile properties of human tendo Achillis: effect of donor age and strain rate. J Foot Ankle Surg 36(6):435–445
Thermann H, Frerichs O, Biewener A, Krettek C, Schandelmaier P (1995) Biomechanical studies of human Achilles tendon rupture. Unfallchirurg 98(11):570–575
Hubbard RP, Soutas-Little RW (1984) Mechanical properties of human tendon and their age dependence. J Biomech Eng 106(2):144–150
Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR (2013) Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age-related tendinopathy? Eur Cells Mater 25:48–60
Lichtwark GA, Wilson AM (2005) In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J Exp Biol 208(Pt 24):4715–4725
Kubo K, Kanehisa H, Miyatani M, Tachi M, Fukunaga T (2003) Effect of low-load resistance training on the tendon properties in middle-aged and elderly women. Acta Physiol Scand 178(1):25–32
Karamanidis K, Arampatzis A (2006) Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech 39(3):406–417
Carroll CC, Dickinson JM, Haus JM et al (2008) Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105(6):1907–1915
Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T (2012) Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol 113(10):1537–1544
Csapo R, Malis V, Hodgson J, Sinha S (2014) Age-related greater Achilles tendon compliance is not associated with larger plantar flexor muscle fascicle strains in senior women. J Appl Physiol 116(8):961–969
Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR (2012) Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface 9(76):3108–3117
Lichtwark GA, Cresswell AG, Ker RF et al (2013) Commentaries on viewpoint: On the hysteresis in the human Achilles tendon. J Appl Physiol 114(4):518–520
Thorpe CT, Klemt C, Riley GP, Birch HL, Clegg PD, Screen HR (2013) Helical sub-structures in energy-storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomater 9(8):7948–7956
Thorpe CT, Riley GP, Birch HL, Clegg PD, Screen HR (2014) Fascicles from energy-storing tendons show an age-specific response to cyclic fatigue loading. Journal of the Royal Society. Interface 11(92): 1–10
Thorpe CT, Godinho MS, Riley GP, Birch HL, Clegg PD (2015) Screen HR. The interfascicular matrix enables fascicle sliding and recovery in tendon, and behaves more elastically in energy storing tendons. J Mech Behav Biomed Mater 52:85–94
Gillis C, Pool RR, Meagher DM, Stover SM, Reiser K, Willits N (1997) Effect of maturation and aging on the histomorphometric and biochemical characteristics of equine superficial digital flexor tendon. Am J Vet Res 58(4):425–430
Parry DA, Craig AS, Barnes GR (1978) Tendon and ligament from the horse: an ultrastructural study of collagen fibrils and elastic fibres as a function of age. Proc R Soc Lond B Biol Sci 203(1152):293–303
Parry DA, Barnes GR, Craig AS (1978) A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci 203(1152):305–321
Franchi M, Ottani V, Stagni R, Ruggeri A (2010) Tendon and ligament fibrillar crimps give rise to left-handed helices of collagen fibrils in both planar and helical crimps. J Anat 216(3):301–309
Patterson-Kane JC, Firth EC, Goodship AE, Parry DA (1997) Age-related differences in collagen crimp patterns in the superficial digital flexor tendon core region of untrained horses. Aust Vet J 75(1):39–44
Wilmink J, Wilson AM, Goodship AE (1992) Functional significance of the morphology and micromechanics of collagen fibres in relation to partial rupture of the superficial digital flexor tendon in racehorses. Res Vet Sci 53(3):354–359
Birch HL, Bailey JV, Bailey AJ, Goodship AE (1999) Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet J 31(5):391–396
Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, Birch HL (2010) Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem 285(21):15674–15681
Couppe C, Hansen P, Kongsgaard M et al (2009) Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107(3):880–886
Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL (1994) Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis 53(6):367–376
Thornton GM, Lemmex DB, Ono Y et al (2015) Aging affects mechanical properties and lubricin/PRG4 gene expression in normal ligaments. J Biomech 48(12):3306–3311
Taguchi M, Sun YL, Zhao C et al (2009) Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J Orthop Res 27(2):257–263
Flannery CR, Hughes CE, Schumacher BL et al (1999) Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun 254(3):535–541
Funakoshi T, Schmid T, Hsu HP, Spector M (2008) Lubricin distribution in the goat infraspinatus tendon: a basis for interfascicular lubrication. J Bone Joint Surg Am 90(4):803–814
Sun Y, Berger EJ, Zhao C, Jay GD, An KN, Amadio PC (2006) Expression and mapping of lubricin in canine flexor tendon. J Orthop Res 24(9):1861–1868
Peffers MJ, Fang Y, Cheung K, Wei TK, Clegg PD, Birch HL (2015) Transcriptome analysis of ageing in uninjured human Achilles tendon. Arthritis Res Thery 17:33
Tavernarakis N (2008) Ageing and the regulation of protein synthesis: a balancing act? Trends Cell Biol 18(5):228–235
Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M (2013) Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J 27(5):2074–2079
Babraj JA, Cuthbertson DJ, Smith K et al (2005) Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab 289(5):E864–E869
Miller BF, Olesen JL, Hansen M et al (2005) Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567(Pt 3):1021–1033
Langberg H, Rosendal L, Kjaer M (2001) Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol 534(Pt 1):297–302
Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M (1999) Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol 521(Pt 1):299–306
Thorpe CT, McDermott BT, Goodship AE, Clegg PD, Birch HL (2015) Ageing does not result in a decline in cell synthetic activity in an injury prone tendon. Scand J Med Sci Sports 26(6):684–693
Peffers MJ, Liu X, Clegg PD (2013) Transcriptomic signatures in cartilage ageing. Arthritis Res Ther 15(4):R98
Peffers MJ, Thorpe CT, Collins JA et al (2014) Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J Biol Chem 289(37):25867–25878
Hwang ES, Yoon G, Kang HT (2009) A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci 66(15):2503–2524
Sethe S, Scutt A, Stolzing A (2006) Aging of mesenchymal stem cells. Ageing Res Rev 5(1):91–116
Arnesen SM, Lawson MA (2006) Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech Aging Dev 127(9):726–732
Tsai WC, Chang HN, Yu TY et al (2011) Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of p27. J Orthop Res 29(10):1598–1603
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Wagner W, Horn P, Castoldi M et al (2008) Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3(5):e2213
Rando TA (2006) Stem cells, ageing and the quest for immortality. Nature 441(7097):1080–1086
Sharpless NE, DePinho RA (2007) How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8(9):703–713
Bi Y, Ehirchiou D, Kilts TM et al (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13(10):1219–1227
Haasters F, Polzer H, Prall WC et al (2011) Bupivacaine, ropivacaine, and morphine: comparison of toxicity on human hamstring-derived stem/progenitor cells. Knee Surg Sports Traumatol Arthrosc 19(12):2138–2144
Lovati AB, Corradetti B, Lange Consiglio A et al (2011) Characterization and differentiation of equine tendon-derived progenitor cells. J Biol Regul Homeost Agents 25(2 Suppl):S75–S84
Tempfer H, Wagner A, Gehwolf R et al (2009) Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers. Histochem Cell Biol 131(6):733–741
Zhou Z, Akinbiyi T, Xu L et al (2010) Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell 9(5):911–915
Williamson KA, Lee KJ, Humphreys WJ, Comerford EJ, Clegg PD, Canty-Laird EG (2015) Restricted differentiation potential of progenitor cell populations obtained from the equine superficial digital flexor tendon (SDFT). J Orthop Res 33(6):849–858
Kohler J, Popov C, Klotz B et al (2013) Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 12(6):988–999
Chen L, Wang GD, Liu JP et al (2015) miR-135a modulates tendon stem/progenitor cell senescence via suppressing ROCK1. Bone 71:210–216
Alberton P, Dex S, Popov C, Shukunami C, Schieker M, Docheva D (2015) Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev 24(5):597–609
Chen L, Liu J, Tao X, Wang G, Wang Q, Liu X (2015) The role of Pin1 protein in aging of human tendon stem/progenitor cells. Biochem Biophys Res Commun 464(2):487–492
Ruzzini L, Abbruzzese F, Rainer A et al (2014) Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surg, Sports Traumatol Arthrosc 22(11):2856–2866
Wu H, Zhao G, Zu H, Wang JH, Wang QM (2015) Aging-related viscoelasticity variation of tendon stem cells (TSCs) characterized by quartz thickness shear mode (TSM) resonators. Sensors Actuators 210:369–380
Franceschi C, Bonafe M, Valensin S et al (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Dakin SG, Dudhia J, Werling NJ, Werling D, Abayasekara DR, Smith RK (2012) Inflamm-aging and arachadonic acid metabolite differences with stage of tendon disease. PLoS One 7(11):e48978
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11(3):298–300
Wang MX, Wei A, Yuan J et al (2001) Antioxidant enzyme peroxiredoxin 5 is upregulated in degenerative human tendon. Biochem Biophys Res Commun 284(3):667–673
Tiku ML, Shah R, Allison GT (2000) Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem 275(26):20069–20076
Machner A, Baier A, Wille A et al (2003) Higher susceptibility to Fas ligand induced apoptosis and altered modulation of cell death by tumor necrosis factor-alpha in periarticular tenocytes from patients with knee joint osteoarthritis. Arthritis Res Ther 5(5):R253–R261
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217
Corps AN, Robinson AH, Harrall RL et al (2012) Changes in matrix protein biochemistry and the expression of mRNA encoding matrix proteins and metalloproteinases in posterior tibialis tendinopathy. Ann Rheum Dis 71(5):746–752
Biemel KM, Friedl DA, Lederer MO (2002) Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem 277(28):24907–24915
Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM (2005) Glucosepane is a major protein cross-link of the senescent human extracellular matrix. Relationship with diabetes. J Biol Chem 280(13):12310–12315
Collier TA, Nash A, Birch HL, de Leeuw NH (2015) Preferential sites for intramolecular glucosepane cross-link formation in type I collagen: A thermodynamic study. Matrix Biol
Fessel G, Li Y, Diederich V et al (2014) Advanced glycation end-products reduce collagen molecular sliding to affect collagen fibril damage mechanisms but not stiffness. PLoS One 9(11):e110948
Li Y, Fessel G, Georgiadis M, Snedeker JG (2013) Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol 32(3–4):169–177
Reddy GK (2003) Glucose-mediated in vitro glycation modulates biomechanical integrity of the soft tissues but not hard tissues. J Orthop Res 21(4):738–743
Reddy GK, Stehno-Bittel L, Enwemeka CS (2002) Glycation-induced matrix stability in the rabbit achilles tendon. Arch Biochem Biophys 399(2):174–180
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Birch, H.L., Peffers, M.J., Clegg, P.D. (2016). Influence of Ageing on Tendon Homeostasis. In: Ackermann, P., Hart, D. (eds) Metabolic Influences on Risk for Tendon Disorders. Advances in Experimental Medicine and Biology, vol 920. Springer, Cham. https://doi.org/10.1007/978-3-319-33943-6_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-33943-6_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33941-2
Online ISBN: 978-3-319-33943-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)