Abstract
Our research group has pioneered the work on somatic embryogenesis of Bixa orellana (annatto), and since then we have directed efforts in understanding several aspects of this morphogenic pathway in annatto. Here, we present a synthetic description of such works, emphasizing anatomical analyzes and the characterization of the cellular alterations that occur in the process, and the association of the SERK gene expression and somatic embryogenesis. These results are unprecedented and contribute to a better understanding of the processes involving somatic embryogenesis in the species. Advances in this area will facilitate the improvement of the mass propagation, genetic manipulation of the carotenoid biosynthetic pathway, and the overall breeding perspectives of the genus.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Worldwide interest in Bixa orellana L. has increased in the past years, because it accumulates two natural dyes at outside the seed coat, named bixin and norbixin carotenoids. Nowadays, these dyes are largely used as substitutes for synthetic dyes in the food and cosmetic industries (Giuliano et al. 2003; Dias et al. 2011; Marcolino et al. 2011; Mala et al. 2015) or as histological plant staining (Akinloye et al. 2010).
There are scientific evidences linking the antioxidant properties of such carotenoids (Bartley and Scolnik 1995; Kiokias and Gordon 2003) with reduction of the reactive oxygen species/nitric oxide induced by diabetes (Rossoni-Júnior et al. 2012) and the risk of cancer and other chronic conditions such as coronary heart disease (Cunningham and Gantt 1998). In addition, carotenoids have important functions in relation to human health and nutrition, since all species that contain a β-ring can be converted into retinol and, therefore, are precursors of vitamin A. The identification of the carotenoid biosynthesis genes in plants and other organisms has offered the foundations to the biotechnological overproduction of carotenoids of interest in crops (Fraser and Bramley 2004). These above mentioned economic and scientific importances have attracted the attention of researchers and in the past 15 years, several basic and applied relevant information on this species have been generated.
Annatto is a cross-pollinated species and thus highly heterozygous. The conventional propagation is mainly through seeds as plant material available to growers. Vegetative propagation via cuttings has limitations because of the intense leaching of a gummy substance and phenolics from the cutting ends, which difficult rooting (D’Souza and Sharon 2001). Therefore, the application of a reliable in vitro clonal propagation system would unquestionably aid in the multiplication of elite types, in especial those with higher carotenoid contents. The main purpose of applying in vitro culture is to establish and optimize a new method of vegetative propagation and the potential advantages of such system would be to reduce the heterogeneity, to increase the production of the pigment bixin, and to provide a foundation for the subsequent genetic manipulation and control of the biosynthesis of specific secondary metabolites like bixin.
In this particular, plant cell and tissue culture techniques have long attracted interest to characterize better and understand the plasticity of somatic plant cells and related morphogenic events resulting from in vitro cell reprogramming toward the acquisition of regeneration competence (Verdeil et al. 2007; Sugimoto 2015; Sugiyama 2015). Notwithstanding, the foundations for the regulation of the in vitro morphogenetic somatic organogenesis and embryogenesis processes at the cellular, molecular, and physiological levels have been increasing (Kurczyńska et al. 2012; Elhiti et al. 2013; Sugiyama 2015).
A prerequisite for any approach relying on tissue culture is the existence of a reliable regeneration system, based either on organogenesis or embryogenesis. However, there have been relatively few studies involving tissue culture of annatto. As a woody perennial species, the genetic improvement aiming to increase the seed pigment contents is very slow and limited by its long life cycle. Thereby, genetic transformation of annatto to overexpress key enzymes of biosynthetic pathways of metabolites like bixin could facilitate the generation of plants with increased pigment accumulation on seed coats (Paiva Neto et al. 2003a).
Annatto is a species in which pluripotency of cells and tissues has been proven from various types of explants derived from seedlings, such as shoot and nodal segments (D’Souza and Sharon 2001), hypocotyl segments (Paiva Neto et al. 2003b; Parimalan et al. 2007), inverted rooted hypocotyls (Paiva Neto et al. 2003/4), cotyledonary nodes (Carvalho et al. 2005), cotyledons (Parimalan et al. 2007), root segments (Cruz et al. 2014), petiole segments (Mohammed et al. 2015), and from nodal and internodal adult stem segments (Siril and Joseph 2013; Cruz et al. 2015). Additionally, several plant growth regulators were tested on these explants, such as auxins, i.e., indole-3-acetic acid (IAA), 2,4-dichlorophenoxyacetic acid (2,4-D), α-naphthalene acetic acid (NAA), and indole-3-butyric acid (IBA) alone or, most commonly, in combination with cytokinins like 6-γ,γ-dimetylalylamino-purine (2-iP), zeatin (ZEA), kinetin (Kin), 6-benzyladenine (BA), and thidiazuron (TDZ)
The acquisition of competence for somatic embryogenesis (Paiva Neto et al. 2003/4; Parimalan et al. 2011) and the expression of totipotency have been rarely reported. Moreover, there are no systematic works covering the different aspects of somatic embryogenesis and gene expression. So, here we will describe some achievements involving the somatic embryogenesis of B. orellana in the past years.
2 Annatto as a Key Species to Understand Metabolic Pathways of Carotenoid Biosynthesis
The aryls of annatto seeds are a rich source of orange–red pigments that are widely used in the food, textile, and cosmetic industries. These pigments are commercially known as annatto (E160b), a mixture of bixin (C25H30O4) as the main color component and traces of norbixin, bixin dimethyl ester, and other minor apocarotenoids. The ability to synthesize bixin and, thereby, its biosynthetic pathway has been the focus of intense interest in some plants like annatto (Mercadante et al. 1997; Mercadante and Pfander 1998; Narváez et al. 2001; Jako et al. 2002; Bouvier et al. 2003; Rodríguez-Ávila et al. 2011a, b; Soares et al. 2011; Rivera-Madrid et al. 2013; Cárdenas-Conejo et al. 2015), Costus pictus (Annadurai et al. 2012), Crocus sativus, and Vitis vinifera (Ramamoorthy et al. 2010). Bixin is a dicarboxylic monomethyl ester apocarotenoid derived from the oxidative cleavage of carotenoids. Based on a heterologous in vitro expression system, it has been proposed that the pathway for bixin biosynthesis in annatto involves the actions of a lycopene cleavage dioxygenase (BoLCD), a bixin aldehyde dehydrogenase (BoBADH), and a norbixin methyltransferase (BonBMT), which catalyze the synthesis of bixin in a series of reactions proceeding sequentially from the C40 carotenoid precursor lycopene (Bouvier et al. 2003). However, the expression and significance of these gene products for the biosynthesis of bixin in annatto have not been confirmed in in vivo studies, so that the elucidation of the later biosynthetic steps and associated genes involved in the biosynthesis of bixin in annatto awaits further investigations. A preliminary analysis of mRNA expression of the genes involved in the early steps of carotenoid biosynthesis, namely DXS (1-deoxy-D-xylulose 5-phosphate synthase), PSY (phytoene synthase), PDS (phytoene desaturase), ε-LCY (lycopene epsilon-cyclase), and β-LCY (lycopene β-cyclase), was investigated in two B. orellana cultivars of contrasting carotenoid and bixin accumulation by reverse transcription-polymerase chain reaction (RT-PCR) analysis, and the expression of three of them (PSY, PDS, and β-LCY) could be associated with pigment accumulation (Rodríguez-Ávila et al. 2011b). Two carotenoid cleavage dioxygenases (CCDs) genes, one encoding a CCD subclass 1 (BoCCD1) (Rodríguez-Ávila et al. 2011a) and another CCD subclass 4 (BoCCD4) (Soares et al. 2011), have been also implicated in the biosynthesis of bixin based on their expression profiles in different tissues of B. orellana, including developing seeds. Recently, a de novo transcriptome sequencing in Bixa orellana has been published (Cárdenas-Conejo et al. 2015), showing that bixin production involves a coordinate expression of genes related to methylerythritol phosphate, carotenoid and bixin biosynthesis in immature seed.
The advent of next-generation high-throughput DNA/RNA sequencing (NGS) and CRISPR/Cas9 genome editing technologies has created unprecedented opportunities toward the elucidation of the metabolic pathway of carotenoid and bixin biosynthesis and their regulation in annatto. The NGS technologies provide high-throughput reads at a relatively low cost as compared to the Sanger method (Mardis 2008), generating highly reproducible and informative data and accurately quantifying transcripts (Marioni et al. 2008; Wang et al. 2009). On the other hand, the CRISPR/Cas9 genome editing technology is an easy and affordable tool that enables the precise manipulation of specific genomic sequences, allowing the generation of targeted knockout mutants for functional characterization of plant genes in a single generation (Belhaj et al. 2015). This simple system consists of a prokaryotic ‘clustered regularly interspaced short palindromic repeat’ (CRISPR)-associated protein 9 (Cas9) endonuclease and a small RNA molecule, the ‘single guide’ RNA (sgRNA), which instructs the nuclease to recognize and cleave a specific DNA target site. The increasing list of plant species that have been successfully used for targeted genome modification by the CRISPR/Cas9 system (see review by Belhaj et al. 2015) and the possibility of assembling multiple sgRNAs and the Cas9 encoded gene into a single delivery vector emphasize both the ease of employing this genome editing tool and the powerful of applying this technology for the rapid elucidation of metabolic pathways and their regulatory mechanisms in plants. And in this sense, somatic embryogenesis may play a key role in allowing adequate and reproducible systems for genetic transformation aiming at unraveling metabolic pathways of carotenoid biosynthesis and functional gene expression studies linked to that.
3 Somatic Embryogenesis as a Key Morphogenic Pathway for Genetic Transformation in Annatto
Due to the great commercial appeal of bixin, the establishment of efficient transformation protocols for Bixa orellana can assist to obtain transgenic lines and potential commercial varieties with high bixin yields (Kumar et al. 2007). To date, surprisingly, genetic transformation of annatto has not been systematically and comprehensively investigated yet. Few studies involving a genetic transformation in annatto have been performed, including direct and indirect somatic embryogenesis-based transformation system (Parimalan et al. 2011) and transient transformation from hypocotyls (Zaldívar-Cruz et al. 2003), all mediated by Agrobacterium tumefaciens. However, there is a need for the establishment of more efficient transformation protocols, where transformed explants could show a higher regenerative rate, which is essential for the success of genetic transformation (Anami et al. 2013).
Somatic embryogenesis is a unique system that has also become an appropriate method for studying the morphophysiological and molecular aspects of cell differentiation. On top of that, there is a growing body of literature that reports the usefulness of the embryogenic pathway in genetic transformation protocols (Jin et al. 2005; Bull et al. 2009; Ribas et al. 2011; Yang et al. 2014; Nyaboga et al. 2015). This topic is extensively reviewed by Ochoa-Alejo in Chap. 23 (this book).
Somatic embryo, a bipolar structure with shoots and root domains, is an efficient system to enable regeneration of transformed plants. The bipolarity linked to the single cell origin of the embryos may reduce the development of chimeras or mixoploidy in the regenerants (Pathi et al. 2013). Somatic embryogenesis may occur directly or in an indirect way. The former occurs without a callus stage, whereas the latter undergoes a callus phase. The direct embryogenesis provides a more efficient transformation because it preserves a higher genetic stability of the plant material (Wang and Wang 2012; Kreis et al. 2015).
In annatto, direct and indirect somatic embryogenesis have been achieved from immature zygotic embryos, hypocotyl, and root segments (Paiva Neto et al. 2003a, b, c; Parimalan et al. 2011). Annatto explants have high plasticity and may exhibit in vitro embryogenic and/or organogenic responses, depending on the growth regulators used, as demonstrated in root segments (Cruz et al. 2014), expanding the possibilities for genetic transformation.
Somatic embryos of annatto may be used for nuclear transformation but also to potentially generate transplastomic plants, as reported for cotton (Kumar et al. 2004a), carrot (Kumar et al. 2004b) and soybean (Dufourmantel et al. 2004). Plastids are important organelles in plant cells, where several biosynthetic pathways occur, such as the production of bixin (Nisar et al. 2015; Louro and Santiago 2016). The plastid genome transformation has become an alternative for the nuclear transformation, because of some advantages such as gene containment—since there is no plastid genome transmission through pollen, precise transgene integration—exclusively by homologous recombination, expression of multiple genes—through the construction of clusters, production of high protein levels, absence of epigenetic effects, and gene silencing (Bock 2015).
Although annatto is an excellent model of study, and highly efficient regeneration protocols have been already established, there is still a lack of studies to develop optimized transformation methods. It is necessary to establish methods ensuring the improvement and enhancement of transformation efficiencies, enabling the generation of plants with phenotypes of interest unattainable by conventional breeding.
4 Somatic Embryogenesis in Annato: Origin and Developmental Stages of Somatic Embryos
Somatic embryogenesis (SE) is a morphogenic route where embryos (bipolar structures) are generated from single cells or from multicellular clusters, which may be formed directly from the original explant tissue or induced after a preceding callus stage. This is an alternative technique with potential applications in the clonal propagation of plants, besides of being excellent tool for basic studies and analysis of molecular and biochemical events that occur during plant embryogenesis (Santos et al. 2005; Cangahuala-Inocente et al. 2009; Kurczyńska et al. 2012; Steiner et al. 2012; Rocha and Dornelas 2013; Steiner et al. 2016). This morphogenic pathway has been widely applied for a variety of species, from basic models to industrial and agronomical crops, as well-documented in this book (Chaps. 11–22) in a variety of applications as outlined in Chaps. 23–27.
In annatto the SE was first reported by Paiva Neto et al. (2003a) from immature zygotic embryos (IZE), where it was possible to observe somatic embryos (SE) in various stages of development few days after the beginning of induction. In addition, Parimalan et al. (2011) obtained somatic embryos from calluses with the intention of establishing a viable system of genetic transformation in annatto using Agrobacterium.
Knowledge of all stages of this process can facilitate the in vitro propagation of the species and the application of genetic breeding techniques. Associated with different techniques, anatomical analyzes are essential to characterize the cellular alterations that occur in the somatic embryogenesis process. They can help us to understand better the factors that lead somatic cells to resume meristematic characteristics and acquire competence to form somatic embryos; furthermore, histodifferentiation in SE can provide the information necessary to understand the embryogenesis process in plants (Corredoira et al. 2006; Moura et al. 2008; von Aderkas et al. 2015). Histochemical analyzes have allowed a better understanding of the mobilization of reserves and energetic demands during the somatic embryos development (Cangahuala-Inocente et al. 2004, 2009; Moura et al. 2010; Rocha et al. 2012; Jariteh et al. 2015). This subject is well-approached in Chap. 23 (this book) by Rocha and co-workers.
4.1 Structural Changes Involved in the Somatic Embryogenesis Program in Annatto
The formation of somatic embryos in annatto is related with plant growth regulators (PGRs) added to the culture medium, the age of the zygotic embryo, besides being dependent on the genotype (Paiva Neto et al. 2003a). Only under optimal conditions embryogenic cells, genetically determined, will develop and form embryos in response to specific signals and also in cells in which the physiological conditions are appropriate (Fehér 2008).
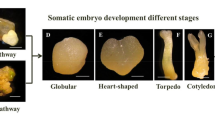
Considering the morphological and anatomical aspects and profiles of gene regulation, it is noticed that there is a need for further studies on somatic embryogenesis. The IZE of annatto consists of two cotyledons and a cylindrical embryonic axis (Fig. 13.1a, b). These embryos show green color and smooth surface which starts to get wrinkled in the initial days of in vitro culture. After the initial days of the induction, in the medium occurs several external and internal changes in IZE leading to the formation of SE (Fig. 13.1c). After around 30 days of culture, somatic embryos at the globular stage are observed on the surface of the IZE (Fig. 13.1c, d). These structures have a spherical shape, smooth surface, and are greenish (Fig. 13.1d). After 52 days of induction, somatic embryos at different stages of development can be viewed throughout the explant, denoting the lack of synchrony of the embryogenic process in annatto (Fig. 13.1f).
Somatic embryogenesis in Bixa orellana L. a Immature zygotic embryo (Z) showing greenish coloring and the presence of bixin at the initiation of culture. b Longitudinal section of Z showing the presence of the procambium in the embryonic axis and phenolic compounds in the cotyledons. c Cross section in Z cotyledon is confirming different origins of a globular somatic embryo (GE). The presence of early GE on the adaxial surface of the Z protoderm. d GE visualized after 30 days of culture. e Cross section of Z with completely individualized somatic embryos (Eb). The presence of procambium and suspensor on Eb and phenolic compounds on Z. f Eb in several stages of development after 52 days cultivation, highlighting the lack of synchronization on somatic embryogenesis system of annatto. g Cross section of the SE. The presence of uniseriate protoderm and individualized SE. GE Globular Embryo. Abbreviations Pc Procambium. Pt Protoderm. Sp Suspensor. Z Immature zygotic embryo. * Phenolic compounds. Eb Somatic embryos
At the beginning of induction, IZEs have uniseriate smooth epidermis with isodiametric cells, uniform and undifferentiated mesophyll and procambium cords distributed in the parenchyma (Fig. 13.1b). Changes continue, and pro-embryogenic zones are evident in certain regions of both sides of the cotyledons standing out initially as protuberances. These zones are formed from the epidermis and the underlying parenchyma (Fig. 13.1c). Epidermal cells divide in an anticlinal way, while in the mesophyll parenchyma cells immediately below the epidermis are divided into different levels, leading to the formation of pro-embryogenic zones (Fig. 13.1c). This sequence of changes leads to SE and, depending on the exposure time, some cells acquire meristematic activity and undergo numerous mitosis (Fehér 2003, 2005, 2008; Paim Pinto et al. 2011; Steiner et al. 2016). In annatto, these changes promote deformations, which are caused by cell walls in different section planes and finish with the formation of the somatic embryos. Rocha et al. (2012) reported the formation of protuberances on the adaxial surface of the cotyledon in passion fruit (Passiflora cincinnata) throughout SE induction. However, these protuberances originated during differentiation of tissue explant did not produce embryogenic callus nor somatic embryo. It occurs in a different way in annatto by the fact that these protuberances observed in this species originate somatic embryos in both the adaxial and abaxial surface of cotyledon.
The SE also has an uniseriate protoderm, ground meristem, and some are connected to the tissue explant through embryo suspensor-like structures (Fig. 13.1e). The observation of the process after 52 days cultivation allows noting the full development of SE in late developmental stages (torpedo and cotyledonary embryo) in addition to being individualized (Fig. 13.1f, g). In these stages, the presence of differentiated procambium can be observed (Fig. 13.1e). It is noticed that the SEs originated in this system have both unicellular and multicellular origin (Fig. 13.1c).
All these events are marked by the presence of phenolic compounds which were stained by toluidine blue (Fig. 13.1b). Phenolic compounds are associated with the antioxidant activity by eliminating free radicals or lessening their effects (Alemanno et al. 2003; Peixoto et al. 2007; Almeida et al. 2012). We note that these compounds are shown in IZE of annatto as the induction time increases. The presence of phenolics in this organ may be associated with the protection of tissues from free radicals since these compounds are present in cells bordering the somatic embryos.
The dedifferentiation of epidermal cells of B. orellana and, therefore, the formation of somatic embryos may also be explained by a possible effect of stress condition on the cultured explants. Whereas the presence of PGRs in the culture medium can be a stress factor, the amount of 2,4-D can be toxic to certain tissues or plants, and the changes that occur in these cells can be justified by stress (Zavattieri et al. 2010). This stressful condition causes changes in the normal endogenous hormonal balance of the cells and leads them to leave a fully differentiated state and to resume meristematic activity, showing the plasticity to hormones in plants (Fehér et al. 2003; Zavattieri et al. 2010; Pandey et al. 2012; Rahman 2013).
In addition to the factors listed before, a new perspective has been considered mutually active in the process of activation/silencing of genes involved in somatic embryogenesis: DNA methylation, histone modifications, and chromatin remodeling (Fehér 2008; Pandey et al. 2012). The subject has been reviewed elsewhere (De-la-Peña et al. 2015; Mahdavi-Darvari et al. 2015) and approached (Chap. 6, this book) by De-la-Peña and colleagues. Activation/silencing of genes is observed in somatic cells acquiring embryogenic competence during the induction period (Rocha and Dornelas 2013). Normally, these cells perceive external signals, often in neighboring cells, which can be cell wall components, or nutrients present in the culture medium, as boron (Pandey et al. 2012). Boron is an essential nutrient in the synthesis and organization of the cell wall and plasma membrane structures (Brown and Hu 1997; Pandey et al. 2012), and it is involved in the induction of somatic embryogenesis by stress signaling pathways.
5 Involvement of SERK Genes Expression During Somatic Embryogenesis of Annatto
The onset of somatic embryogenesis is dependent on a complex network of interactions that will modulate the expression of several genes. Among the genes expressed in somatic embryogenesis, the Somatic Embryogenesis Receptor-Like Kinases family has been the most studied. Its expression pattern in several embryogenic systems has been linked to the acquisition of embryogenic competence from differentiated cells. It has a wide and remarkable involvement in several developmental signal transduction pathways. Despite extensive studies since its discovery, there are several scientific gaps regarding the particular interactions in the complex network of the somatic embryogenesis that still limits to unravel a more comprehensive and definitive role for this gene family. The latest achievements report that it is activated or upregulated in response to endogenous or exogenous signals, particularly by auxin exposition and stress-related responses. It has been hypothesized that SERK gene expression may be associated to cellular reprogramming that triggers a new developmental program (Nolan et al. 2003; Fehér 2008; Nolan et al. 2009; Fehér 2015). Additionally, studies showed that the constitutive overexpression of SERK increased the embryogenic responses (Hecht et al. 2001).
The first homolog of SERK was isolated from Daucus carota (DcSERK) in a subpopulation of competent embryogenic cells, derived from the proliferation of provascular elements of hypocotyls in the presence of 2,4-D (Schmidt et al. 1997). In the Arabidopsis genome, five distinct members were identified (SERK1 to SERK5) (Hecht et al. 2001). Along two decades it has been demonstrated that these genes are present in the genome of all higher plant groups, monocots and eudicots, gymnosperms, and lower plants as well (Sasaki et al. 2007; Steiner et al. 2012; Aan den Toorn et al. 2015).
Genes of this family encode a transmembrane protein belonging to the LRR-RLK II group of the superfamily of receptor-like kinases and contain a highly conserved structural organization. The extracellular domain is constituted by a signal peptide followed by a leucine zipper, 4.5-5 LRR, and a proline-rich region that contains the SPP motif. In the intracellular domain are the catalytic serine/threonine or tyrosine kinase domain and the c-terminal region (Hecht et al. 2001; Shiu and Bleecker 2001a, b; Albrecht et al. 2008; Aan den Toorn et al. 2015).
We have isolated two putative members of the SERK family expressed during somatic embryogenesis in annatto by amplifying cDNA from embryogenic callus using degenerated primers (Baudino et al. 2001) followed by Rapid Amplification of cDNA Ends (RACE). Results of BLAST search with the deduced amino acids sequence in the National Center for Biotechnology Information (NCBI) and UNIPROT (http://www.uniprot.org/) databases revealed high similarity of both sequences with homologs of SERK proteins of other species, such as Theobroma cacao, Coffea canephora, Ricinus comunis, Carica papaya, Citrus sinensis, G ossipium hirsutum, among others. The contig1 exhibited a partial coding sequence of 340 amino acids that showed identity between 96 and 98 % with annotated sequences as SERK1 and SERK2 in the databases. The contig2, in turn, had a complete coding sequence of 589 amino acids that showed identity between 75 and 84 % with annotated homologs as SERK3/BAK1 of various eudicots. In relation to Arabidopsis thaliana it was found through BLAST search from TAIR database (www.arabidopsis.org) that contig1 showed 74–76 % identity with AtSERK1 and AtSERK2, whereas the contig2 showed an identity of 72 % with AtSERK3/BAK1. Due to the identity of sequences, the contigs 1 and 2 were named, respectively, as BoSERK-Like1 and BoSERK-Like3.
The identity of conserved intra and extracellular domains was verified using the Pfam program and by alignment of sequences with related species (data not shown). The partial sequence of BoSERK-LIKE1 encodes part of the leucine zipper, the five LRR and SPP motifs, the transmembrane domain and part of the kinase domain. BoSERK-Like3 contains all structural domains characteristic of this gene family (data not shown).
A phylogenetic relationship was investigated (Fig. 13.2) based on plant lineages (Aan den Toorn et al. 2015). According to the authors, evolutionary changes in the extracellular and intracellular domains were important to specify different members of the SERK family. The analysis of our data led to the separation of the SERK proteins in four major clusters: non-vascular SERK proteins, monocot SERK proteins, and two clusters of eudicot SERK proteins formed by SERK dicots S1/S2 and S3/S4 (Fig. 13.2). Here, we aligned the BoSERK sequences with the same 67 sequences of SERK proteins and six further proteins that showed homology with BoSERK-Like1 and BoSERK-Like2 in the BLAST results. Two other LRR-RLK groups (NIK proteins and two of unknown function) were included as outliers. For eudicots, there was a bifurcation forming a cluster for SERK1/SERK2 and other for SERK3/SERK4. For monocots, a single group was formed and another cluster of the non-vascular SERK proteins. The contig BoSERK-Like1 grouped together to the cluster corresponding to SERK1/SERK2 of the Eudicotyledons, closely to sequences of Carica papaya, Theobroma cacao, Gossypium hirsutum, and Ricinus communis, whereas BoSERK-Like3 was grouped into the cluster corresponding to SERK3/SERK4, being more closely related to SERK3-BAK1 proteins. These data suggest that the contigs named BoSERK-Like are indeed the possible orthologs of SERK1/SERK2 and SERK3 in B. orellana.
Evolutionary history of SERK genes inferred using the Neighbor-Joining method (Saitou and Nei 1987). The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The branches with a percentage below 50 % were cut. The evolutionary distances were computed using the Dayhoff matrix-based method (Schwarz and Dayhoff 1979) and are in the units of the number of amino acid substitutions per site. The analysis involved 77 amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 782 positions in the final dataset. Four distinct groups of SERK proteins are inferred, according to Aan den Toorn et al. (2015): SERK dicots 1/2 that grouped with AtSERK1 and AtSERK2 (blue); SERK monocots (green); SERK dicots 3/4 that grouped together with AtSERK3, AtSERK4 and AtSERK5 and SERK Non-vascular plants (yellow). The black branches refer to a clade of LRR proteins that grouped together with the A. thaliana non-SERKs. BoSERK-Like1 was grouped together to SERK dicots 1/2 while BoSERK-Like3 was positioned into the SERK dicots, 3/4 group. Evolutionary analyzes were carried out in MEGA6 (Tamura et al. 2013)
5.1 Localization of BoSERK-Like Transcripts by In Situ Hybridization
In situ hybridization assay has been an important tool of functional genomics applied to studies of the development of plants. This technique associated with cytological and structural studies has been largely used in attempts to understand in vitro morphogenesis, providing information on the localization of key transcripts and changes in the transcriptional state of the tissues subjected to different inducing conditions.
Here, in situ hybridization analysis was performed in order to locate the patterns of temporal and spatial expression of BoSERK-Like during the embryogenic callus and somatic embryos differentiation from immature zygotic embryos in B. orellana. For this, sense and antisense digoxigenin labeling RNA probe were synthetized from a cloned fragment characterized as BoSERK-LIKE1. Transcripts of BoSERK-Like were observed spread throughout the entire immature zygotic embryo, revealing the occurrence of a basal expression of genes from this family previously to embryogenesis induction and the meristem nature of the young tissues in the development of the embryo. However, a strong signal was detected in the protoderm cells, procambium region and initial cells of the shoot apical meristem during the induction time in the presence of 2,4-D and kinetin. The expression of SERK was maintained in the cells of epidermis and mesophyll in the division. These cells have given origin to pro-embryogenic mass and subsequently to somatic embryos asynchronously. After 30 days of culture, proembryos, and globular somatic embryos showed a strong signal of hybridization. Finally, after 52 days of culture, somatic transcripts of SERK were detected in all phases of development of the somatic embryos. Somatic embryos in advanced stages showed weak signal in comparison to early stages, reflecting a pattern of basal expression similar to that observed in zygotic embryos before induction. No signal of expression was observed in tissues hybridized with the sense probe (Fig. 13.3).
Localization of BoSERK-Like transcripts during somatic embryogenesis in Bixa orellana by means of in situ hybridization. a Cross section of an immature zygotic embryo showing transcripts spread throughout all the cotyledons and shoot apical meristem before induction. A strong signal was detected in the protodermal cells and procambial region (arrow). b Cross section of the cotyledons after 20 days of induction did not hybridize with sense probe (negative control). c Cross section of cotyledons after 20 days with signal expression in all the explant. d Somatic embryo being formed from epidermal cells showing strong hybridization signal (arrow). BoSERK-Like expression was also observed in cells of the apical dome of the zygotic embryo (arrow). e, f, and g Somatic embryos at different developmental stages after 52 days of induction are shown. Hybridization signal was visualized by the formation of a pink or purple precipitate. Abbreviations Eb Somatic embryo; Do Apical dome; Sp Suspensor; Pc Procambium; Pt Protodermal. Bars = 50 µm
6 Concluding Remarks and Future Road Map
The comprehension of the developmental events during the induction phase as well as the development of somatic embryos is essential to regulate each stage of the somatic embryogenesis developmental program. Additionally, the development of efficient protocols of somatic embryogenesis in annatto may be useful for applications in genetic transformation systems with the final aim to obtain annatto plants with increased carotenoids synthesis and accumulation. The advent of NGS and CRISPR/Cas9 genome editing technologies has created unprecedented opportunities towards the elucidation of the metabolic pathway of carotenoid and bixin biosynthesis and their regulation in annatto. We are willing to expand the number of genes involved in somatic embryogenesis by means of a transcriptome-based dataset. Also, proteomics- and metabolomics-associated aspects will be instrumental approaches to be looked, and to explore further possibilities on the arabinogalactan and pectin epitopes and the competence acquisition for somatic embryogenesis in this species.
References
Akinloye AJ, Illoh HC, Olagoke AO (2010) Screening of some indigenous herbal dyes for use in plant histological staining. J For Res 21:81–84. doi:10.1007/s11676-010-0014-2
Albrecht C, Russinova E, Kemmerling B et al (2008) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol 148:611–619. doi:10.1104/pp.108.123216
Alemanno L, Ramos T, Gargandec A et al (2003) Localization and identication of phenolics compounds in Theobroma cacao L. somatic embryogenesis. Ann Bot 92:613–623. doi:10.1093/aob/mcg177
Anami S, Njuguna E, Coussens G et al (2013) Higher plant transformation: principles and molecular tools. Int J Dev Biol 57:483–494
Aan den Toorn M, Albrecht C, de Vries SC (2015) On the origin of SERKS: bioinformatics analysis of the somatic embryogenesis receptor kinases. Mol Plant 8:762–782. doi:10.1016/j.molp.2015.03.015
Almeida PP, Mezzomo N, Ferreira SRS (2012) Extraction of Mentha spicata L. volatile compounds: evaluation of process parameters and extract composition. Food Bioprocess Technol 5:548–559. doi: 10.1007/s11947-010-0356-y
Annadurai RS, Jayakumar V, Mugasimangalam RC et al (2012) Next generation sequencing and de novo transcriptome analysis of Costus pictus D. Don, a non-model plant with potent anti-diabetic properties. BMC Genomics 13:663. doi:10.1186/1471-2164-13-663
Bartley GE, Scolnik PA (1995) Plant carotenoids: pigments for photoprotection, visual attraction, and human health. Plant Cell 7:1027–1038. doi:10.1105/tpc.7.7.1027
Baudino S, Hansen S, Brettschneider R et al (2001) Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK family. Planta 213:1–10. doi:10.1007/s004250000471
Belhaj K, Chaparro-Garcia A, Kamoun S et al (2015) Editing plant genomes with CRISPR/Cas9. Curr Opin Biotechnol 32:76–84. doi:10.1016/j.copbio.2014.11.007
Bock R (2015) Engineering plastid genomes: methods, tools, and applications in basic research and biotechnology. Ann Rev Plant Biol 66:211–224. doi:10.1146/annurev-arplant-050213-040212
Bouvier F, Dogbo O, Camara B (2003) Biosynthesis of the food and cosmetic plant pigment bixin (annatto). Science 300:2089–2091. doi:10.1126/science.1085162
Brown PH, Hu H (1997) Does boron play only a structural role in the growing tissues of higher plants? Plant Soil 196:211–215. doi:10.1023/A:1004245823080
Bull SE, Owiti JA, Niklaus M et al (2009) Agrobacterium-mediated transformation of friable embryogenic calli and regeneration of transgenic cassava. Nat Protoc 4:1845–1854. doi:10.1038/nprot.2009.208
Cangahuala-Inocente GC, Steiner N, Santos M, Guerra MP (2004) Morphological analysis and histochemistry of Feijoa sellowiana somatic embryogenesis. Protoplasma 224:33–40. doi:10.1007/s00709-004-0055-5
Cangahuala-Inocente GC, Steiner N, Maldonado SB, Guerra MP (2009) Patterns of protein and carbohydrates accumulation during somatic embryogenesis of Acca sellowiana. Pesqui Agropecu Bras 44:217–224. doi:10.1590/S0100-204X2009000300001
Cárdenas-Conejo Y, Carballo-Uicab V, Lieberman M, Aguilar-Espinosa M, Comai L, Rivera-Madrid R (2015) De novo transcriptome sequencing in Bixa orellana to identify genes involved in methylerythritol phosphate, carotenoid and bixin biosynthesis. BMC Genomics 16:877. doi:10.1186/s12864-015-2065-4
Carvalho JFRP, Carvalho CR, Otoni WC (2005) Regeneração in vitro de urucum (Bixa orellana L.) a partir de diferentes tipos de explantes. Rev Árvore 29:887–895. doi:10.1590/S0100-67622005000600007
Corredoira E, Valladares S, Vieitez AM (2006) Morphohistological analysis of the origin and development of somatic embryos from leaves of mature Quercus robur. In Vitro Cell Dev Biol-Plant 42:525–533. doi:10.1079/IVP2006827
Cruz ACF, Pinheiro MVM, Xavier A et al (2015) In vitro regeneration of annatto (Bixa orellana L.) plantlets from nodal and internodal adult stem segments. Acta Hortic 1083:335–346. doi:10.17660/ActaHortic.2015.1083.42
Cruz ACF, Rocha DI, Iarema L et al (2014) In vitro organogenesis from root culture segments of Bixa orellana L. (Bixaceae). In Vitro Cell Dev Biol-Plant 50:76–83. doi:10.1007/s11627-013-9580-2
Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583. doi:10.1146/annurev.arplant.49.1.557
De-la-Peña C, Nic-Can GI, Galaz-Ávalos RM et al (2015) The role of chromatin modifications in somatic embryogenesis in plants. Front Plant Sci 6:635. doi:10.3389/fpls.2015.00635
Dias VM, Pilla V, Alves LP et al (2011) Optical characterization in annatto and commercial colorific. J Fluoresc 21:415–421. doi:10.1007/s10895-010-0730-1
D’Souza MC, Sharon M (2001) In vitro clonal propagation of annatto (Bixa orellana L.). In Vitro Cell Dev Biol-Plant 37:168–172. doi:10.1007/s11627-001-0029-7
Dufourmantel N, Pelissier B, Garçon F et al (2004) Generation of fertile transplastomic soybean. Plant Mol Biol 55:479–489. doi: 10.1007/s11103-004-0192-4
Elhiti M, Hebelstrup KH, Wang A et al (2013) Function of type–2 Arabidopsis hemoglobin in the auxin-mediated formation of embryogenic cells during morphogenesis. Plant J 74:946–958. doi:10.1111/tpj.12181
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org 74:201–228. doi:10.1023/A:1024033216561
Fehér A (2005) Why somatic plant cells start to form embryos? In: Mujid A., Samaj J. (eds) Somatic Embryogenesis. Plant Cell Monographs, vol 2. Springer, Berlin/Heidelberg, pp 85–101. doi:10.1007/7089_019
Fehér A (2008) The initiation phase of somatic embryogenesis: what we know and what we don’t. Acta Biol Szeged 52:53–56
Fehér A (2015) Somatic embryogenesis—stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849:385–402. doi:10.1016/j.bbagrm.2014.07.005
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265. doi:10.1016/j.plipres.2003.10.002
Giuliano G, Rosati C, Bramley PM (2003) To dye or not to dye: biochemistry of annatto unveiled. Trends Biotechnol 21:513–516. doi:10.1016/j.tibtech.2003.10.001
Hecht V, Vielle-Calzada JP, Hartog MV et al (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816. doi:10.1104/pp.010324
Jako C, Coutu C, Roewer I et al (2002) Probing carotenoid biosynthesis in developing seed coats of Bixa orellana (Bixaceae) through expressed sequence tag analysis. Plant Sci 163:141–145. doi:10.1016/S0168-9452(02)00083-3
Jariteh M, Ebrahimzadeh H, Niknam V et al (2015) Developmental changes of protein, proline and some antioxidant enzymes activities in somatic and zygotic embryos of Persian walnut (Juglans regia L.). Plant Cell Tiss Org 122:101–115. doi:10.1007/s11240-015-0753-z
Jin S, Zhang X, Liang S, Nie Y et al (2005) Factors affecting transformation efficiency of embryogenic callus of upland cotton (Gossypium hirsutum) with Agrobacterium tumefaciens. Plant Cell Tiss Org 81:229–237. doi:10.1007/s11240-004-5209-9
Kiokias S, Gordon MH (2003) Antioxidant properties of annatto carotenoids. Food Chem 83:523–529. doi:10.1016/S0308-8146(03)00148-1
Kreis W, Haug B, Yücesan B (2015) Somaclonal variation of cardenolide content in Heywood’s foxglove, a source for the antiviral cardenolide glucoevatromonoside, regenerated from permanent shoot culture and callus. In Vitro Cell Dev Biol-Plant 51:35–41. doi:10.1007/s11627-014-9642-0
Kumar S, Dhingra A, Daniell H (2004a) Stable transformation of the cotton plastid genome and maternal inheritance of transgenes. Plant Mol Biol 56:203–216. doi:10.1007/s11103-004-2907-y
Kumar S, Dhingra A, Daniell H (2004b) Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol 136:2843–2854. doi:10.1104/pp.104.045187
Kumar V, Ramakrishna A, Ravishankar GA (2007) Influence of different ethylene inhibitors on somatic embryogenesis and secondary embryogenesis from Coffea canephora P ex Fr. In Vitro Cell Dev Biol-Plant 43:602–607. doi:10.1007/s11627-007-9067-0
Kurczyńska EU, Potocka I, Dobrowolska I et al (2012) Cellular Markers for Somatic Embryogenesis. In: Sato, K-I (ed) Embryogenesis. InTech, Rijeka—Croatia, p 307–332
Louro RP, Santiago LJM (2016) Development of carotenoid storage cells in Bixa orellana L. seed arils. Protoplasma 253:77–86. doi:10.1007/s00709-015-0789-2
Mahdavi-Darvari F, Noor NM, Ismanizan I (2015) Epigenetic regulation and gene markers as signals of early somatic embryogenesis. Plant Cell Tiss Org 120:407–422. doi:10.1007/s11240-014-0615-0
Mala KS, Rao PP, Prabhavathy MB, Satyanarayana A (2015) Studies on application of annatto (Bixa orellana L.) dye formulations in dairy products. J Food Sci Technol 52:912–919. doi:10.1007/s13197-013-1038-3
Marcolino VA, Zanin GM, Durrant LR et al (2011) Interaction of curcumin and bixin with β-cyclodextrin: complexation methods, stability, and applications in food. J Agric Food Chem 59:3348–3357. doi:10.1021/jf104223k
Mardis ER (2008) The impact of next-generation sequencing technology on genetics. Trends Genet 24:133–141. doi:10.1016/j.tig.2007.12.007
Marioni JC, Mason CE, Mane SM et al (2008) RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res 18:1509–1517. doi:10.1101/gr.079558.108
Mercadante AZ, Pfander H (1998) Carotenoids from annatto: a review. Rec Res Dev Agric Food Chem 2:79–91
Mercadante AZ, Steck A, Pfander H (1997) Isolation and identification of new apocarotenoids from annatto (Bixa orellana) seeds. J Agric Food Chem 45:1050–1054. doi:10.1021/jf960412k
Mohammed A, Chiruvella KK, Namsa ND, Ghanta RG (2015) An efficient in vitro shoot regeneration from leaf petiolar explants and ex vitro rooting of Bixa orellana L.—a dye yielding plant. Physiol Mol Biol Plants 21:417–424. doi:10.1007/s12298-015-0297-z
Moura EF, Ventrella MC, Motoike SY et al (2008) Histological study of somatic embryogenesis induction on zygotic embryos of macaw palm (Acrocomia aculeata (Jacq.) Lodd. ex Martius). Plant Cell Tiss Org 95:175–184. doi:10.1007/s11240-008-9430-9
Moura EF, Ventrella MC, Motoike SY (2010) Anatomy, histochemistry and ultrastructure of seed and somatic embryo of Acrocomia aculeata (Arecaceae). Sci Agric 67:399–407. doi:10.1590/S0103-90162010000400004
Narváez JA, Canto Canché BB, Pérez PF, Madrid RR (2001) Differential expression of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) during flower and fruit development of Bixa orellana. J Plant Physiol 158:1471–1477. doi:10.1078/0176-1617-00614
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8:68–82. doi:10.1016/j.molp.2014.12.007
Nolan KE, Kurdyukov S, Rose RJ (2009) Expression of the SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. J Exp Bot 60:1759–1771. doi:10.1093/jxb/erp046
Nolan KE, Irwanto RR, Rose RJ (2003) Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol 133:218–230. doi:10.1104/pp.103.020917
Nyaboga EN, Njiru JM, Tripathi L (2015) Factors influencing somatic embryogenesis, regeneration, and Agrobacterium-mediated transformation of cassava (Manihot esculenta Crantz) cultivar TME14. Front Plant Sci 6:411. doi:10.3389/fpls.2015.00411
Paim Pinto DL, Almeida AMR, Rêgo MM et al (2011) Somatic embryogenesis from mature zygotic embryos of commercial passionfruit (Passiflora edulis Sims) genotypes. Plant Cell Tiss Org 107:521–530. doi:10.1007/s11240-011-0003-y
Paiva Neto VB, Carvalho CR, Otoni WC (2003/4) Mannose: a potential selection system for genetic transformation of annatto. Biol Plant 47:441–444. doi:10.1023/B:BIOP.0000023891.16785.fc
Paiva Neto VB, Botelho MN, Aguiar R, Silva EAM, Otoni WC (2003a) Somatic embryogenesis from immature zygotic embryos of annatto (Bixa orellana L.). In Vitro Cell Dev Biol-Plant 39:629–634. doi:10.1079/IVP2003465
Paiva Neto VB, Mota T, Otoni WC (2003b) Direct organogenesis from hypocotyl-derived explants of annatto (Bixa orellana). Plant Cell Tiss Org 75:159–167. doi:10.1023/A:1025063906822
Pandey DK, Singh AK, Chaudhary B (2012) Boron-mediated plant somatic embryogenesis: a provocative model. J Bot Article ID 375829. doi:10.1155/2012/37582:9
Parimalan R, Venugopalan A, Giridhar P, Ravishankar GA (2011) Somatic embryogenesis and Agrobacterium-mediated transformation in Bixa orellana L. Plant Cell Tiss Org 105:317–328
Parimalan R, Giridhar P, Gururaj HB, Ravishankar GA (2007) Organogenesis from cotyledon and hypocotyl-derived explants of japhara (Bixa orellana L.). Acta Bot Croatica 66:153–160. doi:10.1007/s11240-010-9870-x
Pathi KM, Tula S, Tuteja N (2013) High frequency regeneration via direct somatic embryogenesis and efficient Agrobacterium-mediated genetic transformation of tobacco. Plant Signal Behav 8:6. doi:10.4161/psb.24354
Peixoto PHP, Pimenta DS, Cambraia J (2007) Alterações morfológicas e acúmulo de compostos fenólicos em plantas de sorgo sob estresse de alumínio. Bragantia 66:17–25
Rahman A (2013) Auxin: a regulator of cold stress response. Physiol Plant 147:28–35. doi:10.1111/j.1399-3054.2012.01617.x
Ramamoorthy S, Doss FP, Kundu K et al (2010) Molecular characterization of bixin—an important industrial product. Ind Crops Prod 32:48–53. doi:10.1016/j.indcrop.2010.03.001
Ribas AF, Dechamp E, Champion A et al (2011) Agrobacterium-mediated genetic transformation of Coffea arabica (L.) is greatly enhanced by using established embryogenic callus cultures. BMC Plant Biol 11:92. doi:10.1186/1471-2229-11-92
Rivera-Madrid R, Burnell J, Aguilar-Espinosa M et al (2013) Control of carotenoid gene expression in Bixa orellana L. leaves treated with norflurazon. Plant Mol Biol Rep 31:1422–1432. doi:10.1007/s11105-013-0604-1
Rocha DI, Vieira LM, Tanaka FA et al (2012) Somatic embryogenesis of a wild passion fruit species Passiflora cincinnata Masters: histocytological and histochemical evidences. Protoplasma 249:747–758. doi:10.1007/s00709-011-0318-x
Rocha DI, Dornelas MC (2013) Molecular overview on plant somatic embryogenesis. CAB Reviews 8:1–17. doi:10.1079/PAVSNNR20138022
Rodríguez-Ávila NL, Narváez-Zapata JA, Aguilar-Espinosa ML, Rivera-Madrid R (2009) Full-length gene enrichment by using an optimized RNA isolation protocol in Bixa orellana recalcitrant tissues. Mol Biotechnol 42:84–90. doi:10.1007/s12033-008-9138-4
Rodríguez-Ávila NL, Narváez-Zapata JA, Ramírez-Benítez JE et al (2011a) Identification and expression pattern of a new carotenoid cleavage dioxygenase gene member from Bixa orellana. J Exp Bot 62:5385–5395. doi:10.1093/jxb/err201
Rodríguez-Ávila NL, Narváez-Zapata JA, Aguilar-Espinosa M, Rivera-Madrid R (2011b) Regulation of pigment-related genes during flower and fruit development of Bixa orellana. Plant Mol Biol Rep 29:43–50. doi:10.1007/s11105-010-0207-z
Rossoni-Júnior JV, Araújo GR, Pádua BC et al (2012) Annato extract and β-carotene modulate the production of reactive oxygen species/nitric oxide in neutrophils from diabetic rats. J Clin Biochem Nutr 50:177–183. doi:10.3164/jcbn.11-49
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Santos MO, Romano E, Yotoko KSC et al (2005) Characterisation of the cacao SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE (SERK) gene expressed during somatic embryogenesis. Plant Sci 168:723–729. doi:10.1016/j.plantsci.2004.10.004
Sasaki G, Katoh K, Hirose N et al (2007) Multiple receptor-like kinase cDNAs from liverwort Marchantia polymorpha and two charophycean green algae, Closterium ehrenbergii and Nitella axillaris: extensive gene duplications and gene shufflings in the early evolution of streptophytes. Gene 401:135–144. doi:10.1016/j.gene.2007.07.009
Schmidt EDL, Guzzo F, Toonen MAJ, de Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competente to form embryos. Development 124:2049–2062
Schwarz R, Dayhoff M (1979) Matrices for detecting distant relationships. In: Dayhoff M (ed) Atlas of protein sequences. National Biomedical Research Foundation, pp 353–358
Shiu S-H, Bleecker AB (2001a) Plant receptor-like kinases gene family: diversity, function, and signaling. Science’s STKE 113:1–13. doi:10.1126/stke.2001.113.re22
Shiu S-H, Bleecker AB (2001b) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci (USA) 98:10763–10768. doi:10.1073/pnas.181141598
Siril EA, Joseph N (2013) Micropropagation of annatto (Bixa orellana L.) from mature tree and assessment of genetic fidelity of micropropagated plants with RAPD markers. Physiol Mol Biol Plants 19:147–155. doi:10.1007/s12298-012-0150-6
Soares VLF, Rodrigues SM, Oliveira TM et al (2011) Unraveling new genes associated with seed development and metabolism in Bixa orellana L. by expressed sequence tag (EST) analysis. Mol Biol Rep 38:1329–1340. doi:10.1007/s11033-010-0234-8
Steiner N, Santa-Catarina C, Guerra MP et al (2012) A gymnosperm homolog of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE-1 (SERK1) is expressed during somatic embryogenesis. Plant Cell Tiss Org 109:41–50. doi:10.1007/s11240-011-0071-z
Steiner N, Farias-Soares F, Schmidt EC et al (2016) Toward establishing a morphological and ultrastructural characterization of proembryogenic masses and early somatic embryos of Araucaria angustifolia (Bert.) O. Kuntze. Protoplasma 253:487–501. doi:10.1007/s00709-015-0827-0
Sugimoto K (2015) Plant cell reprogramming as an adaptive strategy. J Plant Res 128:345–347. doi:10.1007/s10265-015-0718-7
Sugiyama M (2015) Historical review of research on plant cell dedifferentiation. J Plant Res 128:349–359. doi:10.1007/s10265-015-0706-y
Tamura K, Stecher G, Peterson D et al (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ (2007) Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci 12:245–252. doi:10.1016/j.tplants.2007.04.002
Von Aderkas P, Teyssier C, Charpentier JP et al (2015) Effect of light conditions on anatomical and biochemical aspects of somatic and zygotic embryos of hybrid larch (Larix × marschlinsii). Ann Bot 115:605–615. doi:10.1093/aob/mcu254
Yang Z, Li C, Wang Y et al (2014) GhAGL15s, preferentially expressed during somatic embryogenesis, promote embryogenic callus formation in cotton (Gossypium hirsutum L.). Mol Genet Genomics 289:873–883. doi: 10.1007/s00438-014-0856-y
Wang Q-M, Wang L (2012) An evolutionary view of plant tissue culture: somaclonal variation and selection. Plant Cell Rep 31:1535–1547. doi:10.1007/s00299-012-1281-5
Wang Z, Gerstein M, Snyder M (2009) RNA-seq: a revolutionary tool for transcriptomics. Nature 10:57–63. doi:10.1038/nrg2484
Zaldívar-Cruz JM, Ballina-Gómez H, Guerrero-Rodríguez C et al (2003) Agrobacterium-mediated transient transformation of annatto (Bixa orellana) hypocotyls with the gus reporter gene. Plant Cell Tiss Org 73:281–284. doi:10.1023/A:1023037108705
Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Shmitt B (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron J Biotechnol 13:1–9. doi:10.2225/vol13-issue1-fulltext-4
Acknowledgments
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Brazil), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (Belo Horizonte, MG, Brazil) and Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) (Salvador, BA, Brazil). The company Chr. Hansen Brasil (Valinhos, SP, Brazil) is acknowledged for kindly supplying annatto seeds to our laboratory.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
de Matos, E.M. et al. (2016). Somatic Embryogenesis in Annatto (Bixa orellana L.). In: Loyola-Vargas, V., Ochoa-Alejo, N. (eds) Somatic Embryogenesis: Fundamental Aspects and Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-33705-0_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-33705-0_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33704-3
Online ISBN: 978-3-319-33705-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)