Abstract
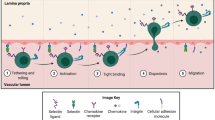
Intestinal inflammation is highly dependent on the recruitment of white blood cells out from the circulation to the mucosal immune system of the gut. Diapedesis and transmigration of activated lymphocytes to the site of inflammation is tightly regulated by a complex interaction between integrins on the leukocyte surface and cell adhesion molecules on microvascular endothelial cells of post-capillary venules (Fig. 36.1). Already in the early nineties, increased expression of vascular cell adhesion molecules was demonstrated in inflammatory bowel disease (IBD) [1–3]. This prompted investigation of anti-adhesion therapy as a therapeutic strategy in IBD. In 1993, Podolsky and coworkers showed that a monoclonal antibody targeting the leukocyte α4 integrin was effective in the treatment of colitis in the cotton-top tamarin [4]. Some years later, Hesterberg et al. found similarly beneficial effects in this model with an antibody against the gut-specific integrin dimer α4β7 [5]. These preclinical studies opened the gate for clinical pilot trials with anti-adhesion agents in IBD. Following a long developmental process, anti-adhesion molecules have now entered the therapeutic armamentarium of IBD. In this chapter, we summarize and discuss the evidence for the use of natalizumab, vedolizumab, and anti-MadCAM in CD.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Inflammatory Bowel Disease
- Progressive Multifocal Leukoencephalopathy
- Clinical Remission
- Progressive Multifocal Leukoencephalopathy
- Fecal Calprotectin
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Intestinal inflammation is highly dependent on the recruitment of white blood cells out from the circulation to the mucosal immune system of the gut. Diapedesis and transmigration of activated lymphocytes to the site of inflammation is tightly regulated by a complex interaction between integrins on the leukocyte surface and cell adhesion molecules on microvascular endothelial cells of post-capillary venules (Fig. 36.1) . Already in the early nineties, increased expression of vascular cell adhesion molecules was demonstrated in inflammatory bowel disease (IBD) [1–3]. This prompted investigation of anti-adhesion therapy as a therapeutic strategy in IBD. In 1993, Podolsky and coworkers showed that a monoclonal antibody targeting the leukocyte α4 integrin was effective in the treatment of colitis in the cotton-top tamarin [4]. Some years later, Hesterberg et al. found similarly beneficial effects in this model with an antibody against the gut-specific integrin dimer α4β7 [5]. These preclinical studies opened the gate for clinical pilot trials with anti-adhesion agents in IBD. Following a long developmental process, anti-adhesion molecules have now entered the therapeutic armamentarium of IBD. In this chapter, we summarize and discuss the evidence for the use of natalizumab, vedolizumab, and anti-MadCAM in CD.
Integrin heterodimers α4β1 and α4β7 on CCR9-expressing gut-homing T-lymphocytes form a stable binding complex with cell adhesion molecules (respectively VCAM-1 and MAdCAM-1) on the endothelium of postcapillary venules in the gut, allowing them to diapedesize into the lamina propria and feed the inflammatory process in IBD. Natalizumab is an anti-α4 antibody blocking both the α4β1-VCAM-1 interaction and the α4β7-MAdCAM-1 interaction. Vedolizumab and anti-MAdCAM-1 only inhibit the gut-specific α4β7-MAdCAM-1 interaction by respectively blocking the α4β7 dimer and MAdCAM-1
Natalizumab
Natalizumab (Perrigo Company plc, Dublin, Ireland) is a recombinant humanized antibody (containing 5 % mouse-derived protein) against the human a4 integrin. In 2001, a first small-scale randomized, double-blind clinical trial was performed comparing a single dose of 3 mg/kg intravenous natalizumab with placebo in active CD patients [6]. Although the primary endpoint (change in the Crohn’s disease activity index at week 2) was not met in this study, the results were promising enough to warrant a large-scale multicenter phase 2 trial with natalizumab in moderate-to-severe CD, published in 2003 [7]. Anti-TNF naïve CD patients were randomly assigned to four different treatment regimens (two IV infusions of placebo or one infusion natalizumab at 3 mg/kg and one infusion of placebo or two infusions of 3 mg/kg natalizumab or two infusions of 6 mg/kg natalizumab). The primary endpoint was clinical remission (CDAI < 150) at week 6. Patients receiving two infusions of 3 mg/kg natalizumab had a significantly higher rate of clinical remission at weeks 4, 6, 8, and 12 compared to the placebo group (27 % versus 44 % respectively at week 6) [7]. A few years later, these results were confirmed in the phase 3 “Efficacy of Natalizumab as Active Crohn’s Therapy” (ENACT-1) [8]. In contrast to the previous studies, previous use of anti-TNF agents was allowed and natalizumab was given at a fixed dose of 300 mg at week 0, 4, and 8. The primary endpoint was clinical response (drop in CDAI of at least 70 points) at week 10 and was not reached in this study [8]. Patients who had a response both a week 10 and week 12 were eligible for the maintenance trial (ENACT-2) in which patients were re-randomized 1:1 to placebo or 300 mg of natalizumab every 4 weeks [8]. The primary endpoint in ENACT-2 was sustained response (with loss of response being defined by an increase in the CDAI score of at least 70 points after week 12 and by an absolute score of at least 220 or the need for intervention after week 12) at week 36 which was observed in 61 % of patients on natalizumab and 28 % on placebo (P = 0.003) [8]. However, ENACT-2 was prematurely halted by the manufacturer because of three cases of JC virus-related progressive multifocal leukoencephalopathy (PML), which was considered to be associated with the study drug All published as case reports. PML was caused by reactivation of the ubiquitous JC virus in combination with impaired immune surveillance in the central nervous system due to blockade of α4 [9–11]. Retrospective analysis showed that an additional patient had died from JC virus-related PML during an open-label extension study of ENACT-2 [8]. As a result of this rare but life-threatening adverse event the European Medicine Agency (EMA) concluded that the benefits of natalizumab in the treatment of CD did not outweigh the risk [12] and refused marketing authorization in Europe for CD although the agent was approved for multiple sclerosis under the name TysabriR. A later second induction trial, “Efficacy of Natalizumab in Crohn’s Disease Response and Remission” (ENCORE), showed that natalizumab was also effective as an induction agent in patients with moderately to severely active CD with an elevated serum CRP as an objective marker of inflammation at baseline with the primary endpoint being induction of response (>70-point decrease from baseline in the Crohn’s Disease Activity Index score at week 8), sustained through week 12 [13]. In 2008 the FDA approved natalizumab (TysabriR) for the treatment of moderate-to-severe Crohn’s disease not responding to, or not tolerating, conventional therapies for CD including inhibitors of TNF-α, albeit under a strict patient safety monitoring program [14, 15]. In the meantime, risk factors for PML have been identified: JCV seropositivity (in approximately 70 % of patients), previous exposure to immunosuppressive drugs, and exposure duration >2 years [16] leading to the recommendation to use the drug without concomitant immunosuppression However, the future of anti-integrin therapy was considered to lie in the development of more selective blockade of the integrin β7 or the combination α4β7 which would ensure higher gut-selectivity.
Vedolizumab
Vedolizumab is a recombinant humanized IgG1 monoclonal antibody targeting the alpha4beta7 integrin on leukocytes. In contrast to natalizumab, vedolizumab only inhibits adhesion of leukocytes to the relatively gut-selective MAdCAM-1 and not to VCAM-1, which is also expressed in the brain endothelium [17].
In 2008, a dose-finding trial with the anti-α4β7 antibody MLN0002 (Millennium, Boston, MA) in 185 moderately active anti-TNF-naïve CD patients [18] did not meet the primary endpoint (clinical response CDAI70 at 8 weeks although the results suggested a dose-dependent clinical benefit of MLN0002 therapy for the induction of remission, necessitating a larger clinical trial [18]. After humanizing the monoclonal antibody to “vedolizumab,” a large phase 3 program was launched. In the GEMINI-2 study, 368 patients with moderately to severely active CD and at least one objective sign of active inflammation (significant endoscopic lesions, elevated CRP or elevated fecal calprotectin + positive findings on imaging) were randomly assigned to treatment with vedolizumab (300 mg IV at week 0 and week 2) or placebo at a 3:2 ratio [19]. The primary endpoints were clinical remission (CDAI < 150) and clinical response (CDAI drop of at least 100 points) as early as at week 6. In contrast to the previous trial, most of the patients were anti-TNF-experienced and concomitant use of corticosteroids and/or immunomodulators (in non-US patients) were allowed at stable dose. In addition, a significant proportion of patients had fistulizing disease and/or previous surgery for their CD. Patients receiving vedolizumab were twice as likely to be in clinical remission at week 6 as compared to patients who had received placebo, although the absolute numbers remained somewhat disappointing (14.5 % versus 6.8 % respectively) [19]. There was no statistically significant difference between vedolizumab- and placebo-treated CD patients with regard to the CDAI-100 response (one of the two primary endpoints) and there was surprisingly little effect of the treatment on serum CRP concentrations [19]. A later study (GEMINI 3) specifically investigated the efficacy of vedolizumab as an induction agent in CD patients with previous anti-TNF failure [20]. The primary efficacy outcome was clinical remission at week 6 (!). Again, vedolizumab treatment was not superior to placebo. However, these relatively poor “early” results may be explained by the slow mode of action, given the observation that vedolizumab significantly increased clinical response and remission rates beyond [20].
Also in GEMINI 2, 747 additional CD patients were treated with open label vedolizumab to in a feeder study for the maintenance study, in which patients with a clinical response (CDAI70) were re-randomized to placebo, vedolizumab 300 mg every 4 weeks or vedolizumab 300 mg every 8 weeks [19]. The primary endpoint was clinical remission at week 52. The effects of vedolizumab in this maintenance trial were quite robust, with 39 % of the patients in clinical remission on vedolizumab compared to 21.6 % on placebo and glucocorticoid-free remission in approximately one third of the vedolizumab-treated patients, irrespective of the dose interval (4 weeks or 8 weeks) [19]. The safety profile of vedolizumab was comparable to placebo and no single case of PML has been observed [19]. As a result, in 2014 both the FDA and the EMA approved vedolizumab (EntyvioR) for the treatment of Crohn’s disease (and ulcerative colitis) that is insufficiently controlled by conventional treatment and/or anti-TNF agents [20, 21]. Further data on mucosal healing, effects on fistula, postoperative recurrence, and pouchitis warrant further dedicated trials.
Anti-MAdCAM-1 Antibodies
The anti-MAdCAM monoclonal antibody PF-0547659 was investigated in a phase-2 dose-finding induction study (OPERA-1) in moderate—severe CD patients intolerant or refractory to anti-TNF and/or immunosuppressant therapy [22]. All patients had objective evidence of active disease (elevated (hs) CRP and mucosal ulcerations on endoscopy) and the primary efficacy parameter was a CDAI-70 response either by week 8 or week 12. Although the active treatment arms, in contrast to the placebo arm, were associated with increased circulating α4β7+ central memory T cells as a clear biological signal of the inhibitory effect of the active agent on MAdCAM-1, the primary endpoint was not met, most likely due to an unusually high placebo-response rate (41 % and 44 % at week 8 and 12, respectively) [22]. However, sub analysis revealed that a higher treatment effect was seen in CD patients with high hsCRP (>7.5 mg/dl) at baseline, with significantly more patients in the treatment arms being in remission at week 8 [22]. In addition, the safety profile was very reassuring. In a open-label induction study (Tosca) immune surveillance in the CNS was studied with repeated lumbar punctures. An induction course of anti-MAdCAM MAb did not affect the cellular determinants of immune surveillance in the central nervous system [22, 23]. In summary, the results with anti-MAdCAM for Crohn’s disease are encouraging. The decision towards further development will depend on the results of the maintenance phase Opera-2 and the outcome of the UC study Turandot (Table 36.1).
The Place Of Anti-adhesion Therapy in the Treatment Algorithm of Crohn’s Disease
Currently, anti-adhesion molecules are being positioned as second line biologics (after anti-TNF) in the therapeutic armamentarium for CD (and IBD in general) in most jurisdictions. The phase 3 trials , however, suggest superior outcomes when the agent is given to patients who are naïve to anti-TNF agents and the mode of action suggests potentially better effects in earlier disease stages. This warrants further investigation and perhaps a head-to-head comparison with anti-TNF agents.
The question is indeed how we could implement this new class of drugs in the most effective way. Based on the results of the completed trials, some general conclusions can be drawn. Firstly, unlike most anti-TNF agents , the drugs seem to have a rather slow onset mode of action in CD [6–8, 13, 18, 19, 22], possibly because of the more pronounced transmural inflammatory infiltrate as compared to UC [24–26], where the inflammation is limited to the mucosa. For daily clinical practice, this means that anti-adhesion monotherapy may not be the ideal monotherapy in CD patients with severe disease that needs rapid remission. Combination with stronger “induction agents ” such as corticosteroids and perhaps anti-TNF agents appears attractive. On the other hand, the integrin-inhibitors were shown to very effective maintenance drugs, with response and remission rates at least as high as with anti-TNF agents [8, 19, 27–30]. Future studies including real “strategy studies” will have to address where this novel class of biological should be positioned in the treatment algorithm of CD. Thus far, only one head-to-head trial is running, comparing vedolizumab IV with adalimumab SC in biological-naïve UC patients [31].
Is there still a place for natalizumab with the advent of the gut-selective integrin-inhibitor vedolizumab? In a recent editorial by Scott and Osterman in Clinical Gastroenterology and Hepatology, the authors state that natalizumab may remain a good option for patients that are JCV antibody negative (roughly one third of the patients) as there has never been a PML case described in this patient subgroup and seroconversion rates also seem to be low [32]. Nonetheless, natalizumab remains only registered without concomitant use of immunosuppressants.
Future studies will also have to address the immunogenicity of the anti-adhesion antibodies and whether combination therapy with immunosuppressant therapy is superior to monotherapy, as it is the case for infliximab [33].
The potential registration/indication of anti-Madcam antibodies in CD will depend on the maintenance phase 2 results and the phase 3 data if such studies will be set up in the future.
In summary, anti-adhesion antibodies are the second group of biologicals for the treatment of IBD. For CD, they seem to be slow-acting for induction but very effective for maintenance treatment. The advent of this new therapeutic option opens a completely new era of clinical trials in which therapeutic strategies will be compared in order to develop the best care for the patients.
References
Schuermann GM, Aber-Bishop AE, Facer P, Lee JC, Rampton DS, Doré CJ, et al. Altered expression of cell adhesion molecules in uninvolved gut in inflammatory bowel disease. Clin Exp Immunol. 1993;94:341–7.
Mishra L, Mishra BB, Harris M, Bayless TM, Muchmore AV. In vitro cell aggregation and cell adhesion molecules in Crohn’s disease. Gastroenterology. 1993;104:772–9.
Koizumi M, King N, Lobb R, Benjamin C, Podolsky DK. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992;103:840–7.
Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B, Sehgal P, et al. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest. 1993;92:372–80.
Hesterberg PE, Winsor-Hines D, Briskin MJ, Soler-Ferran D, Merrill C, Mackay CR, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996;111:1373–80.
Gordon FH, Lai CW, Hamilton MI, Allison MC, Srivastava ED, Fouweather MG, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology. 2001;121:268–74.
Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, et al. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348:24–32.
Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–25.
Kleinschmidt BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74.
Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81.
Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–8.
European Medicines Agency. Refusal CHMP assessment report for natalizumab Elan Pharma. Doc. Ref: EMEA/CHMP/8203/2008.
Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672–83.
Honey K. The comeback kid: TYSABRI now FDA approved for Crohn disease. J Clin Invest. 2008;118:825–6.
Biogen Idec. Tysabri risk evaluation and mitigation strategy (REMS). http://www.fda.gov/downloads/Drugs/Drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/.
Lichtenstein GR, Hanauer SB, Sandborn WJ. Risk of biologic therapy-associated progressive multifocal leukoencephalopathy: use of the JC virus antibody assay in the treatment of moderate-to-severe Crohn’s disease. Gastroenterol Hepatol (NY). 2012;8:1–20.
Tilg H, Kaser A. Vedolizumab, a humanized mAb against the α4β7 integrin for the potential treatment of ulcerative colitis and Crohn’s disease. Curr Opin Investig Drugs. 2010;11:1295–304.
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–7.
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21.
US Food and Drug Administration. BLA approval (BLA 125476/0). 2014. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/125476Orig1s000ltr.pdf.
Takeda. Takeda receives European commission marketing authorisation for Entyvio (vedolizumab) for the treatment of ulcerative colitis and Crohn’s disease (media release); 28 May 2014. http://www.takeda.com/news/2014/20140528_6590.html.
D'Haens G, Lee S, Tarabar D, Louis E, Klopocka M, Park DI, et al. Anti-MAdCAM-1 antibody (PF-00547659) for active refractory Crohn’s disease: results of the OPERA study. ECCO Congress Barcelona 2015;OP022.
D'Haens G, Vermeire S, Cataldi F, Vogelsang H, Allez M, Desreumaux P, et al. Anti-MAdCAM monoclonal antibody PF-00547659 does not affect immune surveillance in the central nervous system of anti-TNF and immunosuppressant experienced Crohn’s disease patients who are anti-TNF inadequate responders: results from the TOSCA study. ECCO Congress Barcelona 2015;OP007.
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–507.
Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710.
Vermeire S, Sandborn W, Danese S, Hebuterne X, Salzberg B, Klopocka M, et al. TURANDOT: a randomized, multicenter double-blind, placebo-controlled study of the safety and efficacy of Anti-MAdCAM Antibody PF-00547659 (PF) in patients with moderate to severe ulcerative colitis (UC). ECCO Congress Barcelona 2015;OP021.
Hanauer SB, Feagan B, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9.
Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–9.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–50.
Takeda. An Efficacy and Safety Study of Vedolizumab Intravenous (IV) Compared to Adalimumab Subcutaneous (SC) in Participants With Ulcerative Colitis. https://clinicaltrials.gov/ct2/show/NCT02497469.
Scott FI, Osterman MT. Natalizumab for Crohn’s disease: down but not out. Clin Gastroenterol Hepatol. 2015:S1542-3565(15)00979-9.
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Hindryckx, P., D’Haens, G. (2017). Biological Therapy of Crohn’s Disease: Natalizumab, Vedolizumab, and Anti-MadCAM. In: Baumgart, D. (eds) Crohn's Disease and Ulcerative Colitis. Springer, Cham. https://doi.org/10.1007/978-3-319-33703-6_36
Download citation
DOI: https://doi.org/10.1007/978-3-319-33703-6_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33701-2
Online ISBN: 978-3-319-33703-6
eBook Packages: MedicineMedicine (R0)