Abstract
Metastatic renal cell carcinoma (RCC) remains largely incurable. Up to 30% of patients show metastasis at the time of the initial diagnosis. Prognostic criteria developed by the IMDC (International Metastatic Renal Cell Carcinoma Database Consortium) and MSKCC (Memorial Sloan Kettering Cancer Center) are used to classify patients based on certain pretreatment factors. The prognosis of patients with metastatic disease varies depending on these risk factors. Anti-angiogenic agents targeting the vascular endothelial growth factor (VEGF) and its receptors are standard treatments based on improved clinical outcomes in randomized phase III trials. Standard of care therapies now include multitargeted tyrosine kinase inhibitors (TKIs) such as sunitinib, axitinib, pazopanib, and cabozantinib, as well as the mTOR inhibitors temsirolimus and everolimus.
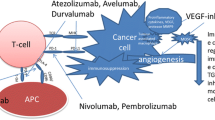
Tumor-associated PD-L1 expression has been detected in RCC and is associated with a worse prognosis. Immune checkpoint inhibitors such as the PD-1 inhibitor nivolumab have shown promising results in treatment of the metastatic disease. Future developments including novel combinations and attempts to find the optimal position of immunotherapy in the disease pathway are subject of ongoing clinical trials.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Metastatic renal cell carcinoma (RCC)
- Anti-angiogenics
- Vascular endothelial growth factor (VEGF)
- Tyrosine kinase inhibitors (TKIs)
- mTOR inhibitors
- Immune checkpoint inhibitors
Introduction
Renal cell cancer represents about 3% of all cancers and is largely incurable. In the European Union, there were approximately 84,400 new cases of RCC and 34,700 kidney cancer-related deaths in 2012 (Ferlay et al. 2013). There is a 1.5:1 male predominance, with a peak incidence between 60 and 70 years. Etiological factors include smoking, obesity, and hypertension (Bergström et al. 2001).
The number of incidentally diagnosed RCCs has increased due to increased detection of tumors by ultrasound as well as magnetic resonance imaging (MRI) and computed tomography (CT). These tumors are usually smaller and of lower stage (Tsui et al. 2000).
Renal cell carcinomas comprise a broad spectrum of histopathological entities. There are three main RCC types: clear cell (ccRCC), papillary (pRCC – type I and II), and chromophobe (chRCC). The RCC type classification has been confirmed by cytogenetic and genetic analyses (Moch et al. 2016). Other forms of kidney cancer tumors constitute the remaining 10–15% of renal cortical tumors, such as the very aggressive renal medullary carcinoma (<0.5% of all RCCs), acquired cystic disease-associated RCC (4% of patients), papillary adenoma, angiomyolipoma, renal oncocytoma, cystic renal tumors, sarcomatoid variants of RCC, and carcinoma of the collecting ducts of Bellini.
Up to 30% of patients show metastasis at the time of the initial diagnosis (Therasse et al. 2000). Patients with metastatic disease have a poor prognosis, with a 5-year survival rate of less than 20% (Itsumi and Tatsugami 2010). Recurrence occurs in approximately 40% of patients after treatment of a localized tumor. High levels of serum lactate dehydrogenase, low hemoglobin level, and high corrected level of serum calcium are the prognostic markers for metastatic RCC (Fig. 1). The average survival of patients with advanced RCC is approximately 12 months.
Angiogenesis and Anti-angiogenics
Drugs targeting angiogenesis are the primary treatment option for such patients. Angiogenesis is the physiological process of the growth of new blood vessels from preexisting blood vessels (Greenblatt and Shubik 1968). Angiogenesis is an important factor for tumor growth and metastasis in humans. Inactivation of the von Hippel-Lindau (VHL) gene is the most common genetic change present in clear cell RCC. In the absence of VHL, hypoxia-inducible factor (HIF) accumulates, leading to the production of several growth factors, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF). One of the most important roles of hypoxia-inducible factor 1 α (HIF-1 α) in cancer is to induce angiogenesis through the synthesis of angiogenesis-related proteins (Kim and Kaelin 2004). HIF-1 α plays an important role in regulating cell cycle and apoptosis as well. The activity of these factors is associated with oncogenesis, growth, and the metastatic potential of RCC cells. Angiogenesis is a process that involves the formation of new blood vessels from the existing vasculature. In addition to its role in tumor growth, angiogenesis is an important step in tumor proliferation and metastasis that offers a route for tumor cells to spread to organs via the bloodstream.
Hypoxia-inducible factor (HIF) is a transcription factor that responds to reduced intracellular oxygen concentration. In the hypoxic condition, HIF accumulates in the cell and is transported to the nucleus where it induces the expression of a wide variety of target gene products such as growth factors, e.g., VEGF, fibroblast growth factor (FGF), transforming growth factor (TGF), etc. These proteins in turn activate different signaling pathways, including PLCγ, PI3K, Smad, Src, etc., so that endothelial cell proliferation, vascular permeability, and cell migration are increased. Extracellular matrix proteases induce tissue matrix remodeling, and new tube formation occurs with the participation of the migrated endothelial cells. Various cytokines play key roles in the process. In addition to hypoxia, the PI3K and Ras pathways can also increase HIF expression by increasing HIF translation (Adams and Alitalo 2007). The growth of any tumor and its metastasis depend on the development of neovasculature in and around the tumor. Angiogenesis facilitates tumor growth progression by supplying adequate oxygen and nutrition to the tumor cells through several interrelated steps. The mechanism regulating angiogenesis is tissue specific (Hanahan et al. 1996). Angiogenic phenotype is regulated by the differential expression of growth factors and cytokines within the microenvironment of the organ.
RCC is one of the most vascular of the solid tumors, which suggests a prominent role for angiogenesis in the pathogenesis of RCC (Bard et al. 1986). VEGF is known as vascular permeability factor and stimulates endothelial cell proliferation in vitro and has got angiogenic activity in vivo. The second secreted angiogenic factor with a role in angiogenesis in RCC is PIGF (Maglione et al. 1993). Elevated VEGF expression is involved in the hypervascularity of RCC and plays an important role in determining the size, stage, and grade of carcinoma. VEGF, PIGF, and bFGF work together to increase angiogenesis in RCC; therefore they can be used as tumor markers, especially in the early stage of the disease (Atsushi et al. 1994).
Like many solid neoplasms, renal tumors are frequently characterized by hypoxic conditions due to local imbalance between oxygen (O2) supply and consumption. Indeed, hypoxia and compensatory hyperactivation of angiogenesis are thought to be particularly important in RCC compared to other tumor types, given the highly vascularized nature of kidney tumors and the specific association of mutation in von Hippel-Lindau (VHL) gene with onset of RCC. Hypoxic signaling is mediated by the hypoxia-inducible factors (HIFs), which regulate the expression of over 200 genes involved in crucial pathways related to tumorigenesis including angiogenesis, invasion, and mitogenesis. In hypoxic RCC tumors, in the absence of VHL, HIF-α proteins remain constitutively expressed, thereby inducing vascular endothelial growth factor (VEGF) and other HIF targets. Increased expression of many of the HIF target genes is implicated in promoting cancer, inducing both changes within the tumor (cell-intrinsic) and changes in the growth of adjacent endothelial cells to promote blood vessel growth. The expression level of VEGF in RCC is known to strongly correlate with microvessel density, a measure of the degree of angiogenesis. A key step in angiogenesis is the upregulation of growth factor receptors on endothelial cells such as vascular endothelial growth factor receptors (VEGFR) and platelet-derived growth factor receptors (Bianconi et al. 2012). As in many other tumors, targeting angiogenesis improves patients’ outcome. Anti-angiogenic drugs targeting the VEGF pathway with proven benefit in RCC include inhibitors of VEGFRs sunitinib, sorafenib, pazopanib, axitinib, cabozantinib, and bevacizumab.
The family of VEGF and VEGFR is a very complex one. The VEGF family members are secreted, dimeric glycoproteins of 40 kDa, consisting of five members, VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor, and binding to specific receptors. The VEGF gene has several alternatively spliced isoforms, and the regulation of expression might differ between normal and tumor tissue. To find these differences, the attention focused on the expression of single-nucleotide polymorphisms (SNP), as it was the case in other carcinomas (Scartozzi et al. 2014). Interestingly, as all identified polymorphisms in VEGF are not in the coding region, alternative mechanisms for their role in gene expression have been proposed. Although many transcription factors bind to the promoter regions of VEGF, none occurs at the common polymorphic sites associated with VEGF expression. Nevertheless, SNPs have been reported to cause changes in VEGF expression levels (Pages and Puyssegur 2005). The SNPs in the VEGF and VEGFR genes have been also correlated with tumor neoangiogenesis through different biological mechanisms.
Sunitinib, pazopanib, and five other agents have been approved by the Food and Drug Administration for the treatment of clear cell, metastatic renal cell carcinoma. Among the tyrosine kinase inhibitors, pazopanib and sunitinib are first-line treatment options.
TKI (Tyrosine Kinase Inhibitors)
Sunitinib is an oral tyrosine kinase (TK) inhibitor with antitumor and anti-angiogenic activity. First-line monotherapy with sunitinib showed significantly longer PFS compared with IFN-α. Overall survival was greater in patients treated with sunitinib (26.4) versus INF-α (21.8 months) despite crossover (Motzer et al. 2009). Sunitinib as second-line monotherapy after cytokines in patients with metastatic renal cell carcinoma demonstrated a partial response in 34–40% and stable disease at >3 months in 27–29% of patients (Motzer et al. 2006). Sunitinib 50 mg/day (4 weeks on/2 weeks off) was compared in the EFFECT trial with continuous uninterrupted sunitinib 37.5 mg/day in patients with clear cell advanced RCC. Median time to progression with sunitinib 50 mg was numerically longer than the 37.5 mg arm (9.9 months vs. 7.1 months). There were no significant differences in overall survival. Toxicity was comparable in both arms. Because of the nonsignificant, but numerically longer time to progression with the standard 50 mg dosage, the authors recommended using this regimen (Motzer et al. 2012). Alternate scheduling of sunitinib (2 weeks on/1 week off) can be used to manage toxicity.
Pazopanib is an oral angiogenesis inhibitor targeting vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and c-Kit. The daily dose is 800 mg. In a trial of pazopanib versus placebo in treatment-naïve metastatic RCC patients and cytokine-treated patients, a significant improvement in progression-free survival and tumor response was observed (Sternberg et al. 2010). Median progression-free survival (PFS) with pazopanib compared with placebo was 9.2 versus 4.2 months in the overall study population, 11.1 versus 2.8 months for the treatment-naïve subpopulation, 7.4 versus 4.2 months for the cytokine-pretreated subpopulation. The COMPARZ trial, which compared pazopanib with sunitinib, established pazopanib as another first-line option. It showed that pazopanib was not associated with significantly worse PFS or overall survival compared to sunitinib. The two drugs had different toxicity profiles, and quality of life was better with pazopanib (Motzer et al. 2013a). The study was limited due to the fact that intermittent therapy (sunitinib) was compared with continuous therapy (pazopanib).
Axitinib is an oral selective second-generation inhibitor of VEGFR1, VEGFR2, and VEGFR3. Axitinib was first evaluated as second-line treatment. The daily dosage is 10 mg, to be taken as 5 mg twice per day. The AXIS trial compared axitinib to sorafenib in patients with previously failed cytokine treatment or targeted agents. The overall median PFS was greater for axitinib than sorafenib. The difference in PFS was greatest in patients in whom cytokine treatment had failed (Rini et al. 2011). In a randomized phase III trial of axitinib versus sorafenib in first-line treatment-naïve clear cell metastatic RCC, a significant difference in median PFS between the treatment groups was not demonstrated (Hutson et al. 2013). As a result of this study, axitinib is not approved for first-line therapy.
Sorafenib is an oral multi-kinase inhibitor. The recommended daily dose is 800 mg. Sorafenib improved progression-free survival in a trial which compared sorafenib and placebo after failure of prior systemic immunotherapy or in patients unfit for immunotherapy (Escudier et al. 2007a). A number of studies have used sorafenib as the control arm in sunitinib-refractory disease versus axitinib, dovitinib, and temsirolimus. None showed superior survival for the study drug compared to sorafenib.
Cabozantinib is an oral inhibitor of TK, including VEGF, and receptor tyrosine kinases MET and AXL. The recommended daily dose is 60 mg. A randomized phase III trial (METEOR) investigated cabozantinib versus everolimus in 658 patients with clear cell RCC failing one or more VEGF-targeted therapies. Cabozantinib delayed PFS compared to everolimus in VEGF-targeted therapy refractory disease by 42%. The median PFS for cabozantinib was 7.4 months versus 3.8 months for everolimus. The median OS was 21.4 months with cabozantinib and 16.5 months with everolimus in VEGF-resistant RCC. Grade 3 or 4 adverse events were reported in 74% with cabozantinib and 65% with everolimus and were managed with dose reductions. Discontinuation due to toxicity was not significantly different for the two drugs (Choueiri et al. 2015).
Lenvatinib is an oral multitarget TKI of VEGFR1, VEGFR2, and VEGFR3, with inhibitory activity against fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3, and FGFR4); platelet growth factor receptor α (PDGFRα), rearranged during transfection (RET); and receptor for stem cell factor (KIT). The recommended daily dose is 24 mg.
Tivozanib is a potent and selective TKI of VEGFR1, VEGFR2, and VEGFR3 and was compared in a phase III trial with sorafenib as initial targeted therapy in patients with mRCC (Motzer et al. 2013b). Tivozanib was approved by the European Medicines Agency in frontline mRCC. It can therefore be prescribed in the European Union. The recommended daily dose is 1.340 μg (3 weeks on/1 week off). The Panel of the European Urological Association feels that it remains an inferior option as compared to other TKIs in this setting without further randomized data; therefore other agents should be used in preference.
Side Effects of Tyrosine Kinase Inhibitors (TKI)
Treatment with anti-VEGFR tyrosine kinase inhibitors is limited by its tolerability, including skin toxicity, which has resulted in rates of discontinuation in some cases exceeding that of conventional cytotoxic chemotherapy (Prasad et al. 2014).
Dermatologic side effects include rash, alopecia, depigmentation, pruritus, xerosis, acneiform rashes, and mucositis (Kamba and McDonald 2007). For most patients the most troublesome cutaneous side effect is a hand-foot skin reaction (HFSR), which is characterized by painful, edematous, erythematous, and keratotic lesions on acral surfaces, particularly weight-bearing sites, usually occurring 1–6 weeks after therapy is initiated (Fischer et al. 2013). Acral dysesthesia and paresthesia commonly precede the lesions (Porta et al. 2007).
HFSR occurring with anti-VEGFR TKIs can be distinguished clinically from hand-foot syndrome (HFS) associated with “cytotoxic” chemotherapeutic agents (Janusch et al. 2006). While HFSR produces localized, hyperkeratotic plaques on acral sites (Yang et al. 2008), HFS is marked by symmetric, desquamative erythema and edema not typically extending beyond volar and plantar surfaces (Lacouture et al. 2008). The mechanism of the HFSR is still poorly understood (Lipworth et al. 2009).
Besides dose reduction and drug discontinuation of oral agents, there are limited treatment options such as topical anti-inflammatory medications which include corticosteroid creams and topical pain relievers, such as lidocaine. These are used as a cream or a patch over painful areas in the palms and soles. There are also useful topical moisturizing exfoliant creams containing urea, salicylic acid, or ammonium lactate. Pain relievers such as ibuprofen, naproxen, and celecoxib can also be helpful.
Bevacizumab plus Interferon (IFN)-α
Bevacizumab is a humanized monoclonal antibody. The double-blind AVOREN study compared bevacizumab plus IFN-α with INF-α monotherapy in mRCC (Escudier et al. 2007b). Overall response was higher in the bevacizumab plus IFN-α group. Median PFS increased from 5.4 months with IFN-α to 10.2 months with bevacizumab plus IFN-α. An open-label trial of bevacizumab plus IFN-α versus IFN-α showed a higher median PFS for the combination group with the objective response rate being higher in the combination group. Overall toxicity was greater for bevacizumab plus IFN-α, with significantly more grade 3 hypertension, anorexia, fatigue, and proteinuria (Rini et al. 2010).
mTOR Inhibitors
Temsirolimus is a specific inhibitor of the mammalian target of rapamycin (mTor). Temsirolimus is an intravenous drug which interferes with the synthesis of proteins that regulate proliferation, growth, and survival of tumor cells. In the INTORSECT trial, temsirolimus versus sorafenib was investigated in patients who had previously failed sunitinib. Although no benefit in PFS was observed, a significant OS benefit for sorafenib was noted (Hutson et al. 2014). Based on these results, temsirolimus is not recommended in patients with VEFG TKI-refractory disease.
Everolimus is an oral mTOR inhibitor, established in the treatment of VEGF-refractory disease. The RECORD-1 study compared everolimus plus best supportive care (BSC) versus placebo plus BSC in patients with previously failed anti-VEGFR treatment (or previously intolerant of VEGF-targeted therapy). It showed a median PFS of 4.9 versus 1.9 months for everolimus and placebo, respectively, in the final analysis (Motzer et al. 2010). Subset analysis of PFS for patients receiving only one previous VEFG TKI was 5.4 months (Calvo et al. 2012). This included some patients who were intolerant rather than progressed on therapy (PFS was also 5.4 months). RECORD-1 included patients who failed multiple lines of VEGF-targeted therapy and received everolimus in a third- and fourth-line setting.
Immunotherapy
Interleukin-2 has been used to treat mRCC since 1985, with response rates ranging from 7% to 27% (McDermott et al. 2005). Complete and durable responses have been achieved with high-dose bolus IL-2; however IL-2 remains the only drug to date that can cure a small percentage of RCC patients (Yang et al. 2003). The toxicity of IL-2 is substantially greater than that of IFN-α.
Several studies showed that interferon (IFN)-α in metastatic RCC resulted in a response rate of 6–15%, a 25% decrease in tumor progression risk and a modest survival benefit compared to placebo (Motzer et al. 2002). However, patients with intermediate-risk disease failed to confirm this benefit (Negrier et al. 2007). Interferon-α may only be effective in some patient subgroups, including patients with ccRCC, favorable-risk criteria, as defined by the Memorial Sloan Kettering Cancer Center (MSKCC), and lung metastases only. Interferon-α has since been superseded by targeted therapy in clear cell metastatic RCC.
Chemotherapy
Chemotherapy should not be offered as first-line therapy in patients with clear cell metastatic RCC since it is moderately effective only if 5-fluorouracil (5-FU) is combined with immunotherapeutic agents (Stadler et al. 2003). In metastatic RCC, chemotherapy is otherwise not effective with the exception of gemcitabine and doxorubicin in sarcomatoid and rapidly progressive disease (Haas et al. 2012).
Future Directions
The introduction of newer immunotherapy, with the immune checkpoint inhibitors such as nivolumab, is a very promising new therapeutical option in kidney cancer treatment.
Immune checkpoint blockade with monoclonal antibodies targets and blocks the inhibitory T-cell receptor PD-1 or cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) signaling to restore tumor-specific T-cell immunity (Ribas 2012). A phase III trial of nivolumab versus everolimus after one or two lines of VEGF-targeted therapy (CheckMate 025) reported a longer OS, better QoL, and fewer grade 3 or 4 adverse events with nivolumab than with everolimus (Motzer et al. 2015). Nivolumab has superior OS to everolimus in VEGF-refractory RCC with a median OS of 25 months for nivolumab and 19.6 months for everolimus. Patients who had failed multiple lines of VEGF-targeted therapy were included in this trial making the results broadly applicable.
The phase III trial CheckMate 214 investigated the combination of nivolumab and ipilimumab versus sunitinib in first-line treatment of treatment-naïve advanced or cc-mRCC. Results showed that a combination of ipilimumab and nivolumab was associated with a significant advantage for both RR and OS. A higher proportion of the patients treated with nivolumab plus ipilimumab achieved durable remissions, justifying their use in unselected patients (including favorable-risk disease). Health-related QoL assessment, based on the NCCN Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI-19), was performed and favored the immunotherapy combination (Escudier et al. 2017).
Tumors which overexpressed the PD-L1 biomarker at baseline were associated with a better RR and PFS with nivolumab plus ipilimumab than sunitinib. This was not the case in the PD-L1-negative cohort, where PFS was almost identical. Therefore, the PD-L1 biomarker appears predictive for PFS. Nivolumab plus ipilimumab was associated with 15% grade 3–5 toxicity and 1.5% treatment-related deaths. It should therefore be administered in centers with experience of immune combination therapy and appropriate supportive care within the context of a multidisciplinary team. Nivolumab plus ipilimumab should not be offered outside of the first-line setting.
Patients who stop nivolumab plus ipilimumab because of toxicity should not be challenged with the same drugs in the future without expert guidance and support from a multidisciplinary team. Further combinations of VEGF-targeted therapy and immune therapy are being compared in phase III trials against sunitinib and may change treatment recommendations soon. These include pembrolizumab plus axitinib and lenvatinib plus everolimus or pembrolizumab.
The combination of nivolumab and ipilimumab is the standard of care in IMDC intermediate- and poor-risk patients (Fig. 2). Alternative agents such as sunitinib, pazopanib, and cabozantinib should be considered where nivolumab plus ipilimumab is not safe or feasible (EAU Guidelines). Sunitinib or pazopanib remains a preferable agent in favorable-risk patients due to the non-inferiority of pazopanib compared to sunitinib (COMPARZ). Key trials have established bevacizumab plus IFN-α as another first-line treatment option in treatment-naïve patients with cc-mRCC and a favorable-to-intermediate risk score. This has not been tested against nivolumab plus ipilimumab, and the evidence for subsequent therapies remains unclear. The same arguments apply for temsirolimus in poor-risk patients. It is therefore more appealing to treat patients with sunitinib or pazopanib, both of which were tested in all three risk groups in pivotal trials, where nivolumab plus ipilimumab is not safe or feasible.
Phase II data which compared sunitinib and cabozantinib for intermediate- and poor-risk RCC favored cabozantinib for RR and PFS, although not OS (Choueiri et al. 2017). It showcases the activity of cabozantinib, but due to missing of a randomized phase III study, it currently cannot be supported above other VEGF-TKIs such as pazopanib or sunitinib.
There is no evidence for sequencing of immune therapies, which remains within the realms of clinical trials. While data with the combination of VEGF-targeted therapy and immune checkpoint inhibition is promising, further randomized data is required prior to any recommendations.
References
Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8:464–478
Atsushi T, Sasald I, Sun JK, Ken-ichi T, Tadao K, Yoshiaki K et al (1994) Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res 54:4233–4237
Bard RH, Mydlo JH, Freed SZ (1986) Detection of tumor angiogenesis factor in adenocarcinoma of kidney. Urology 27:447–450
Bergström A, Hsieh CC, Lindblad P, Lu CM, Cook NR, Wolk A (2001) Obesity and renal cell cancer – a quantitative review. Br J Cancer 85(7):984–990
Bianconi M, Scartozzi M, Faloppi L et al (2012) Angiogenetic pathway as a therapeutic target in renal cell carcinoma. Anal Quant Cytol Histol 34(1):15–22
Calvo E, Escudier B, Motzer RJ, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Ravaud A, Kim D, Panneerselvam A, Anak O, Figlin RA (2012) Everolimus in metastatic renal cell carcinoma: subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer 48(3):333–339
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Géczi L, Keam B, Maroto P, Heng DY, Schmidinger M, Kantoff PW, Borgman-Hagey A, Hessel C, Scheffold C, Schwab GM, Tannir NM, Motzer RJ, METEOR Investigators (2015) Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1814–1823
Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, Feldman DR, Olencki T, Picus J, Small EJ, Dakhil S, George DJ, Morris MJ (2017) Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol 35(6):591–597
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group (2007a) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N, AVOREN Trial Investigators (2007b) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370(9605):2103–2111
Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, Gruenvald V, Horwich A (2017) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv167–iv168
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403
Fischer A, Wu S, Ho AL, Lacouture ME (2013) The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Investig New Drugs 31:787–797
Greenblatt M, Shubik P (1968) Tumor angiogenesis: trans filter diffusion studies by the transparent chamber technique. J Natl Cancer Inst 41:111–124
Haas NB, Lin X, Manola J, Pins M, Liu G, McDermott D, Nanus D, Heath E, Wilding G, Dutcher J (2012) A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol 29(2):761–767
Hanahan D, Christofori G, Naik P, Arbeit J (1996) Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur J Cancer 32A:2386–2393
Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, Rosbrook B, Chen C, Kim S, Vogelzang NJ (2013) Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 14(13):1287–1294
Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, Senico P, Niethammer A, Lu DR, Hariharan S, Motzer RJ (2014) Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 32(8):760–767
Itsumi M, Tatsugami K (2010) Immunotherapy for renal cell carcinoma. Clin Dev Immunol 2010:284581
Janusch M, Fischer M, Marsch WC, Holzhausen HJ, Kegel T, Helmbold P (2006) The hand-foot syndrome – a frequent secondary manifestation in antineoplastic chemotherapy. Eur J Dermatol 16:494–499
Kamba T, McDonald DM (2007) Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96:1788–1795
Kim WY, Kaelin WG (2004) Role of VHL gene mutation in human cancer. J Clin Oncol 22:4991–5004
Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP (2008) Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist 13:1001–1011
Lipworth AD, Robert C, Zhu AX (2009) Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology 77:257–271
Maglione D, Guerriero V, Viglieno O, Ferraro MG, Aprelikova O, Alitalo IL et al (1993) Two alternative mRNAs coding for the angiogenic factor, placenta growth factor (PIGF), are transcribed from a single gene of chromosome 14. Oncogene 8:925–931
McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 23(1):133–141
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol 70(1):93–105
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20(1):289–296
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24(1):16–24
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27(22):3584–3590
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Kay A, Ravaud A, RECORD-1 Study Group (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116(18):4256–4265
Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, Wilding G, Agarwal N, Thompson JA, Cella D, Bello A, Korytowsky B, Yuan J, Valota O, Martell B, Hariharan S, Figlin RA (2012) Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol 30(12):1371–1377
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK (2013a) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369(8):722–731
Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, Tomczak P, Lyulko O, Alyasova A, Harza M, Kogan M, Alekseev BY, Sternberg CN, Szczylik C, Cella D, Ivanescu C, Krivoshik A, Strahs A, Esteves B, Berkenblit A, Hutson TE (2013b) Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 31(30):3791–3799
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate 025 Investigators (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813
Negrier S, Perol D, Ravaud A, Chevreau C, Bay JO, Delva R, Sevin E, Caty A, Escudier B, French Immunotherapy Intergroup (2007) Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer 110(11):2468–2477
Pages G, Puyssegur J (2005) Transcriptional regulation of the vascular endothelial growth factor gene – a concert of activating factors. Cardiovasc Res 65:564–573
Porta C, Paglino C, Imarisio I, Bonomi L (2007) Uncovering Pandora’s vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med 7:127–134
Prasad V, Massey PR, Fojo T (2014) Oral anticancer drugs: how limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol 32(15):1620–1629
Ribas A (2012) Tumor immunotherapy directed at PD-1. N Engl J Med 366(26):2517–2519
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2010) Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 28(13):2137–2143
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378(9807):1931–1939
Scartozzi M, Faloppi L, Svegliati Baroni G, Loretelli C, Piscaglia F, Iavarone M, Toniutto P, Fava G, De Minicis S, Mandolesi A, Bianconi M, Giampieri R, Granito A, Facchetti F, Bitetto D, Marinelli S, Venerandi L, Vavassori S, Gemini S, D'Errico A, Colombo M, Bolondi L, Bearzi I, Benedetti A, Cascinu F (2014) VEGF and VEGFR genotyping in the prediction of clinical outcome for hcc patients receiving sorafenib: the alice-1 study. Int J Cancer 135(5):1247–1256
Stadler WM, Huo D, George C, Yang X, Ryan CW, Karrison T, Zimmerman TM, Vogelzang NJ (2003) Prognostic factors for survival with gemcitabine plus 5-fluorouracil based regimens for metastatic renal cancer. J Urol 170(4 Pt 1):1141–1145
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarbá JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28(6):1061–1068
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Tsui KH, Shvarts O, Smith RB, Figlin R, de Kernion JB, Belldegrun A (2000) Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol 163(2):426–430
Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA (2003) Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 21(16):3127–3132
Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, Kuo TT, Hsieh JJ, Chang JW (2008) Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 158:592–596
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Rübben, H., Panic, A. (2019). Anti-angiogenics in Kidney Cancer Therapy. In: Marmé, D. (eds) Tumor Angiogenesis. Springer, Cham. https://doi.org/10.1007/978-3-319-33673-2_27
Download citation
DOI: https://doi.org/10.1007/978-3-319-33673-2_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33671-8
Online ISBN: 978-3-319-33673-2
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences