Abstract
Tuberous sclerosis complex (TSC) consists of autosomal dominant dysgenetic disorders characterized by hamartomas and neoplasms involving the central nervous system (CNS) and various other tissues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bourneville disease
- Hamartin
- Subependymal nodule
- TSC
- Tuberin
- Tuberous sclerosis
- Subependymal giant cell astrocytoma
1 Overview
-
Tuberous sclerosis complex (TSC) is an autosomal dominant dysgenetic disorder characterized by hamartomas and neoplasms involving the central nervous system (CNS) and various other tissues.
-
The causative mutatio ns are in the TSC1 and TSC2 genes located on 9q and 16p, respectively.
-
Worldwide, 1–2 million individuals are affected by TSC. The estimated prevalence is 1 out of every 5000–6000 births.
-
The definitive diagn osis of TSC is established if either the genetic or the clinical criteria are met [1]:
-
◦ Genetic diagnostic criteria: The identification of either a TSC1 or TSC2 pathogenic mutation in DNA from normal tissue is sufficient to make a definite diagnosis of TSC. A pathogenic mutation is defined as a mutation that clearly inactivates the function of the TSC1 or TSC2 proteins.
-
◦ Clinical diagnostic criteria: Definite diagnosis implies two major features or one major feature with two or more minor features:
-
Major features
-
Hypomelanotic macules (≥3, at least 5 mm diameter)
-
Angiofibromas (≥3) or fibrous cephalic plaque
-
Ungual fibromas (≥2)
-
Shagreen patch
-
Multiple retinal hamartomas
-
Cortical dysplasias
-
Subependymal nodules
-
Subependymal giant cell astrocytoma (SEGA)
-
Cardiac rhabdomyoma
-
Lymphangioleiomyomatosis (LAM)
-
Angiomyolipomas (≥2)
-
-
Minor features
-
“Confetti” skin lesions
-
Dental enamel pits (>3)
-
Intraoral fibromas (≥2)
-
Retinal achromic patch
-
Multiple renal cysts
-
Nonrenal hamartomas
-
-
-
2 Clinical Features
-
About half of TSC patients have a positive family history.
-
Children with TSC can present with seizures, and seizures are present in the majority of pediatric patients [2].
-
Patients with SEGA may present with signs of hydrocephalus associated with blockage of cerebrospinal fluid (CSF) circulation, but early diagnosis of SEGA may be difficult because smaller tumors are often asymptomatic.
-
Cognitive and mental dysfunction and developmental delay are commonly seen in children with TSC.
3 Neuroimaging
-
Several characteristic lesions can be seen in patients with TSC. Neuroimaging may underestimate the extent of neuropathological changes in TSC patients [3]. More recently, 7-T imaging was able to identify lesions not recognized with lower-resolution magnets [4].
-
Subependymal nodules often protrude into the ventricle; they are often isointense to white matter on T1-weighted images, and are hypointense on T2-weighted images. They show limited and variable enhancement after contrast administration.
-
SEGA is an almost entirely intraventricular tumor, which rarely demonstrates a parenchymal component. Tumors are isointense to hypointense on T1-weighted images and isointense to hyperintense on T2-weighted images; they show strong, diffuse enhancement on gadolinium administration.
-
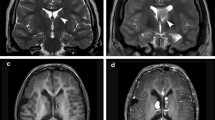
Cortical tubers are often multiple cerebral lesions with variable calcifications, sometimes in a gyriform fashion. The MR appearance of cortical tubers varies with the age of the patient. The signal changes over time that slowly become isointense to white matter should be interpreted with caution. Tubers are often hyperintense on T2 and fluid-attenuated inversion recovery (FLAIR) modalities and typically do not enhance [5]. Calcifications often accompany cortical tubers as T2 dark foci (Fig. 28.1).
4 Histopathological Features
-
Subependymal nodules are often calcified and partially covered by a layer of ependymal cells. They are mostly composed of large cells with glial phenotype and multinucleated cells with extensive glial processes. There is little or no cytological difference between the predominant cell in subependymal nodule and the cells of SEGA. Most cells in subependymal nodules have ambiguous expression of glial and neuronal markers, as in SEGA.
-
SEGA is a well-cir cumscribed neoplasm and is consider ed World Health Organization (WHO) grade I. The tumor is characterized by gemistocyte-like cells with ample glassy, eosinophilic cytoplasm and eccentric nuclei with p rominent nucleoli in a “streaming” pattern (Fig. 28.2). This pattern portends a more spindled morphology for some of the tumor cells, and some tumors may display a striking pleomorphism. There is a striking glial background with rich fibrillarity. Binucleated or occasionally mul tinucleated cells may be seen within the neoplasm, as well as rare mast cells. Some tumors demonstrate perivascular lymph ocytic infiltrates. Typically, the tumor cells are positive for glial fibrillary acidic protein (GFAP), but similar to subependymal nodules, many cells in SEGA demonstrate an ambiguous glial/neuronal immunohistochemical pattern. The proliferation indices are often very low. Rare mitotic figures can be encountered in SEGA. Exceptional cases demonstrate necrosis, but this is often in the form of geographic, and not palisaded necrosis.
-
Cortical tubers ar e firm, wartlike protrusions that are composed of jumbled-up elements of neuropil with bizarre ganglionlike cells with short processes (Fig. 28.3). These bizarre cells are often found in clusters with a gliotic background and large collections of glial processes. Microcalcifications are often present.
Fig. 28.3 High-magnification image of balloon cells in a cortical tuber from the patient in Fig. 28.1. The typical cortical tuber s hows a markedly disorganized cortex, with abnormal glial proliferations, bizarre neurons, and balloon cells
5 Immunohistochemistry
-
SEGAs are often positive with the neuronal antibodies such as neurofilament proteins, class III beta-tu bulin, synaptophysin, and NeuN. The latter is only focally positive in a small percentage of cells, but the staining is variable among tumors.
-
SEGA is variably positive for staining with GFAP and S-100 protein antibodies.
-
Recent studies suggest thyroid transcription factor 1 (TTF-1) positivity in SEGAs [6].
-
Olig-2, which is positive with most glial neoplasms, is almost entirely negative in SEGAs [7]. CD34 (positive in most glioneuronal tumors) is also negative.
6 Electron Microscopy
-
Electron micros copy shows evidence of glial as well as neuronal differentiation.
-
Microtubules, occasional dense-core granules, and, rarely, synapses can be observed.
7 Molecular Pathology
-
Linkage studies have provided evidence for two distinct TSC loci on chromosome 9q34 (TSC1) and on chromosome 16p13.3 (TSC2).
-
TSC1 and TSC2 gene product s are components of a heterodimer that is critical in regulation of a number of cellular functions including proliferation; they are considered to be tumor suppressor genes. Gene products tuberin and hamartin form a complex that integrates and transmits cellular growth factor and stress signals to the PI3K/PKB signaling pathway, and negatively regulates mTOR activity.
-
Loss of either TSC1 or TSC2 seems to result in a similar clinical picture, supporting the suggestion that both genes are involved in the same regulatory pathway [8].
-
Mutations of the TSC2 gene are more common than TSC1 gene mutations.
-
Virtually all mutations of TSC1 seem to result in a truncated gene product.
-
Mutations in the TSC2 gene are more varied and include deletions, missense and nonsense mutations, frameshift deletions or insertions, and splice junction mutations.
8 Prognosis
-
SEGA is essentially a benign neoplasm and often can be cured by resection.
-
In recent years, rapamycin therapy has been suggested for cases where a gross total resection cannot be achieved [9].
-
A last-resort treatment is gamma knife surgery for incompletely resected or chemotherapy-resistant tumors.
-
Even though the prognosis of SEGA is quite favorable, the overall prognosis of TSC patients is dependent on the type and extent of CNS and extra-CNS lesions.
References
Northrup H, Krueger DA. International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243–54.
Kotulska K, Jurkiewicz E, Domanska-Pakiela D, Grajkowska W, Mandera M, Borkowska J, et al. Epilepsy in newborns with tuberous sclerosis complex. Eur J Paediatr Neurol. 2014;18:714–21.
Ruppe V, Dilsiz P, Reiss CS, Carlson C, Devinsky O, Zagzag D, et al. Developmental brain abnormalities in tuberous sclerosis complex: a comparative tissue analysis of cortical tubers and perituberal cortex. Epilepsia. 2014;55:539–50.
Chalifoux JR, Perry N, Katz JS, Wiggins GC, Roth J, Miles D, et al. The ability of high field strength 7-T magnetic resonance imaging to reveal previously uncharacterized brain lesions in patients with tuberous sclerosis complex. J Neurosurg Pediatr. 2013;11:268–73.
Pinto Gama HP, da Rocha AJ, Braga FT, da Silva CJ, Maia Jr AC, de Campos Meirelles RG, et al. Comparative analysis of MR sequences to detect structural brain lesions in tuberous sclerosis. Pediatr Radiol. 2006;36:119–25.
Hewer E, Vajtai I. Consistent nuclear expression of thyroid transcription factor 1 in subependymal giant cell astrocytomas suggests lineage-restricted histogenesis. Clin Neuropathol. 2015;34:128–31.
Barrows BD, Rutkowski MJ, Gultekin SH, Parsa AT, Tihan T. Evidence of ambiguous differentiation and mTOR pathway dysregulation in subependymal giant cell astrocytoma. Turk Patoloji Derg. 2012;28:95–103.
Chan JA, Zhang H, Roberts PS, Jozwiak S, Wieslawa G, Lewin-Kowalik J, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63:1236–42.
Jozwiak S, Mandera M, Mlynarski W. Natural history and current treatment options for subependymal giant cell astrocytoma in tuberous sclerosis complex. Semin Pediatr Neurol. 2015;22:274–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this chapter
Cite this chapter
Tihan, T., Adesina, A.M. (2016). Tuberous Sclerosis Complex. In: Adesina, A., Tihan, T., Fuller, C., Poussaint, T. (eds) Atlas of Pediatric Brain Tumors. Springer, Cham. https://doi.org/10.1007/978-3-319-33432-5_28
Download citation
DOI: https://doi.org/10.1007/978-3-319-33432-5_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33430-1
Online ISBN: 978-3-319-33432-5
eBook Packages: MedicineMedicine (R0)