Abstract
Carotid endarterectomy (CEA) is the treatment of choice for symptomatic carotid stenosis and selected asymptomatic lesions. CEA is most often performed via a longitudinal anterior arteriotomy in the common carotid artery (CCA) extending through the carotid bulb and into the internal carotid artery (ICA). Many surgeons feel that this so-called “standard” technique (sCEA) allows for optimal visualization and excision of the plaque, and maximally facilitates arterial reconstruction. An alternative technique, known as “eversion endarterectomy” (eCEA) has also been popularized. eCEA is performed by transecting the carotid bulb just below the bifurcation and removing the plaque by everting the media/adventitia using the plaque as a mandrill over which to establish a cleavage plane. The purpose of this chapter is to describe, review and compare these methods of CEA, and make recommendations as to the optimal technique of the operation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Standard Carotid Endarterectomy (sCEA)
The hallmark of the standard technique of CEA (sCEA) is longitudinal carotid arteriotomy. It was originally performed by Michael DeBakey in 1953 in a patient with stroke and carotid occlusion [1], and first described by Felix Eastcott and Charles Rob in 1954 who explored and then resected the diseased carotid bifurcation of a woman who was found to have critical stenosis after sustaining 33 separate transient ischemic attacks [2].
In the modern era, sCEA is performed via a cervical skin incision parallel and anterior to the sternocleidomastoid muscle. Following incision, the platysma muscle is divided, the internal jugular vein retracted laterally and the facial vein ligated. The carotid sheath is incised and the common carotid (CCA), external carotid (ECA), internal carotid (ICA) and superior thyroid arteries identified and controlled. In order to expose the proximal ICA, the descending branch of the ansa cervicalis is divided facilitating superomedial retraction of the hypoglossal nerve. The patient is systemically anticoagulated and the internal, common and external carotid arteries sequentially clamped. A longitudinal arteriotomy is made in the CCA extending onto the ICA, and a cleavage plane developed within the arterial media to extract the plaque under direct vision (Fig. 28.1). The endarterectomy proceeds in the proximal-to-distal (caudal-to-cranial) direction. When the ECA is reached, it is endarterectomized via eversion by temporarily releasing its clamp. Finally, the distal portion of the plaque lining the proximal ICA is excised under direct vision, with care to make its endpoint smooth and free of residual stenosis.
Standard carotid endarterectomy (Adapted from Zarins and Gewertz [3]. With permission from Elsevier)
The sCEA technique is ubiquitous, being utilized in the majority of the more than 100,000 CEA procedures performed annually [4]. It was the primary technique employed by investigators demonstrating the efficacy of the procedure in multicenter randomized trials [5, 6]. The contemporary clinical results of CEA are, perhaps, best illustrated by the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST) trial which randomized 2502 patients with carotid stenosis to undergo either CEA or carotid artery stenting in 117 North American centers [7]. Although the use of the sCEA wasn’t mandatory, the fact that 62 % of patients underwent patch angioplasty suggests that it was the dominant technique. For the 1240 patients undergoing CEA in the trial, the overall risks of death, major ipsilateral stroke and any stroke were 0.3 %, 0.3 % and 2.3 %, respectively. It’s instructive to note that the overall incidence of periprocedural stroke in patients undergoing primary CEA (2.3 %) was statistically significantly lower than in patients undergoing primary stenting (4.1 %; p = 0.01).
Proponents of the sCEA technique point to its proven clinical utility and the fundamental surgical advantage of direct visualization of the distal extent of the plaque. Other potential benefits include the relative ease of intraluminal shunt insertion, the option to provide enhanced luminal size through the use of patch angioplasty, the avoidance of circumferential dissection with minimal disturbance of the carotid baroreceptors, and the efficiency with which the procedure can be taught to surgical trainees.
The technical success of sCEA technique depends, in some measure, upon successful eversion endarterectomy of the ECA. Indeed, the ease of ECA eversion was likely the stimulus for the rise in popularity of the alternative technique: eversion CEA (eCEA).
Eversion Carotid Endarterectomy (eCEA)
ECEA was originally described by Michael DeBakey in his classic clinical review of extracardiac vascular surgery published in 1959 [8–10]. It is performed via a similar incision as sCEA, although some would argue that eCEA requires only a limited operative field and can be performed through a smaller incision. Surgical exposure of the carotid bifurcation proceeds identically to sCEA, except that the arteries should be freed from the surrounding tissues circumferentially, and division of the superior thyroid artery should be performed as a matter of routine.
Once the arteries have been exposed and clamped, the CCA is transected just proximal to the bifurcation. Figure 28.2 depicts a CCA that is transected transversely, although many authors recommend oblique transection as a means to facilitate visualization and closure. Following division of the CCA, endarterectomy of the ICA and ECA is performed by developing a cleavage plane within the arterial media and everting it over the plaque (Fig. 28.2b). The plaque acts as a natural mandrill over which to fold the artery; it is gently retracted caudad to facilitate the dissection. Eversion CEA of the ECA is performed in an identical fashion as in sCEA. Because primary carotid plaques are localized to the bulb and proximal ICA, downward retraction and careful blunt withdrawal of the plaque will cause it to “pop” out of the ICA once it reaches its natural endpoint. The result is complete excision of the plaque from the both the ECA and ICA with achievement of a smooth residual lumens that contain no suture lines (Fig. 28.2c).
Eversion carotid endarterectomy (Adapted from Black et al. [11]. With permission from Elsevier)
Attention is then turned toward the CCA which is everted in a similar fashion through, theoretically, the same cleavage plane. There’s rarely a natural proximal endpoint of the plaque within the CCA so it’s transected sharply after a distance of approximately 2 cm. If the ICA is redundant, a rim of CCA can be resected to accomplish its straightening. End-to-end anastomosis of the two ends of the transected CCA is facilitated by rotating the “freely floating” artery within the field (Fig. 28.2e). The completed reconstruction bears no suture line within the ICA (Fig. 28.2f). In the 1990s, several surgeons described modifications to the above technique, most notably Vanmaele et al. who proposed transection, eversion and reimplantation of the ICA at its origin [12], and Reigner et al. who described oblique transaction of the ICA distal to the lesion followed by eCEA through longitudinal incision of the CCA and ECA [13].
Proponents of the eCEA technique point to the more limited dissection it requires, the rapidity in which it can be performed, the ease to which arterial redundancy can be addressed, the advantages of placing sutures in the widest part of the bifurcation, the avoidance of patches leading to better fluid dynamics [14], the fact that the reconstruction is accomplished without tacking stitches or suture lines in the ICA [15, 16] and, potentially, reduced restenosis [17]. Some even advocate the procedure for recurrent stenoses [18, 19].
Search Strategy
A search of the University of Chicago Articles Plus + database was conducted for the years 1997–2015 to identify published data regarding open surgical approaches to treat carotid artery disease using the PICO outline (Table 28.1). The University of Chicago Articles Plus + is a database and search tool that allows simultaneous searching of a broad range of articles, books, and other collections. An Articles Plus + search includes hundreds of the Library’s article databases, including MEDLINE, Science Direct and Academic Search Premier, over 40,000 journals and periodicals, the University of Chicago library catalog, and digitized collections of documents and images from a variety of organizations.
Eight comparative trials, and four single arm studies, were included in the analysis (see Tables 28.2 and 28.3).
Single-Arm Studies of eCEA
The clinical results of several large, single-arm series of eCEA are shown in Table 28.2; they demonstrate that excellent results have been achieved by surgeons devoted to this technique. Including over 12,000 eCEA procedures, the (weighted) averages of reported cranial/cervical nerve injury, reoperation for hemorrhage and peri-operative stroke/death were 1.3 %, 1.9 % and 1.1 %, respectively. After long-term follow-up (4–12 years), the risk of late ipsilateral stroke was only 0.3 %. The majority of patients in this analysis were culled from the experience of Radak, et al. whose series of 9,897 eCEAs in the Republic of Serbia represents the largest series reported to date [22].
Comparative Studies
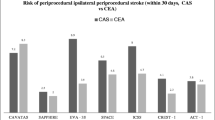
The results of several prospective and retrospective studies directly comparing sCEA to eCEA are shown in Table 28.3. The studies encompass over 5,000 procedures in a variety of geographies and institutions. About half of all patients were symptomatic. The preference for performing eCEA without shunting is evident in the data as shunts were utilized in only 8 % of eCEA procedures compared to 24 % of sCEA procedures. Cross-clamp time was generally shorter for eCEA procedures, although only by a few minutes. Accepting that methods of reporting varied fairly widely among these studies, there appeared to be little discernable differences in short-term outcome measures including cranial/cervical nerve injury, the need to re-explore for hemorrhage and peri-operative stroke/death (weighted average 2.6 % for sCEA and 2.3 % for eCEA). Interestingly, some studies and meta-analyses conclude that peri-operative stroke/death is significantly lower after eCEA [16, 17], while others draw an opposite, but no less convincing, conclusion [28]. In the aggregate, these data appear to demonstrate clinical equipoise between sCEA and eCEA.
Long-term anatomic and clinical results are also shown in Table 28.3. After median follow-up intervals ranging from 1 to 2.8 years, the incidences of restenosis for sCEA and eCEA were 5.4 % and 1.6 %, respectively. However, one study reported the rate of >50 % restenosis of sCEA as 38 % which should probably be considered an outlier [23]. If this study is excluded from the analysis, the difference in the rates of restenosis is small, if present at all. Similarly, although not every study reported numeric rates for patients sustaining an ipsilateral stroke in the follow-up period; those that did generally found the risks to be comparable for the two procedures.
Interestingly, several authors have suggested that eCEA induces more post-operative hemodynamic liability than sCEA, the purported mechanism being that circumferential dissection of the blub denervates the terminal afferent fibers of the Nerve of Hering within the carotid sinus [28, 30–33]. Although some studies have shown that post-operative blood pressure control is more problematic after eCEA compared to sCEA, no differences in clinical outcome have been conclusively demonstrated to date.
Recommendation
Carotid endarterectomy can be safely and reliably performed using either standard or eversion techniques (Quality of Evidence, strong; level of recommendation, strong).
Personal View of the Data
Carotid endarterectomy (CEA) is the treatment of choice for symptomatic carotid stenosis and selected asymptomatic lesions. It can be safely and reliably performed using either standard or eversion techniques. Although the differences in these techniques have been exhaustively studied over the past decade, this author agrees with the overall conclusion reached by Piergiorgio Cao in 2002 after his comprehensive review of this same subject: “Until data are available, the choice of the surgical technique for CEA should depend on the experience and preference of the operating surgeon” [19].
References
DeBakey ME. Successful carotid endarterectomy for cerebrovascular insufficiency. JAMA. 1975;233:1083–5.
Eastcott HHG, Pinching GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954;2:994–6.
Zarins CK, Gewertz BL. Carotid endarterectomy. In: Zarins CK, Gewertz BL, editors. Atlas of vascular surgery. New York: Churchill Livingstone; 1989.
Tendera M, Aboyans V, Bartelink M-L, Baumgartner I, Clement D, Collet J-PC A, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur Heart J. 2011;32:2851–906.
The North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53.
Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, et al. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–8.
Brott TG, Hobson RWI, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23.
DeBakey ME, Crawford ES, Cooley DA, Morris Jr GC. Surgical considerations of occlusive disease of innominate, carotid, subclavian and vertebral arteries. Ann Surg. 1959;149:690–710.
DeBakey ME. A technique of eversion carotid endarterectomy. Postgrad Med. 1959;26:227–37.
DeBakey ME. Regarding “A randomized study on eversion versus standard carotid endarterectomy: study design and preliminary results: the Everest trial”. J Vasc Surg. 1998;28:753.
Black III JH, Ricotta JJ, Jones CE. Long-term results of eversion carotid endarterectomy. Ann Vasc Surg. 2010;24:92–9.
Vanmaele RG, Van Schil PE, DeMaeseneer MG, Meese G, Lehert P, Van Look RF. Division- endarterectomy-anastomosis of the internal carotid artery: a prospective randomized comparative study. Cardiovasc Surg. 1994;2:573–81.
Reigner B, Reveilleau P, Gayral M, Papon X, Enon B, Chevalier J-M, Chevalier JM. Eversion endarterectomy of the internal carotid artery: midterm results of a new technique. Ann Vasc Surg. 1995;9:241–6.
Demirel S, Chen D, Mei Y, Partovi S, Von Tengg-Kobligk H, Dadrich M, et al. Comparison of morphological and rheological conditions between conventional and eversion carotid endarterectomy using computational fluid dynamics – a pilot study. Vascular. 2015;23:474–82.
Green RM, Greenberg R, Illig K, Shortell C, Ouriel K. Eversion endarterectomy of the carotid artery: technical considerations and recurrent stenoses. J Vasc Surg. 2000;32:1052–61.
Shah D, Darling III RC, Chang BB, Paty PSK, Kreienberg PB, Lloyd WE, et al. Carotid endarterectomy by eversion technique: its safety and durability. Ann Surg. 1998;228:471–8.
Antonopoulos CN, Kakisis JD, Sergentanis TN, Liapis CD. Eversion versus conventional carotid endarterectomy: a meta-analysis of randomised and non-randomised studies. Eur J Vasc Endovasc Surg. 2011;42:751–65.
Mehta M, Roddy SP, Darling III RC, Paty PSK, Kreienberg PB, Ozvath KJ, et al. Safety and efficacy of eversion carotid endarterectomy for the treatment of recurrent stenosis: 20-year experience. Ann Vasc Surg. 2005;19:492–8.
Cao P, De Rango P, Zannetti S. Eversion vs. conventional carotid endarterectomy: a systematic review. Eur J Vasc Endovasc Surg. 2002;23:195–201.
Ballotta E, Toniato A, Da Giau G, Lorenzeti R, Da Roit A, Baracchini C. Durability of eversion carotid endarterectomy. J Vasc Surg. 2014;59:1274–81.
Ballotta E, Giau GD. Selective shunting with eversion carotid endarterectomy. J Vasc Surg. 2003;38:1045–50.
Radak D, Tanaskovic S, Matic P, Babic S, Aleksic N, Ilijevski N. Eversion carotid endarterectomy – our experience after 20 years of carotid surgery and 9897 carotid endarterectomy procedures. Ann Vasc Surg. 2012;26:924–8.
Brothers TE. Initial experience with eversion carotid endarterectomy: absence of a learning curve for the first 100 patients. J Vasc Surg. 2005;42:429–34.
Cao P, Giordano G, De Rango P, Caporali S, Lenti M, Ricci S, et al. Eversion versus conventional carotid endarterectomy: a prospective study. Eur J Vasc Endovasc Surg. 1997;14:96–104.
Cao P, Giordano G, De Rango P, Zannetti S, Chiesa R, Coppi G, et al. A randomized study on eversion versus standard carotid endarterectomy: study design and preliminary results: the Everest trial. J Vasc Surg. 1998;27:595–605.
Cao P, Giordano G, De Rango P, Zannetti S, Chiesa R, Coppi G, et al. Eversion versus conventional carotid endarterectomy: late results of a prospective multicenter randomized trial. J Vasc Surg. 2000;31:19–30.
Crawford RS, Chung TK, Hodgman TA, Pedraza JD, Corey M, Cambria RP. Restenosis after eversion vs. patch closure carotid endarterectomy. J Vasc Surg. 2007;46:41–8.
Demirel S, Attigah N, Bruijnen H, Ringleb P, Eckstein H-H, Fraedrich G, et al. Multicenter experience on eversion versus conventional carotid endarterectomy in symptomatic carotid artery stenosis; observations from the stent-protected angioplasty versus carotid endarterectomy (SPACE-1) trial. Stroke. 2012;43:1865–71.
Yasa H, Akyuz M, Yakut N, Aslan O, Akyuz D, Ozcem B, et al. Comparison of two surgical techniques for carotid endarterectomy: conventional and eversion. Neurochirurgie. 2014;60:33–7.
Demirel S, Attigah N, Bruijnen H, Hakimi M, Burgmer B, Böckler D. Perioperative blood pressure alterations after eversion and conventional carotid endarterectomy sustain in the midterm. Langenbecks Arch Surg. 2013;398:303–12.
Demirel S, Attigah N, Bruijnen H, Hakimi M, Mavek H, Bockler D. Eversion carotid endarterectomy is associated with impaired postoperative hemodynamic stability compared with the conventional technique. Ann Vasc Surg. 2012;26:755–65.
Demirel S, Attigah N, Bruijnen H, Macek L, Hakimi M, Able T, et al. Changes in baroreceptor sensitivity after eversion carotid endarterectomy. J Vasc Surg. 2012;55:1322–8.
Demirel S, Bruijnen H, Attigah N, Hakimi M, Böckler D. The effect of eversion and conventional- patch technique in carotid surgery on postoperative hypertension. J Vasc Surg. 2011;54:80–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Schwartz, L.B. (2017). In Patients Undergoing Carotid Endarterectomy, Is the Eversion Technique Superior to a Patch Technique to Reduce Restenosis?. In: Skelly, C., Milner, R. (eds) Difficult Decisions in Vascular Surgery. Difficult Decisions in Surgery: An Evidence-Based Approach. Springer, Cham. https://doi.org/10.1007/978-3-319-33293-2_28
Download citation
DOI: https://doi.org/10.1007/978-3-319-33293-2_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33291-8
Online ISBN: 978-3-319-33293-2
eBook Packages: MedicineMedicine (R0)