Abstract
Electrolysis and electroporation technologies have been utilized to provide several valuable nonthermal tissue ablation modalities. The synergistic combination of electroporation and electrolysis (SEE) has produced a new method of tissue ablation that has distinct advantages over electrolysis or electroporation alone. Electrolysis uses a low-magnitude direct electric current to create chemical species at the electrode-tissue interface which then diffuse through the tissue, resulting in extreme pH changes and cell death. Electroporation, on the other hand, is used to create permeabilizations in the cell membrane that can be used to induce cell death by several different mechanisms: through electrochemotherapy, cytotoxic drugs are introduced to the cell interior, and irreversible electroporation results in cell death by loss of cell homeostasis. When electrolysis is combined with electroporation, a new mode of tissue ablation is achieved that results in a very effective method of cell death. This mechanism of action is likely due to the ability of the electrolytic products to penetrate the cell membrane through the permeabilizations created by electroporation. Here, fundamental principles of electrolysis and electroporation are presented, the mechanism of ablation by SEE is discussed, and different types of SEE protocols are examined with respect to their effect on the tissue. This chapter hopes to serve as a foundation and starting point for further research into this ablation modality as well as for the development of new types of SEE protocols that may be used to address specific clinical needs.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Synergistic combination of electroporation and electrolysis
- Electroporation
- Reversible electroporation
- Irreversible electroporation
- Electrolytic ablation
- Tissue ablation
Introduction
Electroporation is a technique which employs pulsed electric fields to create permeabilized cell membranes. This phenomenon occurs when an electric field is applied across the cell, destabilizing the electric potential maintained by the cell membrane and resulting in the formation of nanoscale defects in the lipid bilayer. Reversible pore formation caused by reversible electroporation (RE) has been utilized as an effective method for introducing molecules such as genes and drugs into the cell while maintaining the cell viability. Changing the electrical parameters can, however, result in cell death by irreversible electroporation (IRE) due to extensive loss of metabolites and loss of homeostasis. Electrical parameters for IRE can be employed such that any Joule heating effects are minimized, resulting in a nonthermal tissue ablation modality that spares important tissue components such as major blood vessels, nerves, and the extracellular matrix. Electrochemotherapy (ECT) uses reversible electroporation protocols to produce temporary pores in the cell membrane, allowing cancer drugs such as bleomycin to be introduced directly into the tumor cells. Protocols have been developed for treatment of metastases to liver, lung, brain, bone, and head and neck cancers. ECT is increasingly becoming integrated with other treatments, guidelines for treatment are beginning to list ECT as an option, and in some locations, ECT is becoming available for treatment on a hospital level. Nonthermal irreversible electroporation (NTIRE) for tumor ablation has also made great strides and is currently seen as a promising nonthermal, minimally invasive tissue ablation modality.
The high voltages employed with irreversible electroporation may induce muscle contractions, requiring general anesthesia and neuroblocking agents to mitigate these effects during the procedure. Such spasms may in turn cause treatment electrodes to shift in location, resulting in incomplete ablation in the target site or damage to nearby vital structures. Managing these effects adds a significant level of complexity to the surgical procedure, resulting in increased surgical time and cost of implementing this type of treatment method. Reversible electroporation does not require as high of voltages and/or number of pulses as irreversible electroporation and thus does not have the same issues with muscle contractions seen in irreversible electroporation. RE has been used with success by combining it with cytotoxic drugs, resulting in tissue ablation by electrochemotherapy. Nonetheless, there would be a significant advantage in being able to obtain tissue ablation without the requirement of introducing the cytotoxic drugs into the body as required for electrochemotherapy.
Tissue ablation by electrolysis (E) (also known as electrochemical therapy ) uses a direct electric current to create tumor ablation due to the creation of chemical species at the electrode-tissue interface as well as extreme pH changes throughout the ablation volume. Electrolytic treatment has been reported to be a simple, safe, and low-cost technique for ablating solid tumors. At the electrode-anode interface with the tissue, electrochemical reactions consist mainly of water decomposition and chloride oxidation. At the cathode surface, hydroxide ions and hydrogen are produced from water decomposition. Chemical species generated at the interfaces of the electrodes diffuse away into the tissue in a process driven by differences in electrochemical potential. These electrolytic reactions yield changes in pH, resulting in an acidic region near the anode and a basic region near the cathode. The changes in pH as well as the presence of the chemical species formed during the electrolytic reactions result in a cytotoxic environment. Electrolytic ablation requires very low direct currents (tens of mA) and very low voltages (single to low tens of volts), thus requiring less expensive instrumentation than needed for tissue ablation by irreversible electroporation. However, for traditional electrolytic ablation, a high level of electrolytic products must diffuse through the tissue to create a cellular environment that is cytotoxic enough to produce cell death. This requires a longer application time (tens of minutes to hours) for the electrical current, increasing the length of the surgical procedure.
When electrolysis is combined with electroporation, a new method of tissue ablation is achieved. It is hypothesized that the pores formed during electroporation allow the electrolytic products to pass through the permeabilized cell membrane and contribute to cell death at low levels of exposure to electrolytic chemical species. This technique combines several advantages of electroporation and electrolysis alone and can enable different modes of tissue ablation. For example, when reversible electroporation is combined with electrolysis, it applies the low current and low voltage required to produce electrolysis along with the high speed of treatment time associated with electroporation. In addition, since the electrolytic products are at low concentrations and only penetrate the electroporated cells, this new method allows for accurate treatment planning and tight margins between the ablated and untreated tissue zones.
The primary goal of this chapter is to supply readers with the understanding necessary to develop synergistic electroporation and electrolysis (SEE) protocols for use in tissue ablation. Fundamental principles of electrolysis and electroporation when applied separately are first presented. The proposed mechanism of ablation by combining electrolysis with electroporation is discussed, and different types of SEE protocols are examined with respect to their effect on the tissue. With this background and knowledge of how SEE can be applied toward tissue ablation, it is hoped that the reader can gain a basic understanding of this new field and apply this foundation toward investigating and developing new SEE protocols.
Fundamental Principles of Electrolysis
Mechanism of Action
Electrolysis is an electrochemical process that takes place at the electrode surface and occurs during the passage of an electric current between two electrodes immersed in an ionic solution. New chemical species generated at the interface of the electrodes diffuse away from the electrodes into the tissue. If the anode is electrically soluble, metal dissolution will be a major part of the resulting chemical reaction occurring at the electrodes. The use of passivated metal electrodes can make this effect negligible. During electrolysis, protons are produced at the anode , according to the following reaction:
The major chemical reaction occurring at the cathode is the decomposition of water into molecular hydrogen and hydroxyl ions:
Sodium chloride is ionized into Na+ and Cl− ions, which move toward the cathode and the anode, respectively. The concentration of Cl− ions is higher at the anode, and additional reactions result in the production of chlorine gas:
The chlorine gas produced can go on to react with water to produce additional chemical substances:
These chemical reactions are illustrated in Fig. 1.
Essential chemical reactions occurring at the anode and cathode during the electrolytic process. NaCl sodium chloride, NaOH sodium hydroxide, Cl 2 chlorine (gas), HCl hydrochloric acid, HClO hypochlorous acid, H 2 O water, OH − hydroxide ion, O 2 oxygen (gas), H 2 hydrogen (gas) (Reprinted from Gravante et al. (2011), copyright 2009, with permission from Elsevier)
Species produced at the anode and cathode are transported to the surrounding tissue due to diffusion from a concentration gradient and migration due to the potential gradient. Negatively charged ions become attracted to the anode, and positively charged ions move toward the cathode. At the anode, destructive species may include metal ions, hydrogen ions, and various chemical species containing oxygen and chlorine (Nilsson et al. 2000). Smaller, more mobile, and abundant ions initially alter the tissue’s local chemistry. Reactions at the anode attract protons (H+), resulting in the formation of an acidic region. At the cathode, hydroxyl ions and molecular hydrogen act as destructive reaction products (Nilsson et al. 2000). Hydrogen is given off as a gas, and a more alkaline region forms at the cathode due to electrochemical reactions and a repulsion of protons (Finch et al. 2002a).
Electrolytic cell death is driven by the presence of a cytotoxic environment that develops due to local changes in pH as well as the presence of the new chemical species formed during electrolysis. Cell necrosis occurs when a pH of less than 6 or greater than 9 is reached (Finch et al. 2002b). The lethal effects of electrolysis on the tissue is a function of the concentration of electrochemical products produced and the time of exposure to these products. The area of necrosis has been shown to depend on the coulomb dosage applied (Colombo et al. 2007; Czymek et al. 2011), although this dependency may be contingent upon the experimental conditions.
Pathology Findings
Electrolytic ablation is characterized by a lesion that begins at the electrodes and propagates outward with increased delivered charge, as expected for a process that is driven by diffusion mechanisms. The resulting lesion has an irregular shape, with a greater volume affected near the tissue surfaces that were in contact with the electrodes. The cell morphology is different at the cathode side of the lesion in comparison to the anode side. Around the anode , the lobular architecture tends to be retained, and the cells become more amorphous and eosinophilic, whereas, at the cathode side, the tissue is more disrupted (Finch et al. 2002a). This dissimilar appearance is caused by different products of electrolysis near the cathode and the anode, resulting in different ablative reactions. Near the anode, the pH is low, and many of the toxic chemical species are related to the various pH-dependent components of Cl. Near the cathode, on the other hand, the pH is high and the toxicity is primarily caused by various components of OH−. In addition, electroosmotic migration of water from the anode to the cathode results in desiccation near the anode and edema at the anode. It has been observed during electrolysis that the resulting lesion near the anode surface is larger than that near the cathode. This may be attributed to the formation of chlorine at the anode, which acts as a more destructive chemical than those produced at the cathode (Finch et al. 2002a).
Advantages and Limitations
Electrolysis has been harnessed for tissue ablation in medicine since the early 1800s (Amory 1886). During the last two decades, this field has seen substantial research advances. From an operational standpoint, electrolysis requires very low voltages and currents, providing advantages relative to other ablation techniques. Low voltages can help avoid muscle contractions during application, and these low voltage and current parameters reduce the need for instrument complexity. One major limitation of electrolytic ablation, however, is that it is a lengthy procedure (requiring current applications for tens of minutes to hours). The time scale for application is controlled by the process of diffusion of the toxic chemical species from the electrodes into the tissue. High concentrations of electrically produced ablative chemical species are required, thus limiting the speed of the procedure.
Fundamental Principles of Electroporation
Mechanism of Action
Electroporation is characterized by the permeabilization of the cell membrane’s lipid bilayer through the application of very brief (nanosecond to millisecond), high-field (in the range of MV/m) electric pulses across the cell. The effects of electroporation depend on the magnitude and duration of the pulsed electric field as well as other factors such as cell size and shape and number of electrical pulses applied. The electric field magnitude triggers pore formation, whereas electric field strength and/or pulses with longer duration and/or number affect the pore expansion process (Rems and Miklavcic 2016).
Though a comprehensive theory has not yet been developed to fully explain the mechanism of electroporation, extensive experimental work and proposed models have developed a strong foundation that has allowed for the development of electroporation for a wide array of applications and is currently being built upon to produce a more detailed understanding of the phenomenon. The essential features of the electroporation process are known and can be briefly summed up as follows:
-
1.
Electroporation utilizes short (on the order of ns to ms) electrical pulses, applying an elevated transmembrane potential. The cell membrane requires a certain charging time before the transmembrane potential reaches a critical threshold. This charging time depends on electrical properties of the cell and solution as well as cell size and shape (Rems and Miklavcic 2016) and may vary from an order of 100 ns to several microseconds.
-
2.
The membrane conductivity increases immediately, and a time-dependent membrane transition occurs as long as the externally applied electric field is held at over above the critical value.
-
3.
Depending on the electrical parameters utilized, once the external electric field is removed, either membrane stabilization and resealing occur for reversible electroporation or loss of cell homeostasis leads to cell death by irreversible electroporation.
For reversible electroporation , once the electric field is lowered below the critical value, a stabilization process occurs over a few microseconds. The transmembrane potential drops quickly to near zero, and the membrane dramatically recovers to a level in which it is permeable to only small molecules. The membrane reseals slowly over seconds or even minutes. For irreversible electroporation, cell death occurs via a number of potential mechanisms such as continued pore growth and membrane rupture, membrane rupture due to colloidal-osmotic swelling, changes in ionic concentrations, and loss of cellular content. This process of pore formation is illustrated in Fig. 2, based on the theory of aqueous pore formation.
Membrane pore formation during electroporation. An idealized schematic of the lipid bilayer membrane of electroporation based on the aqueous pore formation theory. (a) Thermal fluctuations occur in the lipid bilayer membrane. (b) After the membrane has charged to threshold value, a hydrophobic pore is created, allowing water molecules to start penetrating the bilayer. (c) The lipids start reorienting to form a more stable hydrophilic pore, enabling more water and other polar molecules and ions to pass through the membrane. (d) The pore will shrink and eventually reseal after the external electric field has been removed. For irreversible electroporation, extensive transport through the cell membrane and slow pore resealing result in cell death
The family of electrical pulses that cause electroporation are divided into three types: in reversible electroporation (RE), the cells survive the permeabilization process, nonthermal irreversible electroporation (NTIRE) results in cell death due to the lipid bilayer destabilization and permeabilization, and irreversible electroporation coupled with thermal effects results when sufficiently strong fields are applied, causing a temperature increase sufficiently high enough for thermal damage to occur. Electroporation is becoming extensively utilized in biotechnology and medicine. In the reversible mode, electroporation has become a central technology for cell manipulation, and, in combination with chemicals, fundamental research and clinical trials have demonstrated its promise for gene therapy (Lambricht et al. 2016), and it is used clinically to target cancerous tissue through electrochemotherapy. Irreversible electroporation has been harnessed to ablate tissue while retaining the structural integrity of blood vessels, nerves, and extracellular matrix (Jourabchi et al. 2014). The ablative modality of NTIRE has been shown to result in a quicker recovery of the biological tissues (Phillips et al. 2012). The ability to apply NTIRE in a minimally invasive manner and the safety of this procedure have led to a recent surge in its clinical use.
Pathology Findings
NTIRE results in focused apoptotic cell death and the creation of sharp boundaries between the ablated cells and the adjacent, normal tissue. NTIRE has been shown to spare the surrounding extracellular tissue structure. Blood vessels, bile ducts, the urethra, and nerves are left intact and continue to function normally (Jourabchi et al. 2014). It is believed that these structures remain undamaged because their high collagenous connective tissue and elastic fiber content lack a cellular membrane that could be targeted by NTIRE. This preserving feature could also be due to the gap junction found within the cellular structure, allowing the electric current produced by NTIRE to travel through the gap junctions without affecting the cell membrane (Jourabchi et al. 2014).
Advantages and Limitations
A major advantage of tissue ablation by electroporation is the relative speed of the procedure in comparison to any other ablation technique. In addition, because the procedure targets the cell membrane, critical features of the extracellular matrix are spared. However, irreversible electroporation usually employs up to one hundred pulses of microsecond length and electric fields up to the several kV/cm range which produce muscle contractions and require the use of paralyzing drugs and may affect the electrical function of the heart. Reversible electroporation for electrochemotherapy employs lower electric fields in the range of 300 V/cm to 1 kV/cm, typically applied over eight pulses. Tissue ablation by RE, however, requires the injection of chemo toxic drugs, such as bleomycin or cisplatin. Finding a way to harness electroporation for tissue ablation without the need for additional drugs, including total-body paralysis agents, would provide great strides in simplifying the use of electroporation for medical applications and increase its ability for use within developing countries.
The Combination of Electrolysis with Electroporation
Presence of Electrolytic Phenomenon During Traditional Electroporation
Electroporation pulses in tissue have been shown to produce some level of electrolysis at the electrodes, generating products of electrolysis that could ablate cells. This phenomenon has been demonstrated through agar gel models (Turjanski et al. 2011), ex vivo experimental work on excised tissue (Maglietti et al. 2013), and in vivo and mathematical models (Olaiz et al. 2014). The resulting cytotoxic environment could prove detrimental to some reversible electroporation processes that depend on the cell to stay alive. For example, cell damage during gene electrotransfer would decrease the overall efficiency of the procedure. Thus, initial efforts sought to reduce and minimize this effect (Olaiz et al. 2014).
Electrolytic effects have been demonstrated to contribute to cell death in applications that were previously attributed solely to irreversible electroporation. Rubinsky et al. (2015) demonstrated that the cytotoxic effects previously attributed to IRE could actually be caused by at least three different mechanisms: reversible electroporation combined with electrolysis, irreversible electroporation combined with electrolysis, or irreversible electroporation without electrolysis. These different mechanisms of cell death may result in distinct effects on the cell and tissue ablation. A schematic to summarize which mode may become relevant, based on electric field magnitude and electric current delivery time, is illustrated in Fig. 3.
Tissue ablation domains as a function of electric field and exposure time. E indicates electrolysis ablation; R + E represents a combination of reversible electroporation and electrolysis ablation; IRE + E represents a combination of irreversible electroporation and electrolysis (Reprinted from Rubinsky et al. (2015) © the Author(s) 2015, permission from Sage Publishing)
The presence of electrolysis during electroporation procedures may also explain a phenomenon that has been observed clinically. During clinical use of NTIRE as well as occasionally during the clinical use of reversible electroporation, loud explosion-like sounds may be produced. These noises may be caused by electric breakdown across gases near the electrodes produced by electrolysis. During NTIRE studies, a bright spot is often produced near the electrodes on ultrasound that increases in size with the number of applied pulses. A bright hyperechoic appearance has also been noted in the region adjacent and between the electrodes when performing electrolysis experiments under the guide of ultrasound (Stehling et al. 2016). As noted by Stehling et al. (2016), it is likely that this bright spot in both cases is caused by the presence of gases produced due to electrolytic reactions at the electrodes. The ultrasonic waves are reflected from the interface between the tissue and gases, resulting in the observed hyperechoic appearance. Guenther et al. (2015) demonstrated that the increase in electrolytic gases around the electrode, as observed on ultrasound, corresponds to an increased electric discharge. This electric discharge occurs primarily at the cathode and follows a pattern typical of electrical breakdown across ionized gases at atmospheric pressures. The electrolytic products produced at the cathode contribute toward the electrons at discharge. When the electric field across the gas layer increases above the breakdown voltage, electric discharge occurs, accompanied by light from the ionized gas and producing the violent sound often observed (Guenther et al. 2015). The electric discharge phenomenon generates high-pressure waves and could be detrimental to the organ being treated (Stehling et al. 2016). A further understanding of the presence of electrolysis during electroporation may help to devise electroporation protocols that minimize this electric breakdown effect.
Overall, these studies indicate that some level of electrolysis may occur in all electroporation protocols and must be considered and taken into account. However, the combination of electrolysis and electroporation is not a detrimental in all cases and indeed may be harnessed as new modality for tissue ablation that is seen to meet many of the shortcomings of electroporation or electrolysis alone.
Combining Electroporation and Electrolysis: Proposed Mechanism of Increased Tissue Ablation
It has been proposed that combining the cell permeabilization effect of electroporation with the effects of the electrolytic products generated from electrostimulation could result in a new ablation modality with an efficiency that is dramatically increased compared to electrolysis alone. Though additional studies are warranted to study this phenomenon, one explanation for this effect is that the electrolytically produced chemicals can pass through the pores formed in the cell membrane during electroporation, as illustrated in Fig. 4.
Proposed mechanisms of combining electrolysis with electroporation. Low levels of electrolysis may not be strong enough to cause cell death on their own, and reversible electroporation on its own causes cell to become temporarily permeabilized followed by recovery. Combining these two phenomena, however, would allow the electrolytic products to pass through the permeabilized cell and cause cell death at much lower concentrations
This interaction could be leveraged to induce cell death using a lower applied electric charge than required for traditional electrolytic ablation. This mechanism of tissue ablation could be harnessed through a variety of methods. New methods that provide support for the proposed mechanism of action and take advantage of this combined effect are being developed. Recent investigation into new synergistic electroporation and electrolysis (SEE) protocols is described in the next section.
Methods for Applying Synergistic Electroporation and Electrolysis (SEE) for Tissue Ablation
New methods and protocols are currently being developed to investigate the effect of SEE and develop methods for tissue ablation that may be used clinically. Though this field is new and much more work is needed for understanding the mechanisms at play and optimizing treatment parameters for clinical use, it is beneficial to look at different methods that have been used thus far to illustrate the SEE effect. These experimental results give new insight into the technology and help develop a foundation for future work. The types of SEE parameters that have been applied experimentally can be divided into four different groups, each of which illustrates a unique way to achieve tissue ablation by enabling electrolytically produced chemical products’ access to the cell interior through electroporation. Each type of SEE protocols may be more applicable for specific applications.
-
1.
SEE by reversible electroporation and low-charge electrolysis in series
-
2.
SEE by irreversible electroporation and low-charge electrolysis in series
-
3.
SEE applied through multiple reversible electroporation-type pulses
-
4.
SEE achieved through the use of a single decay pulse
Each of these methods illustrates a unique way to achieve tissue ablation by enabling electrolytically produced chemical products’ access to the cell interior through electroporation. Overall, they help to gain a stronger foundation of understanding around the technology that can be used for further development in this field.
SEE by Reversible Electroporation and Low-Dose Electrolysis in Series
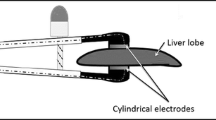
An ablation protocol that combines reversible electroporation with low-charge electrolysis would allow electrolytic products’ direct access to the cell interior, requiring a much lower electric field than irreversible electroporation, reducing the treatment time and level of electrolytic products required during electrolysis, and avoiding the need for drug injection as practiced with electrochemotherapy. The synergistic electroporation and electrolysis effect has been demonstrated as a new tissue ablation modality by combing reversible electroporation and low-dose electrolysis together in series. This was first proven using a simple parallel-plate electrode configuration to apply electrical parameters across the rat liver in vivo (Fig. 5f) (Phillips et al. 2015a). Though the experimental setup is less clinically relevant, the simple experimental design allows for results that effectively illustrate the result of SEE . Hematoxylin and eosin (H&E) cross sections of the treated livers are shown in Fig. 5. Parameters of typical electrolytic ablation (8 mA applied for 60 s) resulted in transverse ablation across the liver thickness, as expected (Fig. 5a). A set of low-charge electrolysis parameters were developed by reducing the current and applied time (4 mA, 30 s), resulting in a decreased charge delivered to the tissue and a much smaller ablation volume, centered around the electrode surfaces (Fig. 5b). Reversible electroporation parameters (electric field of 250 V/cm, 100 μs pulse length, and 8 pulses applied at a 1 Hz frequency) were also shown to result in minimal tissue ablation, with the volume of ablation located near the electrode surfaces only (Fig. 5c). When the low-charge electrolysis was combined with reversible electroporation, however, transverse tissue ablation was achieved (Fig. 5d, e).
Demonstration of the effectiveness of combining electrolysis and electroporation for increased ablation volume. Electrical parameters are applied across the liver lobe thickness using plate electrodes (f). The anode is adjacent to the top surface of the liver and the cathode is adjacent to the bottom surface. H&E staining is used to visualize the volume of ablation, and the region of ablation is outlined using dashed lines. Typical parameters used for electrolysis (E) result in transverse ablation across the liver cross section (a), delivering a total charge per unit area of 1.24 C/cm2. Decreasing the delivered charge to 0.31 C/cm2 results in non-transverse electrolytic ablation located near the electrode surfaces (b). Typical parameters used for reversible electroporation (RE) deliver a charge per unit area of 0.0001 C/cm2 and result in some minimal tissue ablation near the electrode surfaces due to electrolytic effects (c). When electrolysis and reversible electroporation are combined in series, however, transverse ablation is achieved (d, e)
During electrolytic ablation , the delivered charge can be used as a quantitative measure of the amount of products of electrolysis generated at the electrodes. Since these products of electrolysis propagate inward into the tissue through diffusion, the extent of tissue ablation depends on the amount of charge delivered at the electrodes as well as the distance of that point from the electrodes. Thus, when comparing SEE protocol results with those of electrolysis and reversible electroporation alone, the delivered charge can be used as a measure of the electrolytic products produced. The delivered charge by reversible electroporation alone was orders of magnitude lower than either electrolysis protocol and thus should have an infinitesimal effect on pure electrolysis. Nonetheless, the results shown in Fig. 5 show that a substantial increase in tissue volume when electrolysis and reversible electroporation are combined.
This hypothesis has been further supported by the use of a more clinically relevant experimental setup, using titanium needle electrodes to apply the electrical parameters to an in vivo porcine liver model (Stehling et al. 2016). When the reversible electroporation parameters were followed by the low-charge electrolysis protocol, the ablated area significantly increased. The treated volume was increased even further by applying reversible electroporation parameters, followed by the low-charge electrolysis protocol, followed by another set of reversible electroporation parameters. The mechanisms at play likely involve the use of conventional electrolysis as the central tissue ablation modality while utilizing electroporation pulses to permeabilize the cell membrane, allowing for cell death to occur at lower amounts of electrolytic products.
SEE Applied as a Combination of Electrolysis and Irreversible Electroporation
The combined use of electrolysis and electroporation has the potential application in extending the ablated tissue volume in conventional NTIRE without increasing the voltage on the electrodes. This would take advantage of the region that develops outside of the irreversible electric field zone in which a lower electric field, typical of reversible electroporation protocols, is present. The presence of even low-level electrolytic products in this region could enable the ablation zone to be extended to the edge of the reversible electroporation threshold of the electric field. Survival of the tissue between electrodes is a critical concern during the use of NTIRE clinically, and developing methods to increase the ablation zone would be greatly beneficial to the field. SEE has been shown to increase this ablation zone (Stehling et al. 2016), using an NTIRE-electrolysis-NTIRE treatment sequence. These results fit the overall hypothesis of SEE. When NTIRE treatment parameters are applied by needle electrodes, the strength of the electric field decreases with distance from the electrodes. Beyond the volume of tissue treated by irreversible electroporation lies a region where electric fields are still strong enough to produce reversible electroporation effects (Fig. 6). It is expected that by combining electrolysis with NTIRE, ablation can now be achieved within the reversible electroporation region via electrolytically produced products.
A mathematical model of two 1-mm-diameter needle electrodes in a volume of tissue. Regions that experience an electric field magnitude over a given threshold (region within inner contour line) experience irreversible electroporation, resulting in tissue ablation. Reversible electroporation is achieved for lower electric fields (region between outer and inner contour line). Results were obtained for an 800-μs, 1331-V pulse. By inducing cell death within the reversible electroporation zone, the volume of treated tissue can be increased (Reprinted from Davalos et al. (2005), © 2005, with permission of Springer)
SEE Applied Through Multiple Reversible Electroporation-Type Pulses
Reversible electroporation on its own can produce a limited electrolytic ablation effect, typically confined to the tissue volume immediately adjacent to the electrode surfaces. A third type of SEE parameters uses a single protocol of multiple reversible electroporation-type pulses to increase the electrolytic effect, enabling tissue ablation by applied electric fields below the threshold for irreversible electroporation (Phillips et al. 2015b). By using delivered charge as a comparative measure, the multiple-pulse SEE protocol can be designed to produce a much more effective method of electrolytic ablation. The SEE protocol is able to produce damage comparable to that of conventional electrolytic protocols while delivering orders of magnitude less charge to the target tissue over much shorter periods of time. This effect is illustrated in Fig. 7, using a simple parallel-plate electrode configuration (same as shown in Fig. 5f) to apply the electrical parameters to liver tissue.
SEE delivered by multiple reversible electroporation-type pulses. Electrical parameters are applied across the liver lobe thickness using plate electrodes (Fig. 5f). The anode is adjacent to the top surface of the liver and the cathode is adjacent to the bottom surface. Masson trichrome staining is used to visualize the volume of ablation, and the region of ablation is outlined using dashed lines. Typical parameters used for electrolysis (E) illustrate the pattern of tissue ablation with increasing charge delivered (a–c). Reversible electroporation-type parameters were chosen to increase the charge delivered (d–f), resulting in a pattern of ablation that is markedly similar to that obtained by typical electrolysis. The volume of ablation achieved with SEE, however, occurs at orders of magnitude less delivered charge than required for typical electrolysis
The ablation zone achieved by applying SEE through multiple low-voltage electroporation pulses extends throughout the reversible electroporation zone surrounding electrodes when using a clinically relevant two-needle electrode setup (Stehling et al. 2016). This further supports the mechanistic explanation for how SEE increases ablation efficiency and can be used to increase the treated volume. In Fig. 8, calculated isoelectric field lines are superimposed on histology images of the ablated zone. When a low number of pulses are applied, the ablation zone extends to electric field lines greater than 500 V/cm corresponding to ablation by IRE and suggesting that the combined effect of electrolysis and electroporation did not occur. However, when the number of pulses is increased, increasing the total charge applied to the tissue, the region of ablation extends to electric field lines of approximately 200 V/cm, indicating that cell death is occurring in the surrounding reversible electroporation zone due to the SEE effect.
Extent of tissue ablation achieved by eight electrochemotherapy-type pulses compared to a high number of electrochemotherapy-type pulses. Eight typical electrochemotherapy magnitude-type pulses (1000 V, 100 μs, 1 Hz) result in a minimal level of ablation, as shown by gross macroscopic section (a) and trichromatic staining (b). This is compared to increased level of ablation achieved by increasing the number of pulses to 297 (c) and (d). Calculated isoelectric field lines are superimposed on the bottom row, showing ablation extents to an electric field of approximately 200 V/cm. These isoelectric field lines are also applicable for the top row as well and would indicate that ablation extents to an electric field of greater than 500 V/cm for the 8-pulse protocol (Reprinted from Stehling et al. (2016) (© Stehling et al. 2016) licensed under CC BY 2.0)
A possible application of this type of SEE parameters is a method of tissue ablation that uses reversible electroporation strength fields to permeabilize the cell membrane to products of electrolysis, thus inducing cell death. These SEE parameters result in cell death without requiring the high voltages of irreversible electroporation while relying on a drastically decreased level of electrolytic product production than required for traditional electrolytic ablation.
A Single Exponential Decay Pulse for Achieving SEE Ablation
A single exponential decay pulse is another type of SEE parameter that can produce ablation with the tissue. A discharge capacitor can deliver a pulse that decays exponentially and is defined by the initial voltage and the time constant. The applied voltage for the exponential decay pulse can be given as:
where V o is the initial voltage, t is time, and τ is the time constant. A set of capacitor discharge parameters can be chosen such that the initial high voltage at the beginning of the pulse serves to generate electroporation, while the rapid decay toward a trailing low voltage generates sufficient charge for the generation of electrolytic products. When the exponential decay pulse delivers a relatively low level of charge, no tissue ablation is observed. Increasing the delivered charge by applying a higher initial voltage and/or longer time constant can be used to increase the ablation volume (Phillips et al. 2016). It should be noted, however, that continued increase in applied voltage will eventually lead to tissue ablation by irreversible electroporation effects rather than SEE.
These single exponential decay SEE parameters indicate a method for tissue ablation that allows for reduced instrument complexity in comparison to that used by irreversible electroporation while requiring shorter application time than traditional electrolysis. In addition, the single exponential decay pulse causes a substantial reduction in muscle contractions in comparison to the same level of ablation achieved by a typical multiple-pulse electroporation protocol. This may pave the way to the development of protocols that do not require the use of general anesthesia and neuroblocking agents during clinical use, an area which is currently seen as a major limitation of current irreversible electroporation protocols.
Pathology Findings
Histological analysis of the lesion produced by SEE ablation reveals that multiple modes of cell death may be present, depending on the type of SEE parameters applied, the electrode configuration, and the location within the tissue relative to the electrodes. The mechanisms of damage can be divided into at least five different categories (Stehling et al. 2016):
-
1.
Dominant anodic ablation
-
2.
Dominant cathodic ablation
-
3.
Combination reversible electroporation and anodic compounds
-
4.
Combination reversible electroporation and cathodic compounds
-
5.
Irreversible electroporation
SEE ablation protocols result in a sharp transition between the untreated cells and the ablated region, marked by the presence of congestion of the sinusoids. The appearance of the ablated cells differs throughout the ablated region, and marked differences are apparent when comparing cell morphology near the cathode to that near the anode . The treated zone is larger near the electrodes and grows outward toward the center tissue, resulting in either two separate lesions or a single dumbbell-shaped lesion. Large blood vessels remain intact and patent (Stehling et al. 2016), a feature that has been also observed for both electrolysis and electroporation when applied in separate protocols.
When SEE was applied across the liver tissue using parallel-plate electrodes both by the method of reversible electroporation and low-charge electrolysis in series (Phillips et al. 2015a) and by the method of using multiple reversible electroporation-type pulses (Phillips et al. 2015b), histological analysis indicated that the mode of tissue ablation maintained key characteristics of electrolytic ablation. The appearance of the treated tissue at the anode, at the cathode, and in the core of the treated tissue was markedly different, caused by different products of electrolysis near the anode versus the cathode that result in different ablative reactions. The general mode of damage is consistent with an electrolysis mechanism of damage; the tissue ablation begins from the vicinity of the electrodes, is different at the anode side in comparison to the cathode side, and increases to encompass the entire tissue between the electrodes with an increased delivered charge. Thus, it is likely that dominant anodic ablation and cathodic ablation occurred immediately adjacent to the electrode surfaces. However, the ablation seen throughout the majority of the liver thickness in Figs. 5 and 6 is likely due to the third and fourth mechanisms of damage, combining reversible electroporation with either electrolysis products produced from the anode or from the cathode. These electrolytic products are able to penetrate the electroporated cells, resulting in electrolytic cell death even when only low levels of electrolytic product are present.
An irreversible electroporation zone, however, can also be present, depending on the parameters used. When applying irreversible electroporation with electrolysis to increase the ablation volume, a volume of the tissue will experience histological effects that can be primarily attributed to cell death by NTIRE. An NTIRE-dominated ablation zone has also been shown to be present in histology analyzed from multiple low-voltage electroporation pulses applied through needle electrodes (Stehling et al. 2016).
Histological analysis for a single decay pulse also demonstrates an ablation pattern similar to that achieved by electrolytic ablation. By increasing the applied charge, the volume of ablation also increases, starting at the electrode surfaces and moving in toward the center of the tissue volume. For the parallel-plate electrode experimental model described by Phillips et al. (2016), initial electric field of 1000 V/cm and 500 V/cm appears to apply a mechanism of tissue ablation that combines electroporation effects with electrolysis (damage modes 1–4). Increasing the electric field to 1500 V/cm, however, results in a mode of ablation is likely dominated by irreversible electroporation effects rather than the combination of electrolysis and electroporation.
Imaging
Imaging methods for combined electrolysis and electroporation protocols may be developed based on imaging success seen when each ablation modality is applied separately. For example, ultrasound monitoring, magnetic resonance imaging , and non-contrast and contrast-enhanced CT have been used to assess the volume of ablation zone after electroporation (Yarmush et al. 2014). In addition, electroporation leads to an immediately detectable impedance decrease, and electrical impedance tomography is a method that is under development, providing a two-dimensional reconstruction of tissue impedance that allows for near-real-time monitoring of tissue electroporation (Yarmush et al. 2014). Local changes in pH can be used to reliably monitor the extent of tissue ablation achieved by electrolytic ablation (Finch et al. 2002b). Discrete pH-measuring devices, however, cannot provide a continuous, spatial image of the pH distribution, and thus an alternative method for real-time monitoring of lesion size is desired. Bubbles of gas produced during electrolysis have made the use of ultrasound monitoring of lesions difficult (Finch et al. 2002a). A technology that can detect pH changes in both time and space is directly applicable for research and clinical applications of electrolysis. Electrical impendence tomography has been shown as one possible mechanism for monitoring lesions created during electrolysis in real time (Meir and Rubinsky 2015). This method takes advantage of the relatively small size of proton ions and hydroxyl ions compared to other physiological ions produced during the electrochemical reaction. These smaller ions not only result in local pH changes but also lead to increased local mobility, which in turn results in increased conductivity around the anode and the cathode. This local change in tissue conductivity can be monitored in real time using principles of electrical impedance tomography. Magnetic resonance imaging (MRI) can also be used to image the process of electrolysis by detecting pH fronts (Meir et al. 2015), resulting in another promising method for monitoring and advancing the use of this technology. Future work wi ll develop these imaging techniques for real-time monitoring of tissue ablation by SEE.
Conclusions
The synergistic combination of electroporation and electrolysis can be used as a new tissue ablation modality, resulting in cell death at much lower levels of electrolytic product than required by traditional electrolysis alone. By combining electrolysis and electroporation, a new method may be achieved that addresses some of the clinical drawbacks of each technology when applied individually. SEE may be applied through a variety of different types of ablation protocols. This new type of treatment method is still in the early stages of development, and substantial additional research and quantitative analysis are necessary to advance this field. Though initial studies appear to support the proposed mechanism of SEE ablation, further research is necessary to gain an in-depth knowledge of this phenomenon. There are various parameter configurations that can be chosen to apply SEE, and the method of choice may depend strongly on the individual application. These different types of SEE protocols need to be further examined and refined in order to choose protocols that are most relevant clinically. In addition, new types of SEE protocols may be developed that allow for increased ablative efficiency, reduced instrument complexity, or serve to meet other specific clinical needs.
Cross-References
-
Current Density Imaging as Means to Follow Tissue Electroporation
-
Mass Transfer of Electrolytic Species During Electric Field-Based Tumor Treatments
-
Modeling of Electrochemical Reactions During Pulsed Electric Field Treatment
-
Principles and Use of Magnetic Resonance Electrical Impedance Tomography in Tissue Electroporation
References
Amory R (1886) A treatise on electrolysis and its therapeutical and surgical treatment in disease. William Woof & Co., New York
Colombo L, Gonzalez G, Marshall G, Molina FV, Soba A, Suarez C, Turjanski P (2007) Ion transport in tumors under electrochemical treatment: in vivo, in vitro and in silico modeling. Bioelectrochemistry 71:223–232
Czymek R, Dinter D, Loffler S, Gebhard M, Laubert T, Lubienski A, Bruch HP, Schmidt A (2011) Electrochemical treatment: an investigation of dose–response relationships using an isolated liver perfusion model. Saudi J Gastroenterol 17(5):335–342
Davalos RV, Mir LM, Rubinsky B (2005) Tissue ablation with irreversible electroporation. Ann Biomed Eng 33(2):223–231
Finch JG, Fosh BG, Maddern GJ (2002a) Direct current electrolysis for local ablation of liver metastases. In: Habid NA (ed) Multi-treatment modalities of liver tumours. Springer, Boston, pp 269–292. doi:10.1007/978-1-4615-0647-1_22
Finch JG, Fosh B, Anthony A, Slimani E, Texler M, Berry D, Dennison A, Maddern G (2002b) Liver electrolysis: pH can reliably monitor the extent of hepatic ablation in pigs. Clin Sci 102:389–395
Gravante G, Ong SL, Metcalfe MS, Bhardwaj N, Maddern GJ, Lloyd DM, Dennison AR (2011) Experimental application of electrolysis in treatment of liver and pancreatic tumours: principles, preclinical and clinical observations and future perspectives. Surg Oncol 20:106–120. doi:10.1016/j.suronc.2009.12.002
Guenther E, Klein N, Mikus P, Stehling M, Rubinsky B (2015) Electrical breakdown in tissue electroporation. Biochem Biophys Res Commun 467:736–741
Jourabchi N, Beroukhim K, Tafti BA, Kee ST, Lee EW (2014) Irreversible electroporation (NanoKnife) in cancer treatment. Gastrointest Interv 3:8–18
Lambricht L, Lopes A, Kos S, Sersa G, Preat V, Vandermeulen G (2016) Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin Drug Deliv 13(2):295–310. doi:10.1517/17425247.2016.1121990
Maglietti F, Michinski S, Olaiz N, Castro M, Suarez C, Marshall G (2013) The role of pH fronts in tissue electroporation based treatments. PLoS One 8(11):e80167
Meir A, Rubinsky B (2015) Electrical impedance tomography of electrolysis. PLoS One 1–16. doi:10.1371/journal.pone.0126332
Meir A, Hjouj M, Rubinsky L, Rubinsky B (2015) Magnetic resonance imaging of electrolysis. Sci Rep 5(8095):1–9. doi:10.1038/srep08095
Nilsson E, von Euler H, Berendson J, Thorne A, Wersall P, Naslund I, Lagerstedt AS, Narfstrom K, Olsson J (2000) Electrochemical treatment of tumours. Bioelectrochemistry 51:1–11
Olaiz N, Signori E, Maglietti F, Soba A, Suarez C, Turjanski P, Michinski S, Marshall G (2014) Tissue damage modeling in gene electrotransfer: the role of pH. Bioelectrochemistry 100:105–111
Phillips M, Raju N, Padath T, Rubinsky B (2012) Irreversible electroporation on the small intestine. Br J Cancer 106(3):490–495
Phillips M, Raju N, Rubinsky L, Rubinsky B (2015a) Modulating electrolytic tissue ablation with reversible electroporation pulses. Technology 3(1):1–9
Phillips M, Rubinsky L, Meir A, Raju N, Rubinsky B (2015b) Combining electrolysis and electroporation for tissue ablation. Technol Cancer Res Treat 14(4):395–410. doi:10.1177/1533034614560102
Phillips M, Krishnan H, Raju N, Rubinsky B (2016) Tissue ablation by a synergistic combination of electroporation and electrolysis delivered by a single pulse. Ann Biomed Eng. doi:10.1007/s10439-106-1624-4, Published online May 4, 2016
Rems L, Miklavcic D (2016) Tutorial: electroporation of cells in complex materials and tissue. J Appl Phys 119:201101. doi:10.1063/1.4949264 (21 pages)
Rubinsky L, Guenther E, Mikus P, Stehling M, Rubinsky B (2015) Electrolytic effects during tissue ablation by electroporation. Technol Cancer Res Treat 1–9. doi:10.1177/1533034615601549
Stehling MK, Guenther E, Mikus P, Klein N, Rubinsky L, Rubinsky B (2016) Synergistic combination of electrolysis and electroporation for tissue ablation. PLoS One 11(2):E0148317. doi:10.1371/JOURNAL.PONE.0148317
Turjanski P, Olaiz N, Maglietti F, Michinski S, Suarez C, Molina FV, Marshall G (2011) The role of pH fronts in reversible electroporation. PLoS One 6(4):e17303. doi:10.1371/journal.pone.0017303.t001
Yarmush ML, Goldberg A, Sersa G, Kotnik T, Miklavcic D (2014) Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng 16:295–320. doi:10.1146/annurev-bioeng-071813-104622
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Ho, M.P. (2017). Combining Electrolysis and Electroporation for Tissue Ablation. In: Miklavčič, D. (eds) Handbook of Electroporation. Springer, Cham. https://doi.org/10.1007/978-3-319-32886-7_63
Download citation
DOI: https://doi.org/10.1007/978-3-319-32886-7_63
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32885-0
Online ISBN: 978-3-319-32886-7
eBook Packages: EngineeringReference Module Computer Science and Engineering