Abstract
Angiogenesis, vasculogenesis, and arteriogenesis are endogenous responses to obstructive arterial disease or trauma causing tissue ischemia such as atherosclerotic plaques obstructing perfusion of the lower limbs or myocardial ischemia. Sufficient growth of collateral vessels is often impaired in patients due to factors such as the underlying atherosclerosis, age, or diabetes. There are many preclinical and clinical studies seeking exogenous angiogenesis induction to alleviate tissue ischemia. Translation of these therapies into clinical applications could address a multitude of ailments including wound healing, tissue grafting, bone and muscle regeneration, and myocardial repair. Gene therapy approaches delivering angiogenic factors have been attempted using various gene delivery methods. This chapter will focus on evaluating preclinical studies investigating application of gene electrotransfer approaches for ischemic tissues, in particular for wound healing, peripheral artery disease, and myocardial infarction. Gene electrotransfer has been utilized to deliver proangiogenic factors such as vascular endothelial growth factor, insulin-like growth factor, basic fibroblast growth factor, angiopoietin, etc. Delivery and expression have been demonstrated minimally invasively and invasively to the skin, to skeletal muscle, and to cardiac muscle. Notable improvement in tissue perfusion was consistently reported in skin flap wound healing models, in hind limb ischemia models, and in cardiac muscle ischemia model.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Gene electrotransfer
- Electroporation
- Angiogenesis
- Gene therapy
- Ischemia
- Peripheral artery disease
- Coronary artery disease
Introduction
Tissue ischemia is often caused by blockage of blood flow as a result from trauma or blood vessel disease such as atherosclerosis. Advanced atherosclerosis can lead to peripheral artery disease (PAD) or coronary artery disease (CAD) often with comorbidities depending on which arteries are affected (Ross 1993). Significant tissue ischemia results in diseased tissue and subsequent remodeling of the normal tissue structure, altering function. Patient quality of life is highly dependent on preserving as much of the original tissue function and tissue repair as possible. Clinical treatment options for patients with severely ischemic tissues focus on re-vascularizing either by circumventing blocked vessels with a bypass graft vessel or attempting to remove the blockage of the existing vessel and maintaining wall integrity with stents (Go et al. 2013). Multiple clinical studies investigating angiogenic therapies aiming to induce collateral vessel development have been reported with conflicting results (Wolfram and Donahue 2013). Main gene therapy approaches involve gene delivery of an angiogenic cytokine or growth factor, primarily vascular endothelial growth factor A (VEGF) or basic fibroblast growth factor (bFGF). The most commonly utilized delivery methods utilize viral-based methods. Adeno-associated virus (AAV) has been used extensively with high efficiency of gene transfer. However, there are considerable immunogenicity and toxicity considerations associated with this delivery method (Wolfram and Donahue 2013). Naked plasmid DNA delivery poses fewer side effects; however, lower gene transfer efficiency is expected (Wolfram and Donahue 2013). Gene electrotransfer (GET) in vivo has been shown to enhance naked DNA delivery, improving expression levels (Ferraro et al. 2009). In this chapter, GET delivery of angiogenic factors to ischemic tissues for wound healing, peripheral artery disease, and coronary artery disease will be evaluated.
Lower Limb Ischemia
Peripheral artery disease (PAD) generally refers to atherosclerosis of the arteries supplying the legs. Depending on the extent of the disease, PAD symptoms include patients experiencing pain, weakness, or numbness during walking; sores on toes, feet, or legs; change in leg color; and weak pulse. Severe PAD leads to tissue injury, possible open sore infections in feet and legs as well as tissue death sometimes requiring limb amputation. Severe PAD is often treated surgically with bypass grafting or percutaneous interventions (Collinson and Donnelly 2004). Patients with mild to moderate disease have few treatment options. Those having recurrent symptoms after already exhausting surgical interventions also have few available treatments (Collinson and Donnelly 2004). Due to the atherosclerosis, endogenous angiogenesis in response to hypoxic/ischemic conditions is impaired (Collinson and Donnelly 2004). Developing collateral circulation may improve patient symptoms; thus, many preclinical therapies are focused on angiogenesis induction. The standard preclinical in vivo model for PAD is the hind limb ischemia model in a small animal like mouse or rat, where a portion of the femoral artery is either ligated or even excised to induce ischemia to the downstream limb. Here, preclinical gene therapy approaches to PAD with gene electrotransfer-enhanced gene delivery in the hind limb ischemia model will be discussed.
Rabinovsky et al. (2004) reported the first gene electrotrasfer of an effector gene for repair of ischemic skeletal muscle. Plasmid DNA encoding insulin-like growth factor-I (IGF-I) was used for its angiogenic properties and potential therapeutic benefits for skeletal muscle repair postischemic damage. An intramuscular injection of DNA was performed to the anterior tibialis muscle. Parallel plate electrodes were placed directly over the injection site of the muscle to deliver pulsed electric fields. Two disease models were utilized. A crushed sciatic nerve model was used to cause muscle atrophy. In a different set of experiments, the femoral artery was ligated in diabetic mice to cause hind limb ischemia. In the case of sciatic nerve damage and subsequent muscle atrophy, increased levels of endogenous VEGF and VEGF receptors were observed after IGF gene transfer. While in the hind limb ischemia model, increased blood flow to the ischemic limb was confirmed with laser Doppler imaging, indicating angiogenesis had occurred (Rabinovsky and Draghia-Akli 2004).
A similar approach was reported by Nishikage et al. (2004). Gene electrotransfer of basic fibroblast growth factor (bFGF) was performed in a rabbit hind limb ischemia model. Ischemia of the hind limb was induced by surgically excising a portion of the femoral artery. GET was performed directly on the muscle 10 days after excision of a portion of the femoral artery. Plasmid DNA encoding bFGF was injected into the ischemic muscle followed by insertion of needle electrodes 1 cm apart. Pulsed electric field was delivered with six pulses of 50 ms, and alternating polarity, 75 V, was applied. Angiogenesis was confirmed with angiography imaging. The vascular tree contained new blood vessels implicating angiogenesis induction. Limb survival compared to controls was also observed, implicating functional blood vessel growth and resolution of ischemia (Nishikage et al. 2004).
A common concern associated with angiogenesis inducing therapies is developing leaky, unstable vessels that never mature to functional vasculature. Immature blood vessels are inefficient at reducing ischemia and promoting tissue repair postischemic damage. Jiang et al. (2006) attempted to address this concern with co-delivery of VEGF and angiopoietin-I (Ang-I) encoding plasmid DNA via electrotransfer. Experiments were conducted in a rat hind limb ischemia model. A portion of the femoral artery was excised to establish distal limb ischemia. GET was performed on the muscle tissue distal to the excision site. Pulses were delivered with a two-needle electrode with a 5 mm gap between the needles. One hundred volts were applied across the needles. Pulse duration was 40 ms. Six pulses were applied. Microvessel density measurements and angiography were performed to evaluate angiogenesis induction. A modified Evans blue assay was performed to indirectly evaluate blood vessel maturation and integrity of the vessel walls. Leakage through the blood vessels was assessed by measurement of the Evans blue in tissue interstitial space. Co-expression of Ang-I and VEGF resulted in increased perfusion relative to single expression controls of either Ang-I or VEGF. Evans blue dye analysis indicated more presence of dye in excised muscle tissue from single expression groups than the co-expression group, indicating leaky vessels, although it is unclear how it was determined that extracted dye from muscle tissue came from interstitial fluid and not blood vessel lumens located within the excised tissue. It was concluded that Ang-I and VEGF have a synergistic effect on angiogenesis and blood vessel maturation, resulting in functional vasculature development in the ischemic muscle (Jiang et al. 2006).
An alternative strategy for inducing angiogenesis and blood vessel maturation, thus reducing leaky, receding vessels, reported co-delivering phage phiC31 integrase with mVEGF164. A mouse hind limb ischemic model was used for these co-delivery experiments. Portlock et al. (2006) hypothesized that transient expression of angiogenic factors resulted in transient perfusion to the ischemic limbs and long-term expression of angiogenic factor may lead to angiogenesis, blood vessel maturation, and stable blood vessel supply to the ischemic tissue. Therefore, site-specific integration of plasmid DNA encoding mVEGF164 was proposed. The phiC31 integrase system with VEGF was injected into the ischemic muscle and was followed by pulsed electric field application to achieve electrotransfer and site-specific delivery. Levels of VEGF expression were measured over a 40 day period. Persistently high VEGF levels were observed throughout the duration of the study up to 40 days. Site-specific integration of plasmid DNA was confirmed, implicating constitutive expression of VEGF (Portlock et al. 2006). While therapeutic merit was demonstrated over 40 days, and further long-term benefits are strongly implicated as the benefits of permanent integration and constitutive expression of VEGF, there are considerable concerns with permanent overexpression of a potent angiogenic factor.
In atherosclerotic disease, established blood vessels harden and become blocked over time, reducing blood flow to distal regions. In patients affected by atherosclerosis, normal angiogenesis in response to hypoxia/ischemia may be impaired. Therefore, preclinical models demonstrating angiogenesis in non-atherosclerotic ischemic disease models may not be representative of clinical outcomes in patients with impaired angiogenesis. In order to investigate angiogenesis in an ischemia model closely resembling atherosclerotic disease, hind limb ischemia was induced in ecNOS-KO mice. These mice have impaired angiogenesis. NOS1177D encoding plasmid DNA was injected into the ischemic skeletal muscle after induction of ischemia via femoral artery occlusion. Caliper electrodes were used to deliver eight pulses of 200 V/cm. Caliper electrodes require pinching the tissue of interest between the plates of the caliper and thus are difficult to translate to clinical applications especially for gene delivery to skeletal muscle. The pulse duration was 20 ms. Qian et al. (2006) reported reversal of hind limb ischemia in angiogenesis impaired mice (Qian et al. 2006).
Another co-expression study was reported by Sacramento et al. (2010) delivering VEGF and granulocyte colony-stimulating factor (G-CSF) encoding DNA in a mouse model of hind limb ischemia. The authors hypothesized that local transient expression of VEGF and stem cell mobilizer G-CSF genes may complement activities in repair of ischemic limbs. Hind limb ischemia was surgically induced in a mouse model. Three pulses were delivered with needle electrodes. An electric field of 75 V/cm was applied with a 20 ms pulse length. Necrosis, capillary density, muscle mass, muscle force, and hematopoietic cell mobilization were measured and analyzed 3–4 weeks post electrotransfer. Animals that were treated with both VEGF and G-CSF genes presented with reduced incidence of limb necrosis. Muscle mass recovery was significantly higher than in animals treated with either VEGF or G-CSF genes individually. The animals in the double transfection group also had higher muscle force than those in single transfection groups, up to 60% of muscle force measured in the nonischemic group. Capillary density was found to be higher in the double transfection group, with reduced degree of fibrosis. Infiltration of Sca1 + cells and neutrophils was also higher in the double transfected group, indicating a possible mechanism for reduction of necrosis. Therefore, a synergistic effect between promoting blood vessel formation and tissue repair upregulation was observed (Sacramento et al. 2010).
Ferraro et al. (2010) reported a noninvasive gene delivery approach to improving hind limb ischemia in a rat model. This noninvasive approach delivers plasmid DNA to the skin overlaying ischemic tissue minimizing the need for surgery to expose ischemic muscle and potentially minimizing the need for general anesthesia. Hind limb ischemia was established in a rat model with excision of a portion of the femoral artery. Fibroblast growth factor-2 (FGF-2) encoding plasmid DNA was delivered intradermally to the skin overlaying the ischemic muscle. A 16-pin, multi-electrode array (MEA) was used to deliver eight pulses at 250 V/cm, with a 150 ms pulse duration. The pins are located 2 mm apart, in a 4x4 array. Additionally, this electrode design does not require pinching tissue between the electrodes, thus enhancing translation to clinical applications. Laser Doppler imaging was performed to monitor perfusion to the ischemic limbs in animals over time. Improved perfusion was observed in the gene transfer group compared to no gene transfer controls. Limb survival was also dramatically improved in the FGF-2 gene transfer group compared to appropriate controls (Ferraro et al. 2010). This report was the first instance of a noninvasive electrotransfer delivery approach to the skin that enhanced translatability of electrotransfer-mediated gene delivery for peripheral artery disease.
Co-expression of VEGF and hepatocyte growth factor (HGF) in a mouse hind limb ischemia model was reported by Makarevich et al. (2012). Hind limb ischemia was established by occluding the femoral artery. Plasmid DNA encoding VEGF and/or plasmid DNA encoding HGF were injected into the ischemic muscle. Tweezer electrodes were used to apply electric pulses directly to the ischemic muscle after plasmid DNA injection. Immunohistochemistry for the endothelial cell marker CD31 revealed higher microvasculature density in the co-expression group compared to single expression control groups. Laser Doppler revealed improved perfusion in VEGF and HGF co-expression group compared to the control groups, indicating an advantage of the co-expression strategy over single expression gene electrotransfer (Makarevich et al. 2012). The advantage of this approach to prior co-expression strategies is unclear; however, this study is consistent with other co-expression studies indicating an advantage over delivery of the VEGF alone for PAD.
Another angiogenic gene therapy mediated with GET delivered a constitutively expressed hypoxia-inducible factor 1-alpha (HIF-1α) in a mouse hind limb ischemia model. Hypoxia was induced in mice assigned to three experimental groups by ligating the femoral artery. HIF-1α with GET, HIF-1α without electrotransfer, and empty vector with GET were the three groups. Plasmid DNA was injected directly into the ischemic muscle. Electrotransfer was performed with a three-electrode array. Two pulses were applied around 0.1 AMP. Limb perfusion was monitored with laser Doppler imaging over 21 days. Muscle movement was scored for muscle function recovery postischemic induction and gene therapy. Adductor muscle was evaluated for necrosis. Capillary density was evaluated by immunohistochemical staining for the endothelial cell marker, CD31. Improved limb perfusion and survival, angiogenesis, muscle function, and necrosis reduction were reported for the HIF-1α GET group compared to control groups (Ouma et al. 2014). While this method was reported as a noninvasive gene delivery strategy, the needles used to inject plasmid DNA into the muscle tissue and electrode needles used to deliver electric pulses still pierce the skin and muscle tissue; therefore, a minimally invasive designation might be more accurate.
Parallel plate electrodes have recently been used for minimally invasive intradermal gene transfer of hCAP-18/LL37 in a mouse hind limb ischemia model. The femoral artery and vein were resected to establish hind limb ischemia. Plasmid DNA encoding hCAP-18/LL37 was injected intradermally into the skin overlaying the ischemic muscle. One high-voltage pulse (700 V/cm, 100 μs) followed by one low-voltage (200 V/cm, 400 ms) pulse were applied to the skin overlaying ischemic muscle after intradermal injection of plasmid DNA. Laser Doppler imaging was used to monitor tissue perfusion. Muscle atrophy was evaluated to assess treatment benefits. Angiogenic chemokine levels were measured for VEGF, SDF-1a, as well as their receptors VEGF-R and CXCR-4. Steinstresser et al. (2014) reported improved limb perfusion. Muscle atrophy was reduced in the gene transfer group compared to control groups. Angiogenic chemokine levels were increased as well as presence of their respective receptors. Therefore, gene transfer of plasmid encoding hCAP-18/LL37 may be a promising approach for treatment of ischemic disease (Steinstraesser et al. 2014). While this is also a minimally invasive approach, it is difficult to translate application of parallel plates to human skin for clinical therapies.
In summary, multiple strategies have utilized electrotransfer as a delivery modality inducing angiogenesis in preclinical animal models of hind limb ischemia. Multiple genes, combinations of genes, multiple pulsing protocols, and invasive and minimally invasive delivery sites have been evaluated. All have shown improved perfusion and limb rescue. No resulting clinical studies investigating electrotransfer-mediated gene therapy for peripheral artery disease have been reported. It is not clear what confounding factors are limiting clinical translation; since toxicity of the therapy is reportedly low, the expression profiles indicate temporary gene expression; and gene integration is unlikely due to the plasmid design and delivery methods. Clinical studies with other delivery methods or with naked plasmid DNA delivery of VEGF and bFGF have been reported with conflicting results (Collinson and Donnelly 2004). Gene electrotransfer may resolve some of those problems by enhancing delivery efficiency and allowing for modulating protein levels to achieve a therapeutic dose of the effector protein.
Wound Healing
Skin grafts and other forms of wound healing require an abundant blood supply. Providing adequate perfusion to grafted tissue is often challenging and is a common limiting factor for successful tissue repair and generation. Angiogenesis stimulating therapies are emerging as promising strategies for improving wound repair and regeneration therapies. Gene electrotransfer, as a physical method for gene expression enhancement with minimal side effects, has gained interest for enhancing transient expression of angiogenic factors for wound healing applications. GET-mediated angiogenic therapies have been evaluated in the flap model by multiple groups with encouraging results. A review of GET for wound healing applications in vivo is presented in this chapter section.
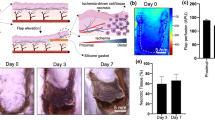
Fujihara et al. (2005) reported using bFGF gene therapy mediated by GET for wound healing in a small animal model. A full-thickness skin flap wound was created surgically on the backs of Sprague-Dawley rats. The dorsal muscle underlying the skin flap was designated as the recipient bed. The recipient bed received an injection of plasmid DNA encoding bFGF or LacZ. Electrotransfer groups received six pulses of 50 ms with an amplitude of 50 V delivered directly to the muscle with a two-needle electrode. The gap between the electrode needles was 1 cm. Three sites received treatment per flap. Micro-angiography was performed to evaluate tissue perfusion. Macroscopic evaluation of necrosis was also performed. GET was performed 2 days post flap surgery. Increased blood vessel density was observed in the electrotransfer of bFGF group compared to control groups. Gene expression of bFGF in the dorsal muscle was confirmed. Increased distal vascularization of the skin flap was also observed in the bFGF-GET group compared to control groups. Necrosis evaluation showed reduced necrosis of distal regions of the skin flaps in the bFGF-GET groups. Together, the study suggests that bFGF gene therapy mediated by electrotransfer yielded a therapeutic benefit for wound healing applications (Fujihara et al. 2005).
A noninvasive electrotransfer-mediated gene therapy approach to wound healing was reported by Ferraro et al. (2009). Plasmid DNA encoding VEGF was delivered intradermally in the flap wound healing model in a rat. A full-thickness skin flap wound was surgically created on a flank of a Sprague-Dawley rat. Four equally spaced sites were identified for treatment on each flank. Plasmid DNA was injected intradermally at each site, and animals randomly assigned to the GET group received electric pulses at each injection site, while control groups received only plasmid DNA injections, without electric pulses. A four-plate electrode was utilized with a 6 mm gap. Eight pulses were delivered per site with a 20 ms pulse duration and a 200 V/cm electric field. Laser Doppler imaging was performed to quantitatively assess perfusion and angiogenesis at different time points during the study. Plasmid VEGF delivery enhanced by electrotransfer resulted in increased perfusion of the skin flap and reduced necrosis of tissue distal to the flap attachment point compared to perfusion and necrosis measures in plasmid DNA injection’s only control group. Intradermal delivery of pVEGF was sufficient to promote healing, induce angiogenesis, increase perfusion, and reduce distal necrosis (Ferraro et al. 2009). The advantage of this approach compared to delivery to the recipient bed is the noninvasive nature, as well as timing of the therapy, which does not require angiogenic therapy prior to injury, and thus has a higher potential for translation to clinical wound healing applications.
Basu et al. (2014) reported a variation of the noninvasive method of plasmid DNA encoding VEGF delivery. The flap wound in a rat model was created as in the previous studies. A multi-electrode array (MEA) composed of 16 blunt tipped pins with a 2 mm gap was utilized in the rat wound healing model. Plasmid DNA was injected intradermally into the skin of the flap at two or four equally space sites. Animals designated to the GET group received pulses of 50 V with a 150 ms pulse width. Each of the nine four-pin regions within the MEA electrode received eight pulses per site. The control groups received plasmid DNA injections without applied pulses. Laser Doppler was used to monitor perfusion through the flap, and necrosis evaluation was performed. Timing of the gene transfer treatment was also studied to determine the optimal window for when treatment could be effective after injury onset. As in the previous study, GET-enhanced VEGF delivery groups had higher perfusion, than corresponding control groups. Two treatment sites per flank were found to be equivalent to four treatment sites in terms of therapeutic benefit. Additionally, it was determined that there is a 2 day delivery window post injury that allowed for increased perfusion and prevention of necrosis (Basu et al. 2014).
Wound healing preclinical studies consistently suggest that GET-mediated angiogenic treatments are feasible for translation to clinical applications and are potentially therapeutically effective. Ischemic skin can recover from injury with increased blood perfusion and reduced necrosis with gene delivery of angiogenic factors such as bFGF or VEGF enhanced by gene electrotransfer. Additionally, these therapies can be administered noninvasively (Ferraro et al. 2009; Basu et al. 2014).
Delivery to Nonischemic and Ischemic Left Ventricular Myocardium
Heart disease accounted for one quarter of all deaths in the United States and is the leading cause of all deaths (Go et al. 2013). The 5-year survival rate for patients diagnosed with heart failure is 50% (Go et al. 2013; Krumholz et al. 2009). Coronary arteries are susceptible to chronic or acute plagues causing blockage and triggering downstream tissue ischemia in advanced states of atherosclerotic disease (Ross 1993). Improving direct revascularization of ischemic tissue distal to myocardial infarction is therefore the main focus of current clinical therapies (Mukherjee et al. 1999). Angiogenesis induction is another strategy currently pursued in clinical and preclinical studies. Gene therapy approaches with exogenous expression of angiogenic agents such as VEGF for collateral vessel development in hypoxic myocardium have been studied extensively with many viral delivery methods (Wolfram and Donahue 2013). These viral delivery methods include adenoviral vectors and adeno-associated vectors with major possible side effects including toxicity and immunogenicity. There are fewer side effects associated with naked plasmid DNA delivery including low immunogenicity, low toxicity, and reduced cost, at the expense of lower gene transfer efficiency (Wolfram and Donahue 2013). GET delivery approaches directly to the ischemic and nonischemic ventricular myocardium have been demonstrated in large and small animal models, respectively. Short-term preclinical studies were conducted for evaluation of feasibility, safety, and efficiency of reporter and effector gene delivery and expression. Long-term preclinical studies were conducted for the evaluation of the therapeutic potential of VEGF to induce angiogenesis and arteriogenesis and repair ischemic myocardium (Marshall et al. 2010; Ayuni et al. 2010; Eigeldinger-Berthou et al. 2012; Hargrave et al. 2013, 2014; Bulysheva 2016).
The first report of successful gene delivery with gene electrotransfer directly to a beating heart was performed in a swine cardiac model (Marshall et al. 2010). In this study, Marshall et al. (2010) reported delivering a reporter gene (luciferase encoding plasmid DNA) and an effector gene (VEGF encoding) plasmid DNA directly to the ventricular myocardium. A sternotomy was performed and the pericardium was surgically removed to expose the epicardial surface of the ventricular myocardium. Plasmid DNA was injected at a predetermined depth into the myocardium. A penetrating four-needle electrode was inserted into the myocardium at the site of DNA injection, and pulses were delivered to enhance gene transfer to the myocardium. The key to making delivery possible and avoiding ventricular fibrillation, while applying electric pulses to the heart, was synchronizing the timing of applied pulses with the absolute refractory period. Additional electrical stimulation of cardiac cells during the absolute refractory period resulted in no additional contraction of those cells; thus, arrhythmias can be avoided. The electrocardiogram (ECG) was used to determine the timing of the absolute refractory period, which occurs during the QRS-wave. Each pulse was electronically synchronized to the rise of the R-wave and was completed before the beginning of the T-wave. Custom pulsing software and hardware were developed to accomplish synchronization of applied pulses and the ECG. Several applied voltages were tested with clear differences between expression levels and pulsing conditions, suggesting ability to modulate expression levels to an optimal level with changing pulse conditions. No damage from electrotransfer was observed (Marshall et al. 2010). This study was followed up with a separate study thoroughly evaluating different electrode designs and different applied voltages to optimize delivery to swine myocardium as evident by reporter gene delivery encoding luciferase and green fluorescence protein (Hargrave et al. 2013).
The first report of GET-mediated gene delivery to the ischemic myocardium in a swine model of acute myocardial infarction detailed variable pulsing conditions to achieve optimal expression profiles and kinetics in ischemic and nonischemic swine myocardium (Hargrave et al. 2014). Acute myocardial infarction was surgically induced by permanently ligating the left anterior descending (LAD) coronary artery. The optimal pulsing conditions were defined by enhanced expression of VEGF documented in both ischemic and nonischemic regions. These pulsing conditions were empirically determined to be 60 V applied, eight pulses, with 20 ms pulse duration. Each applied pulse was synchronized with the rise of the R-wave of the ECG. The optimal gene delivery pulsing electrode had a four-needle array. The gap between the four penetrating needles was 5 mm. The needles were 7 mm long, with DNA injection port injecting DNA at a depth of 3.5 mm from the epicardium. VEGF expression was evaluated over time in ischemic and nonischemic treatment sites via ELISA quantitation of harvested myocardial tissue homogenate. This study also reported evaluating perfusion through the heart 2 weeks after onset of acute myocardial infarction and gene transfer. Animals assigned to the GET groups received four treatment sites with plasmid DNA injections followed by electrotransfer application at those sites. The control animals received plasmid DNA injections, without electrotransfer. Fluorescence perfusion imaging was performed to quantitatively evaluate blood perfusion through the ischemic myocardium post myocardial infarction onset and gene transfer treatment. Perfusion was significantly improved in the GET-enhanced VEGF delivery groups at the 2-week time point compared to corresponding controls (Hargrave et al. 2014).
Bulysheva et al. reported a long-term study evaluating safety and effectiveness of pVEGF GET-mediated delivery in a swine myocardial infarction model (Bulysheva 2016). Acute myocardial infarction was induced surgically with a permanent occlusion of the LAD coronary artery in swine. Animals were randomly assigned to either the GET group delivering plasmid DNA encoding VEGF or a control group with plasmid DNA injection without electrotransfer application. Angiography, echocardiography, dobutamine stress test, and infarct size measures were assessed to evaluate cardiac repair 7 weeks post onset of myocardial infarction (MI) and gene transfer treatment. A smaller infarct size and arteriogenesis were observed relative to controls at the treatment sites 7 weeks post MI. Also there was a notable improvement of contractility and relaxation of the left ventricle as evident from the dobutamine stress test. Together, this study suggests repair of myocardium is possible due to delivery of pVEGF via GET in a swine model of acute myocardial infarction (Bulysheva 2016).
Shortly after the first report of GET to swine nonischemic myocardium (Marshall et al. 2010), Ayuni et al. (2010) reported GET plasmid DNA encoding GFP or luciferase to rat myocardium (Ayuni et al. 2010). Electroporation was achieved without synchronization with the ECG; however, no fibrillation was observed. Plasmid DNA was injected into temporarily occluded coronary sinus, while the entire heart was pulsed by fitting the organ between parallel plate electrodes. Histological analysis for damage and GFP expression revealed minimal damage and higher expression of GFP and luciferase compared to controls; therefore, GET enhanced GFP expression (Ayuni et al. 2010). This was followed up by a similar study also in nonischemic rat myocardium but with direct injection of plasmid DNA into the myocardium (Eigeldinger-Berthou et al. 2012). GFP expression was evaluated to assess delivery efficiency, which was significantly higher than respective controls (Eigeldinger-Berthou et al. 2012).
In summary, application of GET to cardiac muscle can be consistently accomplished in large and small animal models (Marshall et al. 2010; Ayuni et al. 2010; Eigeldinger-Berthou et al. 2012; Bulysheva, 2016). Reporter gene delivery studies indicate that changing gene transfer conditions such as applied voltage or electrode design can modulate gene expression (Hargrave et al. 2013, 2014). It was also determined that ischemic myocardial repair is similar to ischemic skeletal muscle repair with GET-mediated VEGF gene delivery; tissue perfusion was improved and some repair of function was observed in both tissues post VEGF delivery and expression.
Conclusions
All studies of gene electrotransfer for tissue ischemia report significant reversal of ischemia in animal models. GET of particular proangiogenic growth factors: bFGF and VEGF consistently alleviated ischemia in the skin, skeletal muscle, and cardiac muscle, as reported by multiple groups in multiple preclinical in vivo studies in small and large animal models. GET can be performed noninvasively to the skin for PAD and wound healing without loss of efficacy compared to intramuscular delivery. Gene delivery to a beating heart mediated by electrotransfer has been shown in small and large animal models. Effector protein levels can be modulated with pulsing parameters; therefore, therapeutic window of expression can be determined. In conclusion, gene electrotransfer for ischemic disease has been shown to be effective in preclinical studies and is a promising gene delivery therapy with clinical applications to wound healing, peripheral artery disease, and even myocardial ischemia. There are currently no clinical studies reporting GET for ischemia; however, all preclinical studies show consistent reversal of ischemia and minimal known safety concerns; therefore, clinical applications are the next logical steps.
References
Ayuni EL, Gazdhar A, Giraud MN, Kadner A, Gugger M, Cecchini M et al (2010) In vivo electroporation mediated gene delivery to the beating heart. PLoS One 5:e14467
Basu G, Downey H, Guo S, Israel A, Asmar A, Hargrave B et al (2014) Prevention of distal flap necrosis in a rat random skin flap model by gene electro transfer delivering VEGF(165) plasmid. J Gene Med 16:55–65
Bulysheva AA, Hargrave B, Burcus N, Lundberg CG, Murray L, Heller R (2016) Vascular endothelial growth factor-A gene electrotransfer promotes angiogenesis in a porcine model of cardiac ischemia. Gene Ther 23:649–656
Collinson DJ, Donnelly R (2004) Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation? Eur J Vasc Endovasc Surg 28:9–23
Eigeldinger-Berthou S, Buntschu P, Fluck M, Frobert A, Ferrie C, Carrel TP et al (2012) Electric pulses augment reporter gene expression in the beating heart. J Gene Med 14:191–203
Ferraro B, Cruz YL, Coppola D, Heller R (2009) Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol Ther 17:651–657
Ferraro B, Cruz YL, Baldwin M, Coppola D, Heller R (2010) Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther 17:763–769
Fujihara Y, Koyama H, Nishiyama N, Eguchi T, Takato T (2005) Gene transfer of bFGF to recipient bed improves survival of ischemic skin flap. Br J Plast Surg 58:511–517
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB et al (2013) Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127:e6–e245
Hargrave B, Downey H, Strange R Jr, Murray L, Cinnamond C, Lundberg C et al (2013) Electroporation-mediated gene transfer directly to the swine heart. Gene Ther 20:151–157
Hargrave B, Strange R Jr, Navare S, Stratton M, Burcus N, Murray L et al (2014) Gene electro transfer of plasmid encoding vascular endothelial growth factor for enhanced expression and perfusion in the ischemic Swine heart. PLoS One 9:e115235
Jiang J, Jiangl N, Gao W, Zhu J, Guo Y, Shen D et al (2006) Augmentation of revascularization and prevention of plasma leakage by angiopoietin-1 and vascular endothelial growth factor co-transfection in rats with experimental limb ischaemia. Acta Cardiol 61:145–153
Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH et al (2009) Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes 2:407–413
Makarevich P, Tsokolaeva Z, Shevelev A, Rybalkin I, Shevchenko E, Beloglazova I et al (2012) Combined transfer of human VEGF165 and HGF genes renders potent angiogenic effect in ischemic skeletal muscle. PLoS One 7:e38776
Marshall WG Jr, Boone BA, Burgos JD, Gografe SI, Baldwin MK, Danielson ML et al (2010) Electroporation-mediated delivery of a naked DNA plasmid expressing VEGF to the porcine heart enhances protein expression. Gene Ther 17:419–423
Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG (1999) Direct myocardial revascularization and angiogenesis–how many patients might be eligible? Am J Cardiol 84:598–600 A8
Nishikage S, Koyama H, Miyata T, Ishii S, Hamada H, Shigematsu H (2004) In vivo electroporation enhances plasmid-based gene transfer of basic fibroblast growth factor for the treatment of ischemic limb. J Surg Res 120:37–46
Ouma GO, Rodriguez E, Muthumani K, Weiner DB, Wilensky RL, Mohler ER (2014) In vivo electroporation of constitutively expressed HIF-1alpha plasmid DNA improves neovascularization in a mouse model of limb ischemia. J Vasc Surg 59:786–793
Portlock JL, Keravala A, Bertoni C, Lee S, Rando TA, Calos MP (2006) Long-term increase in mVEGF164 in mouse hindlimb muscle mediated by phage phiC31 integrase after nonviral DNA delivery. Hum Gene Ther 17:871–876
Qian HS, Liu P, Huw LY, Orme A, Halks-Miller M, Hill SM et al (2006) Effective treatment of vascular endothelial growth factor refractory hindlimb ischemia by a mutant endothelial nitric oxide synthase gene. Gene Ther 13:1342–1350
Rabinovsky ED, Draghia-Akli R (2004) Insulin-like growth factor I plasmid therapy promotes in vivo angiogenesis. Mol Ther 9:46–55
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
Sacramento CB, da Silva FH, Nardi NB, Yasumura EG, Baptista-Silva JC, Beutel A et al (2010) Synergistic effect of vascular endothelial growth factor and granulocyte colony-stimulating factor double gene therapy in mouse limb ischemia. J Gene Med 12:310–319
Steinstraesser L, Lam MC, Jacobsen F, Porporato PE, Chereddy KK, Becerikli M et al (2014) Skin electroporation of a plasmid encoding hCAP-18/LL-37 host defense peptide promotes wound healing. Mol Ther 22:734–742
Wolfram JA, Donahue JK (2013) Gene therapy to treat cardiovascular disease. J Am Heart Assoc 2:e000119
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Bulysheva, A.A., Heller, R. (2017). Gene Electrotransfer for Ischemic Tissue. In: Miklavčič, D. (eds) Handbook of Electroporation. Springer, Cham. https://doi.org/10.1007/978-3-319-32886-7_58
Download citation
DOI: https://doi.org/10.1007/978-3-319-32886-7_58
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32885-0
Online ISBN: 978-3-319-32886-7
eBook Packages: EngineeringReference Module Computer Science and Engineering