Abstract
Pregnancy during adolescence often occurs before peak bone mass has been fully consolidated. This may have short or long-term implications for maternal, fetal and neonatal skeletal health. Optimal calcium and vitamin D intake are essential for bone health, but data regarding metabolism of these nutrients in relation to skeletal health among pregnant teens remains limited. This review summarizes calcium and vitamin D homeostasis in relation to bone outcomes in the pregnant teen and her neonate, and adult pregnancy is used as a reference for comparison throughout. The conclusion highlights key gaps in knowledge and challenges for future work in this area.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vitamin D
- Parathyroid Hormone
- Calcium Absorption
- Pregnancy
- Adolescence
- Bone
- Secondary Hyperparathyroidism
- Fetal Bone
Adolescent pregnancy continues to be a significant public health problem in the United States. Statistics from the Centers for Disease Control in 2011 indicated that nearly 1,100 adolescents gave birth each day in the United States, 1 in 10 new mothers was an adolescent and teen childbearing cost U.S. taxpayers more than 9 billion dollars annually [1]. In addition to the social, economic and emotional concerns associated with early childbearing, pregnancy during adolescence often occurs before adolescents have consolidated their peak bone mass or stopped growing in linear height. This may have short and/or long-term implications for skeletal health and subsequent risk of low bone mass, but data on this topic are limited given the challenges associated with research in this population. To complicate matters, many pregnant adolescents give birth more than once during their teenage years as fully 1 in 5 births to teenage mothers’ ages 15–19 years is a repeat birth [2]. This review compares and contrasts calcium (Ca) and vitamin D homeostasis during pregnancy in adolescents to data obtained in adults and discusses possible effects of early childbearing on maternal and fetal/neonatal bone outcomes in the pregnant teen.
Females accrue approximately 40 % of their skeletal mass between Tanner Stages 2 and 5 [3], such that by the age of 16 years, females have a bone mass that is nearly equal to that of their premenopausal mothers [4]. When adolescent skeletal accretion is interrupted by a pregnancy, the adolescent must divert nearly 30 g of Ca to consolidate the fetal skeleton. If this loss is expressed on a daily basis, during late gestation approximately 300 mg of Ca per day are accrued by the fetus [5]. This amount is comparable to the peak daily skeletal calcium deposition observed in adolescent females (284 mg/day at age 11.8 years) [6].
Racial differences in bone mass are known to occur with African-Americans having the lowest risk of osteoporosis at maturity and significantly higher rates of skeletal Ca deposition and Ca absorption, along with lower urinary Ca excretion and alterations in calcitropic hormones (lower 25-hydroxyvitamin D and higher parathyroid hormone and calcitriol) [7, 8]. This point is important to mention as teen pregnancy disproportionately impacts minorities; Hispanic and Black teens have more than double the birth rate (per 1,000 girls ages 15–19 years) when compared to White adolescents [9].
At this time the calcium and vitamin D intake requirements for pregnant adolescents (<19 years of age) are no different than those for their age-matched non-pregnant peers, but the need for more data on the metabolism and role of Ca and vitamin D in this population has been highlighted by the Institute of Medicine [10]. Even without any increase in Ca or vitamin D requirements it is unlikely that pregnant teens will achieve the Estimated Average Requirements (EAR) for these nutrients. Nationally representative data indicate that 87 % of US females age 14–18 years consume less than the EAR for vitamin D and 77 % consume less than the EAR for Ca when considering the combined intake from both food and supplements [11]. Many believe the intake recommendations for vitamin D are too low and this issue remains controversial. The Endocrine Society published guidelines for use by clinicians treating or preventing vitamin D deficiency. These guidelines suggest that the RDA of 600 international units (IU) of vitamin D per day is insufficient to prevent vitamin D deficiency during pregnancy and instead advocate daily vitamin D intakes between 1,500 and 2,000 IU in order to achieve target 25-hydroxyvitamin D (25(OH)D) concentrations of 30 ng/mL [12].
Calcium and Vitamin D Physiology Across Gestation in Adults
Calcium and vitamin D physiology are closely inter-related to maintain circulating Ca and P concentrations at the supersaturated concentrations needed to support skeletal homeostasis and overall health. This is accomplished with the assistance of several calcitropic hormones including parathyroid hormone (PTH), calcitonin, 1,25-dihydroxyvitamin D (calcitriol) and the phosphatonin FGF23. These hormones interact to regulate renal Ca and phosphorus (P) reabsorption, intestinal Ca and P absorption and bone turnover of Ca and phosphorus.
Many studies have characterized changes in Ca dynamics across gestation in adults. Early in gestation the efficiency of Ca absorption increases, well before the period of rapid fetal bone growth [13]. By the end of pregnancy, Ca absorption averages ~50 % in US women consuming ~1,000 mg of Ca per day. This represents an approximate doubling of the pre-pregnancy absorption efficiency [13, 14]. Urinary Ca excretion also increases markedly across gestation and offsets some of the gains in Ca retention that occur due to the increased absorption of this mineral. Pregnancy associated increases in urinary Ca excretion have been reported even among adult Brazilian women ingesting habitual intakes of only ~500 mg of Ca per day [15]. In contrast data from Gambian women ingesting ~350 mg Ca/day found a substantially lower urinary Ca excretion at 20 weeks (~60 mg/day) compared to other published pregnancy data in other geographical locations and this loss did not significantly differ in Gambian women receiving Ca supplementation across pregnancy (1,500 mg Ca/day) [16].
Marked changes in calcitropic hormones occur to support the Ca demands of pregnancy. Concentrations of 25(OH)D have typically been found to remain static or decrease across gestation in those receiving standard prenatal supplements containing 400 IU of vitamin D [17, 18]. Increased attention has been placed on not only the total concentration of this prohormone but also on the biological activity of 25(OH)D. Bioavailable (free) 25(OH)D is impacted by concurrent concentrations of vitamin D binding protein (DBP) and this binding protein is also known to increase significantly across gestation [19]. Data on bioavailable 25(OH)D are constrained by challenges associated with the techniques utilized to quantify free 25(OH)D. A new assay that is purported to have more sensitivity in quantifying free 25(OH)D found that while DBP concentrations increased across gestation, free 25(OH)D concentrations remained unchanged from the 2nd to the 3rd trimester perhaps as a consequence of alterations in the affinity of DBP for 25(OH)D [20].
Alterations in bioavailable 25(OH)D across pregnancy would impact the cellular use of this prohormone for intracrine functions. Genetic polymorphisms in DBP may also influence bioavailable vitamin D concentrations. The prevalence of individual genetic polymorphisms in DBP has been found to significantly differ between African Americans and Caucasians, with African-Americans having an increased prevalence of a polymorphism that is associated with lower concentrations of DBP [21]. Additional studies are needed to identify how these polymorphisms impact the amount of free versus bound 25(OH)D, to ensure that assays utilized for DBP equally recognize all DBP polymorphisms and to evaluate some of the assumptions that are utilized in the equations used to quantify bioavailable vitamin D [22].
Calcitriol, the hormonal form of vitamin D, significantly increases within the first trimester of pregnancy [14, 23]. In women consuming typical diets and standard prenatal supplements, final concentrations of calcitriol in late gestation are increased ~2–2.5 fold compared to pre-pregnancy values [18, 23]. Vitamin D supplementation with 2,000 or 4,000 IU of D3/day leads to even greater increases in calcitriol as well as gains in 25(OH)D across gestation [17, 24]. Other longitudinal studies have reported slight decreases in calcitriol from late gestation until term in pregnant teens who did not receive high dose vitamin D supplementation [25].
Data on pregnancy associated changes in intact PTH (iPTH) remain controversial. Some reviews report that iPTH is low to undetectable across pregnancy in most women except for those from Asia or the Gambia [26], but recent studies contradict this finding. Pregnancy associated elevations in PTH have now been reported in several US populations including women ingesting average Ca intakes of ~1,000 mg/day [17], low income and predominantly minority women ingesting Ca intakes of 1,085 mg/day [27], and pregnant adolescents (predominantly minority) consuming Ca intakes averaging 913 mg/day [25].
Evaluation of serum analytes in pregnant women is challenging as these must be evaluated in relation to the marked hemodilution that is known to occur across pregnancy. Decreases in concentrations of serum biomarkers may be due to plasma volume expansion while constant concentrations may actually reflect increased synthesis or failure to appropriately expand the plasma volume. Total Ca concentrations decrease across pregnancy a finding that may be driven by hemodilution given the increase in plasma volume and concurrent decreases in serum albumin. Ionized Ca concentrations, in contrast, do not decrease between early and late pregnancy [18].
The net impact of pregnancy-associated alterations in Ca retention and calcitropic hormones on bone turnover has been examined using stable Ca isotopes. These studies have reported significant increases in bone deposition and resorption rates during pregnancy compared to values evident in the non-pregnant state [28]. Some of these studies have also found that higher Ca intake across gestation is associated with an improvement in Ca balance [28].
Calcium and Vitamin D Physiology Across Gestation in Adolescents
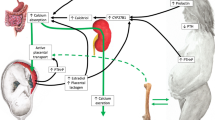
The growing literature on gestational changes in Ca physiology in pregnant adolescent populations indicates that the physiological adaptations to pregnancy in adolescents largely mirror those observed among adults. Adolescents experience a similar magnitude of increase in Ca absorption, similar elevations in urinary Ca losses, and comparable hormonal changes across gestation [29]. Because adolescent pregnancy disproportionately impacts minorities, and darker skin tones are a risk factor for lower 25(OH)D, it is perhaps not surprising that vitamin D insufficiency is prevalent in this population. We found in a recent study of 168 pregnant teens ≤18 years of age (65 % were African-American and 25 % were Hispanic) residing in Rochester, NY (latitude of 43° N), that at delivery 47 %, 31 %, and 18 % of teens had 25(OH)D under 20 ng/mL, 16 ng/mL and 12 ng/mL respectively [25]. In these adolescents fully 25 % of teens exhibited elevated PTH at delivery (>60 pg/mL) while ingesting mean Ca intakes of 900 mg/day [25]. A depiction of the typical temporal changes in Ca and vitamin D parameters across gestation in adults and adolescents is presented in Figure 26.1.
Typical temporal changes in markers of calcium and vitamin D metabolism are presented across trimesters as fold changes relative to pre-pregnancy values. The patterns of change depicted are based on published data from observational and experimental studies conducted in U.S. pregnant adults. The y-axis reflects a 2× (double the non-pregnant value, i.e. a 100 % increase) or 3× increase (triple the non-pregnant value, i.e. a 200 % increase). Arrows presented below the x-axis depict the direction and magnitude of changes in these same parameters based on data obtained in pregnant adolescent study populations. No data on vitamin D binding protein have been published in pregnant adolescents to date. Data on PTH concentrations across gestation are mixed with some studies finding no change, a decrease, or an increase in this hormone by late gestation. Several recent U.S. studies in individuals consuming average Ca intake of ~1000 mg/day have found that from 11 to 25 % of women studied exhibit elevated PTH (>60 pg/mL) during pregnancy
Secondary Hyperparathyroidism During Pregnancy
The prevalence of secondary hyperparathyroidism in our study of pregnant adolescents at mid-gestation was 6.6 % (intact PTH (iPTH) >60 pg/mL) at 26.3 ± 3.6 weeks of gestation and 25 % at delivery [25]. This can be compared to data from another study of 1,116 low-income and minority pregnant subjects, in which 11 % of women studied exhibited iPTH >62 pg/mL at 13.8 weeks of gestation [27]. Furthermore, in a vitamin D supplementation trial by Hollis et al., women in the lowest supplementation dose group (400 IU) had iPTH concentrations of 18.1 pg/mL at baseline; this increased by ~17 % to 21.0 pg/mL by 1-month prior to delivery [17].
Factors associated with those who exhibit secondary hyperparathyroidism across pregnancy have not been fully elucidated. In the vitamin D supplementation study by Hollis et al., PTH elevations were blunted in African Americans receiving higher dose vitamin D supplementation (2,000 and 4,000 IU D3/day), and higher 25(OH)D concentrations also resulted in increased calcitriol [17]. A similar vitamin D supplementation study by Wagner et al., which compared 2,000 IU vs 4,000 IU of vitamin D3, found that women in the 2,000 IU group had an increase in PTH across gestation while those in the 4,000 IU group experienced a net 1.0 pg/mL decrease, a difference between treatment groups that was significant (P = 0.03) [24]. In our adolescent study, one-quarter of teens studied had elevated PTH at delivery. PTH was inversely associated with maternal 25(OH)D concentrations in the cohort as a whole and in teens with 25(OH)D < 20 ng/mL. Of note this relationship did not remain significant when examined only in the sub-group of teens with 25(OH)D concentrations over 20 ng/mL. Mid-gestation 25(OH)D was also inversely associated with calcitriol at delivery, irrespective of Ca intake [25].
The secondary hyperparathyroidism observed in some women during pregnancy suggests that a subset of adult and adolescent women experience greater Ca stress across gestation. Additional research is needed to identify those at risk for secondary hyperparathyroidism and to determine if this response is associated with adverse maternal, fetal or neonatal outcomes. Towards this goal, Scholl et al. recently measured serum iPTH, 25(OH)D and Ca intake in relation to birth outcomes in a group of 1,116 low-income pregnant women and adolescents from Camden, NJ (23 % of the population was <19 years of age). In these women, 11 % exhibited elevated PTH (>62 pg/mL) at entry into prenatal care (13.8 weeks of gestation), the only time point that serum was obtained [27]. The authors categorized women as having calcium metabolic stress if they exhibited elevated PTH in combination with either a low dietary Ca intake (<60 % of the EAR) or vitamin D insufficiency (defined as 25(OH)D < 20 ng/mL). Concentrations of PTH in early gestation were independently associated with both 25(OH)D and Ca intake. However, elevated PTH was common in those with insufficient 25(OH)D, even in the face of Ca intakes that met or exceeded the recommended dietary allowance (RDA). Women with elevated PTH and either or both low Ca intake and low 25(OH)D status had a 2–3 fold increased risk of small for gestation age birth (SGA) and gave birth to infants with significantly lower birth weight, birth length and head circumference [27]. Alterations in serum PTH were highly predictive of adverse fetal outcomes; women with insufficient 25(OH)D or low Ca intake who did not have elevated PTH did not experience reductions in fetal growth. Stable Ca isotope studies have also found that rates of bone calcium deposition and resorption in late pregnancy are significantly positively associated with PTH [28]. If Ca economy is strained, then there may be insufficient substrate to fully support maternal and fetal skeletal health and both maternal and fetal mineralization may be adversely impacted. Interactions between PTH, 25(OH)D and calcitriol in a pregnant adolescent population may well differ from those identified to date among pregnant adults due to the rapid bone deposition that typically occurs during adolescence and the increased competition for calcium between the pregnant adolescent and her developing fetus.
Teen Pregnancy and Bone Mass
A 2012 literature review of all total body and site specific bone mineral data across gestation found net bone changes ranged from losses of −2.0 % to non-significant gains of 0.5 % [30]. These authors noted that a 2 % loss in total body bone mineral in adult women reflects a net loss of ~25 g of Ca, an amount approaching the 30 g of Ca that are thought to be present in the neonate at birth [30]. This same 30 g loss would reflect nearly 4 % of an adolescents’ total body Ca content given their lower bone mass [29].
Stable Ca isotope absorption studies in pregnant adolescents (ages 13.5–18.3 years) have reported average percent Ca absorption values of ~53 % in teens ingesting Ca intakes averaging 1,200 mg/day. Using concurrent measures of urinary Ca excretion and estimates of endogenous fecal Ca secretion, this translated to an estimated Ca balance of 240 mg/day during the third trimester. This amount is comparable to reported peak rates of fetal Ca accretion but would not be sufficient to achieve the combined peak rates of fetal and adolescent bone Ca accretion [29].
Data on the degree to which Ca or vitamin D status influences maternal bone outcomes across gestation are mixed. Many studies have utilized heel ultrasound measures to track changes across pregnancy since this method does not involve radiation exposure. These studies report significant declines in calcaneal measures across gestation in both adults and adolescents [31–33]. In one study that recruited both pregnant teens (n = 45) and pregnant adults (n = 199), teens experienced significantly greater deficits in the quantitative ultrasound index outcome (−5.5 % vs. −1.9 %) across the interval from 16 ± 7 weeks gestation to 6 ± 1 week postpartum [31].
We recently assessed possible associations between adolescent heel ultrasound measures across gestation (n = 156 adolescents ≤18 years at entry into the study) in relation to maternal dietary Ca and vitamin D intake and maternal vitamin D status. In pregnant adolescents, maternal bone loss across pregnancy was not significantly associated with maternal Ca or vitamin D intake, nor was it impacted by 25(OH)D status [32]. Loss of calcaneal bone among UK pregnant women was also not found to be correlated with milk intake or Ca supplement use, except that women ingesting less than a pint of milk per day before pregnancy were found to experience a greater loss in calcaneal bone (measured by speed of sound) across pregnancy [33].
Of interest, while Ca intake and vitamin D status did not impact maternal bone loss across pregnancy, adolescent pre-pregnancy body mass index (ppBMI) was a significant determinant of calcaneal bone outcomes across gestation. Teens entering pregnancy with a lower BMI experienced significantly greater decreases in all bone quality outcomes across gestation [32]. Body composition was also found to be a key determinant of gestational bone losses in a group of 307 pregnant adults; reductions in heel ultrasound measures across gestation were attenuated in women with greater fat stores (based on maternal mid upper arm circumference in late pregnancy) [33]. Similarly, pre-gravid weight and weight gain across pregnancy were predictive of reductions in heel ultrasound measures in pregnant adults and adolescents [31].
Site specific alterations in calcaneal bone quality across pregnancy may or may not translate to net changes in total body or site specific bone mineral content or density. This question is challenging to evaluate in pregnant adolescents as the peak bone mass that would have been achieved had the pregnancy not occurred is unknown, it is difficult to obtain pre-pregnancy data from teens that subsequently become pregnant and this group is challenging to follow in the post-partum period to evaluate postpartum gains in bone mass once menses resume.
Given the well-known effects of estrogen on closure of the epiphyseal growth plate [34], it is possible that the elevated estrogen concentrations that occur during pregnancy may accelerate epiphyseal senescence and fusion of the growth plate [35]. Studies in adolescents have found lower cortical BMD and whole body BMC in the early post-partum period compared to age and ethnicity matched controls [29, 36], but these studies are generally small and must rely on estimates of expected peak bone mass. Supplementation studies in Brazilian adolescents (14–19 years) found that Ca (600 mg) and D supplementation (200 IU) over the last 14 weeks of pregnancy resulted in significantly higher lumbar spine bone mineral content and bone area at 5 weeks post-partum when compared to the placebo group [37].
Using other approaches, epidemiological studies have evaluated determinants of bone mass in women with and without histories of early childbearing. Studies have found an adverse effect of early childbearing on postmenopausal BMD at several sites [35, 38]. These findings remain controversial and the long-term impact of any acute bone losses on peak bone mass and subsequent risk of low bone mass and osteoporosis at maturity has not been sufficiently characterized.
Teen Pregnancy and Fetal Skeletal Growth
Nutrients necessary for bone mineralization may be disproportionately partitioned between the growing adolescent and her fetus. Adolescent sheep models have frequently been utilized to study the perturbed nutrient partitioning to the fetus that is often evident in animals that are still growing at the time of conception [39]. In humans, many studies have found average birth weights of neonates born to pregnant teens are lower than birth weights observed among adult populations even when adjusting for race to account for the smaller birth weights evident among African-Americans compared to Caucasians [40].
In our adolescent studies, while maternal Ca and D intake were not associated with changes in maternal bone outcomes, increased maternal dairy intake was found to have a significant positive impact on fetal femur length [41]. A limitation of this whole food based approach is that the observed associations with dairy intake cannot be attributed to any one individual nutrient. In a subsequent study, the impact of adolescent Ca and vitamin D intake and 25(OH)D concentrations across gestation was evaluated in relation to longitudinal measures of fetal femur and humerus length [42]. Although maternal 25(OH)D and intakes of Ca and D were not significantly associated with changes in maternal calcaneal outcomes [32], longer fetal femur and humerus length were present in the adolescents consuming Ca intakes ≥1,050 mg/day. Significant positive associations were also evident between fetal femur and humerus z-scores in teens with 25(OH)D >20 ng/mL [42]. In this same study, a significant association was observed between dietary Ca intake and fetal femur z-scores and birth length, but only in teens with vitamin D insufficiency (25(OH)D < 20 ng/mL). These findings highlight the interactions that are evident between adequate Ca and vitamin D status and the negative impact that insufficiency of either of these nutrients can have on fetal bone growth (Fig. 26.2) [42].
Optimal maternal calcium (Ca) intake and adequate 25-hydroxyvitamin D (25(OH)D) status are integral to optimal fetal bone growth. In a group of 169 pregnant adolescents, maternal dietary calcium intake ≥ 1050 mg/d was associated with a significantly more positive fetal femur and humerus length z score. Similarly, pregnant teens with 25(OH)D concentrations ≥ 20 ng/mL exhibited significantly higher fetal femur and humerus length z scores. Interactions between maternal Ca intake and 25(OH)D status were evident. Maternal Ca intakes and vitamin D status (as determined by 25(OH)D) were only significantly associated with fetal femur and humerus length z scores when intake/status of the other nutrient was inadequate. In a pregnant adolescent population, consuming either an adequate Ca intake or achieving sufficient 25(OH)D status was found to attenuate deficits in fetal long-bone growth that occurred in those with sub-optimal status/intake of both nutrients
In contrast, other recent data in pregnant adolescents habitually ingesting ~600 mg Ca/day found that additional Ca supplementation (600 mg/day) over the last trimester of pregnancy did not significantly impact fetal bone measures or infant BMC, BMD or BA at 5 weeks post-partum [43]. The authors concluded that early infant bone mass in those born to this age group is largely met by maternal bone mobilization when Ca intake is low [37, 43].
Data on the effects of maternal Ca intake and fetal bone outcomes remains controversial. Calcium supplementation (1,500 mg/day Ca) across the second half of pregnancy to women habitually ingesting only ~350 mg of Ca per day was associated with lower maternal bone mineral at the hip and a significant increase in maternal bone loss over the following 12 months of lactation compared to the placebo group [44]. In these Ca supplemented women no changes in fetal growth (based on birth weight and crown-heel length within 5 days of birth) or neonatal whole body BMC at 2 weeks of age were noted. The rates of total body bone mineral content and bone area accumulation at 52 weeks of age were slower in babies born to Ca supplemented women compared to the placebo group [45]. Given that the majority of bone mass is genetically determined, more studies are needed in different population groups of women with variable combinations of Ca, P and vitamin D status to more fully identify environmental and genetic determinants of maternal, fetal and neonatal bone health.
Gaps in Knowledge
Until recently it was assumed that phosphorus homeostasis was largely maintained by serum PTH but it is now known that the phosphatonin, fibroblast growth factor 23 (FGF23), is integral to systemic phosphorus regulation [46]. At this time little is known about FGF23 homeostasis across human pregnancy and how FGF23 interacts with the dynamic gestational changes in calcitriol and PTH that may also occur at this time. Similarly, there are few data on vitamin D kinetics during pregnancy and more data on basic aspects of production and catabolism of calcidiol and calcitriol during pregnancy are needed in order to better inform nutritional requirements and clinical practice. Concurrent data on DBP concentrations are needed when measures of calcidiol and calcitriol are obtained in order to estimate how net concentrations are related to free concentrations of vitamin D metabolites.
Because there are no easily applied methods to quantify plasma volume it is assumed that plasma volume expansion is comparable between individuals when interpreting biochemical indicators at a given stage of gestation. This has not been sufficiently evaluated in adolescent or adult pregnancies. For iron it is known that there is a U-shaped distribution with respect to hemoglobin concentrations and adverse birth outcomes in adolescents [47] and adults [48]. The increased risks evident in women with high hemoglobin concentrations may be associated with a failure to appropriately expand plasma volume across gestation. Similar perturbations in plasma volume expansion would also influence the interpretation of any calcitropic serum biomarker; greater attention to this issue is warranted when evaluating outcomes and nutrient requirements across gestation.
Optimal maternal nutrition during pregnancy must support health in both the adolescent and her developing fetus. Studies evaluating Ca and vitamin D requirements in pregnant women often do not concurrently consider fetal and neonatal outcomes. The nutrient intakes required to prevent maternal deficiency may not be identical to those necessary to optimize maternal health or the fetal environment as it relates to developmental programming. Additional studies on this point are needed.
Finally, when evaluating the possible benefits of vitamin D, non-bone health outcomes should be considered given the recently identified role of vitamin D in immune system function and the numerous associations linking vitamin D status to increased risk of adverse birth outcomes [49].
Conclusion
Adolescence is a time of rapid bone acquisition that culminates in the fusion of the epiphyseal growth plate and cessation of linear growth. When adolescence is interrupted by pregnancy, females that should be consolidating their own skeletal mass must divert substantial amounts of Ca to their developing fetus. The physiological adaptations that occur to support this process may not be sufficient to fully support maximum maternal and fetal skeletal growth and consolidation. Further data are needed in order to evaluate acute and long-term implications of early childbearing on maternal and offspring bone health. Optimal Ca and vitamin D requirements in this age group are challenging to identify given that the peak bone mass that would have been achieved is unknown. Additional research is needed to fully characterize changes in calcitropic hormones across gestation in pregnant adolescents and adults in order to quantify bioactive concentrations of vitamin D metabolites and their interaction with total and ionized Ca and phosphorus while including measures of serum albumin to account for possible differences in plasma volume.
Accumulating data indicates that a subset of pregnant women exhibit elevated parathyroid hormone combined with low 25(OH)D status and/or low Ca intake, and this may increase the risk of adverse birth outcomes. While genetic factors are known to be responsible for the majority of bone mass, many of these factors have yet to be identified, and additional work is needed to identify genotypes that predispose pregnant women and their developing fetuses to mineral insufficiency. Finally, greater focus on the placental interface will help identify factors that influence placental trafficking of Ca and vitamin D in support of fetal demands.
References
Control CfD. Vital signs. CDC [Internet]; 2011.
Prevention USCfDCa. Preventing repeat teen births. Vital signs [Internet]. 2013 06/15/15 [cited 2015 06/15/2015].
Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799–808.
Matkovic V, Fontana D, Tominac C, Goel P, Chesnut CH. Factors that influence peak bone mass formation: a study of calcium balance and the inheritance of bone mass in adolescent females. Am J Clin Nutr. 1990;52(5):878–88.
Forbes GB. Calcium accumulation by the human fetus. Pediatrics. 1976;57(6):976–7.
Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15(11):2245–50.
Abrams SA, O’Brien KO, Liang LK, Stuff JE. Differences in calcium absorption and kinetics between black and white girls aged 5–16 years. J Bone Miner Res. 1995;10(5):829–33.
Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88(2):545S–50.
Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
Institute of Medicine FaNB. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011.
Berner LA, Keast DR, Bailey RL, Dwyer JT. Fortified foods are major contributors to nutrient intakes in diets of US children and adolescents. J Acad Nutr Diet. 2014;114(7):1009–22.e8.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Heaney RP, Skillman TG. Calcium metabolism in normal human pregnancy. J Clin Endocrinol Metab. 1971;33(4):661–70.
Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67(4):693–701.
O’Brien KO, Donangelo CM, Zapata CL, Abrams SA, Spencer EM, King JC. Bone calcium turnover during pregnancy and lactation in women with low calcium diets is associated with calcium intake and circulating insulin-like growth factor 1 concentrations. Am J Clin Nutr. 2006;83(2):317–23.
Jarjou LM, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. Am J Clin Nutr. 2010;92(2):450–7.
Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;226(10):2341–57.
Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Ann Rev Nutr. 2011;31:89–115.
Zhang JY, Lucey AJ, Horgan R, Kenny LC, Kiely M. Impact of pregnancy on vitamin D status: a longitudinal study. Br J Nutr. 2014;112(7):1081–7.
Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631–7.
Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):880–1.
Hollis BW, Bikle DD. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):879–80.
Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61(3):514–23.
Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208(2):137.e1–13.
Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O’Brien KO. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with parathyroid hormone and calcitriol in pregnant adolescents. J Bone Miner Res. 2012;27(1):177–86.
Kovacs CS. The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Ann Rev Nutr. 2012;32:97–123.
Scholl TO, Chen X, Stein TP. Maternal calcium metabolic stress and fetal growth. Am J Clin Nutr. 2014;99(4):918–25.
O’Brien KO, Donangelo CM, Ritchie LD, Gildengorin G, Abrams S, King JC. Serum 1,25-dihydroxyvitamin D and calcium intake affect rates of bone calcium deposition during pregnancy and the early postpartum period. Am J Clin Nutr. 2012;96(1):64–72.
O’Brien KO, Nathanson MS, Mancini J, Witter FR. Calcium absorption is significantly higher in adolescents during pregnancy than in the early postpartum period. Am J Clin Nutr. 2003;78(6):1188–93.
Olausson H, Goldberg GR, Laskey MA, Schoenmakers I, Jarjou LM, Prentice A. Calcium economy in human pregnancy and lactation. Nutr Res Rev. 2012;25(1):40–67.
Sowers MF, Scholl T, Harris L, Jannausch M. Bone loss in adolescent and adult pregnant women. Obstet Gynecol. 2009;96(2):189–93.
Whisner CM, Young BE, Pressman EK, Queenan RA, Cooper EM, O’Brien KO. Maternal diet but not gestational weight gain predicts central adiposity accretion in utero among pregnant adolescents. Int J Obes. 2015;39(4):565–70.
Javaid MK, Crozier SR, Harvey NC, Taylor P, Inskip HM, Godfrey KM, Cooper C, Southhampton Women’s Survey Study Group. Maternal and seasonal predictors of change in calcaneal quantitative ultrasound during pregnancy. J Clin Endocrinol Metab. 2015;90(9):5182–7.
Shim KS. Pubertal growth and epiphyseal fusion. Ann Pediatr Endocrinol Metab. 2015;20(1):8–12.
Lloyd T, Lin HM, Eggli DF, Dodson WC, Demers LM, Legro RS. Adolescent caucasian mothers have reduced adult hip bone density. Fertil Steril. 2002;77(1):136–40.
Ward KA, Adams JE, Mughal MZ. Bone status during adolescence, pregnancy and lactation. Curr Opin Obestet Gynecol. 2005;17(4):435–9.
Diogenes ME, Bezerra FF, Rezende EP, Taveira MF, Pinhal I, Donangelo CM. Effect of calcium plus vitamin D supplementation during pregnancy in Brazilian adolescent mothers: a randomized, placebo-controlled trial. Am J Clin Nutr. 2013;98(1):82–91.
Cho GJ, Shin JH, Yi KW, Park HT, Kim T, Hur JY, Kim SH. Adolescent pregnancy is associated with osteoporosis in postmenopausal women. Menopause. 2012;19(4):456–60.
Wallace JM, Luther JS, Milne JS, Aitken RP, Redmer DA, Reynolds LP, Hay Jr WW. Nutritional modulation of adolescent pregnancy outcome – a review. Placenta. 2006;27(A):S61–8.
Chang SC, O’Brien KO, Nathanson MS, Mancini J, Witter FR. Characteristics and risk factors for adverse birth outcomes in pregnant black adolescents. J Pediatr. 2003;43(2):250–7.
Chang SC, O’Brien KO, Nathanson MS, Caulfield LE, Mancini J, Witter FR. Fetal femur length is influenced by maternal dairy intake in pregnant African American adolescents. Am J Clin Nutr. 2003;77(5):1248–54.
Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O’Brien KO. Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. Am J Clin Nutr. 2012;95(5):1103–12.
Diogenes ME, Bezerra FF, Rezende EP, Donangelo CM. Calcium plus vitamin D supplementation during third trimester of pregnancy in adolescents accustomed to low calcium diets did not affect infant bone mass at early lactation in a randomized controlled trial. J Nutr. 2015;145(7):1515–23.
Jarjou LM, Sawo Y, Goldberg GR, Laskey MA, Cole TJ, Prentice A. Unexpected long-term effects of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake: a follow-up study. Am J Clin Nutr. 2013;98(3):723–30.
Jarjou LM, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, Cole TJ. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr. 2006;83(3):657–66.
Razzaque MS. Bone-kidney axis in systemic phosphate turnover. Arc Biochem Biophys. 2014;561:154–8.
Chang SC, O’Brien KO, Nathanson MS, Mancini J, Witter FR. Hemoglobin concentrations influence birth outcomes in pregnant African-American adolescents. J Nutr. 2003;133(7):2348–55.
Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao XG, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am J Epidemiol. 1988;148(10):998–1006.
Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12(10):976–89.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
O’Brien, K.O., Best, C.M. (2016). Nutrition, Adolescent Pregnancy and Bone. In: Weaver, C., Daly, R., Bischoff-Ferrari, H. (eds) Nutritional Influences on Bone Health. Springer, Cham. https://doi.org/10.1007/978-3-319-32417-3_26
Download citation
DOI: https://doi.org/10.1007/978-3-319-32417-3_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32415-9
Online ISBN: 978-3-319-32417-3
eBook Packages: MedicineMedicine (R0)