Abstract
Antiepileptic drugs (AEDs) are commonly prescribed for both neurologic and psychiatric indications throughout the life span. However, taking AEDs during pregnancy is associated with potential risks since in utero AED exposure may incur increased risks of major congenital malformations and negative cognitive or behavioral effects. Cognitive and behavioral risks do not appear uniform across all medications. For the AEDs that have been adequately studied, fetal valproate (VPA) exposure is associated with the greatest risks including decreased cognition and risk of autism spectrum disorder, a fact that has been recognized by the US Food and Drug Administration. Phenobarbital also has risk based on limited data. In utero exposure to other AEDs including carbamazepine, lamotrigine, levetiracetam, or phenytoin appear to have less cognitive and behavioral risks than VPA, although it is unlikely that these medications are risk-free. Little or no information exists on newer medications. Women with epilepsy of child bearing potential should take folate as a supplement. Since AED exposure from breast milk appears low, there is no reason to discourage mothers from breast feeding their children.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antiepileptic drugs
- AEDs
- Epilepsy
- In utero risk
- Fetal valproate syndrome
- NEAD study
- Neurodevelopmental effects
- Valproate
- Carbamazepine
- Lamotrigine
Definitional Issues and Prevalence
Antiepileptic drugs (AEDs) are the primary therapeutic option for seizure control in patients with epilepsy. Women with epilepsy wanting to start families, however, face difficult decisions about how to best manage their epilepsy care while simultaneously minimizing risks to their unborn children from AED exposure. If a woman’s epilepsy is mild (e.g., infrequent sensory seizures without altered awareness), she may choose to discontinue AEDs before attempting to become pregnant. For most women, however, AED discontinuation produces significant risks to both the mother (e.g., Sudden Unexplained Death in Epilepsy or SUDEP) and unborn child (e.g., fetal hypoxia; Edey, Moran, & Nashef, 2014; Viguera et al., 2007). For example, there is an approximate 10× mortality increase in pregnancy and the postpartum period in women with epilepsy compared to baseline population rates (Cantwell et al., 2011). The increase in mortality is assumed to be related to SUDEP (Edey et al., 2014). In addition to treating epilepsy, AEDs are also commonly used for psychiatric considerations (e.g., bipolar disorder, mood stabilization) and pain indications, and in fact, the number of AED prescriptions for non-epilepsy indications during pregnancy is higher than those for epilepsy (Bobo et al., 2012). Approximately 2 % of women during their pregnancy take AEDs for either psychiatric or neurologic indications (Bobo et al., 2012).
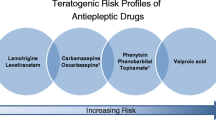
In utero AED risk has traditionally been operationalized by major congenital malformation risk, and it has been suggested that AED monotherapy doubles , and AED polytherapy triples , the risk of developing major congenital malformation s (Hill, Wlodarczyk, Palacios, & Finnell, 2010). Although estimates vary based upon sampling characteristics, major congenital malformations are observed in 6.1 % of children born to women with epilepsy taking AEDs during pregnancy, and 2.8 % among children born to mothers with epilepsy who were not taking AEDs. This compares to 2.2 % in control mothers (Tomson & Battino, 2012). Valproate (VPA) has a higher major congenital malformation risk than either taking no AEDs or to other AEDs, with a 1–2 % risk of neural tube deficits, representing a 10–20× increase over the general population (Meador, Reynolds, Crean, Fahrbach, & Probst, 2008). Several AEDs including carbamazepine (CBZ), lamotrigine (LTG), phenytoin (PHT), or levetiracetam (LEV) have smaller risks of major congenital malformation s (Campbell et al., 2014; Harden, 2014; Hernandez-Diaz et al., 2012; Mawhinney et al., 2013; Vajda, O’Brien, Lander, Graham, & Eadie, 2014). Phenobarbital and topiramate (TPM) exposures are associated with intermediate risks (Harden, 2014; Hernandez-Diaz et al., 2012; Margulis et al., 2012; Mines et al., 2014). In this chapter, we focus on cognitive and behavioral developmental outcomes of children exposed to AEDs during pregnancy that are necessary to inform treatment decision making, and briefly discuss the role of folate and breast feeding in these patients.
Impact on the Developing Child
Because AEDs have different mechanisms of action, neurodevelopmental risks of in utero exposure will vary. In this section, we review developmental impact across the medications for which adequate research and clinical data have been collected. Risks of AED exposure have only been partially described, however, and even when specific AED risks have been formally investigated, there are often methodological issues limiting the confidence of drug-specific findings including absence of assessor blinding during assessment, follow-up that begins at birth or later rather than during pregnancy, or failure to control for potential confounding factors such as maternal IQ, seizure type and frequency, or AED dose/blood levels.
Valproate (VPA)
Multiple studies have confirmed that among AEDs adequately studied, VPA is the AED associated with the greatest risk of cognitive and behavioral teratogenesis . VPA exposed children are more likely to miss developmental milestones and have decreased IQ scores. In addition, verbal skills appear particularly vulnerable to VPA effects. VPA exposed children also have poorer adaptive skills, with increased risks of Attention Deficit Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD ).
Cognitive Effects
Despite a variety of methodological limitations, VPA exposure has consistently been associated with developmental delay and decreased general level of function. A meta-analysis of developmental outcomes of children of women with epilepsy estimates that VPA exposure is associated with a 6-point reduction in Full Scale IQ (Banach, Boskovic, Einarson, & Koren, 2010). Whether used as monotherapy or part of a polytherapy regimen, VPA exposure is associated with a higher likelihood of either intellectual disabilit y (i.e., FSIQ < 70, 10 %) or borderline cognition (i.e., FSIQ = 70–79; 14 %) than other AEDs studied (Eriksson et al., 2005). Demonstrating the importance of potential confounds, mothers taking VPA in this study had lower FSIQ and also had lower levels of education (p = 0.035; Eriksson et al., 2005). A retrospective study controlling for maternal IQ demonstrated a lower verbal IQ (approximately ten points) in children exposed in utero to VPA (n = 41) versus other monotherapy groups and an unexposed group (Adab, Jacoby, Smith, & Chadwick, 2001) . The decrease in IQ in the VPA group was dose-dependent.
An alternative way of characterizing AED risk is the frequency of “additional educational needs” during schooling, which reflects the provision of additional assistance during mainstream education. Children exposed in utero to VPA had an odds ratio of 3.4 (95 % CI, 1.6–7.1) of being provided with additional educational services compared to unexposed children (Adab et al., 2001). VPA exposure has been associated with an increased risk of delayed early development in comparison to controls (n = 230; Bromley et al., 2010). In an observational cohort study , there was developmental delay in 40 % of VPA exposed children (OR 26.1, 95 % CI, 4.9–139; p < 0.001), 20 % for CBZ exposed children (OR 7.7, 95 % CI, 1.4–43.1; p < 0.01), but only 3 % for LTG exposure. This contrasts with 4.5 % in controls (Cummings, Stewart, Stevenson, Morrow, & Nelson, 2011).
The NEAD study (Neurodevelopmental Effects of Antiepileptic Drugs ) characterized developmental outcomes of children exposed to either valproate (VPA), carbamazepine (CBZ), lamotrigine (LTG), or phenytoin (PHT) monotherapy during their mothers’ pregnancy in a prospective patient cohort, controlling for multiple potential confounding factors. The NEAD study demonstrated increased risk of VPA exposure compared to other AEDs (Meador et al., 2009, 2013). When evaluated at age 6 years, children exposed to VPA had General Conceptual Ability (GCA) scores that were 7–10 points lower than children exposed to other AEDs demonstrating that additional time following exposure does not allow them to “catch up.” This effect was seen in correlational analyses in which the strong relationship between maternal IQ and child GCA scores in all AED groups with the exception of VPA. VPA exposure was associated with decreased GCA, decreased memory, and decreased executive function. Of the various domains affected including GCA verbal abilities were most severely affected by VPA. A dose-dependent adverse effects across all cognitive domains assessed was also described.
Although risk of drug-induced major malformations are related to first trimester exposures, the risk for cognitive-behavioral problems, similar to alcohol, appears greatest during third trimester exposure. A dose relationship for VPA has been observed across trimesters, although this likely reflects that AED dose for epilepsy tends to be constant, with higher doses of VPA correlated with lower GCA, verbal and non-verbal indices, and also lower memory and executive function (Meador et al., 2013). This dose-relationship reflects VPA’s neurodevelopmental toxicity , but does not indicate whether any fully safe VPA doses without any risk exists.
Because the behavioral risk of VPA occurs in the first trimester, women taking VPA often incur exposure risk since it will often occur prior to a woman knowing that she is pregnant. High dose VPA exposure (>800 mg/day) has been associated with an GCA that was nearly ten points lower than healthy controls, which was accompanied by an eightfold increased need of educational intervention (Baker et al., 2015). Lower VPA levels (≤800 mg/day) was not associated with reduced GCA, but it was associated with impaired verbal abilities and a sixfold increase in the need for educational intervention. Again, despite this dose-related effect, a completely safe dose of VPA has not been established.
Behavioral Effect s
Early reports identified an association between autism and VPA exposure (Christianson, Chesler, & Kromberg, 1994; Rasalam et al., 2005; Williams et al., 2001; Williams & Hersh, 1997). In addition to ASD, 20 % of ASD affected children also experienced major congenital malformations (Rasalam et al., 2005). Even when excluding major congenital malformations, impaired social and adaptive functioning in VPA children has been described with an increased risk autism or ASD diagnosis (Christensen et al., 2013). The absolute risk was 2.5 % for autism and 4.4 % for ASD in the VPA exposed children cohort compared with 0.5 and 1.5 % in the general population. The rates of autism and ASD in children of mothers taking CBZ, clonazepam, oxcarbazepine (OXC), or lamotrigine (LTG) monotherapy were not increased.
When characterizing poor outcomes using a combined neurodevelopmental disorders (NDD) outcome to account for low incidence of specific events including autism, ASD, ADHD, or dyspraxia, a NDD was present in 6 of 50 (12 %) children exposed to VPA monotherapy and 3 of 20 (15 %) children exposed to VPA in polytherapy (33). These rates are higher than in 214 controls (1.9 %) (Bromley et al., 2013).
VPA exposed children have lower daily living and socialization skills than do children exposed to other AEDs. In the prospective NEAD cohort at both 3 and 6 years, VPA exposed children had poorer scores for adaptive functioning than children exposed to phenytoin or lamotrigine, but not carbamazepine (Cohen et al., 2011; Cohen et al., 2013). VPA exposed children were significantly more likely to receive poor scores for adaptive functioning compared to children exposed to phenytoin or lamotrigine, but not carbamazepine. VPA dose correlated with poorer scores for adaptive functioning, and VPA exposure correlated with a greater number of atypical (socially immature) behavior and inattention signs than did either PHT or LTG.
Carbamazepine (CBZ)
The evidence addressing CBZ exposure risks has been conflicting, although most reports describe no adverse cognitive or development effect s (Adab et al., 2004; Gaily et al., 2004; Thomas, Sukumaran, Lukose, George, & Sarma, 2007; Vinten et al., 2005, 2009). Nevertheless, several studies describe increased rates of developmental delay (Cummings et al., 2011; Dean et al., 2002; Mawer, Clayton-Smith, Coyle, & Kini, 2002; Ornoy & Cohen, 1996). Although a meta-analysis surveying studies conducted before 2005 described adverse negative effects of CBZ exposure on Performance IQ, not all of these studies controlled for maternal IQ. A negative correlation between CBZ dose with verbal performance and motor functioning was observed in the NEAD study at 3 years but not when tested at 6 years (Cohen et al., 2011, 2013; Meador et al., 2009). No IQ difference for CBZ exposed children was described at age 6 years compared to controls, although the verbal index was 4.2 points lower (Baker et al., 2015). This was associated with an increased risk of having an IQ < 85. Although CBZ is associated with a smaller risk of poor cognitive outcomes compared to VPA, there appears to be increased risk relative to controls. No increased risk of diagnosed neurodevelopmental disorders for CBZ exposed children was observed (Bromley et al., 2013).
CBZ exposed children may have decreased fine motor and social skills at 18 months, and have been characterized as having more aggressive behaviors at 36 months (Veiby et al., 2013). Adaptive scores for VPA exposed children at age 6 years in the NEAD study were significantly lower than that of children exposed to LTG or PHT but did not differ from CBZ exposed children (Cohen et al., 2013). CBZ exposure was also associated with an increased risk for ADHD by parental, but not teacher, behavior reports. A retrospective study found no difference between parentally reported adaptive behavior of CBZ exposed children age 6–16 years compared with unexposed children (Vinten et al., 2009). In summary, although there are some inconsistencies in the literature, CBZ appears to have less cognitive and behavioral risk than VPA, and appears comparable to controls regarding overall level of general function.
Lamotrigine (LTG)
Risks of LTG exposure appear low, with developmental outcomes that do not differ from controls (Bromley et al., 2010; Cummings et al., 2011). The NEAD study described GCA scores in LTG exposed children that did not differ from either CBZ or PHT exposed children, while being significantly higher than children exposed to VPA (Cohen et al., 2011; Meador et al., 2009, 2013). In the 6-year-old NEAD cohort, both VPA and LTG exposure were associated with poorer verbal indices than non-verbal indices, and both had higher incidence of atypical handedness (Meador et al., 2013) raising the possibility of the AEDs altering the process of normal cerebral language development and lateralization . In contrast to the NEAD findings, an independent report described no IQ score differences between VPA and LTG exposed children (Rihtman, Parush, & Ornoy, 2013). However, LTG and VPA were both associated with poorer non-verbal IQs compared with controls, although the non-verbal IQ in the control group was seven points higher than verbal IQ.
In one study, LTG exposure has been reported to be associated with increased frequency of autistic traits based on a parental questionnaire (Veiby et al., 2013). Parents of children exposed to LTG in the NEAD study rated children as being at increased risk for ADHD, although this pattern was not reported by teachers (Cohen et al., 2013). This pattern of neurodevelopmental disorders has not been observed, however, in other reports (Bromley et al., 2013).
Levetiracetam (LEV)
In a subset from the UK pregnancy register, 51 children exposed to levetiracetam were found not to differ from controls at either 3–24 months or 36–54 months, but were better than VPA exposed children (Shallcross et al., 2011, 2014). The developmental scores of the exposed children did not differ from those of controls at either time point, but were better than VPA. This is the only current report of developmental outcomes for LEV exposure.
Phenobarbital (PB)
In several prospective studies, phenobarbital exposure is associated with lower IQ scores (Reinisch, Sanders, Mortensen, & Rubin, 1995) which appears primarily reflected with decreased verbal IQ. Exposure during the third trimester appears to be associated with the greatest negative effect. This relationship has been described in a separate prospective study (Thomas et al., 2007). Retrospective studies, however, have produced inconsistent results (Dean, Colantonio, Ratcliff, & Chase, 2000; Dessens et al., 2000; van der Pol, Hadders-Algra, Huisjes, & Touwen, 1991). Although phenobarbital is infrequently prescribed in North America and Europe, its low cost makes phenobarbital a common first-line treatment option in less well developed regions (Zhang, Zeng, & Li, 2011).
Phenytoin (PHT)
Several reports of PHT exposure have noted no differential cognitive outcome compared to controls (Adab et al., 2004; Koch et al., 1999; Thomas et al., 2007; Wide, Winbladh, Tomson, Sars-Zimmer, & Berggren, 2000). Even in the absence of generalized cognitive impairment, decreased locomotor performance has been observed (Wide et al., 2000). One study describing decreased FSIQ associated with PHT exposure (Scolnik et al., 1994) was criticized due to significant differences in maternal IQs across groups (Loring, Meador, & Thompson, 1994). Other reports have described increased risk of developmental delay in children exposed to PHT, but also associated with VPA and CBZ exposure (Dean et al., 2002). The NEAD study reported no differences between PHT exposed children from either CBZ or LTG exposed children on FSIQ or verbal IQ, with PHT scores higher than with VPA exposure. No differences in adaptive behavior following PHT exposure as reported by parents compared to controls have been noted (Vinten et al., 2009).
Topiramate (TPM)
TPM exposure was associated with lower IQ scores in a very small sample of nine children, which was accompanied by poorer motor and visual spatial skills, and high rates of speech, occupational or physical therapy (Rihtman, Parush, & Ornoy, 2012).
Other AEDs
There is little or no information on the developmental outcomes associated with exposure to other AEDs including benzodiazepines, eslicarbazepine, ezogabine, felbamate, gabapentin, lacosamide, oxcarbazepine, perampanel, pregabalin, rufinamide, vigabatrin, or zonisamide.
Polytherapy
Multiple studies described an increased risk of poor developmental outcomes for children exposed to multiple AEDs during pregnancy (Adab et al., 2001; Dean et al., 2002; Dessens et al., 2000; Gaily et al., 2004; Koch et al., 1999; Nadebaum, Anderson, Vajda, Reutens, & Wood, 2012; Thomas et al., 2007; Veiby et al., 2013), although several reports have not found an increased risk (Adab et al., 2004; Bromley et al., 2010; Gaily, Kantola-Sorsa, & Granstrom, 1988; Wide et al., 2000). This discrepancy likely reflects different polytherapy combinations examined. When polytherapy combinations include VPA, an increased cognitive risk is described whereas polytherapy combinations with AEDs other than VPA are not associated with a similar risk. Polytherapy exposures including VPA are associated with lower FSIQ and verbal scores compared with either VPA monotherapy or polytherapy combinations with AEDs other than VPA (Baker et al., 2015; Nadebaum et al., 2012). Nevertheless, the effects of various polytherapy AED combinations remain uncertain and need to be more fully established.
Prognosis and Moderating Factors
Long-term studies of developmental outcomes have not been performed. Unfortunately, there is no research as yet that has identified specific moderating factors or long term outcomes. At the same time, there is no reasons to suspect that children with neurodevelopmental deficits or ASD from AED exposure in untero would differ from others with similar neurobehavioral disorders simply as a function of etiology.
Possible Mechanisms of Action
AED-induced cognitive and behavioral deficits have been described in the animal literature at dosages lower than what is necessary to increase risks of physical malformation (Adams, Vorhees, & Middaugh, 1990). Phenobarbital, for example, produces neuronal deficits, reduces brain weight and brain catecholamine levels, and impairs development of normal behaviors (Forcelli, Janssen, Vicini, & Gale, 2012). Prenatal phenytoin has been associated with dose-dependent impairment of coordination and learning (Ogura et al., 2002). Behavioral deficits have been described not only with older AEDs (e.g., PB, PHT, VPA), but also impaired rotorod performance for adult rats with neonatal-exposure to lamotrigine (Forcelli et al., 2012). Behavioral and anatomical defects likely involve different mechanisms—anatomical risks are related to first trimester exposure, and functional deficits appear primarily related to third trimester exposure. Third trimester exposure association interferes with the development of functional properties and connectivity, and susceptible to AED-induced apoptosis and altered synaptogenesis (Forcelli et al., 2012). Possible mechanisms associated with functional AED teratogenicity include folate deficiency, reactive intermediates (e.g. epoxides or free radicals), ischemia, apoptosis-related mechanisms, and neuronal suppression (Gedzelman & Meador, 2012).
The leading hypothesis for the mechanism behind behavioral/cognitive dysfunction involves AED induced apoptosis and altered synaptogenesis. Similar to alcohol, exposure of the immature brain to some AEDs is associated with widespread neuronal apoptosis (Turski & Ikonomidou, 2012). NMDA antagonist and GABA mimetic traits of ethanol have been postulated to produce apoptogenic action since other drugs that block NMDA glutamate receptors also trigger apoptosis in the developing brain. This may be clinically relevant because many AEDs used therapeutically in humans have NMDA antagonist or GABA mimetic properties (e.g., barbiturates and benzodiazepines; Olney et al., 2002). The associated cognitive deficits are likely more related to dysfunction in the surviving neurons than to the actual neuronal loss; exposure of the immature brain to AEDs impairs synaptic maturation in neurons that survive the initial exposure (Forcelli et al., 2012). Genetic predisposition likely also plays a role involving interaction of teratogens with multiple liability genes (Finnell, Shields, Taylor, & Chernoff, 1987). Genetic predisposition may explain the observed individual variability.
Considerations to Minimize Risk and Maximize Cognitive Outcome
Folate
Folate supplementation for women of reproductive age is now standard of care based on population studies demonstrating decreased neural tube defect risk (Blencowe, Cousens, Modell, & Lawn, 2010), although the optimal dose has not yet been established (Aguglia et al., 2009; Kjaer et al., 2008; Wilson et al., 2003). Several studies have demonstrated a similar relationship between folate levels or supplementation and decreased major congenital malformations in women with epilepsy although larger studies are needed (Harden et al., 2009; Kaaja, Kaaja, & Hiilesmaa, 2003; Kjaer et al., 2008).
In the NEAD study, children whose mothers reported periconception folate supplementation had higher Developmental Abilities Scale (DAS) GCA scores at 6 years of age than children whose mother did not take folate during their pregnancy (Meador et al., 2013). When excluding VPA, there was a dose-related effect of folate on conceptual abilities scores. This suggests that folate may have a positive effect on cognitive development, although others have failed to observe a similar beneficial effect of folate supplementation on cognitive outcome (Baker et al., 2015). Whether there is a more general beneficial effect of folate on cognitive outcome is unknown since, in the case of the NEAD study, the positive effect may simply have occurred from mitigating the negative AED effects. However, other studies report a beneficial effect of periconceptional folic acid supplementation for cognitive and behavioral outcomes in the general population (Chatzi et al., 2012; Roth et al., 2011; Schmidt et al., 2012; Suren et al., 2013). There is presently insufficient evidence to conclude that folate supplementation mitigates the structural or developmental teratogenic effects of AEDs, but it appears to provide a more general benefit and should be used in women of childbearing age being treated with AEDs.
Breast Feeding
Breast feeding provides important health benefits for both mother and child (Ip, Chung, Raman, Trikalinos, & Lau, 2009) that may include improved cognitive development (Heikkila, Kelly, Renfrew, Sacker, & Quigley, 2014; Kramer et al., 2008; Quigley et al., 2012). This benefit also appears to be present in children exposed to AEDs who are breast-fed. In the NEAD study, AED exposed children who were breast-fed had higher DAS GCA scores and Verbal Scores when tested at age 6 years than children who were not breast-fed (Meador et al., 2014). Although a similar pattern was described in a separate cohort of children when tested up to age 18 months, the benefit was no longer observed when tested at age 36 months (Veiby et al., 2013). Importantly, however, both studies indicate an absence of increased cognitive risk for children being breast-fed by mothers taking AEDs. Breast feeding by women taking AEDs has often been discouraged due to concerns that the child will be exposed to AED through breast milk. Unlike in utero exposure, in which the fetal levels are similar to maternal plasma concentrations, AEDs are differentially secreted into human milk. Although additional studies are needed, the current data suggest that the theoretical concern of infant breast milk AED exposure likely does not outweigh the known long-lasting benefits of breast feeding.
School Accommodations
There has been no systematic research demonstrating the specific benefit of school accommodations in this group of children. However, as with other developmental disorders, children with AED induced language delay or attentional impairment will likely benefit based upon their specific needs from modification of assignment length, the provision of extended time for assignments and tests, the assistant of a peer tutor/study partner, and private location for testing as needed. Similarly, children with ASD related to AED exposure may benefit from strategies to develop social skills and teaching social rules, with small group (or one-to-one) instruction and interventions generally appropriate for those with ASD.
Conclusions
Women of reproductive age taking AEDs for any indication should be counseled about exposure risks. These risks should also be discussed with teens and preteens and their parents before the girls become sexually active. Because roughly half of pregnancies in women with epilepsy are unplanned (Davis, Pack, Kritzer, Yoon, & Camus, 2008), not discussing risks until a woman is planning pregnancy is often too late. Contraceptive counseling is also extremely important for all women taking AEDs since they have significant drug–drug interactions with hormonal contraception (Gaffield, Culwell, & Lee, 2011).
Even with effective contraception, however, medication management of a reproductive-age woman should involve AEDs with lowest teratogenic risk that effectively manage their epilepsy. Thus, VPA is a poor first choice for females of child-bearing potential. Unfortunately, some women will have inadequate control of seizures or mood symptoms with medications other than VPA. In these cases, the smallest effective dose of VPA should be employed. Although the dose-related exposure risks appear to be the greatest for VPA, women taking other AEDs should be managed with the smallest effective doses as well. Polytherapy should be avoided if possible. Finally, it may also be necessary to establish that AEDs are appropriately indicated since some patients with non-AED responsive seizures or atypical histories may have non-epileptic seizures, and appropriate monitoring in an epilepsy unit may be necessary to establish this diagnosis.
An area of debate is whether AED changes should ever be contemplated during pregnancy. When characterization of AED teratogenesis was limited to major congenital malformations, this was generally not considered since by the time pregnancy was confirmed, the risks of cross-titrating AEDs was thought to simply expose the fetus to polytherapy and increased the risk of seizures after the major congenital malformation risk had already occurred. We now know that cognitive AED risks likely occur throughout pregnancy and, in particular, during the third trimester. Consequently, some have suggested that it is reasonable to consider AED changes during pregnancy, particularly if the woman is taking VPA. This consideration should include seizure history or severity of her mood disorder, history of prior medication trials and effectiveness, likelihood of responding to a different medication, and the ability to closely monitor her and her child. This is strictly a clinical consideration since there is insufficient evidence to argue for or against this approach.
Women taking AEDs should receive folate supplementation since it decreases risk of neural tube defects, may reduce the risk of other malformations, and may have a positive effect on cognitive development. Although it is unclear if folate provides benefit to women taking AEDs beyond that seen in the general population, supplementation is recommended by multiple professional organizations.
Most women taking AEDs should not be discouraged from breast-feeding their child. For women taking CBZ, LTG, PHT, or VPA in monotherapy, there is no evidence to suggest risk to the child. Women taking other AEDs or being treated with polytherapy may consider the risks vs. benefits of breast feeding since the AED risks remain primarily theoretical. Further, pediatricians treating children exposed to AEDs in utero should also monitor the child’s development closely and consider early intervention for children exposed to AEDs (especially VPA) who demonstrate any signs of developmental delay.
References
Adab, N., Jacoby, A., Smith, D., & Chadwick, D. (2001). Additional educational needs in children born to mothers with epilepsy. Journal of Neurology, Neurosurgery & Psychiatry, 70, 15–21.
Adab, N., Kini, U., Vinten, J., Ayres, J., Baker, G., Clayton-Smith, J., … Chadwick, D. W. (2004). The longer term outcome of children born to mothers with epilepsy. Journal of Neurology, Neurosurgery & Psychiatry, 75(11), 1575–1583.
Adams, J., Vorhees, C. V., & Middaugh, L. D. (1990). Developmental neurotoxicity of anticonvulsants: human and animal evidence on phenytoin. Neurotoxicology & Teratology, 12(3), 203–214.
Aguglia, U., Barboni, G., Battino, D., Cavazzuti, G. B., Citernesi, A., Corosu, R., … Verrotti, A. (2009). Italian consensus conference on epilepsy and pregnancy, labor and puerperium. Epilepsia, 50 Suppl 1, 7–23.
Baker, G. A., Bromley, R. L., Briggs, M., Cheyne, C. P., Cohen, M. J., Garcia-Finana, M., … Clayton-Smith, J. (2015). IQ at 6 years after in utero exposure to antiepileptic drugs: A controlled cohort study. Neurology, 84(4), 382–390.
Banach, R., Boskovic, R., Einarson, T., & Koren, G. (2010). Long-term developmental outcome of children of women with epilepsy, unexposed or exposed prenatally to antiepileptic drugs: A meta-analysis of cohort studies. Drug Safety, 33(1), 73–79.
Blencowe, H., Cousens, S., Modell, B., & Lawn, J. (2010). Folic acid to reduce neonatal mortality from neural tube disorders. International Journal of Epidemiology, 39(Suppl 1), i110–i121.
Bobo, W. V., Davis, R. L., Toh, S., Li, D. K., Andrade, S. E., Cheetham, T. C., … Cooper, W. O. (2012). Trends in the use of antiepileptic drugs among pregnant women in the US, 2001–2007: A medication exposure in pregnancy risk evaluation program study. Paediatric and Perinatal Epidemiology, 26(6), 578–588.
Bromley, R. L., Mawer, G. E., Briggs, M., Cheyne, C., Clayton-Smith, J., Garcia-Finana, M., … Baker, G. A. (2013). The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. Journal of Neurology, Neurosurgery & Psychiatry, 84(6), 637–643.
Bromley, R. L., Mawer, G., Love, J., Kelly, J., Purdy, L., McEwan, L., … Baker, G. A. (2010). Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia, 51(10), 2058–2065.
Campbell, E., Kennedy, F., Russell, A., Smithson, W. H., Parsons, L., Morrison, P. J., … Morrow, J. (2014). Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. Journal of Neurology, Neurosurgery & Psychiatry, 85(9), 1029–1034.
Cantwell, R., Clutton-Brock, T., Cooper, G., Dawson, A., Drife, J., Garrod, D., … Springett, A. (2011). Saving mothers’ lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG, 118 Suppl 1, 1–203.
Chatzi, L., Papadopoulou, E., Koutra, K., Roumeliotaki, T., Georgiou, V., Stratakis, N., … Kogevinas, M. (2012). Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: the mother-child cohort ‘Rhea’ study in Crete, Greece. Public Health Nutrition, 15(9), 1728–1736.
Christensen, J., Gronborg, T. K., Sorensen, M. J., Schendel, D., Parner, E. T., Pedersen, L. H., & Vestergaard, M. (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA, 309(16), 1696–1703.
Christianson, A. L., Chesler, N., & Kromberg, J. G. (1994). Fetal valproate syndrome: Clinical and neuro-developmental features in two sibling pairs. Developmental Medicine & Child Neurology, 36(4), 361–369.
Cohen, M. J., Meador, K. J., Browning, N., Baker, G. A., Clayton-Smith, J., Kalayjian, L. A., … Loring, D. W. (2011). Fetal antiepileptic drug exposure: motor, adaptive, and emotional/behavioral functioning at age 3 years. Epilepsy & Behavior, 22(2), 240–246.
Cohen, M. J., Meador, K. J., Browning, N., May, R., Baker, G. A., Clayton-Smith, J., … Loring, D. W. (2013). Fetal antiepileptic drug exposure: Adaptive and emotional/behavioral functioning at age 6 years. Epilepsy Behav, 29(2), 308–315.
Cummings, C., Stewart, M., Stevenson, M., Morrow, J., & Nelson, J. (2011). Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Archives of Disease in Childhood, 96(7), 643–647.
Davis, A. R., Pack, A. M., Kritzer, J., Yoon, A., & Camus, A. (2008). Reproductive history, sexual behavior and use of contraception in women with epilepsy. Contraception, 77(6), 405–409.
Dean, S., Colantonio, A., Ratcliff, G., & Chase, S. (2000). Clients’ perspectives on problems many years after traumatic brain injury. Psychological Reports, 86(2), 653–658.
Dean, J. C., Hailey, H., Moore, S. J., Lloyd, D. J., Turnpenny, P. D., & Little, J. (2002). Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. Journal of Medical Genetics, 39(4), 251–259.
Dessens, A. B., Cohen-Kettenis, P. T., Mellenbergh, G. J., Koppe, J. G., van De Poll, N. E., & Boer, K. (2000). Association of prenatal phenobarbital and phenytoin exposure with small head size at birth and with learning problems. Acta Paediatrica, 89(5), 533–541.
Edey, S., Moran, N., & Nashef, L. (2014). SUDEP and epilepsy-related mortality in pregnancy. Epilepsia, 55(7), e72–e74.
Eriksson, K., Viinikainen, K., Monkkonen, A., Aikia, M., Nieminen, P., Heinonen, S., & Kalviainen, R. (2005). Children exposed to valproate in utero—population based evaluation of risks and confounding factors for long-term neurocognitive development. Epilepsy Research, 65(3), 189–200.
Finnell, R. H., Shields, H. E., Taylor, S. M., & Chernoff, G. F. (1987). Strain differences in phenobarbital-induced teratogenesis in mice. Teratology, 35(2), 177–185.
Forcelli, P. A., Janssen, M. J., Vicini, S., & Gale, K. (2012). Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Annals of Neurology, 72(3), 363–372.
Gaffield, M. E., Culwell, K. R., & Lee, C. R. (2011). The use of hormonal contraception among women taking anticonvulsant therapy. Contraception, 83(1), 16–29.
Gaily, E., Kantola-Sorsa, E., Hiilesmaa, V., Isoaho, M., Matila, R., Kotila, M., … Granstrom, M. L. (2004). Normal intelligence in children with prenatal exposure to carbamazepine. Neurology, 62(1), 28–32.
Gaily, E., Kantola-Sorsa, E., & Granstrom, M. L. (1988). Intelligence of children of epileptic mothers. Journal of Pediatrics, 113(4), 677–684.
Gedzelman, E. R., & Meador, K. J. (2012). Neurological and psychiatric sequelae of developmental exposure to antiepileptic drugs. Frontiers in Neurology, 3, 182.
Harden, C. L. (2014). Pregnancy and epilepsy. Continuum (Minneap, MN), 20(1 Neurology of Pregnancy), 60–79.
Harden, C. L., Hopp, J., Ting, T. Y., Pennell, P. B., French, J. A., Hauser, W. A., … Le Guen, C. (2009). Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology, 73(2), 126–132.
Heikkila, K., Kelly, Y., Renfrew, M. J., Sacker, A., & Quigley, M. A. (2014). Breastfeeding and educational achievement at age 5. Maternal & Child Nutrition, 10(1), 92–101.
Hernandez-Diaz, S., Smith, C. R., Shen, A., Mittendorf, R., Hauser, W. A., Yerby, M., & Holmes, L. B. (2012). Comparative safety of antiepileptic drugs during pregnancy. Neurology, 78(21), 1692–1699.
Hill, D. S., Wlodarczyk, B. J., Palacios, A. M., & Finnell, R. H. (2010). Teratogenic effects of antiepileptic drugs. Expert Review of Neurotherapeutics, 10(6), 943–959.
Ip, S., Chung, M., Raman, G., Trikalinos, T. A., & Lau, J. (2009). A summary of the agency for healthcare research and quality’s evidence report on breastfeeding in developed countries. Breastfeeding Medicine, 4(Suppl 1), S17–S30.
Kaaja, E., Kaaja, R., & Hiilesmaa, V. (2003). Major malformations in offspring of women with epilepsy. Neurology, 60(4), 575–579.
Kjaer, D., Horvath-Puho, E., Christensen, J., Vestergaard, M., Czeizel, A. E., Sorensen, H. T., & Olsen, J. (2008). Antiepileptic drug use, folic acid supplementation, and congenital abnormalities: A population-based case-control study. BJOG, 115(1), 98–103.
Koch, S., Titze, K., Zimmermann, R. B., Schroder, M., Lehmkuhl, U., & Rauh, H. (1999). Long-term neuropsychological consequences of maternal epilepsy and anticonvulsant treatment during pregnancy for school-age children and adolescents. Epilepsia, 40(9), 1237–1243.
Kramer, M. S., Aboud, F., Mironova, E., Vanilovich, I., Platt, R. W., Matush, L., … Shapiro, S. (2008). Breastfeeding and child cognitive development: new evidence from a large randomized trial. Archives of General Psychiatry, 65(5), 578–584.
Loring, D. W., Meador, K. J., & Thompson, W. O. (1994). Neurodevelopment after in utero exposure to phenytoin and carbamazepine. JAMA, 272(11), 850–851.
Margulis, A. V., Mitchell, A. A., Gilboa, S. M., Werler, M. M., Mittleman, M. A., Glynn, R. J., & Hernandez-Diaz, S. (2012). Use of topiramate in pregnancy and risk of oral clefts. American Journal of Obstetrics & Gynecology, 207(5), 405.e401–405.e407.
Mawer, G., Clayton-Smith, J., Coyle, H., & Kini, U. (2002). Outcome of pregnancy in women attending an outpatient epilepsy clinic: Adverse features associated with higher doses of sodium valproate. Seizure, 11(8), 512–518.
Mawhinney, E., Craig, J., Morrow, J., Russell, A., Smithson, W. H., Parsons, L., … Hunt, S. J. (2013). Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology, 80(4), 400–405.
Meador, K.J., Baker, G.A., Browning, N., Clayton-Smith, J, Combs-Cantrell, D.T., Cohen, M., … Loring, D.W. (2009). Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. New England Journal of Medicine, 360(16), 1597–1605.
Meador, K. J., Baker, G. A., Browning, N., Cohen, M. J., Bromley, R. L., Clayton-Smith, J., … Loring, D. W. (2014). Breastfeeding in children of women taking antiepileptic drugs: Cognitive outcomes at age 6 years. JAMA Pediatrics, 168(8), 729–736.
Meador, K. J., Baker, G. A., Browning, N., Cohen, M J., Bromley, R. L., Clayton-Smith, J. , … Loring, D. W. (2013). Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): A prospective observational study. The Lancet Neurology, 12(3), 244–252.
Meador, K., Reynolds, M. W., Crean, S., Fahrbach, K., & Probst, C. (2008). Pregnancy outcomes in women with epilepsy: A systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Research, 81(1), 1–13.
Mines, D., Tennis, P., Curkendall, S. M., Li, D. K., Peterson, C., Andrews, E. B., … Chan, K. A. (2014). Topiramate use in pregnancy and the birth prevalence of oral clefts. Pharmacoepidemiology & Drug Saftey, 23(10), 1017–1025.
Nadebaum, C., Anderson, V., Vajda, F., Reutens, D., & Wood, A. (2012). Neurobehavioral consequences of prenatal antiepileptic drug exposure. Developmental Neuropsychology, 37(1), 1–29.
Ogura, H., Yasuda, M., Nakamura, S., Yamashita, H., Mikoshiba, K., & Ohmori, H. (2002). Neurotoxic damage of granule cells in the dentate gyrus and the cerebellum and cognitive deficit following neonatal administration of phenytoin in mice. Journal of Neuropathology and Experimental Neurology, 61(11), 956–967.
Olney, J. W., Wozniak, D. F., Jevtovic-Todorovic, V., Farber, N. B., Bittigau, P., & Ikonomidou, C. (2002). Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathology, 12(4), 488–498.
Ornoy, A., & Cohen, E. (1996). Outcome of children born to epileptic mothers treated with carbamazepine during pregnancy. Archives of Disease in Childhood, 75(6), 517–520.
Quigley, M. A., Hockley, C., Carson, C., Kelly, Y., Renfrew, M. J., & Sacker, A. (2012). Breastfeeding is associated with improved child cognitive development: A population-based cohort study. Journal of Pediatrics, 160(1), 25–32.
Rasalam, A. D., Hailey, H., Williams, J. H., Moore, S. J., Turnpenny, P. D., Lloyd, D. J., & Dean, J. C. (2005). Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Developmental Medicine & Child Neurology, 47(8), 551–555.
Reinisch, J. M., Sanders, S. A., Mortensen, E. L., & Rubin, D. B. (1995). In utero exposure to phenobarbital and intelligence deficits in adult men. JAMA, 274(19), 1518–1525.
Rihtman, T., Parush, S., & Ornoy, A. (2012). Preliminary findings of the developmental effects of in utero exposure to topiramate. Reproductive Toxicology, 34(3), 308–311.
Rihtman, T., Parush, S., & Ornoy, A. (2013). Developmental outcomes at preschool age after fetal exposure to valproic acid and lamotrigine: Cognitive, motor, sensory and behavioral function. Reproductive Toxicology, 41, 115–125.
Roth, C., Magnus, P., Schjolberg, S., Stoltenberg, C., Suren, P., McKeague, I. W., … Susser, E. (2011). Folic acid supplements in pregnancy and severe language delay in children. JAMA, 306(14), 1566–1573.
Schmidt, R. J., Tancredi, D. J., Ozonoff, S., Hansen, R. L., Hartiala, J., Allayee, H., … Hertz-Picciotto, I. (2012). Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. American Journal of Clinical Nutrition, 96(1), 80–89.
Scolnik, D., Nulman, I., Rovet, J., Gladstone, D., Czuchta, D., Gardner, H. A., … Einarson, T. (1994). Neurodevelopment of children exposed in utero to phenytoin and carbamazepine monotherapy. JAMA, 271(10), 767–770.
Shallcross, R., Bromley, R. L., Cheyne, C. P., Garcia-Finana, M., Irwin, B., Morrow, J., & Baker, G. A. (2014). In utero exposure to levetiracetam vs valproate: Development and language at 3 years of age. Neurology, 82(3), 213–221.
Shallcross, R., Bromley, R. L., Irwin, B., Bonnett, L. J., Morrow, J., & Baker, G. A. (2011). Child development following in utero exposure: Levetiracetam vs sodium valproate. Neurology, 76(4), 383–389.
Suren, P., Roth, C., Bresnahan, M., Haugen, M., Hornig, M., Hirtz, D., … Stoltenberg, C. (2013). Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA, 309(6), 570–577.
Thomas, S. V., Sukumaran, S., Lukose, N., George, A., & Sarma, P. S. (2007). Intellectual and language functions in children of mothers with epilepsy. Epilepsia, 48(12), 2234–2240.
Tomson, T., & Battino, D. (2012). Teratogenic effects of antiepileptic drugs. The Lancet Neurology, 11(9), 803–813.
Turski, C. A., & Ikonomidou, C. (2012). Neuropathological sequelae of developmental exposure to antiepileptic and anesthetic drugs. Frontiers in Neurology, 3, 120.
Vajda, F. J., O'Brien, T. J., Lander, C. M., Graham, J., & Eadie, M. J. (2014). The teratogenicity of the newer antiepileptic drugs: An update. Acta Neurologica Scandinavica, 130(4), 234–238.
van der Pol, M. C., Hadders-Algra, M., Huisjes, H. J., & Touwen, B. C. (1991). Antiepileptic medication in pregnancy: Late effects on the children's central nervous system development. American Journal of Obstetrics & Gynecology, 164(1 Pt 1), 121–128.
Veiby, G., Daltveit, A. K., Schjolberg, S., Stoltenberg, C., Oyen, A. S., Vollset, S. E., … Gilhus, N. E. (2013). Exposure to antiepileptic drugs in utero and child development: a prospective population-based study. Epilepsia, 54(8), 1462–1472.
Viguera, A. C., Whitfield, T., Baldessarini, R. J., Newport, D. J., Stowe, Z., Reminick, A., … Cohen, L. S. (2007). Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. American Journal of Psychiatry, 164(12), 1817–1824.
Vinten, J., Adab, N., Kini, U., Gorry, J., Gregg, J., & Baker, G. A. (2005). Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology, 64(6), 949–954.
Vinten, J., Bromley, R. L., Taylor, J., Adab, N., Kini, U., & Baker, G. A. (2009). The behavioral consequences of exposure to antiepileptic drugs in utero. Epilepsy & Behavior, 14(1), 197–201.
Wide, K., Winbladh, B., Tomson, T., Sars-Zimmer, K., & Berggren, E. (2000). Psychomotor development and minor anomalies in children exposed to antiepileptic drugs in utero: A prospective population-based study. Developmental Medicine & Child Neurology, 42(2), 87–92.
Williams, P. G., & Hersh, J. H. (1997). A male with fetal valproate syndrome and autism. Developmental Medicine & Child Neurology, 39(9), 632–634.
Williams, G., King, J., Cunningham, M., Stephan, M., Kerr, B., & Hersh, J. H. (2001). Fetal valproate syndrome and autism: Additional evidence of an association. Developmental Medicine & Child Neurology, 43(3), 202–206.
Wilson, R. D., Davies, G., Desilets, V., Reid, G. J., Summers, A., Wyatt, P., & Young, D. (2003). The use of folic acid for the prevention of neural tube defects and other congenital anomalies. Journal of Obstetrics and Gynaecology Canada, 25(11), 959–973.
Zhang, L. L., Zeng, L. N., & Li, Y. P. (2011). Side effects of phenobarbital in epilepsy: A systematic review. Epileptic Disorders, 13(4), 349–365.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Loring, D.W., Gerard, E.E., Meador, K.J. (2016). Neurodevelopmental Considerations with Antiepileptic Drug Use During Pregnancy. In: Riccio, C., Sullivan, J. (eds) Pediatric Neurotoxicology. Specialty Topics in Pediatric Neuropsychology. Springer, Cham. https://doi.org/10.1007/978-3-319-32358-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-32358-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32356-5
Online ISBN: 978-3-319-32358-9
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)