Abstract
Carbohydrate composition varies between different fruiting species and is the balance between the carbon supply to the fruit and its storage via a cascade of biochemical reactions. In kiwifruit, a starch-storing fruit, soluble solids content (SSC) during fruit development is determined by both the partitioning of carbohydrates into soluble and insoluble components, and the conversion of starch to sugars. The seasonal patterns of carbohydrate concentrations show great dissimilarities in Actinidia depending on species and tissue. However, ripe fruits of Actinidia chinensis var. chinensis and Actinidia chinensis var. deliciosa contain glucose and fructose as the predominant soluble sugars and sucrose in smaller amounts, while Actinidia arguta differs significantly, as its fruit contain mainly sucrose and great quantities of myo-inositol during the early phases of sugar accumulation. Here, we report an overview of the recent developments in the study of pathways controlling carbohydrate metabolism in kiwifruit, specifically focusing on the genes encoding the biosynthetic enzymes sucrose-phosphate synthase (SPS), l-myo-inositol-1-phosphate synthase (MIPS), ADP-glucose pyrophosphorylase (AGPase), and the degradative enzymes, such as sucrose synthase (SUS), invertases, and amylases. A brief outline of sugar transport and signaling has also been presented, helping to indicate the complexity of the genetic variation that underpins kiwifruit compositional differences. The availability of the Actinidia genome sequence represents an important starting point for the identification and characterization of new genes, providing a valuable tool for genetic improvement.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
15.1 Introduction
The regulation of carbon partitioning at the whole plant level is strictly dependent on the cellular pathways of assimilate transport, metabolism, and allocation of sugars, in source leaves and sink organs such as roots and fruit. This phenomenon, of considerable general importance for plant growth and development, assumes a particular relevance in commercial fruit crops, where sugar metabolism affects quality attributes such as the sugar–acid balance and starch accumulation. In addition, a large body of evidence shows that sugars also function as signaling molecules in regulating gene expression and plant development.
In higher plants, two enzymes catalyze the cleavage of sucrose: sucrose synthase (SUS) and invertase (INV). The former degrades sucrose in the presence of UDP to UDP-glucose and fructose, whereas the latter hydrolyzes sucrose to glucose and fructose. Current understanding indicates that SUS is mainly involved in the biosynthesis of sugar polymers, including starch and cellulose, and the generation of energy (ATP). On the other hand, INV appears to have a wide range of regulatory functions in plant growth and development in addition to its major role in primary carbon metabolism (Ruan et al. 2010).
Carbohydrate composition varies between different fruiting species and reflects the balance between carbon supply to the fruit and its storage via a cascade of biochemical reactions; for instance, tomato and peach accumulate mainly soluble sugars during fruit development (Bertin et al. 2009; Falchi et al. 2013; Zanon et al. 2015), whereas banana, apple, pear, and kiwifruit are starch-storing fruit (Nardozza et al. 2010a). Therefore, the changing soluble solids content (SSC) of kiwifruit during fruit development is determined both by the partitioning of carbohydrates into soluble and insoluble components, and by the conversion of starch to sugars.
Besides health benefits and appearance, taste and flavor, largely determined by the concentration and balance of sugars and acids, are critical desirable attributes of kiwifruit for consumer acceptance. The pattern, or the rate, of soluble solids accumulation is likely to be a more robust indicator of the physiological state of the fruit, and therefore postharvest performance, than a single SSC value. Studies have shown that there is a stronger association between softening in storage and soluble solids accumulation rate than with either SSC or firmness values at harvest (Burdon et al. 2013).
Currently, the green-fleshed kiwifruit, Actinidia deliciosa var. deliciosa, the closely related yellow-fleshed Actinidia chinensis var. chinensis, and the kiwiberry, Actinidia arguta, are the most important Actinidia taxa being grown commercially. Cultivated kiwifruit are mainly seedling selections, as owing to dioecy and the variation in ploidy of Actinidia, systematic breeding is still difficult. Consequently, there are still many characteristics within the genus that could be incorporated into commercial cultivars and to this aim a better knowledge of the regulation of these traits is required (Crowhurst et al. 2008).
15.2 Photoassimilate Metabolism in Actinidia
Carbohydrate metabolism in kiwifruit has been described both during fruit growth, in terms of metabolism of imported carbon and sink strength (Moscatello et al. 2011), and during ripening and the postharvest period (MacRae et al. 1992).
Fruit of Actinidia are strong sinks for accumulation of photosynthates produced by the canopy in the form of sucrose. During kiwifruit development, there are three main stages in terms of the predominating metabolism: (1) cell division, (2) starch accumulation, and (3) fruit maturation (Richardson et al. 2004). In the first stage, from 0 to 45 days after full bloom (DAFB), there is a remarkable increase of glucose in the outer pericarp. During the second stage, lasting from about 45 DAFB to about 120 DAFB, cell division slows and starch accumulates even to exceed more than 40 % of the fruit dry weight (DW). Rapid starch accumulation follows the rise in glucose, suggesting that glucose may play a role of signaling that contributes to a dramatic change in the allocation of imported carbon toward starch, a sharp change that marks the switch from the first to the second phase of fruit growth in kiwifruit (Moscatello et al. 2011). Kiwifruit are always commercially harvested when sufficient starch has degraded for soluble solids to reach a minimum Brix value, but starch is still the major carbohydrate present in the fruit (in the order of 5–7 % fresh weight (FW) or 40–50 % dry weight) (MacRae et al. 1989). Analysis of several A. chinensis var. deliciosa breeding families found consistent genetic differences in fruit starch concentration between genotype classes with contrasting dry matter content, explicable by differences in the relative volumes of small and large cells in the outer pericarp. This finding is significant because it means that selective breeding based on gross compositional traits, such as dry matter or flesh starch concentration, may inadvertently result in the selection of loci controlling anatomical traits. Changes in cellular anatomy have potential consequences for fruit quality traits that are not directly related to composition, such as postharvest storability, softening patterns, texture, or juiciness (Nardozza et al. 2010b).
In the last stage, from about 120 DAFB to harvest, starch accumulation ends, and the soluble sugar content increases remarkably, in the form of equimolar amounts of glucose and fructose. By the time the fruit are edible, starch is no longer present and the sugar content is approximately fivefold higher than that at harvest. These events take place over an extended period after harvest, and several studies indicate that at least some starch degradation has to occur prior to the climacteric peak. Hence, kiwifruit provide a potential contrast to bananas, where starch degradation appears to overlap temporally with the climacteric (MacRae et al. 1992).
Big differences in SSC and sugars have been found between and within Actinidia taxa. The seasonal patterns of carbohydrate concentrations in Actinidia taxa, in leaf, fruit, and fine root tissue samples from A. arguta, A. chinensis var. chinensis, A. chinensis var. deliciosa, Actinidia eriantha, and Actinidia polygama, have been determined (Boldingh et al. 2000). All five taxa transiently accumulated starch, and the onset of net starch degradation coincides with the onset of net sugar accumulation. Hexose sugars transiently increased in all taxa, between 25 DAFB in A. arguta and 45–60 DAFB in A. chinensis var. deliciosa. During the developmental period, A. polygama accumulated more than twice as much sugar as A. arguta or A. chinensis var. chinensis and more than three times the concentrations found in A. eriantha (Boldingh et al. 2000).
The sugar composition of A. chinensis var. chinensis fruit resembles that of A. chinensis var. deliciosa, glucose and fructose being the predominant soluble sugars with sucrose present in smaller amounts (Esti et al. 1998), while A. arguta greatly differs as its fruit contain sucrose as the predominant soluble sugar. All taxa show maximal myo-inositol concentrations during the early accumulation of sugars, but in A. arguta myo-inositol content represents about 60 % of all sugars (33 % of total non-structural carbohydrate) at that time, whereas myo-inositol contributes only about 10 % in A. chinensis var. deliciosa, 20 % in A. chinensis var. chinensis, and 5 % of the total sugar accumulated in A. polygama (Boldingh et al. 2000). Even so, the myo-inositol level in the ‘Hayward’ fruit (A. chinensis var. deliciosa), which is reported to be 153 mg/100 g FW, is higher than commonly consumed fruits, including orange, grapefruit, and mandarin orange (Klages et al. 1997; Sanz et al. 2004).
Klages et al. (1997) suggested that part of the myo-inositol in the fruit might be synthesized in situ, whereas some of myo-inositol might be translocated from the phloem as a minor component (Nishiyama 2007). The polyol myo-inositol, synthesized from d-glucose-6-phosphate through myo-inositol-1-phosphate, is ubiquitously present in higher plants, playing a central role in several biochemical pathways, being a precursor in the synthesis of phosphoinositides, phytoglycolipid, inositol phosphates, auxin conjugates, the raffinose series of sugars, and ascorbate (Klages et al. 2004). It has been suggested that the sugar functions in Actinidia fruit to maintain cellular turgor, especially during rapid cell enlargement. At that time, myo-inositol may also act as an osmoprotectant and as a substrate for cell wall precursors. However, carbohydrate accumulation in A. eriantha seems to be insufficient to fulfill those cellular functions. Therefore, the relatively higher activity of myo-inositol-synthesizing enzymes in that species when the fruits are taking up water quickly may be indicative of a rapid transformation from glucose to myo-inositol. It might also reflect the large requirement for myo-inositol during that time, perhaps acting as a precursor for phosphatidylinositol, inositol phosphates, or members of the raffinose family (Cui et al. 2013). As fruit begins to ripen, net starch breakdown starts and glucose, fructose, and sucrose accumulate in fruit of all species. In A. eriantha, A. chinensis var. chinensis, and A. chinensis var. deliciosa, sucrose accumulation is delayed compared to net starch breakdown and hexose accumulation, while in A. arguta sucrose accumulates faster than the hexose sugars combined (Boldingh et al. 2000).
The fresh weight of leaves increases in all species over the first 80 days after bud break, and maximum leaf weight differs markedly between the species, with those with the heaviest fruit also having the heaviest leaves. Total carbohydrate, sucrose, and starch accumulate in leaves of all species until the time around flowering. Maximum starch concentrations are lower in A. arguta than in A. chinensis var. deliciosa, but are maintained for longer after flowering. A. polygama leaves contain more starch before flowering than leaves of any of the other species and have the lowest sugar–starch ratio. In all taxa, leaf starch is lowest toward the end of the growing season, prior to leaf fall. Glucose, fructose, and myo-inositol are present in leaves of all taxa, while A. chinensis var. deliciosa has the highest concentrations of leaf myo-inositol (Boldingh et al. 2000). In contrast to starch and sucrose, myo-inositol concentrations are similar before and after flowering, but contributing 14–19 % of the carbohydrate pool in sink leaves and only 7–10 % in source leaves, showing after flowering a circadian behavior (Klages et al. 2004).
The main sugar detected in all A. arguta leaves is a trisaccharide, planteose , which exceeds sucrose concentrations in all samples measured. Planteose is similar to raffinose, but with galactose attached to a different carbon of sucrose. While oligosaccharides of the raffinose series represent a major component of both temporary storage carbohydrate in leaves and translocated carbon in the phloem of a variety of plant species, planteose appears to be less common (Sprenger and Keller 2000). Planteose has been reported from cyclamen (Rothe et al. 1999), ash (Jukes and Lewis 1974), and sesame seed (Dey 1980). Planteose represents 45–65 % of the total sugar fraction in leaves of seedling and fruiting A. arguta plants. In A. arguta leaves, there is more planteose than sucrose with clear diurnal patterns in concentration—opposite to those for sucrose—and it forms the major storage form from new photosynthate. Sucrose concentrations also show diurnal patterns, but these differ according to the species and to the presence of fruit. Most importantly, experimental data indicate distinct times for the synthesis of planteose and sucrose. Starch is, as expected, synthesized during the day and metabolized during the night, but its overall variation is much less dramatically than that of planteose (Klages et al. 2004). Recent studies have shown that planteose is also present in phloem exudates and is translocated from leaves, through shoots to fruit (Boldingh et al. 2015).
The main carbohydrates identified in fine roots (2 mm diameter) of several Actinidia species are starch and sucrose, characterized by a similar seasonal pattern of declining throughout late winter and spring to reach the lowest concentrations in summer (Boldingh et al. 2000).
Modification of the photoassimilate supply substantially affects fruit development and size through the modulation of cell number and cell size (Bohner and Bangerth 1988; Bertin et al. 2002); therefore, the regulation of enzymes involved in primary carbon metabolism and photosynthesis is expected to have an impact on fruit growth (Azzi et al. 2015).
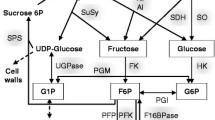
In order to shed light on the genetic control of fruit development, genes regulating the dynamics of starch metabolism and sugar homeostasis have been studied in different species and tissues of the genus Actinidia (Fig. 15.1).
Schematic diagram of the genes involved in sugar metabolism and transport processes contributing to the flow of assimilates through the source–sink pathway in Actinidia. Sucrose synthesis is catalyzed by sucrose-phosphate synthase (SPS). Degradation of sucrose by sucrose synthase (SUS) and vacuolar, cell wall, and neutral invertases (VI, CWI, NI) generates hexoses which, after phosphorylation mediated by hexokinase (HXK) and fructokinase (FK), enter various biosynthetic pathways including starch synthesis. ADP-glucose pyrophosphorylase (AGPase) and α-/β-amylases (AMY, BAM) are involved in starch synthesis and degradation, respectively. Myo-inositol (synthesized by l-myo-inositol-1-phosphate synthase (MIPS)) and planteose are carbohydrates peculiarly accumulated in kiwifruit tissues. Sucrose and hexose transporters (SUC, STP) are involved in sugar transport and partitioning. The genes specifically studied in leaf or fruit tissues are shown in bold
15.2.1 Sucrose Synthase
Among the different cleavage enzymes, sucrose synthase (SUS) mobilizes sucrose into multiple pathways involved in metabolic, structural, and storage functions, for example, by producing precursors for polysaccharide synthesis and/or as a substrate for respiration (Koch 2004). SUS is a glycosyltransferase, which, in the presence of uridine 5′-diphosphate (UDP), converts sucrose into UDP-glucose and fructose in a reversible manner. Genes encoding this enzyme are highly expressed in storage organs such as seed, fruit, and taproots, and expression is often positively associated with starch synthesis and fruit size (Hennen-Bierwagen et al. 2009; Baroja-Fernández et al. 2012), being generally considered as a biochemical marker of sink strength (Ruan 2014). Despite conflicting results reported for tomato (Chengappa et al. 1999; D’Aoust et al. 1999), the ectopic expression of StSUS4 in maize resulted in seeds with both higher starch content and amylose/amylopectin balance (Li et al. 2013).
A small multigene family encodes SUS isoforms in many of the plant species examined to date. Studies of the predicted amino acid sequences and gene structure have shown that the Arabidopsis SUS family consists of six SUS genes displaying different developmental expression patterns (Baud et al. 2004). In kiwifruit, expression of SUS genes appears tightly regulated spatially and temporally. In A. chinensis var. deliciosa, two different isoforms were identified (Richardson et al. 2004; Nardozza et al. 2013), the expression of which changed markedly during fruit growth. SUSA transcript level increased with fruit development, representing the dominant SUS in mature fruit as also in mandarin (Komatsu et al. 2002) and melon (Dai et al. 2011). Analysis of expression in several A. chinensis var. deliciosa genotypes indicates a positive correlation between SUSA, starch, and dry matter (Nardozza et al. 2013). The expression of this gene is also environmentally regulated as it responds to temperature (Richardson et al. 2004) and to different treatments to elicit ripening responses (Tanou et al. 2015).
Transcript levels of another sucrose synthase, SUS1, homologous to sucrose synthases postulated to play a role in sucrose unloading in storage organs, peak early in fruit growth (40–50 DAFB), concomitantly with high glucose content, and then progressively decrease as fruit develops (Richardson et al. 2004; Nardozza et al. 2013). In maize, suppression of SUS1 expression results in reduced starch biosynthesis (Chourey et al. 1998), while in potato tubers, the orthologous gene, StSUS4, is predominantly expressed in the vascular and storage tissues (Fu and Park 1995). Conversely, in A chinensis var. chinensis fruit, SUSA expression increased starting from 150 DAFB (harvest) and remained high until senescence (Richardson et al. 2011).
The genotype- and development-dependent differential expression patterns of SUS genes suggest that each isoform may have evolved into specialized functions to regulate efficiently sucrose cleavage, under the different metabolic conditions that characterize fruit developmental stages as cell division, starch accumulation, or (climacteric) respiration (Bahaji et al. 2014). Furthermore, SUS is subjected to both transcriptional and post-transcriptional regulation (Kleczkowski et al. 2010).
15.2.2 Invertase
This enzyme, which catalyzes the irreversible hydrolysis of sucrose to glucose and fructose, is present in plants in three different isoforms with specific biochemical properties and subcellular localizations, namely acid vacuolar (VI), acid cell wall-bound (CWI), and neutral (cytoplasmic) invertases (NI) (Roitsch and González 2004). An association between a major QTL controlling fruit weight and sugar content, and a gene coding for a cell wall-bound invertase has been identified in tomato (Fridman et al. 2000, 2004). In A. chinensis var. deliciosa, a single gene coding CWI identified in the Actinidia expressed sequence tag (EST) database (Crowhurst et al. 2008) was not expressed in fruit, but only in vegetative tissue (Nardozza et al. 2013). This is consistent with the lack of CWI enzyme activity in fruit (Moscatello et al. 2011; Nardozza et al. 2013). In tomato, silencing a CWI (LIN5) resulted in a reduction of fruit yield and fruit size (Zanor et al. 2009). Comparable results were obtained by silencing a tomato VI (TIV1), the production of smaller fruits being related to lower levels of glucose and fructose, and to higher sucrose accumulation during the final phase of development (Klann et al. 1996). This suggests that the concentration of osmotically active sugars is linked to water influx, which is an important cue driving fruit enlargement (Azzi et al. 2015). In fact, in A. chinensis var. deliciosa fruit, the expression of a vacuolar invertase gene specifically increases during cell expansion, while the cytoplasmic isoform sharply diminishes after the first phase of fruit growth and cell division (Nardozza et al. 2013). Three genes coding NI were identified in the Actinidia EST database (Crowhurst et al. 2008), and high transcript levels of one of these genes were determined in the very early stages of A. chinensis var. deliciosa fruit development (Nardozza et al. 2013). These results, together with measurements of enzyme activity, suggest a role for NI in the transition from a phase characterized by cell division and high levels of glucose to a phase of net starch accumulation.
15.2.3 Sucrose-Phosphate Synthase
Sucrose-phosphate synthase (SPS) catalyzes the chemical conversion of UDP-glucose and d-fructose to sucrose-6-phosphate and UDP, and is a key enzyme of sucrose synthesis playing a role in regulating the starch/sucrose balance in photosynthetic (autotrophic) tissues. In addition, SPS is also present in heterotrophic organs, such as fruit, where it leads to sucrose accumulation.
A small SPS family of at least four genes was identified in A. chinensis var. deliciosa (Langenkämper et al. 1998), and their expression pattern was studied during fruit development. SPS mRNA increased near fruit maturity, concomitantly with the beginning of starch degradation and the increasing level of substrate for disaccharide synthesis, as confirmed by Nardozza et al. (2013).
In apple fruit, SPS mirrored the reduction in starch level during ripening, suggesting a role for this enzyme in starch degradation (Brookfield et al. 1997); an association between SPS transcription and sucrose content was also reported in wheat (Xue et al. 2013). In the Chinese bayberry, upregulated expression of SPS was correlated with an increase in fruit sweetness (Feng et al. 2012).
Characterization of the SPS gene family in A. chinensis var. chinensis allowed classification of the genes into two clades: three belonged to Family A and one to Family B (Fung et al. 2003). The high similarity between the A. chinensis var. chinensis and the A. chinensis var. deliciosa sequences, notably in the 3′UTR region, led Fung et al. (2003) to the suggestion that these genes are paralogues deriving from gene duplication events. SPSA1 appeared highly expressed in senescent leaves, stem, and flower buds. In fruit, transcript levels increased during ripening and were upregulated by ethylene, while it did not respond to low temperature. SPSA2 and SPSA3 expression was ubiquitously present in all plant tissues and in fruit during development. As far as Family B is concerned, SPSB mRNA was measured in leaves, stem, flower, and root tissue, while in fruits transcript level was detectable only in early development and in ripe ethylene-treated fruit. SPS genes belonging to the Family B are thought to be involved in response to environmental stress (Langenkämper et al. 1998); indeed, Actinidia SPSB gene is upregulated in fruit stored at low temperature (Fung et al. 2003).
15.2.4 l-Myo-Inositol-1-Phosphate Synthase
l-Myo-inositol-1-phosphate synthase (MIPS) is the rate-limiting enzyme in myo-inositol biosynthesis. MIPS has been observed to exist as a gene family in several plant species, and different isoforms may serve specific roles. A gene coding MIPS was isolated from several wild and cultivated Actinidia taxa (A. chinensis var. chinensis, A. chinensis var. deliciosa, A. arguta, A. rufa, and A. eriantha) displaying diverse inositol contents (Cui et al. 2013). Comparison of obtained sequences indicated that the gene is conserved among taxa with a high level of similarity (98.94 % identity). MIPS gene was expressed, albeit to different degrees depending on the taxon, in vegetative and reproductive tissues. In developing fruit, the maximum transcript level was detected at early stages, but as expression did not parallel myo-inositol content, the presence of other regulatory mechanisms in its biosynthesis was hypothesized (Cui et al. 2013).
In addition, several studies have demonstrated that MIPS expression levels relate tightly to normal embryo formation and that MIPS transcripts can be detected in the seed tissues of different species, such as Arabidopsis, soybean, common bean, and rice (Yoshida et al. 1999; Chiera and Grabau 2007; Mitsuhashi et al. 2008). These data suggest that, almost certainly, more isoforms of MIPS are present in Actinidia and at least one of these could be involved in the coordinated regulation of normal seed development.
15.2.5 Hexokinase–Fructokinase
In plants, hexokinase (HXK) and fructokinase (FK) are enzymes normally involved in hexose phosphorylation, and as glucose and fructose must be phosphorylated before entering any metabolic process, HXK and FK coordinate sugar availability with plant physiology and development (Granot et al. 2014). Specific sugar-sensing roles (in addition to the metabolic function) have been hypothesized for several HKs, but, to date, there is no strong evidence that FK plays any direct role in sugar-sensing (Rolland et al. 2006; Granot et al. 2014). In A. chinensis var. deliciosa, three genes encoding hexokinase (HK1, HK3, and HKL1) and three encoding fructokinase (FK4, FK6, and FK8) have been identified (Nardozza et al. 2013). The high expression level of FK4 and HK3 during the early phase of fruit growth was linked to the phosphorylation of hexoses derived from NI activity, while FK6 expression increased during cell expansion (Fig. 15.2). In addition, FK4 transcription was shown to be associated with SUS1 expression, and with FW and DW relative growth rates. In tomato plants, the overexpression of an Arabidopsis Hexokinase 1 (AtHXK1) gene induced marked phenotypic and biochemical modifications in developing fruits, such as reduced fruit size and a decrease in cell expansion (Menu et al. 2004).
Schematic representation of hexokinase (HK) and fructokinase (FK) activity and effects on fruit tissues. Sucrose can be cleaved by invertase intracellularly, and the resulting hexoses are converted to hexose phosphates by FK and HK. These enzymes have an undeniable effect on the metabolic status of the cells and on the availability of sugars, which in turn can contribute to regulate transcriptionally other physiological processes. HK3, FK4, and FK6 refer to kiwifruit genes specifically expressed in different phases of fruit development
15.2.6 ADP-Glucose Pyrophosphorylase
ADP-glucose pyrophosphorylase (AGPase) catalyzes the first committed step in starch biosynthesis, converting glucose-1-phosphate and ATP to inorganic pyrophosphate (PPi) and ADP-glucose which, in turn, acts as the glucosyl donor for several classes of starch synthase (Geigenberger 2011). AGPase is a heterotetramer composed of two large and two smaller subunits, encoded in Arabidopsis by four genes (APL1–APL4) and two genes (APS1 and APS2), respectively (Crevillen et al. 2003, 2005). Genes coding for the large subunits are specifically tissue- and development-expressed and are subjected to stringent regulation by internal (e.g., photoassimilate availability) and environmental constraints (e.g., light and biotic stress) (Scheible et al. 1997; Tiessen et al. 2003; Tetlow et al. 2004; Bahaji et al. 2014). However, owing to the frequent lack of correspondence between AGPase transcription and enzyme activity, the presence of regulatory mechanisms at post-transcriptional level was hypothesized (Geigenberger 2011; Bahaji et al. 2014). Allosteric regulation of AGPase activity was described in A. chinensis var. deliciosa fruit (Moscatello et al. 2011). The transcription of two genes coding for the AGPase large subunit (APL2 and APL4) and one for the small subunit (APS1) in A. chinensis var. deliciosa genotypes with fruit of different sizes and starch concentrations provided confirmation of the key role of the enzyme in starch metabolism and dry matter accumulation in this taxon (Nardozza et al. 2013). APL4 and APS1 expression was correlated with higher fruit starch and dry matter content.
It would be interesting to study the involvement of AGPase in the putative contribution of early fruit photosynthesis to fruit development, quality, and yield as shown in other fruits (Cocaliadis et al. 2014).
15.2.7 Amylase
The α- and β-amylases are enzymes that play a role in starch metabolism and homeostasis. In the Actinidia EST database (Crowhurst et al. 2008), a small family of amylases was identified, consisting of three genes coding for α-amylase (AMY1–AMY3) and four coding for β-amylase (BAM1–BAM3 and BAM9), and these exhibited diverse transcription patterns during fruit development (Nardozza et al. 2013). It was hypothesized that BAM9/AMY2 was involved in cytosolic starch turnover, while the high expression of AMY3 and BAM3 (both plastid) in mature fruit could elicit the start of the fruit ripening process. BAM3 showed a similar behavior also in A. chinensis var. chinensis fruit during ripening (Atkinson et al. 2011; Richardson et al. 2011). In apple, the high level of transcription of a β-amylase during the early phase of fruit growth was considered a distinct starch degradation pathway as compared with that operating at later stages of development (Janssen et al. 2008).
15.3 Sugar Transport
It is well accepted that phloem unloading and metabolism of sugars from source to sink organs play a key role in the partitioning of photoassimilates, the unloading pathway (symplastic or apoplastic) being dependent on the particular sink involved and its developmental stage (Ludewig and Flügge 2013). In a number of fruits, such as grape, peach, and tomato, the main phloem-unloading route is modified during development (Ruan and Patrick 1995; Zhang et al. 2006; Zanon et al. 2015). The apoplastic phloem unloading relies on the concomitant presence in the tissue of specific sugar transporters and CWI, whereas symplastic unloading requires plasmodesmatal connections between phloem and parenchyma cells. As a rule, the main sugar translocated in the phloem is sucrose, but in A. arguta planteose was identified as a short-term carbohydrate in leaves (Klages et al. 2004) and, recently, as the transported carbon form (Boldingh et al. 2015).
In kiwifruit, long-distance transport of carbohydrate to the fruit (principally sucrose) occurs in the phloem, but once at the fruit, the unloading of assimilates from the phloem, and transport through the fruit, occurs via a series of short-distance events that have been recently clarified. Gould et al. (2013) reported the first attempt to define the phloem-unloading pathway in kiwifruit, by means of the symplastically isolated fluorescent dye 5(6)-carboxyfluorescein (CF). The experiments revealed that the sieve element–companion cell complexes in the phloem of vascular bundles in the outer pericarp and inner pericarp unload symplastically early in development. However, in the subsequent stages of fruit development, the spread of CF dye from the vascular bundles was much reduced compared with fruit earlier in development, the dye being confined to areas surrounding phloem strands in both the ventromedian and median dorsal carpellary bundles. However, the reduction in CF unloading was not only a result of a transition to an apoplastic unloading pathway, but also a part of a general trend in declining phloem function, although dry matter accumulation and thus solute import to the fruit continued through to 150 DAFB.
15.3.1 Sugar Transporter Encoding Genes
In A. chinensis var. deliciosa, a suite of genes encoding sugar transporters was identified thanks to the Actinidia EST collection (Crowhurst et al. 2008), and the expression pattern of two sucrose (SUC3 and SUC4) and two hexose transporters (STP1 and STP14) studied in developing fruit (Nardozza et al. 2013). This analysis, together with the lack of CWI expression and activity in fruit and the predominance of cytosolic sucrose cleavage enzymes (NI and SUS), suggested that in kiwifruit phloem unloading is mainly symplastic, at least in earlier fruit development. Results were confirmed by a study using a fluorescent phloem tracer (Gould et al. 2013). Supporting this conclusion, the transport of planteose, likewise galactosyl-sucrose oligosaccharides, is supposed to be symplastic (Boldingh et al. 2015) via ‘polymer trapping’ (Haritatos et al. 1996).
The recent kiwifruit genome sequencing allows identification of a greater number of sucrose transporters, and the predicted proteins grouped into the three clades belonging to the dicots, as proposed by Kühn and Grof (2010) (Fig. 15.3).
Unrooted phylogenetic dendrogram of representative sucrose transporters from monocotyledonous and dicotyledonous species. The tree was inferred using the UPGMA method and by means of MEGA6 software (Tamura et al. 2013). The analysis involved 23 amino acid sequences from Solanum lycopersicum (LeSUT1, CAA57726; LeSUT2, AAG12987; LeSUT4, AAG09270), Arabidopsis thaliana (AtSUC1, At1g71880; AtSUC2, At1g22710; AtSUT2, At2g02860; AtSUT4, At1g09960; AtSUC9, At5g06170), Oryza sativa (OsSUT1, AAF90181; OsSUT3, BAB68368; OsSUT5, BAC67165), and Actinidia chinensis var. chinensis (marked with green circles). Sucrose transporter proteins from Actinidia were identified as in Table 15.1, but incomplete sequences were excluded
15.4 Genetic Variability and Heritability of Sugar Content
Evidence from isozyme and nuclear DNA-RFLP analyses supports the conclusion that A. chinensis var. deliciosa is an allohexaploid originating from hybridization of at least two diploid progenitors, one of which is A. chinensis var. chinensis. A. chinensis var. chinensis is itself probably an ancient polyploid, based on the high number of chromosomes compared with other related genera. Given the complicated provenance and ploidy of Actinidia, single analyses on specific genes (e.g., MIPS or SPS) have provided limited information. Therefore, the divergence of A. chinensis var. deliciosa and A. chinensis var. chinensis requires further investigation that should involve a considerable gene pool and a larger number of Actinidia species (Fung et al. 2003; Cui et al. 2013). Although Actinidia is a challenging genus—dioecious, polyploid, taxonomically difficult because of reticulate evolution—the process of utilizing molecular genetics and genomics to predict outputs from crosses is under way (McNeilage et al. 2011).
Commercial kiwifruit cultivars are often only a few generations removed from their wild relatives (Ferguson 2007); however, the goals for improvement of kiwifruit are similar to those for other species, including the selection of large-fruited cultivars with consistently good quality (sweetness and flavor). In this context, the knowledge of the processes of sucrose unloading and conversion and of the role of each component along the transport pathway in delivering carbon to the fruit cells is a necessary requirement for the formulation of modern predictive growth and dry matter accumulation models, but also a high priority for understanding the genetic variation in kiwifruit composition. Consequently, a more precise approach to selection requires information of genetic parameters, such as heritabilities and correlations among characters under selection, very useful for predicting genetic progress in breeding programs and developing efficient breeding strategies. The high heritabilities of SSC (highly genetically correlated with the main sugars), dry matter, vitamin C, and titratable acidity (TA) suggest that these characters will be amenable to change through selection in this population. Conversely, the low heritabilities of glucose, quinic acid, and fruit number indicate that these characters will be more difficult to change (Cheng et al. 2004).
Putative quantitative trait loci (QTLs) for fruit characters and components from co-analyzing phenotypic data with marker genotype data have been hypothesized, e.g., a QTL for SSC (highly dependent on the levels of fructose, sucrose, and TA) and dry matter occurring without an association with fruit weight (Cheng et al. 2004).
Despite the availability of an extensive EST database (Crowhurst et al. 2008) and several genetic maps (Testolin et al. 2001; Fraser et al. 2009), until recently, the whole-genome sequence resources for the kiwifruit, which are critical for its breeding and improvement, were very limited. The genome sequence of a heterozygous kiwifruit, ‘Hongyang’ (A. chinensis var. chinensis), is now available (Huang et al. 2013) and represents an important tool to get insight into the molecular basis of specific agronomically important traits of kiwifruit. This important work makes available RNA-seq data from A. chinensis var. chinensis leaves and fruit and reveals that the 337 flesh fruit-specific families include genes associated with fruit quality related to flavonoid, phenylpropanoid, anthocyanin, and oligosaccharide metabolism. In this context, the diploid genotypes of A. chinensis var. chinensis could be, instead of hexaploid, green-fleshed A. chinensis var. deliciosa kiwifruit, good models for understanding the molecular processes of this genus, the regulation of metabolism-associated genes being a means to induce variation in fruit composition and size.
15.5 Conclusion
The current breeding aims focus on an increase in yield potential and fruit quality, mainly through improvements in allocation efficiency into harvestable organs. The knowledge about carbohydrate metabolism and partitioning in Actinidia is still incomplete, and further efforts are needed to clarify the number and the pivotal role of all gene family members involved in these processes.
Additional evidence is emerging about other mechanisms regulating source–sink partitioning. For example, a gene encoding a putative chaperone protein (SPA—sugar partitioning affecting) appears to be associated with changes in primary metabolites during tomato fruit development (Bermúdez et al. 2014) and to interact with phosphoglucomutase, sugar kinase, and invertase enzyme activities regulating the harvest index (Azzi et al. 2015). Furthermore, new proteins, named SWEETs, have been identified, as a class of sugar transporters that facilitate diffusion of sugars across cell membranes down a concentration gradient (Baker et al. 2012).
From a broader perspective, SWEETs and SPA encoding genes add an exciting new dimension to our knowledge and could shed light on processes such as sugar accumulation in fruit, filling the gaps only partially explained to date.
In this context, the Actinidia genome sequence (Huang et al. 2013) represents an important resource, enabling the identification and characterization of new genes (Table 15.1) and providing valuable tools for genetic improvement.
References
Atkinson RG, Gunaseelan K, Wang MY, Luo L, Wang T, Norling CL et al (2011) Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using a 1-aminocyclopropane-1-carboxylic acid oxidase knockdown line. J Exp Bot 62(11):3821–3835
Azzi L, Deluche C, Gévaudant F, Frangne N, Delmas F, Hernould M et al (2015) Fruit growth-related genes in tomato. J Exp Bot 66(4):1075–1086
Bahaji A, Li J, Sánchez-López ÁM, Baroja-Fernández E, Muñoz FJ, Ovecka M et al (2014) Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnol Adv 32(1):87–106
Baker RF, Leach KA, Braun DM (2012) SWEET as sugar: new sucrose effluxers in plants. Mol Plant 5(4):766–768
Baroja-Fernández E, Muñoz FJ, Li J, Bahaji A, Almagro G, Montero M et al (2012) Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proc Natl Acad Sci USA 109(1):321–326
Baud S, Vaultier MN, Rochat C (2004) Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J Exp Bot 55(396):397–409
Bermúdez L, de Godoy F, Baldet P, Demarco D, Osorio S, Quadrana L et al (2014) Silencing of the tomato sugar partitioning affecting protein (SPA) modifies sink strength through a shift in leaf sugar metabolism. Plant J 77(5):676–687
Bertin N, Gautier H, Roche C (2002) Number of cells in tomato fruit depending on fruit position and source-sink balance during plant development. Plant Growth Regul 36(2):105–112
Bertin N, Causse M, Brunel B, Tricon D, Génard M (2009) Identification of growth processes involved in QTLs for tomato fruit size and composition. J Exp Bot 60(1):237–248
Bohner J, Bangerth F (1988) Effects of fruit set sequence and defoliation on cell number, cell size and hormone levels of tomato fruits (Lycopersicon esculentum Mill.) within a truss. Plant Growth Regul 7(3):141–155
Boldingh H, Smith GS, Klages K (2000) Seasonal concentrations of non-structural carbohydrates of five Actinidia species in fruit, leaf and fine root tissue. Ann Bot 85(4):469–476
Boldingh H, Richardson A, Minchin P, MacRae E (2015) Planteose is a major sugar translocated in Actinidia arguta ‘Hortgem Tahi’. Sci Hortic 193:261–268
Brookfield P, Murphy P, Harker R, MacRae E (1997) Starch degradation and starch pattern indices; interpretation and relationship to maturity. Postharvest Biol Technol 11(1):23–30
Burdon J, Lallu N, Pidakala P, Barnett A (2013) Soluble solids accumulation and postharvest performance of ‘Hayward’ kiwifruit. Postharvest Biol Technol 80:1–8
Cheng CH, Seal AG, Boldingh HL, Marsh KB, MacRae EA, Murphy SJ et al (2004) Inheritance of taste characters and fruit size and number in a diploid Actinidia chinensis (kiwifruit) population. Euphytica 138(2):185–195
Chengappa S, Guilleroux M, Phillips W, Shields R (1999) Transgenic tomato plants with decreased sucrose synthase are unaltered in starch and sugar accumulation in the fruit. Plant Mol Biol 40(2):213–221
Chiera JM, Grabau EA (2007) Localization of myo-inositol phosphate synthase (GmMIPS-1) during the early stages of soybean seed development. J Exp Bot 58(8):2261–2268
Chourey PS, Taliercio EW, Carlson SJ, Ruan Y-L (1998) Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol Gen Genet 259(1):88–96
Cocaliadis MF, Fernández-Muñoz R, Pons C, Orzaez D, Granell A (2014) Increasing tomato fruit quality by enhancing fruit chloroplast function. A double-edged sword? J Exp Bot 65(16):4589–4598
Crevillen P, Ballicora MA, Merida A, Preiss J, Romero JM (2003) The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme. J Biol Chem 278(31):28508–28515
Crevillen P, Ventriglia T, Pinto F, Orea A, Merida A, Romero JM (2005) Differential pattern of expression and sugar regulation of Arabidopsis thaliana ADP-glucose pyrophosphorylase-encoding genes. J Biol Chem 280(9):8143–8149
Crowhurst RN, Gleave AP, MacRae EA, Ampomah-Dwamena C, Atkinson RG, Beuning LL et al (2008) Analysis of expressed sequence tags from Actinidia: applications of a cross species EST database for gene discovery in the areas of flavor, health, color and ripening. BMC Genom 9(1):351
Cui M, Liang D, Ma F (2013) Molecular cloning and characterization of a cDNA encoding kiwifruit l -myo-inositol-1-phosphate synthase, a key gene of inositol formation. Mol Biol Rep 40(1):697–705
D’Aoust M-A, Yelle S, Nguyen-Quoc B (1999) Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell 11(12):2407–2418
Dai N, Cohen S, Portnoy V, Tzuri G, Harel-Beja R, Pompan-Lotan M et al (2011) Metabolism of soluble sugars in developing melon fruit: a global transcriptional view of the metabolic transition to sucrose accumulation. Plant Mol Biol 76(1–2):1–18
Dey PM (1980) Biosynthesis of planteose in Sesamum indicum. FEBS Lett 114(1):153–156
Esti M, Messia MC, Bertocchi P, Sinesio F, Moneta E, Nicotra A et al (1998) Chemical compounds and sensory assessment of kiwifruit (Actinidia chinensis (Planch.) var. chinensis): electrochemical and multivariate analyses. Food Chem 61(3):293–300
Falchi R, Zanon L, De Marco F, Nonis A, Pfeiffer A, Vizzotto G (2013) Tissue-specific and developmental expression pattern of abscisic acid biosynthetic genes in peach fruit: possible role of the hormone in the coordinated growth of seed and mesocarp. J Plant Growth Regul 32(3):519–532
Feng C, Chen M, Xu CJ, Bai L, Yin XR, Li X et al (2012) Transcriptomic analysis of Chinese bayberry (Myrica rubra) fruit development and ripening using RNA-Seq. BMC Genom 13:19
Ferguson AR (2007) The need for characterisation and evaluation of germplasm: kiwifruit as an example. Euphytica 154(3):371–382
Fraser LG, Tsang GK, Datson PM, De Silva HN, Harvey CF, Gill GP et al (2009) A gene-rich linkage map in the dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex-determining chromosomes. BMC Genom 10:102
Fridman E, Pleban T, Zamir D (2000) A recombination hotspot delimits a wild-species quantitative trait locus for tomato sugar content to 484 bp within an invertase gene. Proc Natl Acad Sci USA 97(9):4718–4723
Fridman E, Carrari F, Liu Y-S, Fernie AR, Zamir D (2004) Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 305(5691):1786–1789
Fu H, Park WD (1995) Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell 7(9):1369–1385
Fung RWM, Langenkämper G, Gardner RC, MacRae E (2003) Differential expression within an SPS gene family. Plant Sci 164(4):459–470
Geigenberger P (2011) Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol 155(4):1566–1577
Gould N, Morrison DR, Clearwater MJ, Ong S, Boldingh HL, Minchin PEH (2013) Elucidating the sugar import pathway into developing kiwifruit berries (Actinidia deliciosa). N Z J Crop Hortic Sci 41(4):189–206
Granot D, Kelly G, Stein O, David-Schwartz R (2014) Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development. J Exp Bot 65(3):809–819
Haritatos E, Keller F, Turgeon R (1996) Raffinose oligosaccharide concentrations measured in individual cell and tissue types in Cucumis melo L. leaves: implications in phloem loading. Planta 198:614–622
Hennen-Bierwagen TA, Lin Q, Grimaud F, Planchot V, Keeling PL, James MG et al (2009) Proteins from multiple metabolic pathways associate with starch biosynthetic enzymes in high molecular weight complexes: a model for regulation of carbon allocation in maize amyloplasts. Plant Physiol 149(3):1541–1559
Huang S, Ding J, Deng D, Tang W, Sun H, Liu D et al (2013) Draft genome of the kiwifruit Actinidia chinensis. Nat Commun 4:2640
Janssen BJ, Thodey K, Schaffer RJ, Alba R, Balakrishnan L, Bishop R et al (2008) Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol 8(1):16
Jukes C, Lewis D (1974) Planteose, the major soluble carbohydrate of seeds of Fraxinus excelsior. Phytochemistry 13(8):1519–1521
Klages K, Smith G, Bieleski R (1997) Myo-inositol is a major carbohydrate in species of Actinidia. Acta Hortic 444:361–368
Klages KU, Boldingh HL, Cooney JM, MacRae EA (2004) Planteose is a short-term storage carbohydrate in Actinidia leaves. Funct Plant Biol 31(12):1205–1214
Klann EM, Hall B, Bennett AB (1996) Antisense acid invertase (TIV1) gene alters soluble sugar composition and size in transgenic tomato fruit. Plant Physiol 112(3):1321–1330
Kleczkowski LA, Kunz S, Wilczynska M (2010) Mechanisms of UDP-glucose synthesis in plants. Crit Rev Plant Sci 29(4):191–203
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7(3):235–246
Komatsu A, Moriguchi T, Koyama K, Omura M, Akihama T (2002) Analysis of sucrose synthase genes in citrus suggests different roles and phylogenetic relationships. J Exp Bot 53(366):61–71
Kühn C, Grof CP (2010) Sucrose transporters of higher plants. Curr Opin Plant Biol 13(3):288–298
Langenkämper G, McHale R, Gardner RC, MacRae E (1998) Sucrose-phosphate synthase steady-state mRNA increases in ripening kiwifruit. Plant Mol Biol 36(6):857–869
Li J, Baroja-Fernández E, Bahaji A, Muñoz FJ, Ovecka M, Montero M et al (2013) Enhancing sucrose synthase activity results in increased levels of starch and ADP-glucose in maize (Zea mays L.) seed endosperms. Plant Cell Physiol 54(2):282–294
Ludewig F, Flügge UI (2013) Role of metabolite transporters in source-sink carbon allocation. Front Plant Sci 4:231
MacRae EA, Lallu N, Searle AN, Bowen JH (1989) Changes in the softening and composition of kiwifruit (Actinidia deliciosa) affected by maturity at harvest and postharvest treatments. J Sci Food Agr 49(4):413–430
MacRae E, Quick WP, Benker C, Stitt M (1992) Carbohydrate metabolism during postharvest ripening in kiwifruit. Planta 188(3):314–323
McNeilage MA, Fraser LG, Tsang GK, Datson PM, De Silva HN, Crowhurst RN et al (2011) Molecular genetics and genomics and kiwifruit breeding. Acta Hortic 913:63–70
Menu T, Saglio P, Granot D, Dai N, Raymond P, Ricard B (2004) High hexokinase activity in tomato fruit perturbs carbon and energy metabolism and reduces fruit and seed size. Plant Cell Environ 27(1):89–98
Mitsuhashi N, Kondo M, Nakaune S, Ohnishi M, Hayashi M, Hara-Nishimura I et al (2008) Localization of myo-inositol-1-phosphate synthase to the endosperm in developing seeds of Arabidopsis. J Exp Bot 59(11):3069–3076
Moscatello S, Famiani F, Proietti S, Farinelli D, Battistelli A (2011) Sucrose synthase dominates carbohydrate metabolism and relative growth rate in growing kiwifruit (Actinidia deliciosa, cv Hayward). Scientia Hortic 128(3):197–205
Nardozza S, Boldingh HL, Richardson AC, Costa G, Marsh H, MacRae EA et al (2010a) Variation in carbon content and size in developing fruit of Actinidia deliciosa genotypes. Funct Plant Biol 37(6):545–554
Nardozza S, Hallett IC, McCartney R, Richardson AC, MacRae EA, Costa G et al (2010b) Is fruit anatomy involved in variation in fruit starch concentration between Actinidia deliciosa genotypes? Funct Plant Biol 38(1):63–74
Nardozza S, Boldingh HL, Osorio S, Höhne M, Wohlers M, Gleave AP et al (2013) Metabolic analysis of kiwifruit (Actinidia deliciosa) berries from extreme genotypes reveals hallmarks for fruit starch metabolism. J Exp Bot 64(16):5049–5063
Nishiyama I (2007) Fruits of the Actinidia genus. Adv Food Nutr Res 52:293–324
Richardson AC, Marsh KB, Boldingh HL, Pickering AH, Bulley SM, Frearson NJ et al (2004) High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit. Plant Cell Environ 27(4):423–435
Richardson AC, Boldingh HL, McAtee PA, Gunaseelan K, Luo Z, Atkinson RG et al (2011) Fruit development of the diploid kiwifruit, Actinidia chinensis ‘Hort16A’. BMC Plant Biol 11:182
Roitsch T, González M-C (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9(12):606–613
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Rothe K, Porzel A, Neumann S, Grimm E (1999) Characteristics of the phloem path: analysis and distribution of carbohydrates in the petiole of Cyclamen. J Exp Bot 50(341):1807–1816
Ruan Y-L (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65:33–67
Ruan Y-L, Patrick JW (1995) The cellular pathway of postphloem sugar transport in developing tomato fruit. Planta 196(3):434–444
Ruan Y-L, Jin Y, Yang YJ, Li GJ, Boyer JS (2010) Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol Plant 3(6):942–955
Sanz ML, Villamiel M, Martı́nez-Castro I (2004) Inositols and carbohydrates in different fresh fruit juices. Food Chem 87(3):325–328
Scheible WR, González-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M (1997) Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9(5):783–798
Sprenger N, Keller F (2000) Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthases. Plant J 21(3):249–258
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis Version 6.0. Mol Biol Evol 30(12):2725–2729
Tanou G, Minas IS, Karagiannis E, Tsikou D, Audebert S, Papadopoulou KK et al (2015) The impact of sodium nitroprusside and ozone in kiwifruit ripening physiology: a combined gene and protein expression profiling approach. Ann Bot 116(4):649–662
Testolin R, Huang WG, Lain O, Messina R, Vecchione A, Cipriani G (2001) A kiwifruit (Actinidia spp.) linkage map based on microsatellites and integrated with AFLP markers. Theor Appl Genet 103(1):30–36
Tetlow IJ, Morell MK, Emes MJ (2004) Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot 55(406):2131–2145
Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG et al (2003) Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J 35(4):490–500
Xue S, Xu F, Li G, Zhou Y, Lin M, Gao Z et al (2013) Fine mapping TaFLW1, a major QTL controlling flag leaf width in bread wheat (Triticum aestivum L.). Theor Appl Genet 126(8):1941–1949
Yoshida KT, Wada T, Koyama H, Mizobuchi-Fukuoka R, Naito S (1999) Temporal and spatial patterns of accumulation of the transcript of myo-inositol-1-phosphate synthase and phytin-containing particles during seed development in rice. Plant Physiol 119(1):65–72
Zanon L, Falchi R, Santi S, Vizzotto G (2015) Sucrose transport and phloem unloading in peach fruit: potential role of two transporters localized in different cell types. Physiol Plant 154(2):179–193
Zanor MI, Osorio S, Nunes-Nesi A, Carrari F, Lohse M, Usadel B et al (2009) RNA interference of LIN5 in tomato confirms its role in controlling Brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol 150(3):1204–1218
Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC et al (2006) A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol 142(1):220–232
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Vizzotto, G., Falchi, R. (2016). Genetics of Sugar and Starch Metabolism. In: Testolin, R., Huang, HW., Ferguson, A. (eds) The Kiwifruit Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-319-32274-2_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-32274-2_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32272-8
Online ISBN: 978-3-319-32274-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)