Abstract
The ovary, the female gonad, serves as the source for the germ cells as well as the major supplier of steroid sex hormones. During embryonic development, the primordial germ cells (PGCs) are specified, migrate to the site of the future gonad, and proliferate, forming structures of germ cells nests, which will eventually break down to generate the primordial follicles (PMFs). Each PMF contains an oocyte arrested at the first prophase of meiosis, surrounded by a flattened layer of somatic pre-granulosa cells. Most of the PMFs are kept dormant and only a selected population is activated to join the growing pool of follicles in a process regulated by both intra- and extra-oocyte factors. The PMFs will further develop into secondary pre-antral follicles, a stage which depends on bidirectional communication between the oocyte and the surrounding somatic cells. Many of the signaling molecules involved in this dialog belong to the transforming growth factor β (TGF-β) superfamily. As the follicle continues to develop, a cavity called antrum is formed. The resulting antral follicles relay on the pituitary gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) for their development. Most of the follicles undergo atretic degeneration and only a subset of the antral follicles, known as the dominant follicles, will reach the preovulatory stage at each reproductive cycle, respond to LH, and subsequently ovulate, releasing a fertilizable oocyte. The remaining somatic cells in the raptured follicle will undergo terminal differentiation and form the corpus luteum, which secretes progesterone necessary to maintain pregnancy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
The ovary, the female gonad, serves as the source for the germ cells as well as the major supplier of steroid sex hormones. During early embryonic development, at 7.25–7.5 dpc (days post coitum) primordial germ cells (PGCs), which are the origin of both oocytes and spermatozoa, are first identified in the extraembryonic mesoderm as a small cluster of alkaline phosphatase (AP)-positive cells. These cells migrate into the posterior portion of the primitive streak (Ginsburg et al. 1990; reviewed by McLaren 2003) and move by active locomotion directly into the endoderm of the developing hindgut (Anderson et al. 2000). Beginning at 9.0–9.5 dpc, PGCs undergo an active, directed migration from the dorsal axis of the hindgut toward the developing genital ridges (Molyneaux et al. 2001), where they reach at around 10.5–11.5 dpc. Throughout their migration, PGCs proliferate rapidly (Ginsburg et al. 1990). In female mice, the PGCs continue to proliferate until 13.5 dpc, forming germ cells clusters known as cysts or nests. At approximately 13.5 dpc, they embark on meiosis, thus becoming oocytes (Jagarlamudi and Rajkovic 2012). The oocytes precede their meiotic division up to the diplotene of the first prophase. Between 17.5 dpc to postnatal day 5 dpp (days post partum), the germ cell cysts break down to form primordial follicles (PMFs), a process accompanied by a massive loss of germ cells (Pepling and Spradling 2001; Menke et al. 2003).
Each PMF contains a prophase-arrested oocyte surrounded by a flattened epithelium (pre-granulosa cells) that will differentiate to form granulosa cells (GC). The PMFs constitute the ovarian quiescent follicle reserve. From this reserve, follicles will be recruited to the growing pool. The transition from a primordial to a primary follicle is characterized by a morphological change in the GC that turn from flattened to cuboidal. During further development to secondary follicle, the oocyte continues to grow, the GC proliferate, and an additional layer of theca cells forms outside the basement membrane that surrounds the follicle. These stages of follicle growth are gonadotropin independent, but require a complex bidirectional communication between the oocyte and the somatic cells (Eppig 2001; reviewed by Edson et al. 2009).
In the subsequent stages of folliculogenesis, small fluid-filled cavities are formed within the follicle, ultimately joining to a single cavity, known as the antrum, thus forming the antral follicle. The antrum defines two separate populations of GC: the cumulus GC, which are adjacent to the oocyte, and the mural GC that line the follicle wall and serve as the major source for steroid hormones (reviewed by Edson et al. 2009). Folliculogenesis at this stage depends on the presence of the pituitary gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). FSH is required for GC survival, proliferation, estradiol production, and LH receptor expression (reviewed by Edson et al. 2009; Richards and Pangas 2010a). Most of the antral follicles will undergo atretic degeneration, whereas only a subset of them will continue to develop to the preovulatory stage. The mural GC of these selected follicles express high concentration of LH receptors, subjecting them to respond to the preovulatory LH surge, which activates a sequence of events culminating in ovulation of the dominant follicles. These events include oocyte meiotic resumption, cumulus expansion, follicle rupture, and the release of a cumulus–oocyte complex that contains a fertilizable oocyte. Once the oocyte is released, the remaining granulosa and theca cells undergo terminal differentiation to create the corpus luteum (CL) (reviewed by Edson et al. 2009). The CL is essential for establishing and maintaining pregnancy, mainly through secretion of progesterone. In case pregnancy has not occurred, the CL degenerates (Stocco et al. 2007).
7.2 Primordial Germ Cells’ Specification and Migration
Prior to gastrulation at 6.25 dpc, the primordial germ cells (PGCs) split off from the proximal region of the pluripotent epiblast and arise, at around 7.25 dpc, as a cluster in the extraembryonic mesoderm (Ginsburg et al. 1990). This process of PGC formation and specification depends on bone morphogenetic protein (BMP) family signaling from the extraembryonic ectoderm (ExE) and visceral endoderm (VE), which surround the epiblast cells. Specifically, BMP4 and BMP8B originate from the ExE and BMP2 is secreted from the VE. Loss of any of these genes results in a reduction or even the absence of PGC (reviewed by Edson et al. 2009). This BMP signal is elicited through Smad1/5/8, which translocate into the nucleus with the common mediator, Smad4 (Hayashi et al. 2007; Ohinata et al. 2009; Saitou et al. 2012). At ~5.5 dpc, BMP4 signaling activates the transcriptional regulators PRDM1 (PRDI-BF1-RIZ domain containing 1; BLIMP1) and PRDM14 in the most proximal epiblast cells. Both BLIMP1 and PRDM14 are essential for PGC specification. BLIMP1 is required for the repression of the somatic program (for example, by turning off Hox gene expression), and both BLIMP1 and PRDM14 are involved in the re-expression of factors of pluripotency (such as Sox2, Oct4, and Nanog) and in preparation for epigenetic reprogramming (Ohinata et al. 2009; Saitou et al. 2012). Interestingly, the abovementioned BMP4 signaling from the ExE is antagonized by signals from the anterior visceral endoderm (AVE). BMP8b from the ExE restricts AVE development, thereby allowing the BMP4 signaling. In addition, Wnt3 expression in the epiblast was shown to be essential for the responsiveness of the epiblast cells to BMP4, thus allowing them to determine the germ cell fate (Ohinata et al. 2009).
One of the earliest genes induced by BMPs is Ifitm3 (interferon-induced transmembrane protein 3; Fragilis), which serves as an early marker for PGCs but does not have an essential role in their differentiation. Additional early markers of PGCs which are not essential for their formation are ALPL (alkaline phosphatase) and STELLA (reviewed by Edson et al. 2009). Starting at ~7.5 dpc, the specified PGCs migrate from the base of the yolk sac through the hindgut and dorsal mesentery to colonize the genital ridge (10.5 dpc; Jagarlamudi and Rajkovic 2012; Saitou et al. 2012). This process probably relays on chemoattractant receptor ligand interactions. One such example is the Kit ligand (reviewed by Edson et al. 2009).
Between 7.5 and 8.5 dpc, PGCs express markers of pluripotency (including POU5F1, SOX2, NANOG) and undergo chromatin changes, resembling the patterns of pluripotent stem cells (specifically, increasing the levels of H3K27me3 and erasing the H3K9me2 methylation). During their migration, they arrest in G2 stage of the cell cycle and transiently become transcriptionally quiescent (Seki et al. 2007). Additional epigenetic events in PGCs are the genome-wide DNA demethylation, which is completed in both sexes by E13.5, erasure of imprinting, and reactivation of the inactive X chromosome in females. Starting ~9.5 dpc, throughout their migration, PGCs undergo active proliferation (for a detailed review, see Saitou et al. 2012). Interestingly, it was recently demonstrated that this process of PGC expansion and migration is done in an incoherent mode, which involves physical mixing of the progenies of dividing PGCs as they migrate before their allocation to different gonads (Reizel et al. 2012).
Upon their arrival at the genital ridge where the future gonad will form, PGCs are subjected to a global change in gene expression, in which the pluripotency program is turned off and sexual specific genes are turned on (reviewed by Hu et al. 2015). Interestingly, the sexual fate of the PGCs is determined by the sexual identity of the fetal gonad to which the PGCs migrate and not by the PGCs’ own identity (Gill et al. 2011). In addition, it was recently shown that the ability of PGCs to undertake sexual specialization requires an active developmental transition to allow them to respond to the surrounding gonad, a process called germ cell licensing (Gill et al. 2011). This process was recently shown to require the formation and signaling of the genital ridges, which induce deleted in azoospermia-like (DAZL) expression in the PGCs (Hu et al. 2015).
7.3 Male and Female Germ Cell Specification
After the entrance of the PGCs to the genital ridges, the bipotential gonad will continue its development in a sexually dependent manner, to form either ovaries or testes. The somatic supporting cells in the gonad, which arise within the urogenital ridge from a common mesodermal progenitor (Jameson et al. 2012), play a critical role in this process. In the XY gonad, expression of Sry gene triggers upregulation of Sox9 and Fgf9, which activate the male pathway and repress signals that promote the female pathway (i.e., Wnt4/Rspo1 and β-catenin). In the XX gonad, in the absence of Sry, WNT4 and RSPO1 promote the female pathway by maintaining β-catenin signaling. Supporting cell precursors commit to granulosa cell fate and control ovarian development by promoting theca cell differentiation and regulating meiotic entry in germ cells (Lin and Capel 2015).
The time of germ cells’ entry into meiosis is sex specific, as XY germ cells arrest in mitosis and do not divide again until postnatally as spermatogonia, whereas the XX germ cells continue to divide and then enter meiosis at approximately 13.5 dpc (Koubova et al. 2006; Lei and Spradling 2013). Therefore, a complex network of signals is required to coordinate this process; the mesonephroi adjacent to the developing ovary and testes contain several aldehyde dehydrogenases that convert retinaldehyde to all-trans-retinoic acid (RA). The enzyme CYP26B1, which degrades RA, is downregulated in the somatic cells of the developing ovary but is upregulated in the somatic cells of the developing testis. Therefore, RA is accessible only to XX germ cells and not to XY germ cells. The binding of RA to its receptor RAR in XX germ cells induces Stra8 expression, which, in turn, induces SYCP3, a component of the synaptonemal complex. SYCP3 is stably translated in the presence of DAZL and loaded on chromosomes, facilitating the pairing of homologous chromosomes, which is required in meiosis (reviewed by Edson et al. 2009).
7.4 Primordial Follicle Formation
After the female PGCs enter the genital ridges and differentiate to oogonia, they proliferate with incomplete cytokinesis, creating clusters (syncytia) of cells connected by intercellular bridges, through which organelles and mitochondria can move. The germ cells in the cysts are synchronized; thus, the number of cells in each cyst is in the power of two (Epifano and Dean 2002; Lei and Spradling 2013; Pepling and Spradling 1998). However, it has been recently demonstrated that these cysts are partially segmented prior to meiosis into smaller cysts and aggregate with cysts from different PGCs to form a nest. From 13.5 dpc, the germ cells within the clusters enter meiosis in an anterior to posterior wave and become oocytes (Lei and Spradling 2013; Menke et al. 2003). Oocytes remain arrested in the diplotene stage of meiosis I, and only after the female reaches sexual maturation, selected oocytes will resume their meiotic division and ovulate (see hereinafter).
Between 17.5 dpc to postnatal day 5 dpp, cyst clusters begin to break down to form PMFs, a process which is accompanied with massive cell lost (Pepling and Spradling 2001). A single layer of squamous somatic pre-granulosa cells encapsulates individual oocytes in the process of follicle formation.
Several factors and signaling pathways were shown to affect follicle assembly:
-
Factor in the germline α (FIGLA), a germ cell-specific bHLH transcription factor. Primordial follicles fail to form in Figlα-null mice, suggesting it is required for initial follicle formation (Soyal et al. 2000).
-
The transcription factor Forkhead box L2 (FOXL2). In mice null for Foxl2, the somatic pre-granulosa cells fail to develop around the growing oocyte, which initiate its growth prematurely and thus undergo atresia (Uda et al. 2004).
-
Members of the TGF-β superfamily. Activin A was reported to increase the number of PMFs (reviewed by Knight et al. 2012); AMH inhibits PMF assembly (Nilsson et al. 2011); Gremlin, a BMP antagonist, was also shown to affect PMF assembly (Myers et al. 2011); however, there is no sufficient indication that BMPs play a role in cyst breakdown (Pangas 2012).
-
Neurotrophins (NTs) and their tyrosine kinase receptor (NTRK). The amount of PMFs is decreased in the absence of nerve growth factor (NGF) and its receptor, NTRK1, and in the absence of NTRK2, which is the receptor of neurotrophin 4 (NTF4) and brain-derived neurotrophic factor (BDNF) (Dissen et al. 2001; Kerr et al. 2009).
-
KITL/KIT signaling. Inhibition of KIT in ovary organ cultures caused a reduction in cyst breakdown and an increase in oocyte numbers, accompanied with reduced cell death. Kit ligand increased levels of phosphorylated MAP kinase. Thus, KIT signaling has a role in cyst breakdown, which may be mediated by MAP kinase (Jones and Pepling 2013).
-
Notch signaling. In the neonatal murine ovary, the Notch ligands, Jagged1 and Jagged2, are expressed in germ cells, whereas the Notch receptors, Notch1 and Notch2, are expressed in pre-granulosa cells. Disruption of Jagged1 or Notch2 leads to altered follicular composition and aberrant follicle formation such as multi-oocytic follicles, which indicates a failure in cyst breakdown (Vanorny et al. 2014).
-
Steroid hormones. An inhibitory involvement of estrogen and progesterone has also been suggested in follicle formation (Chen et al. 2007; Kezele and Skinner 2003).
Although there is mounting evidence to the contrary (Tilly and Telfer 2009), the current consensus is that female germ cells are not formed later in life; therefore, the PMFs constitute a fixed pool known as the ovarian reserve.
7.5 Primordial Follicle Activation
The PMFs are expected to endure one of three possible fates: (1) remain quiescent, (2) be activated and join the growing pool of follicles and then either go through atresia or finish their developmental process up till ovulation, and (3) die directly from their dormant state (Zheng et al. 2012). Most of the PMFs are kept dormant and only a selected subpopulation is activated and joins the growing pool of follicles throughout life until menopause. The transition from primordial to primary follicle is marked histologically by a change in the GC morphology, from squamous to cuboidal and the initiation of oocyte growth (Pedersen and Hannah 1968). By utilizing transgenic mice models, several studies from the last years have demonstrated that inhibitory signals from the oocyte itself and signals from the somatic cells maintain the PMF pool in its dormant state.
7.5.1 Suppression of PMF Activation by Intra-oocyte Inhibitory Signals
The most central signaling pathway identified to this date that controls PMFs activation is the PI3K pathway: oocyte deletion of Pten, a PI3K-negative regulator, caused activation of the entire PMF pool and thus premature ovarian failure (POF) (Reddy et al. 2008). A similar phenotype was also observed in oocyte deletion of FOXO3a, a substrate of AKT and a central downstream target of the of the PI3K pathway (Castrillon et al. 2003). In addition, hyper-phosphorylation and nuclear export of FOXO3a were observed in Pten-null oocytes (John et al. 2009). Therefore, it was suggested that FOXO3a acts downstream of PI3K/PTEN–AKT to negatively regulate follicular activation (Adhikari et al. 2009a; John et al. 2009; Reddy et al. 2010).
Overactivation of the PMF pool was also demonstrated in oocyte-specific knockout of either TSC1 or TSC2 (Adhikari et al. 2009a, b). The TSC1–TSC2 complex suppresses the activation of mTORC1. Indeed, it was shown that in both mutants, elevated activation of mTORC1 increased the activation of p70 S6 kinase 1 (S6K1)–ribosomal protein S6 (rpS6) signaling that promoted protein translation and ribosome biogenesis in oocytes (Reddy et al. 2010). Interestingly, although both PTEN and TSC1/2 regulate the activation of the PMFs by negatively regulating S6K1 and rpS6 activation in oocytes, it appears that they do so by phosphorylating different residues of S6K1. Thus, their similar regulation on the PMF pool is carried in distinct ways (Adhikari et al. 2009b).
An important regulator of the PI3K signaling pathway is PDK1, which can phosphorylate AKT and S6K1. In mice with specific deletion of PDK1 in oocytes, PMFs were depleted directly from the dormant state, causing premature ovarian failure (POF) in early adulthood. This indicates that the PDK1–Akt–S6K1–rpS6 pathway also plays a central role in maintaining the survival of PMFs (Reddy et al. 2009, 2010).
Premature ovarian failure was also observed in mice null to p27 Kip1, an inhibitor of the cell cycle, which is reported to be expressed in the nuclei of oocytes of primordial, primary, and secondary follicles (Rajareddy et al. 2007). However, loss of p27 and Foxo3a leads to synergistically accelerated primordial follicle activation, indicating that they maintain PMF dormancy by different signaling pathways (Rajareddy et al. 2007).
7.5.2 Activation of PMFs by Extra-oocyte Signals
A recently published article shed light on the important role that the pre-GC of the PMF play in its activation. Inhibition of mTORC1 signaling in the GC of PMFs prevents their differentiation, arresting the dormant oocytes in their quiescent state, leading to oocyte death. Overactivation of mTORC1 signaling in GC of PMF by specific TSC1 deletion accelerates their differentiation and causes premature activation of all PMF. It was further demonstrated that the differentiation of the GC in the PMF triggers the awakening of dormant oocytes through KIT ligand (KITL), which subsequently activates the PI3K signaling pathway in the oocytes. Hence, a communication network exists between the GC and the germ cells, which is based on mTORC1-KITL signaling that initiates the KIT-PI3K signaling pathway in oocytes, inducing their activation (Zhang et al. 2014).
This model accounts for a previous report that KITL can activate PMF (Hutt et al. 2006). Additional reports demonstrated the influence of other paracrine factors on primordial follicle activation, including leukemia inhibiting factor (LIF) (Nilsson et al. 2002), basic fibroblast growth factor (BFGF) (Nilsson et al. 2001), keratinocyte growth factor (KGF) (Kezele et al. 2005), platelet-derived growth factor (PDGF) (Nilsson et al. 2006), connective tissue growth factor (CTGF) (Schindler et al. 2010), and Neurotrophins (Nilsson et al. 2009). Interestingly, at least part of these factors are known to activate the PI3K signaling cascade (Pangas 2012).
Another paracrine factor, which affects PMF activation, is anti-Müllerian hormone (AMH), a TGF-β superfamily member, which is expressed by GC of growing follicles. In AMH-null mice, the PMF pool was depleted in an earlier stage, indicating AMH suppresses or prevents PMFs from being activated (Durlinger et al. 1999). In addition, aberrant premature activation of PMFs was also recently indicated in the absence of Notch signaling. However, a link between the Notch and the PI3K signaling is yet to be examined (Vanorny et al. 2014). Finally, BMP4 (Nilsson and Skinner 2003) and BMP7 (Lee et al. 2004) have also been shown to be important for the transition from primordial to primary follicles (Pangas 2012).
Interestingly, although most PMFs lie dormant until they are activated, PMFs in the medulla of the ovary are activated immediately after their assembly, shortly after birth. It was recently demonstrated that these follicles contain GC originating from surface epithelium cells of the gonad at 11.5 dpc. In contrast, primordial follicles in the cortex of the ovary, which are activated during adult life, are populated by GC that arise from the surface epithelium around birth (Mork et al. 2012).
7.6 Pre-antral Folliculogenesis
Primary follicles further develop to form secondary follicles, which contain oocytes in midgrowth stages surrounded by two or more layers of GC. A basement membrane forms around the outermost GC layer and an additional layer of somatic cells called theca cells encapsulates the follicle. These cells differentiate into two cell layers, the theca interna and externa, which have ultrastructural features, such as mitochondria with tubular cristae, which allows them to function as the source of androgens for the conversion to estrogens executed by GC (Magoffin 2005). The inner theca layer (theca interna) becomes increasingly vascularized as the follicle continues to develop, while the GC layer remains avascular (Knight et al. 2012). The oocyte relays on the GC to support its development, but it also has a critical role in regulating the rate of follicular growth by controlling proliferation and differentiation of granulosa and theca cells (Edson et al. 2009; Eppig et al. 2002; Liu et al. 2006, 2015). Indeed, although growth of pre-antral follicles is gonadotropin independent, it is governed by bidirectional communication between the oocyte and the surrounding somatic cells. Many of the signaling molecules involved in this dialog belong to the transforming growth factor β (TGF-β) superfamily (Harlow et al. 2002; Ingman and Robertson 2009; Knight et al. 2012; Matzuk and Burns 2012; Pangas and Matzuk 2004; reviewed by Richards and Pangas 2010a).
7.6.1 TGF-β Superfamily and Their Role in Pre-antral Folliculogenesis
The TGF-β superfamily consists of a structurally conserved group of proteins ubiquitously expressed, which function as extracellular ligands in various physiological processes, such as cell proliferation, differentiation, apoptosis, and cell migration (Wotton and Massague 2000). This superfamily is comprised of several subfamilies, which include TGF-β, bone morphogenetic protein (BMP), growth and differentiation factor (GDF), activin/inhibin, glial cell-derived neurotrophic factor (GDNF), and several additional members such as AMH, also known as Müllerian-inhibiting substance (MIS) (Knight et al. 2012). Members of the TGF-β family bind to specific receptor complexes composed of type I and type II serine/threonine receptor kinases, initiating a signaling cascade that causes the nuclear translocation of the SMAD proteins, resulting in transcriptional activation of target genes. Based on their activated signaling pathways, the proteins can be divided into two major groups: the BMPs, which activate SMAD1-5-8, and activins/TGF-βs/GDF9, which activate SMAD2–3 (Chang et al. 2002; Myers et al. 2009). Several members of the TGF-β superfamily are produced by different cell types in the follicle: GC produce activins, theca cells BMP4 and BMP7, granulosa and theca cells both express TGF-β, and oocytes express GDF9 and BMP15 (Oktem and Urman 2010). The latter two factors, which are selectively expressed by oocytes, have a central role in the dialog between the oocyte and the surrounding somatic cells. In GDF9-null mice, folliculogenesis is blocked at the primary stage (Dong et al. 1996). Furthermore, it was recently demonstrated that GDF9 originating from the oocyte is responsible for the production of protein signals by the GC, which in turn induce theca cell differentiation (Liu et al. 2015). BMP15 is not required during pre-antral folliculogenesis in mice, though BMP15 and GDF9 may have redundant roles (Yan et al. 2001). However, the importance of GDF9 and BMP15 in pre-antral folliculogenesis may be species specific, as BMP15 missense mutation in sheep causes similar phenotypes as in mouse GDF9-null mutation (Hanrahan et al. 2004).
Activin A produced by GC was shown to promote pre-antral follicle growth (Oktem and Urman 2010). TGF-β promotes proliferation of GC and pre-antral follicle growths (Liu et al. 1999) as well as progesterone production and FSH-induced estrogen synthesis (Dodson and Schomberg 1987). It also appears that TGF-β suppresses steroidogenesis in thecal cells from most species (Juengel et al. 2005).
Another signaling pathway involved in the progress from primary to secondary follicles involves the neurotrophins NTF5 and BDNF, which signal thorough NTRK2.
In null Ntrk2 mice, as well as in double knockout of Ntf5 and Bdnf, there is a significant reduction in the number of secondary follicles, which is apparently caused by a signaling pathway other than the GDF9 (Edson et al. 2009).
In addition to paracrine signaling, direct cell-to-cell communication via gap junctions also plays a significant role in pre-antral growth, as evident from mice with deficiencies of connexin43 (CX43) and connexin37 (CX37), two of the core proteins that form the gap junctions in the ovary (Granot and Dekel 1994; Teilmann 2005). In CX43-deficient ovaries, follicles fail to develop beyond the primary stage (Ackert et al. 2001), whereas CX37-deficient mice fail to develop mature (Graafian) follicles (Simon et al. 1997).
7.7 Folliculogenesis Toward Ovulation
As the follicle continues to develop, an antrum is formed and separates the GC population into two functional cell types, the mural GC, which are responsible for steroidogenesis, and the cumulus cells, which are adjacent to the oocyte (reviewed by Edson et al. 2009). Antral follicles relay on the pituitary gonadotropins FSH and LH for their development and ovulation. FSH is required for antral follicle survival, GC proliferation, LH receptor expression, and estradiol production. FSH binds to its G-protein coupled receptor, FSHR, and initiates the classical adenylyl cyclase (AC)/cAMP/protein kinase A (PKA) pathway that results in phosphorylation and activation of the transcription factor cAMP-response element-binding protein (CREB), thus upregulating a number of target genes such as aromatase and the LH receptor. FSH also activates several PKA-independent pathways such as AKT via PI3K and SRC tyrosine kinase-dependent pathway (Ulloa-Aguirre et al. 2007). FSH and estradiol affect GC proliferation by modulating their cell cycle (Edson et al. 2009).
7.7.1 Follicular Steroidogenesis: The Two-Cell Two-Gonadotropin System
The theca cells are capable of de novo production of androgens from cholesterol. The key enzymes of the synthesis, namely StAR (transporter of cholesterol to the inner mitochondrial membrane), CYP11A1 (which converts the cholesterol to pregnenolone), CYP17A1 (which converts pregnenolone to dehydroepiandrosterone—DHEA), and 3β-hydroxysteroid dehydrogenase (3β-HSD, which converts DHEA into androstenedione), are under LH control (reviewed by Magoffin 2005). However, the enzymes necessary for the conversion of androstenedione to estradiol, CYP19A1 (aromatase) and 17β-hydroxysteroid dehydrogenase (17β-HSD), are expressed by the GC and are regulated by FSH (reviewed by Magoffin 2005). Theca cells express LH receptor from the secondary follicle stage, before estradiol is necessary for follicular growth. Therefore, androgen biosynthesis is modulated in pre-antral and small antral follicles by secretion of inhibitory factors such as activins by the GC as well as autocrine inhibition of factors secreted by the theca cells themselves, including TGF-β, TGF-α, and FGF7 (Magoffin 2005; reviewed by Edson et al. 2009). In addition to signaling pathways activated by FSH and LH, locally produced factors such as insulin-like growth factor 1 (IGF-1) also take part in the GC proliferation and differentiation and thus are necessary for the survival of antral follicles and promoting ovulation (Ulloa-Aguirre et al. 2007).
Importantly, the majority of follicles in the growing pool will undergo atresia. Only a subset population of the antral follicles will reach the preovulatory stage and will respond to LH by activating downstream signal transduction pathways mediated by AC and cAMP and will eventually ovulated (Salomon et al. 1977). These are the dominant follicles.
7.8 Dominant Follicle Selection
In mice, dominant follicle (DF) selection is known to occur 36 h after the release of FSH (Salomon et al. 1977). The DF possess several characteristic features. It produces more estrogen than the subordinate follicle (SF) and by that is more effective in eliciting the negative feedback on FSH production, which results in reduced circulation of FSH. Additionally, the DF contains more GC and a higher level of FSHR as compared to the SF (Louvet and Vaitukaitis 1976; Rao et al. 1978; Van Santbrink et al. 1995). Upon stimulation by FSH, GC express anti-apoptotic factors such as x-linked inhibitor of apoptosis (XIAP) and flice-like inhibitory protein (FLIP). In the absence of FSH, GC express the death receptor and ligand FAS and FAS ligand (Jiang et al. 2003). Therefore, the DF is able to thrive in a declining FSH environment, while the SF cannot. Furthermore, the estrogen production in DF mediates the effect of FSH on the expression of LH receptors on granulosa cells. While SF only have LH receptors on theca cells, DF become less dependent on FSH and acquire further responsiveness to LH by expressing LH receptors on GC. In addition, insulin-like growth factor II mRNA levels and bioavailability are increased in the DF, stimulating steroidogenesis (Baerwald et al. 2012). A leading hypothesis regarding the mechanism of DF selection suggests that its advantage over the SF is achieved by the development of richer vascularization that allows the exposure to higher gonadotropin concentrations.
7.8.1 The Involvement of Angiogenesis in the Ovary and DF Selection
The ovary is one of the only organs in the adult that conserves the capacity to remodel vascular networks under tight regulation during each reproductive cycle. Primordial and primary follicles lack their own vasculature and use diffusion for oxygen and metabolite transfer. During their development, follicles acquire a two-capillary vascular network located in the theca interna and externa layers. At each given time point, hundreds of growing follicles recruit and remodel a vascular bed necessary to sustain their changing metabolic needs (Brown and Russell 2014). However, the follicular blood vessels will never penetrate to the GC layers due to a barrier of basement membrane that is located between them and the theca cells. After ovulation, upon the formation of the CL, the basement membrane breaks down and a massive angiogenic process, involving penetration of blood vessels into the inner parts of the follicle, takes place.
Several lines of evidence from different animal model systems suggest that DFs as compared to SFs are highly vascularized, thus being effectively exposed to higher gonadotropins level. Specifically, increased vascularization of individual follicles in rhesus monkeys resulted in preferential delivery of gonadotropins (Zeleznik et al. 1981). Moreover, in vivo studies in cattle showed that the maintenance of follicular vasculature and appropriate blood supply to follicles are essential for the establishment of follicular dominance (Acosta 2007). In mares, differential blood flow and blood flow velocity are early parameters for identification of DF (Acosta and Miyamoto 2004).
Several known pro- and anti-angiogenic factors were shown to participate during folliculogenesis and may be involved in DF selection:
-
Vascular endothelial growth factor A (VEGFA) is expressed in ovaries of numerous species including human, bovine, ovine, monkeys, and rodents. It has been shown that follicular growth caused by hormonal stimulation induces inner hypoxia that causes an elevation in VEGFA expression (Neeman et al. 1997). Along this line, injection of VEGFA to rat ovaries increased the number of antral follicles (Danforth et al. 2003) and elevated the number of ovulating oocytes (Iijima et al. 2005). Additionally, the blockage of VEGF receptor 2 (VEGFR2) by neutralizing antibodies attenuated the formation of pre-antral follicles (Zimmermann et al. 2003). VEGF receptor 1 (VEGFR1), which delivers the VEGF signal intracellularly, is upregulated in theca cells of large follicles after pregnant mare serum gonadotropin (PMSG) administration in gilts (Shimizu et al. 2002). Interestingly, it has been shown that soluble Flt (sFlt), the truncated and soluble form of the same receptor, which inhibits VEGF activity, is expressed by the bovine dominant follicle (Macias et al. 2012).
-
The PI3K/AKT signaling pathway, known to mediate angiogenesis, proliferation metabolism, and other cellular processes, also seems to participate in follicular growth and DF selection. In cattle, the PI3K/AKT signaling pathway is known to be an early marker for follicular dominance (Ryan et al. 2007).
-
Angiopoietin 2 (ANG2), a competitive inhibitor of the pro-angiogenic factor angiopoietin 1 (ANG1) for the TIE2 receptor, is expressed in large but not small follicles of the ewe (Chowdhury et al. 2010).
-
Thrombospondin 1 (TSP1), an anti-angiogenic factor that induces endothelial apoptosis, known to promote follicular atresia in the primate and in the rat ovary (Garside et al. 2010; Thomas et al. 2008), is expressed in pre-antral and early-antral follicles of the rat (Petrik et al. 2002). The knockout of TSP1 receptor, CD36, in mice resulted in hypervascularized ovaries, increased levels of VEGF and VEGFR2, an elevation on the number of preovulatory follicles, a decrease in the number of CL, and a reduction in the number of offspring (Osz et al. 2014). Treatment with a mimetic protein of TSP1 in marmoset monkeys did not block the emergence of DF, but reduced the pre-antral and early-antral follicles survival and angiogenesis (Garside et al. 2010).
7.9 Ovulation
Dominant follicle selection is followed by ovulation. This multistep process occurs in response to the preovulatory LH surge, which is generated by the hypothalamus/pituitary/ovary feedback system as follows. As mentioned above, FSH induces antral follicle growth, associated with elevated estradiol production. The high estradiol levels produced by the follicular GC increase the pulses of the hypothalamic gonadotropin-releasing hormone (GnRH) that trigger the LH surge (reviewed by Richards and Pangas 2010b).
The ovulatory response to LH consists of the resumption of meiosis in the oocyte, the expansion/mucification of the cumulus, the rupture of the follicle, and the release of a cumulus–oocyte complex (COC) that contains a fertilizable oocyte. Once the oocyte is released, the residual follicle cells, the granulosa and thecal cells, undergo reprogramming of terminal differentiation to create the corpus luteum (CL) through a process defined as luteinization. Consequently, the gene expression program initiated by FSH is turned off and is replaced by genes that control matrix formation and luteinization (reviewed by Edson et al. 2009; Richards and Pangas 2010a, b).
Several transcriptional regulators, necessary for ovulation, are induced in response to LH. The expression of the progesterone receptor (PR) is rapidly induced in the GC of the preovulatory follicle and is responsible for the expression of several key enzymes, including Adamts1, cathepsin L (Ctsl) proteases, endothelin 2 (Edn2), and Snap25, which regulates vesicle secretion and seems to be important for the release of potent cytokines from the GC as part of the inflammatory and immune-like response of ovulation (see hereinafter). Additional transcriptional regulators that mediate the ovulatory response to LH include C/EBP, the nuclear receptor NR5A2 also known as liver receptor homolog 1 (LRH1), and NR5A1 also known as steroidogenic factor 1 (SF1) (reviewed by Edson et al. 2009).
The binding of LH to its G-protein coupled receptor in the GC activates the AC/cAMP/PKA pathway, as well as the PI3K/AKT and RAS signaling cascades, each of which is critical for ovulation (Richards and Pangas 2010b). Downstream to the AC/cAMP/PKA pathway, the CG express the EGF-like factors amphiregulin (AREG), beta-cellulin (BTC), and epiregulin (EREG) in GC. These EGF-like factors undergo proteolytic cleavage—by ADAM17/TACE in porcine (Yamashita and Shimada 2012) or by matrix metalloproteinases MMP2 and MMP9 in mice (Light and Hammes 2015). Subsequently, the EGFR is activated and initiates the ERK1/2 pathway, thus inducing expression of downstream target genes responsible for cumulus expansion, steroidogenesis, oocyte maturation, and ovulation. Disruption of the EGF ligand/receptor signaling pathway in mice caused impaired cumulus expansion and inhibited ovulation (Hsieh et al. 2007). Disruption of ERK1/2 in GC resulted in failure of the follicles to ovulate and luteinize (Fan et al. 2009). Interestingly, ERK1/2 were also shown to be essential in the female mouse pituitary to initiate the LH surge (Bliss et al. 2009).
7.9.1 Oocyte Resumption of Meiosis
As described extensively above, in rodents the germ cells, oogonia, enter the meiotic prophase asynchronously starting on 13.5 dpc and proceed until 18.5 dpc, through leptotene, zygotene, pachytene, and diplotene, a stage at which they arrest. Meiotically arrested oocytes are characterized by the large nucleus known as “germinal vesicle” (GV). Alongside with the progress of folliculogenesis described above, the oocyte grows in size but persists in the GV stage throughout infancy and for variable periods beyond puberty. In sexually mature females, during each reproductive cycle a number of fully grown oocytes, characteristic of the species, reenter meiosis as manifested by “germinal vesicle breakdown” (GVBD). These oocytes complete the first round of meiosis with extrusion of the first polar body (PBI) and immediately thereafter progress to the second meiotic metaphase. Resumption of meiosis and its progression to the metaphase of the second meiotic division is usually referred to as “oocyte maturation.” At this stage, the meiotic process is arrested again, being completed upon fertilization, by the extrusion of the second polar body (reviewed by Tsafriri and Dekel 2010).
Fully grown oocytes remain arrested in the first prophase due to low activity of the maturation-promoting factor (MPF), which is a complex consisting of cyclin-dependent kinase 1 CDK1 and cyclin B that regulates the G2/M transition of the cell cycle. Low activity of CDK1 in meiotically arrested oocytes is maintained by relatively high concentrations of cAMP. Under these conditions, the active PKA stimulates the Wee1 kinase, inactivating, in turn, the Cdc25B phosphatase, required for CDK1 dephosphorylation and its resultant activation. In addition, in meiotically arrested oocytes, APCCdh1 constantly degrades cyclin B1 (reviewed by Adhikari and Liu 2014).
The intra-oocyte cAMP is contributed by the somatic compartment of the ovarian follicle that supplies this inhibitor to the oocyte by cell-to-cell communication mediated by gap junctions (Dekel et al. 1981). In addition, local production of cAMP by the oocyte itself has been suggested (Mehlmann et al. 2002; Kalinowski et al. 2004). Maintenance of the high levels of cAMP in the oocyte is further executed by the prevention of its degradation by phosphodiesterase 3A (PDE3A). Inhibition of PDE3A is achieved by cGMP, which is synthesized in the cumulus cells by the guanylyl cyclase natriuretic peptide receptor 2 (NPR2) and enters the oocyte via gap junctions (reviewed by Zhang and Xia 2012). Interestingly, NPR2, which is the natriuretic peptide precursor type C (NPPC) receptor, is activated in response to NPPC generated by mural granulosa cells (Zhang et al. 2010).
Activation of CDK1 at the onset of meiosis is dependent on the reduction in intra-oocyte cAMP. The preovulatory LH surge closes the gap junctions, thus preventing entrance of both cAMP and cGMP to the oocyte. Cessation of its supply, together with its increased hydrolysis by PDE3A, lowers the intra-oocyte cAMP levels (Tsafriri and Dekel 2010). The resulting inactivated PKA can no longer inhibit Cdc25B, which now dephosphorylates CDK1, turning on its catalytic activity (Adhikari and Liu 2014).
7.9.2 Cumulus Expansion
Cumulus expansion, also known as cumulus mucification, represents the secretion and apposition of hyaluronan-rich extracellular matrix in the COC (Dekel and Kraicer 1978; Eppig 1980). Hyaluronan, which forms the structural backbone of the COC extracellular matrix, is the product of hyaluronan synthase 2 (Has2), the expression of which is upregulated by LH. This gonadotropin also elevates the expression of TNF-α-induced protein 6 (Tnfaip6), which links the HA covalently to the heavy chain of serum-derived inter-α-trypsin inhibitor (IαI) upon its interaction with multimers of pentraxin 3 (PTX3). Another protein that interacts with HA to stabilize the COC matrix is Versican, a complex matricellular proteoglycan, which is also rapidly induced in GC by the LH surge (Dunning et al. 2015). Prostaglandin synthase 2 (PTGS2; also known as COX2), the rate-limiting enzyme in the synthesis of prostaglandins, is also essential for cumulus expansion and may function upstream of TNFAIP6 (reviewed by Edson et al. 2009).
Interestingly, many of these matrix-associated genes that are upregulated in the follicle after the LH surge are also found at sites of inflammation. In fact, ovulation and inflammation share several common attributes, including increased vascular permeability and prostaglandin synthesis as well as massive generation of reactive oxygen species (ROS), which were shown to be indispensable for ovulation (Shkolnik et al. 2011). Moreover, in the process of follicle rupture, there is enhanced ovarian blood flow and participation of proteinases, bradykinins, and histamine, which are all features of acute inflammatory reaction. Furthermore, the involvement of immune cells such as dendritic cells was also demonstrated in the ovulatory process (Cohen-Fredarow et al. 2014).
7.10 Corpus Luteum Formation
Ovulation culminates in the release of an ovum from the ovarian follicle to the oviduct, where it can be fertilized by sperm. The remaining granulosa and theca cells in the ruptured follicle undergo terminal differentiation to luteinizing cells and create the CL. The CL is essential for establishing and maintaining pregnancy mainly through secretion of progesterone. In case pregnancy has not occurred, the CL degenerates.
As opposed to the FSH-stimulated GC proliferation, LH-induced luteinization is characterized by cell cycle arrest. This involves the elevated expression of endogenous Cdk inhibitors (e.g., p21cip1 and p27kip1) and loss of positive cell cycle regulators, including cyclins and Cdk2 (Stocco et al. 2007).
During luteinization, the cellular responsiveness to external hormonal signals is altered due to changes in the expression pattern of the associated receptors. The FSHR is silenced and an activation and thereafter desensitization of the LHR occur. A sustained stimulation of the prolactin (PRL) receptor (PRLR) as well as a short rapid increase in progesterone receptor (PR) expression and a shift in the expression of the estrogen receptor (ER) from the predominance of ERβ to that of ERα also take place (Stocco et al. 2007).
The formation of the CL involves extensive tissue remodeling, including changes in the ECM that allows cell migration and neovascularization. The CL is extremely vascularized and has one of the highest rates of blood flow in the organism. The dense capillary network that is formed is important for efficient supply of nutrients, hormones, and lipoprotein-bound cholesterol to the luteal cells facilitates the efficient production of progesterone and its secretion. The process of CL vascularization is greatly controlled by VEGF, which promotes endothelial cell migration and proliferation, as well as angiopoietins (Ang), which appear to be important for vessel maturation and/or stabilization (Ferrara et al. 1998; Wulff et al. 2000). The transcription factor induced by LH, C/EBPβ, has a central role in the process of luteinization, as evident from the fact that in response to gonadotropin stimulation, C/EBPβ-null mice ovulate fertilizable eggs, but luteinization does not take place, and the CL is not formed (Sterneck et al. 1997). Double knockout of CEBPα and CEBPβ specifically in GC (Cebpα/β gc–/–) resulted in sterility, and microarray analyses identified numerous gene targets, including genes associated with neovascularization and endothelial cell functions (Fan et al. 2011).
As mentioned previously, the central role of the CL is the production and secretion of steroids, mainly progesterone. The major source of cholesterol used for steroid production is plasma lipoproteins (HDL and LDL) (Azhar and Menon 1982; Tureck and Strauss 1982). The HDL receptor scavenger receptor class B type I (SR-BI, Scarb1) is considered the central receptor responsible for cholesterol uptake in these cells. However, SR-BI-null mice synthesize normal amounts of progesterone during pseudopregnancy, suggesting the presence of additional pathways, such as de novo synthesis (Miettinen et al. 2001; Trigatti et al. 1999). Cholesterol is transported to the inner mitochondrial membrane by the sterol carrier protein 2 (SCP2) and steroidogenic acute regulatory protein (StAR). There, it is converted to pregenolone by cholesterol side chain cleavage P450 (P450scc), which is later converted in the endoplasmic reticulum to progesterone by 3β hydroxysteroid dehydrogenase (HSD). Both enzymes are highly expressed in the CL throughout pregnancy (Bachelot and Binart 2005; Stocco et al. 2007).
In mice, progesterone levels drop before parturition due to expression of the enzyme 20αHSD that catabolizes progesterone into the inactive progestin, 20α-DHP, and the enzyme P450c26, which catalyzes the conversion of cholesterol to 26-hydroxycholesterol thus reduces cholesterol availability to be turned into progesterone (Stocco et al. 2007).
In rodents and humans, the CL also produces androgens and estrogens in addition to progesterone. The weak androgen, androstenedione, is produced from progesterone by the enzyme P45017α-hydroxylase/C17–20 lyase (P450c17 or CYP17). Androstenedione is converted to estradiol by Cyp19 and 17βHSD-7 (Stocco et al. 2007).
To allow normal reproductive function, the CL must be regularly eliminated. In rodents, regression of the CL occurs in two phases. The first phase is functional and is associated with marked decrease in progesterone production. This is followed by a structural regression phase, characterized by programmed cell death of the luteal cells. The CL decreases in size and weight and eventually becomes a scar known as corpus albicans (Stocco et al. 2007).
7.11 Conclusion
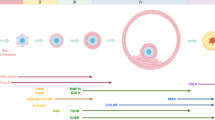
The ovary is a highly dynamic organ, which undergoes massive developmental changes up to sexual maturation, which are followed by periodic functional modifications at each reproductive cycle. The ovary executes a diverse repertoire of major roles. It houses the entire reservoir of the female oocytes encapsulated in dormant PMFs constantly generating growing follicles from which selected ones will ovulate a mature oocyte ready for fertilization. It also serves as the source for female sex hormones, thus functioning as an endocrine gland. We herein present the strict and complex regulatory mechanisms that orchestrate the successful accomplishment of these functions, as schematically described in Fig. 7.1.
Ovarian folliculogenesis in the mouse: a schematic presentation of the stages in ovarian folliculogenesis and the major factors and signaling pathways involved in this process. Primordial germ cells (PGCs) are specified by the BMP signaling pathway, then proliferate and migrate to the gonad, and form germ cells clusters. These clusters break down to form primordial follicles (PMFs), each containing a single oocyte (purple) surrounded by a flattened layer of pre-granulosa cells (blue). This process is negatively regulated by estradiol (E2) and AMH and is controlled by activin, neurotrophins (NTs), Figlα, Kitl/kit, FOXL2, and notch signaling (see text for details). Most of the PMFs remain quiescent, but a selected subpopulation is activated to further develop into primary follicles in a process regulated by both intra- and extra-oocyte factors (see text for details). The transition from primary to secondary follicle is accompanied by GC proliferation and encapsulation of the follicle by the theca cells (green). Secondary pre-antral follicles depend greatly on TGF-β signaling. Antral follicles depend on the gonadotropin hormones FSH and LH for their survival and ovulation. The LH surge triggers ovulation, while the remaining GC undergo terminal differentiation to form the CL (see text for details)
References
Ackert CL, Gittens JE, O’Brien MJ et al (2001) Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 233:258–270. doi:10.1006/dbio.2001.0216
Acosta TJ (2007) Studies of follicular vascularity associated with follicle selection and ovulation in cattle. J Reprod Dev 53:39–44. doi:10.1262/jrd.18153
Acosta TJ, Miyamoto A (2004) Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci 82–83:127–140. doi:10.1016/j.anireprosci.2004.04.022
Adhikari D, Liu K (2014) The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol 382:480–487. doi:10.1016/j.mce.2013.07.027
Adhikari D, Flohr G, Gorre N et al (2009a) Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod 15:765–770. doi:10.1093/molehr/gap092
Adhikari D, Zheng W, Shen Y et al (2009b) Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 19:397–410. doi:10.1093/hmg/ddp483
Anderson R, Copeland TK, Scholer H et al (2000) The onset of germ cell migration in the mouse embryo. Mech Dev 91:61–68. doi:10.1016/S0925-4773(99)00271-3
Azhar S, Menon KM (1982) Receptor mediated gonadotropin action in gonadal tissues: relationship between blood cholesterol levels and gonadotropin stimulated steroidogenesis in isolated rat Leydig and luteal cells. J Steroid Biochem 16:175–184. doi:10.1016/0022-4731(82)90165-0
Bachelot A, Binart N (2005) Corpus luteum development: lessons from genetic models in mice. Curr Top Dev Biol 68:49–84. doi:10.1016/S0070-2153(05)68003-9
Baerwald AR, Adams GP, Pierson RA (2012) Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 18:73–91. doi:10.1093/humupd/dmr039
Bliss SP, Miller A, Navratil AM et al (2009) ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol 23:1092–1101. doi:10.1210/me.2009-0030
Brown HM, Russell DL (2014) Blood and lymphatic vasculature in the ovary: development, function and disease. Hum Reprod Update 20:29–39. doi:10.1093/humupd/dmt049
Castrillon DH, Miao L, Kollipara R et al (2003) Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301:215–218. doi:10.1126/science.1086336
Chang H, Brown CW, Matzuk MM (2002) Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev 23:787–823. doi:10.1210/er.2002-0003
Chen Y, Jefferson WN, Newbold RR et al (2007) Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 148:3580–3590. doi:10.1210/en.2007-0088
Chowdhury MWH, Scaramuzzi RJ, Wheeler-Jones CPD, Khalid M (2010) The expression of angiogenic growth factors and their receptors in ovarian follicles throughout the estrous cycle in the ewe. Theriogenology 73:856–872
Cohen-Fredarow A, Tadmor A, Raz T et al (2014) Ovarian dendritic cells act as a double-edged pro-ovulatory and anti-inflammatory sword. Mol Endocrinol 28:1039–1054. doi:10.1210/me.2013-1400
Danforth DR, Arbogast LK, Ghosh S et al (2003) Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod 68:1736–1741. doi:10.1095/biolreprod.101.000679
Dekel N, Kraicer PF (1978) Induction in vitro of mucification of rat cumulus oophorus by gonadotrophins and adenosine 3′,5′-monophosphate. Endocrinology 102:1797–1802
Dekel N, Lawrence TS, Gilula NB, Beers WH (1981) Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev Biol 86:356–362. doi:10.1016/0012-1606(81)90193-7
Dissen GA, Romero C, Hirshfield AN, Ojeda SR (2001) Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology 142:2078–2086. doi:10.1210/endo.142.5.8126
Dodson WC, Schomberg DW (1987) The effect of transforming growth factor-beta on follicle-stimulating hormone-induced differentiation of cultured rat granulosa cells. Endocrinology 120(2):512–516
Dong J, Albertini DF, Nishimori K et al (1996) Growth differentiation factor 9 is required during early ovarian folliculogenesis. Nature 383(6600):531–535
Dunning KR, Watson LN, Zhang VJ et al (2015) Activation of mouse cumulus-oocyte complex maturation in vitro through EGF-like activity of versican. Biol Reprod 92:1–10. doi:10.1095/biolreprod.114.127274
Durlinger AL, Kramer P, Karels B et al (1999) Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140:5789–5796. doi:10.1210/en.140.12.5789
Edson MA, Nagaraja AK, Matzuk MM et al (2009) The mammalian ovary from genesis to revelation. Endocr Rev 30:624–712. doi:10.1210/er.2009-0012
Epifano O, Dean J (2002) Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab 13:169–173. doi:10.1016/S1043-2760(02)00576-3
Eppig JJ (1980) Regulation of cumulus oophorus expansion by gonadotropins in vivo and in vitro. Biol Reprod 23:545–552. doi:10.1095/biolreprod23.3.545
Eppig JJ (2001) Review Oocyte control of ovarian follicular development and function in mammals. Reproduction 122(6):829–838
Eppig JJ, Wigglesworth K, Pendola FL (2002) The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A 99:2890–2894. doi:10.1073/pnas.052658699
Fan H, Liu Z, Shimada M et al (2009) MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:1–4
Fan H-Y, Liu Z, Johnson PF, Richards JS (2011) CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol 25:253–268. doi:10.1210/me.2010-0318
Ferrara N, Chen H, Davis-Smyth T et al (1998) Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 4:336–340
Garside SA, Henkin J, Morris KD et al (2010) A thrombospondin-mimetic peptide, ABT-898, suppresses angiogenesis and promotes follicular atresia in pre- and early-antral follicles in vivo. Endocrinology 151:5905–5915. doi:10.1210/en.2010-0283
Gill ME, Hu Y-C, Lin Y, Page DC (2011) Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc Natl Acad Sci U S A 108:7443–7448. doi:10.1016/j.juro.2011.10.069
Ginsburg M, Snow MH, McLaren A (1990) Primordial germ cells in the mouse embryo during gastrulation. Development 110:521–528
Granot I, Dekel N (1994) Phosphorylation and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem 269(48):30502–30509
Hanrahan JP, Gregan SM, Mulsant P et al (2004) Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod 70:900–909. doi:10.1095/biolreprod.103.023093
Harlow CR, Davidson L, Burns KH et al (2002) FSH and TGF-beta superfamily members regulate granulosa cell connective tissue growth factor gene expression in vitro and in vivo. Endocrinology 143:3316–3325. doi:10.1210/en.2001-211389
Hayashi K, de Sousa Lopes SM, Surani MA (2007) Germ cell specification in mice. Science 316(5823):394–396
Hsieh M, Lee D, Panigone S et al (2007) Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924. doi:10.1128/MCB.01919-06
Hu Y-C, Nicholls PK, Soh YQS et al (2015) Licensing of primordial germ cells for gametogenesis depends on genital ridge signaling. PLoS Genet 11:e1005019. doi:10.1371/journal.pgen.1005019
Hutt KJ, McLaughlin EA, Holland MK (2006) Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod 12:61–69. doi:10.1093/molehr/gal010
Iijima K, Jiang J-Y, Shimizu T et al (2005) Acceleration of follicular development by administration of vascular endothelial growth factor in cycling female rats. J Reprod Dev 51:161–168. doi:10.1262/jrd.51.161
Ingman WV, Robertson SA (2009) The essential roles of TGFB1 in reproduction. Cytokine Growth Factor Rev 20:233–239. doi:10.1016/j.cytogfr.2009.05.003
Jagarlamudi K, Rajkovic A (2012) Molecular and cellular endocrinology oogenesis: transcriptional regulators and mouse models. Mol Cell Endocrinol 356:31–39. doi:10.1016/j.mce.2011.07.049
Jameson SA, Natarajan A, Cool J et al (2012) Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet 8:e1002575. doi:10.1371/journal.pgen.1002575
Jiang J-Y, Cheung CKM, Wang Y, Tsang BK (2003) Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci 8:222–237
John GB, Gallardo TD, Shirley LJ, Castrillon DH (2009) Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 321:197–204. doi:10.1016/j.ydbio.2008.06.017.Foxo3
Jones RL, Pepling ME (2013) KIT signaling regulates primordial follicle formation in the neonatal mouse ovary. Dev Biol 382:186–197. doi:10.1016/j.ydbio.2013.06.030
Juengel JL, Mcnatty KP, Animal W et al (2005) The role of proteins of the transforming growth factor-b superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 11:144–161. doi:10.1093/humupd/dmh061
Kalinowski RR, Berlot CH, Jones TLZ et al (2004) Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein-mediated pathway. Dev Biol 267:1–13. doi:10.1016/j.ydbio.2003.11.011
Kerr B, Garcia-Rudaz C, Dorfman M et al (2009) NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction 138:131–140. doi:10.1530/REP-08-0474
Kezele P, Skinner MK (2003) Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 144:3329–3337. doi:10.1210/en.2002-0131
Kezele P, Nilsson EE, Skinner MK (2005) Keratinocyte growth factor acts as a mesenchymal factor that promotes ovarian primordial to primary follicle transition. Biol Reprod 73:967–973. doi:10.1095/biolreprod.105.043117
Knight PG, Satchell L, Glister C (2012) Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol 359:53–65. doi:10.1016/j.mce.2011.04.024
Koubova J, Menke DB, Zhou Q et al (2006) Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 103:2474–2479. doi:10.1073/pnas.0510813103
Lee W-S, Yoon S-J, Yoon T-K et al (2004) Effects of bone morphogenetic protein-7 (BMP-7) on primordial follicular growth in the mouse ovary. Mol Reprod Dev 69:159–163. doi:10.1002/mrd.20163
Lei L, Spradling AC (2013) Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development 140:2075–2081. doi:10.1242/dev.093864
Light A, Hammes SR (2015) LH-induced steroidogenesis in the mouse ovary, but not testis, requires matrix metalloproteinase 2 and 9-mediated cleavage of upregulated EGF receptor ligands. Biol Reprod. doi:10.1095/biolreprod.115.130971
Lin Y-T, Capel B (2015) Cell fate commitment during mammalian sex determination. Curr Opin Genet Dev 32:144–152. doi:10.1016/j.gde.2015.03.003
Liu X, Andoh K, Abe Y et al (1999) A comparative study on transforming growth factor-beta and activin A for preantral follicles from adult, immature, and diethylstilbestrol-primed immature mice. Endocrinology 140:2480–2485. doi:10.1210/en.140.6.2480
Liu K, Rajareddy S, Liu L et al (2006) Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol 299:1–11. doi:10.1016/j.ydbio.2006.07.038
Liu C, Peng J, Matzuk MM, Yao HH-C (2015) Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat Commun 6:6934. doi:10.1038/ncomms7934
Louvet JP, Vaitukaitis JL (1976) Induction of follicle stimulating hormone (FSH) receptors in rat ovaries by estrogen priming. Endocrinology 99:758–764
Macias V, Pinzón C, Fierro F et al (2012) Identification of soluble forms of vascular endothelial growth factor receptors, sVEGFR-1 and sVEGFR-2, in bovine dominant follicles. Reprod Domest Anim 47:e39–e42. doi:10.1111/j.1439-0531.2011.01919.x
Magoffin DA (2005) Ovarian theca cell. Int J Biochem Cell Biol 37:1344–1349. doi:10.1016/j.biocel.2005.01.016
Matzuk MM, Burns KH (2012) Genetics of mammalian reproduction: modeling the end of the germline. Annu Rev Physiol 74:503–528. doi:10.1146/annurev-physiol-020911-153248
McLaren A (2003) Primordial germ cells in the mouse. Dev Biol 262:1–15. doi:10.1016/S0012-1606(03)00214-8
Mehlmann LM, Jones TLZ, Jaffe LA (2002) Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 297:1343–1345. doi:10.1126/science.1073978
Menke DB, Koubova J, Page DC (2003) Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 262:303–312. doi:10.1016/S0012-1606(03)00391-9
Miettinen HE, Rayburn H, Krieger M (2001) Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest 108:1717–1722. doi:10.1172/JCI200113288
Molyneaux KA, Stallock J, Schaible K, Wylie C (2001) Time-lapse analysis of living mouse germ cell migration. Dev Biol 240:488–498. doi:10.1006/dbio.2001.0436
Mork L, Maatouk DM, McMahon JA et al (2012) Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod 86:37. doi:10.1095/biolreprod.111.095208
Myers M, Middlebrook BS, Matzuk MM, Pangas SA (2009) Loss of inhibin alpha uncouples oocyte-granulosa cell dynamics and disrupts postnatal folliculogenesis. Dev Biol 334:458–467. doi:10.1016/j.ydbio.2009.08.001
Myers M, Tripurani SK, Middlebrook B et al (2011) Loss of gremlin delays primordial follicle assembly but does not affect female fertility in mice. Biol Reprod 85:1175–1182. doi:10.1095/biolreprod.111.091728
Neeman M, Abramovitch R, Schiffenbauer YS, Tempel C (1997) Regulation of angiogenesis by hypoxic stress: from solid tumours to the ovarian follicle. Int J Exp Pathol 78(2):57–70
Nilsson EE, Skinner MK (2003) Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 69:1265–1272. doi:10.1095/biolreprod.103.018671
Nilsson E, Parrott JA, Skinner MK (2001) Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol 175:123–130. doi:10.1016/S0303-7207(01)00391-4
Nilsson EE, Kezele P, Skinner MK (2002) Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol 188:65–73. doi:10.1016/S0303-7207(01)00746-8
Nilsson EE, Detzel C, Skinner MK (2006) Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction 131:1007–1015. doi:10.1530/rep.1.00978
Nilsson E, Dole G, Skinner MK (2009) Neurotrophin NT3 promotes ovarian primordial to primary follicle transition. Reproduction 138:697–707. doi:10.1530/REP-09-0179
Nilsson EE, Schindler R, Savenkova MI, Skinner MK (2011) Inhibitory actions of anti-Müllerian hormone (AMH) on ovarian primordial follicle assembly. PLoS One. doi:10.1371/journal.pone.0020087
Ohinata Y, Ohta H, Shigeta M et al (2009) A signaling principle for the specification of the germ cell lineage in mice. Cell 137:571–584. doi:10.1016/j.cell.2009.03.014
Oktem O, Urman B (2010) Understanding follicle growth in vivo. Hum Reprod 25:2944–2954. doi:10.1093/humrep/deq275
Osz K, Ross M, Petrik J (2014) The thrombospondin-1 receptor CD36 is an important mediator of ovarian angiogenesis and folliculogenesis. Reprod Biol Endocrinol 12:1–10. doi:10.1186/1477-7827-12-21
Pangas SA (2012) Regulation of the ovarian reserve by members of the transforming growth factor beta family. Mol Reprod Dev 79(10):666–679. doi:10.1002/mrd.22076
Pangas SA, Matzuk MM (2004) Genetic models for transforming growth factor beta superfamily signaling in ovarian follicle development. Mol Cell Endocrinol 225:83–91. doi:10.1016/j.mce.2004.02.017
Pedersen T, Hannah P (1968) Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 17:555–557
Pepling ME, Spradling AC (1998) Female mouse germ cells form synchronously dividing cysts. Development 125:3323–3328
Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234(2):339–351. doi:10.1006/dbio.2001.0269
Petrik JJ, Gentry PA, Feige J et al (2002) Expression and localization of thrombospondin-1 and -2 and their cell-surface receptor, CD36, during rat follicular development and formation of the corpus luteum. Biol Reprod 67:1522–1531. doi:10.1095/biolreprod.102.007153
Rajareddy S, Reddy P, Du C et al (2007) p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol 21:2189–2202. doi:10.1210/me.2007-0172
Rao MC, Midgley AR, Richards JS (1978) Proliferation of ovarian cellular. Cell 14:71–78
Reddy P, Liu L, Adhikari D et al (2008) Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319:611–613. doi:10.1126/science.1152257
Reddy P, Adhikari D, Zheng W et al (2009) PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet 18:2813–2824. doi:10.1093/hmg/ddp217
Reddy P, Zheng W, Liu K (2010) Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab 21:96–103. doi:10.1016/j.tem.2009.10.001
Reizel Y, Itzkovitz S, Adar R et al (2012) Cell lineage analysis of the mammalian female germline. PLoS Genet 8:e1002477. doi:10.1371/journal.pgen.1002477
Richards JS, Pangas SA (2010a) Fertility control. Handb Exp Pharmacol 198:3–27. doi:10.1007/978-3-642-02062-9
Richards JS, Pangas SA (2010b) The ovary: basic biology and clinical implications, Review series. J Clin Invest 120:963–972. doi:10.1172/JCI41350, Review
Ryan KE, Casey SM, Canthy MJ et al (2007) Akt and Erk signal transduction pathways are early markers of differentiation in dominant and subordinate ovarian follicles in cattle. Reproduction 133:617–626. doi:10.1530/REP-06-0130
Saitou M, Kagiwada S, Kurimoto K (2012) Epigenetic reprogramming in mouse pre-implantation development and primordial germ cells. Development 139:15–31. doi:10.1242/dev.050849
Salomon Y, Yanovsky A, Mintz Y et al (1977) Synchronous generation of ovarian hCG binding sites and LH-sensitive adenylate cyclase in immature rats following treatment with pregnant mare serum gonadotropin. J Cyclic Nucleotide Res 3:163–176
Schindler R, Nilsson E, Skinner MK (2010) Induction of ovarian primordial follicle assembly by connective tissue growth factor CTGF. PLoS One 5:e12979. doi:10.1371/journal.pone.0012979
Seki Y, Yamaji M, Yabuta Y et al (2007) Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development 134:2627–2638. doi:10.1242/dev.005611
Shkolnik K, Tadmor A, Ben-Dor S et al (2011) Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A 108(4):1462–1467. doi:10.1073/pnas.1017213108
Shimizu T, Jiang J, Sasada H, Sato E (2002) Changes of messenger RNA expression of angiogenic factors and related receptors during follicular development in gilts. Biol Reprod 67:1846–1852. doi:10.1095/biolreprod.102.006734
Simon AM, Goodenough DA, Li E, Paul DL (1997) Female infertility in mice lacking connexin 37. Nature 385:525–529
Soyal SM, Amleh A, Dean J (2000) FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development 127:4645–4654
Sterneck E, Tessarollo L, Johnson PF (1997) An essential role for C/EBPbeta in female reproduction. Genes Dev 11:2153–2162. doi:10.1101/gad.11.17.2153
Stocco C, Telleria C, Gibori G (2007) The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149. doi:10.1210/er.2006-0022
Teilmann SC (2005) Differential expression and localisation of connexin-37 and connexin-43 in follicles of different stages in the 4-week-old mouse ovary. Mol Cell Endocrinol 234:27–35. doi:10.1016/j.mce.2004.10.014
Thomas FH, Wilson H, Silvestri A, Fraser HM (2008) Thrombospondin-1 expression is increased during follicular atresia in the primate ovary. Endocrinology 149:185–192. doi:10.1210/en.2007-0835
Tilly JL, Telfer EE (2009) Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol Hum Reprod 15:393–398. doi:10.1093/molehr/gap036
Trigatti B, Rayburn H, Viñals M et al (1999) Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A 96:9322–9327. doi:10.1073/pnas.96.16.9322
Tsafriri A, Dekel N (2010) Intra- and intercellular molecular mechanisms in regulation of meiosis in murid rodents. In: Tosti E, Boni R (eds) Oocyte maturation and fertilization: a long history for a short event. Bentham, Dubai, pp 38–63
Tureck RW, Strauss JF (1982) Progesterone synthesis by luteinized human granulosa cells in culture: the role of de novo sterol synthesis and lipoprotein-carried sterol. J Clin Endocrinol Metab 54:367–373. doi:10.1210/jcem-54-2-367
Uda M, Ottolenghi C, Crisponi L et al (2004) Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 13:1171–1181. doi:10.1093/hmg/ddh124
Ulloa-Aguirre A, Zariñán T, Pasapera AM et al (2007) Multiple facets of follicle-stimulating hormone receptor function. Endocrine 32:251–263. doi:10.1007/s12020-008-9041-6
Van Santbrink E, Hop W, Van Dessel T et al (1995) Decremental follicle-stimulating hormone and dominant follicle development during the normal menstrual cycle. Fertil Steril 1(64):37–43
Vanorny DA, Prasasya RD, Chalpe AJ et al (2014) Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol 28:499–511. doi:10.1210/me.2013-1288
Wotton D, Massague J (2000) Transcriptional control by the TGF-b/Smad signaling system. EMBO J 19:1745–1754
Wulff C, Wilson H, Largue P et al (2000) Angiogenesis in the human corpus luteum: localization and changes in angiopoietins, tie-2, and vascular endothelial growth factor messenger ribonucleic acid. J Clin Endocrinol Metab 85:4302–4309. doi:10.1210/jcem.85.11.6942
Yamashita Y, Shimada M (2012) The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J Reprod Dev 58(5):510–514
Yan C, Wang P, DeMayo J et al (2001) Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15:854–866. doi:10.1210/mend.15.6.0662
Zeleznik AJ, Schuler HM, Reichert LE (1981) Gonadotropin-binding sites in the rhesus monkey ovary: role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology 109:356–362
Zhang M, Xia G (2012) Hormonal control of mammalian oocyte meiosis at diplotene stage. Cell Mol Life Sci 69:1279–1288. doi:10.1007/s00018-011-0867-3
Zhang M, Su Y-Q, Sugiura K et al (2010) Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330:366–370
Zhang H, Risal S, Gorre N et al (2014) Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol 24(21):2501–2508. doi:10.1016/j.cub.2014.09.023
Zheng W, Nagaraju G, Liu Z, Liu K (2012) Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol 356:24–30. doi:10.1016/j.mce.2011.05.027
Zimmermann RC, Hartman T, Kavic S et al (2003) Vascular endothelial growth factor receptor 2—mediated angiogenesis is essential for gonadotropin-dependent follicle development. J Clin Invest 112:659–669. doi:10.1172/JCI200318740
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rimon-Dahari, N., Yerushalmi-Heinemann, L., Alyagor, L., Dekel, N. (2016). Ovarian Folliculogenesis. In: Piprek, R. (eds) Molecular Mechanisms of Cell Differentiation in Gonad Development. Results and Problems in Cell Differentiation, vol 58. Springer, Cham. https://doi.org/10.1007/978-3-319-31973-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-31973-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31971-1
Online ISBN: 978-3-319-31973-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)