Abstract
Molecular dynamics (MD) simulation has been done for the melting temperature of gold–copper bimetallic nanostructure with 55 total gold and copper atom numbers and its bulk alloy. The trend of melting temperature for gold–copper bimetallic nanocluster is not the same as the melting temperature for gold–copper bulk alloy. MD simulation shows that the melting temperature of gold–copper bimetallic nanocluster increases with copper atom fraction. Semi-empirical potential within the tight-binding second moment approximation as new application potential model regarding the melting temperature for gold–copper bulk structure shows better result in comparison with EAM, Sutton–Chen potential and quantum Sutton–Chen potential models.
This chapter book is dedicated to my parents due to their endless support and encouragement
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Molecular dynamics
- Quantum Sutton–Chen potential
- Semi-empirical potential
- Gold–copper bimetallic nanostructure
6.1 Introduction
Nanoclusters are aggregates of atoms or molecules of nanometric size, containing a number of constituent particles ranging from 10 to 106 [1]. There are unusual chemical and physical properties in metallic nanocluster in comparison with metal bulk properties. Due to the extended applications of metallic nanoclusters in heterogeneous catalysts, electronic devices and microelectronics, investigation of their structural and dynamical properties are topics of interest in previous literature [2, 3]. Applications of metal nanoclusters to heterogeneous catalysis have shown that good selectivity can be achieved toward specific reactions by controlling the catalyst surface composition [4]. Gold nanoparticle catalysts are used mainly in selective oxidation reactions and in a broad range of organic transformations besides hydrogenation [5]. Copper nanoparticles are known to be active in the oxidation of alcohols to aldehydes [6]. Therefore, by mixing the two metals, Au–Cu bimetallic nanoclusters offer a way to fine-tune the catalytic properties of these two metals. Nevertheless, understanding their structural–thermal stability relationship is a difficult task.
Molecular dynamics (MD) simulation is an ideal tool for investigating the thermal physical properties of such small clusters. Among different bimetallic alloy nanoparticle catalysts, Au–Cu catalysts have recently attracted interest for catalytic oxidation of CO, benzyl alcohol, and propene [7–9] and for the partial oxidation of methanol to produce hydrogen fuels. Some recent experimental results indicate that the icosahedral phase structure can be stabilized with the addition of some transition or noble metal to a pure metallic cluster [10]. It is known that for pure 55-atom coinage clusters, only the Ag55 and Cu55 exhibit the icosahedral structure, whereas the Au55 cluster has a low-symmetry C 1 structure [11–13]. Gao and co-workers recently studied doping a single metal atom to the Au55 cluster. They found that a single-atom dopant is unlikely to convert the low-symmetry parent structure to the icosahedral (I h ) structure [14]. It is found that doping of Au55 with a single Cu atom can sharply raise the melting point of the cluster. It is well known that the melting temperature of Au55 (380 K) is much lower than that of Cu1Au54 (530 K) [15]. A Cu–Au cluster does not experience a smooth transition from a pure Cu-like behavior to a pure Au cluster with a decrease in the concentration of Cu. The thermal behavior of Cu–Au is mainly influenced by the presence of Cu atoms in the bimetallic structure [16]. Studies of phase conformations and thermal behavior of Au–Cu binary clusters by classical MD simulations showed that the cluster size, the concentration in the alloy, and the annealing temperature have a dominant effect on the morphology reconstruction process [17]. For example, when the starting morphology is of a cuboctahedral type, by changing the concentration of copper from 50 to 10 %, an optimum stability of an icosahedral-type morphology is found at a bimetallic concentration of gold 75 % and copper 25 %, in fair agreement with experimental reports [18]. In the small size of nanostructure, as the surface-to-volume ratio is large, the surface effect has a significant contribution on physical quantities such as transport properties, melting point, and thermal energy [19, 20]. One of the most commonly used parameters for the description of the energetic situation on the surface of a solid is the surface energy. The surface energy may be defined as the excess energy of the surface of a material compared to its bulk. Joseph and co-workers have investigated the relative surface energy changes with temperature for two bimetallic alloys. At low temperatures, they found the segregation to be dependent primarily on the surface energy difference for Pt–Pd and Cu–Ni systems. In the Pt–Pd system, the surface energy difference between pure Pt and Pd increases with an increase of temperature. This behavior was different from that of a Cu–Ni bimetallic alloy where the surface energy difference decreases with an increase of temperature [15]. Knowledge of diffusion behavior is of interest for the production of metallic materials and for their practical use in the near phase transition. On this basis, investigations were performed on the Pt–Pd [15], Cu–Ni [19], Ag–Pt [21], Au–Pd [22], and Fe–Al [23] systems. In all cases, self-diffusion coefficient was found to increase with temperature. Self-diffusion coefficient decreases with pressure [24–26]. Near the melting temperature, the spin multiplicity of bimetallic nanocluster changes in comparison with its solid state completely [25, 26]. In the present study, we show that the presence of impurity at the pure cluster affects the melting temperature for gold–copper bimetallic nanostructure as well as the bulk structure. In this work, we study the structure details and dynamic behavior of Au–Cu nanoclusters for the specific case of icosahedral geometry and the variation of the configurational energy with temperature.
6.2 MD Simulation Details
In classical MD, each particle is treated as a point mass and the motion is governed by Newton’s second law. The thermodynamic properties are obtained from time averages over the ensembles of particles.
MD simulations were done in the constant temperature ensemble (NVT) using the Verlet algorithm for the integration of Newton’s equations of motion, with a time step of one femto second resulting in long simulation runs of 500 picoseconds (ps). Ensemble averaging has been done after 400 ps. The Nose–Hoover thermostat is used for controlling the temperature of MD simulation with DL POLY package [27]. The quantum Sutton–Chen (QSC) many-body inter-atomic potential leads to an accurate description of many properties of I h metals and their alloys. In this work, we have applied the QSC inter-atomic potential to model the interaction in the Au–Cu alloy clusters. A cut-off ratio for the range of the interaction of 7.0 Å was used in all the cases.
6.3 Potential Function
MD simulations using DL POLY were used to gain insights into the melting process at the atomistic level. All the thermodynamic and transport properties were obtained as time averages over the particle positions and velocities. The local electronic density is included to account for the many-body terms [15]. Based on the Sutton–Chen potential, the potential energy of the finite system is given by
where r ij is the distance between atoms i and j, c a positive dimensionless parameter and ε a parameter with the dimensions of energy. Parameter \( V(r_{ij} ) \) is a pair potential for including the repulsion of Pauli’s exclusion principle:
The local density ρ i accounting for cohesion associated with any atom i is given by
where a is a length parameter scaling all spacing (leading to dimensionless V and ρ); n and m are both positive integer parameters with condition n > m.
Sutton and Chen restricted values of m to be greater than 6 and fitted it to give close agreement with the bulk modulus and the elastic constants. The Sutton–Chen potential predicts properties involving defects, surfaces, and interfaces poorly. The quantum Sutton–Chen potential includes quantum corrections and takes into account the zero-point energy allowing better prediction of temperature-dependent properties. The QSC potential function was found to be better suited to melting and phase transformation studies of bulk Cu–Ni [28], Cu–Au [29], and Pd–Ni [30] alloys. The QSC parameters for Au and Cu are listed in Table 6.1 [29].
Semi-empirical potential derived within the tight-binding second moment approximation as in Eq. 6.4 by Cleri and Rosato was applied to describe interactions for bulk systems.
where \( E_{j}^{b} , { }E_{j}^{r} \) are bonding and repulsion of Born–Mayer term and can be written as Eqs. 6.5 and 6.6, respectively.
r ji is the distance between the atoms at sites, i.e., j; s = A, B is the chemical species of the atom j, while w = A, B is the species of the atom i, \( r_{sw}^{0} \) is the nearest-neighbors distance. The parameters A sw , p sw , q sw , ξ sw are fitted to several bulk properties of the Au0.25Cu0.75 cubic alloy. The parameters of mentioned empirical potential can be defined with the exact work of Cleri and Rosato [31] which is presented at Table 6.2.
6.4 Results and Discussion
6.4.1 Solid–Liquid Phase Transition in Bulk Au–Cu Alloy
The effects of impurity on the melting point of Cu x Au55−x are investigated. Sutton–Chen (SC) [32], quantum Sutton–Chen (Q-SC) many-body [32] and embedded atom method (EAM) [33] potentials are used. For comparison of the different potential models we have chosen semi-empirical potential models for investigation of the melting temperature of copper–gold alloy in bulk structure. Many techniques such as distortion parameter [34–37], thermal conductivity [19, 20], self-diffusion coefficient [38, 39], heat capacity [18, 40, 41], phase-field-crystal models [42], local pressure tensor [43] and Lindemann index [44] can be used for recognition of solid–liquid phase transition [34].
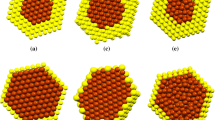
On the basis of Fig. 6.1, the structural configuration at 300 K for 0.250 mol fraction of gold in copper is more stable than other doping fractions such as 0.75 and 0.50 mol fraction of copper in gold due to more interaction between gold and copper atoms. Chemical ordering effect is investigated on the phase transition of solid–liquid bimetallic nanocluster. Heat capacity is used for investigation of solid–liquid phase of gold–copper alloy transition as well. The results of melting temperature with Gupta potential model are represented in Table 6.3. According to Table 6.3 the following order for melting temperature is observed:
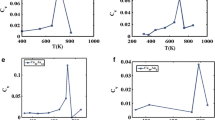
On the basis of Table 6.3, the bulk melting temperature with Gupta potential model shows good agreement with available experimental data in comparison with other potential models such as quantum Sutton–Chen, EAM and Sutton–Chen. The results of heat capacity for investigation of solid–liquid phase transition of pure bulk gold and copper are given in Fig. 6.2. There is a peak in the heat capacity graph, which is proportional to the melting temperature for pure and gold bulk structure.
On the basis of Fig. 6.2, our results for copper and gold in bulk, solid–liquid phase transition are 1338 and 1325 K, respectively, and are consistent with the corresponding experimental results of 1356 and 1337 K.
6.4.2 Solid–Liquid Phase Transition in Bimetallic Nanocluster Au–Cu
MD simulation has been used for studying the melting temperature of bimetallic Au–Cu system with total atom 55. The most direct way to analyze the thermal behavior is to consider the configuration energy versus temperature. For this purpose, we calculated the configuration energy at different temperatures; the melting temperature (T m) of the system would be located at the point where the thermal behavior line presents a change in the slope. On the basis of configuration energy, the slope is not sharp and seems relatively wide. We observe that the configuration energy shows a simple jump as a function of temperature at the melting point of the solid. The results of configuration energy for different chemical compositions namely Cu6Au49, Cu12Au43, Cu18Au37, Cu27Au28, Cu41Au14, and Cu49Au6 gold–copper bimetallic nanoclusters are presented in Fig. 6.3.
The melting point of bimetallic Au–Cu 55 atom nanoclusters is examined using the quantum Sutton–Chen many-body potential function. The impurity effect on the melting point for Cu6Au49, Cu12Au43, Cu18Au37, Cu41Au14, Cu49Au6 and Cu55 bimetallic nanoclusters is considered. The results of melting temperature as a function of doping are presented in Fig. 6.4.
According to Fig. 6.4, the melting temperature of gold–copper bimetallic nanocluster increases with copper atom fraction. The trend of melting temperature for bulk gold–copper is not the same as the melting temperature trend for gold–copper bimetallic nanocluster. The surface energy effect is an important phenomenon which is observed in nanosystem but not in bulk structure [40, 41]. Previous literature shows that the surface energy of gold nanoparticle is less than that of copper nanoparticle [40, 41]. As a result, most of the gold atoms move on the surface. The strength of metallic bond for copper is greater than that of gold, thus the melting temperature of gold–copper bimetallic nanocluster increases with higher copper atom fraction due to surface effect. The melting temperature of bimetallic gold–copper nanoclusters has been calculated by Cheng et al. [14]. The results indicate that the melting temperature of Au55 increases with doping of copper as impurity. Canonical Monte Carlo simulations [14], based on the second-moment approximation of the tight-binding potentials, are consistent with our results. On the basis of Fig. 6.4, the melting temperature for gold–copper bimetallic nanoclusters is lower than the melting temperature of bulk structure significantly.
6.5 Conclusions
Configurational energy analysis has been used for the determination of melting temperature of gold–copper bimetallic nanoclusters. MD simulation with semi-empirical potential within the tight-binding second moment approximation has been used as a new application potential model for the melting temperature of gold–copper bulk structure. Optimum structure of gold–copper bulk structure has been obtained with MD simulation as well. The following order for the melting temperature of gold–copper alloy in bulk structure was observed:
Semi-empirical potential model for the melting temperature shows good agreement with available experimental data in comparison with EAM, quantum Sutton–Chen and Sutton–Chen potential models. MD simulation regarding the melting temperature of gold–copper bimetallic nanoclusters with a total of 55 atoms shows that the melting temperature increases with copper atom fraction due to lower surface energy and metallic bond energy of gold. MD simulation with Quantum Sutton–Chen potential model estimates the melting temperature of gold–copper bimetallic nanoclusters around 600−800 K temperature range which is significantly lower than its bulk structure.
References
Baletto F, Ferrando R (2005) Structural properties of nanoclusters: energetic, thermodynamic, and kinetic effects. Rev Mod Phys 77:371–423
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910
Huang S-P, Mainardi DS, Balbuena PB (2003) Structure and dynamics of graphite-supported bimetallic nanoclusters. Surf Sci 545:163–179
Bracey CL, Ellis PR, Hutchings GJ (2009) Application of copper–gold alloys in catalysis: current status and future perspectives. Chem Soc Rev 38:2231–2243
Della Pina C, Falletta E, Prati L, Rossi M (2008) Selective oxidation using gold. Chem Soc Rev 37:2077–2095
Yoo W-J, Li C-J (2007) Copper-catalyzed oxidative esterification of alcohols with aldehydes activated by Lewis acids. Tetrahedron Lett 48:1033–1035
Liu X, Wang A, Wang X, Mou C-Y, Zhang T (2008) Au–Cu alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem Commun 27:3187–3189
Zhang L (2012) A molecular dynamics study of thermal behavior of melting an Au54Cu1 cluster. Procedia Eng 36:207–211
Zhang L, Zhang C-B, Qi Y (2008) Local structure changes of 54-, 55-, 56-atom copper clusters on heating. Phys Lett A 372:2874–2880
Huang W, Ji M, Dong C-D, Gu X, Wang L-M, Gong XG, Wang L-S (2008) Relativistic effects and the unique low-symmetry structures of gold nanoclusters. ACS Nano 2:897–904
Gao Y, Shao N, Pei Y, Zeng XC (2010) Icosahedral crown gold nanocluster Au43Cu12 with high catalytic activity. Nano Lett 10:1055–1062
Häkkinen H, Moseler M, Kostko O, Morgner N, Hoffmann MA, Issendorff BV (2004) Symmetry and electronic structure of noble-metal nanoparticles and the role of relativity. Phys Rev Lett 93:093401
Xing X, Danell RM, Garzón IL, Michaelian K, Blom MN, Burns MM, Parks JH (2005) Size-dependent fivefold and icosahedral symmetry in silver clusters. Phys Rev B 72:081405
Cheng D, Huang S, Wang W (2006) Thermal behavior of core-shell and three-shell layered clusters: melting of Cu1Au54 and Cu12Au43. Phys Rev B 74:064117
Sankaranarayanan SK, Bhethanabotla VR, Joseph B (2005) Molecular dynamics simulation study of the melting of Pd–Pt nanoclusters. Phys Rev B 71:195415
Rodrıguez-López J, Montejano-Carrizales J, José-Yacamán M (2003) Molecular dynamics study of bimetallic nanoparticles: the case of AuxCuy alloy clusters. Appl Surf Sci 219:56–63
Yin J, Shan S, Yang L, Mott D, Malis O, Petkov V, Cai F, Shan Ng M, Luo J, Chen BH (2012) Gold–copper nanoparticles: nanostructural evolution and bifunctional catalytic sites. Chem Mater 24:4662–4674
Taherkhani F, Akbarzadeh H, Abroshan H, Fortunelli A (2012) Dependence of self-diffusion coefficient, surface energy, on size, temperature, and Debye temperature on size for aluminum nanoclusters. Fluid Phase Equilib 335:26–31
Huang S-P, Balbuena PB (2002) Melting of bimetallic Cu–Ni nanoclusters. J Phys Chem B 106:7225–7236
Taherkhani F, Rezania H (2012) Temperature and size dependency of thermal conductivity of aluminum nanocluster. J Nanopart Res 14:1–8
Negreiros F, Taherkhani F, Parsafar G, Fortunelli A (2012) Kinetics of chemical ordering in a Ag–Pt nanoalloy particle via first-principles simulations. J Chem Phys 137:194302
Mejía-Rosales SJ, Fernández-Navarro C, Pérez-Tijerina E, Montejano-Carrizales JM, José-Yacamán M (2006) Two-stage melting of Au–Pd nanoparticles. J Phys Chem B 110:12884–12889
Mehrer H, Eggersmann M, Gude A, Salamon M, Sepiol B (1997) Diffusion in intermetallic phases of the Fe–Al and Fe–Si systems. Mater Sci Eng A 239:889–898
Holzapfel C, Chakraborty S, Rubie D, Frost D (2009) Fe–Mg interdiffusion in wadsleyite: the role of pressure, temperature and composition and the magnitude of jump in diffusion rates at the 410 km discontinuity. Phys Earth Planet Inter 172:28–33
Lott K, Nirk T, Volobujeva O (2002) Chemical self-diffusion in undoped ZnS and in undoped CdSe. Cryst Eng 5:147–153
Palagin D, Doye PK (2014) Ni-based nanoalloys: towards thermally stable highly magnetic materials. J Chem Phys 141:214302
Smith W, Todorov IT (2006) A short description of DL_POLY. Mol Simul 32:935–943
Qi Y, Çağın T, Kimura Y, Goddard WA III (1999) Molecular-dynamics simulations of glass formation and crystallization in binary liquid metals: Cu–Ag and Cu–Ni. Phys Rev B 59:3527–3533
Qi Y, Çağin T, Kimura Y, Goddard WA III (2001) Viscosities of liquid metal alloys from non-equilibrium molecular dynamics. J Comput Aided Mater 8:233–243
Özdemir KS, Tomak M, Uludoğan M, Çağın T (2004) Liquid properties of Pd–Ni alloys. J Non-Cryst Solids 337:101–108
Cleri F, Rosato V (1993) Tight-binding potentials for transition metals and alloys. Phys Rev B 48:22
Kart H, Tomak M, Çağin T (2005) Thermal and mechanical properties of Cu–Au intermetallic alloys. Model Simul Mater Sci 13:657–669
Lv YJ, Chen M (2011) Thermophysical properties of undercooled alloys: an overview of the molecular simulation approaches. Int J Mol Sci 12:278–316
Akbarzadeh H, Yaghoubi H, Shamkhali AN, Taherkhani F (2013) Effects of gas adsorption on the graphite-supported Ag nanoclusters: a molecular dynamics study. J Phys Chem C 117:26287–26294
Akbarzadeh H, Yaghoubi H, Shamkhali AN, Taherkhani F (2014) CO adsorption on Ag nanoclusters supported on carbon nanotube: a molecular dynamics study. J Phys Chem C 118:9187–9195
Taherkhani F, Freshteh Seresht P (2015) Doping effect on the Janus-like structure of a copper–iron bimetallic nanocluster and its solid–liquid phase transition. PTEP 4:043I01, 1–12
Taherkhani F, Parviz Z, Akbarzadeh H, Fortunelli A (2015) Temperature and doping effect on thermal conductivity of copper–gold icosahedral bimetallic nanoclusters and bulk structures. J Phys Chem C 119:7922–7932
Ala-Nissila T, Ferrando R, Ying S (2002) Collective and single particle diffusion on surfaces. Adv Phys 51:949–1078
Taherkhani F, Negreiros FR, Parsafar G, Fortunelli A (2010) Simulation of vacancy diffusion in a silver nanocluster. Chem Phys Lett 498:312–316
Taherkhani F, Akbarzadeh H, Rezania H (2014) Chemical ordering effect on melting temperature, surface energy of copper–gold bimetallic nanocluster. J Alloy Compd 617:746–750
Akbarzadeh H, Taherkhani F (2013) Cluster size dependence of surface energy of Ni nanocluster: a molecular dynamics study. Chem Phys Lett 558:57–61
Heinonen V, Achim CV, Elder KR, Buyukdagli S, Ala-Nissila T (2014) Phase-field-crystal models and mechanical equilibrium. Phys Rev E 89:032411
Laasonen K, Panizon E, Bochicchio D, Ferrando R (2013) Competition between icosahedral motifs in AgCu, AgNi, and AgCo nanoalloys: a combined atomistic−DFT study. J Phys Chem C 117:26405–26413
Alavi S, Thompson DL (2006) Molecular dynamics simulations of the melting of aluminum nanoparticles. J Phys Chem A 110:1518–1523
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Taherkhani, F. (2016). Doping Effect on Melting Temperature for Gold–Copper Bimetallic Nanocluster and Dependency on Bulk Melting Temperature to the Potential Model. In: Ramasami, P., Gupta Bhowon, M., Jhaumeer Laulloo, S., Li Kam Wah, H. (eds) Crystallizing Ideas – The Role of Chemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-31759-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-31759-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31758-8
Online ISBN: 978-3-319-31759-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)