Abstract
Tuber species in the Puberulum group sensu lato (s.l.) produce small and light-colored ascomata with alveolate-reticulated ascospores. Members of this group are commonly called “whitish truffles”. Puberulum group s.l. is the most widely distributed group, has the highest species richness within Tuber genus, and includes commercially valuable species which are becoming increasingly popular in the marketplace. This chapter aimed to investigate the phylogenetic relationships and the diversity within Puberulum group s.l. based on the recent findings and the screening of the internal transcribed spacer (ITS) rDNA sequences available in GenBank database. We attempted to select an ITS reference sequence and, consequently, to assess the current extent of misidentified entries for each whitish truffle species. Further, we reported the geographical distribution and intraspecific variability of each member of the Puberulum group s.l. as well as the description of mycorrhizas formed by this group of fungi.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Tuber species in the Puberulum group sensu lato (s.l.) produce small and light-colored ascomata with alveolate-reticulated ascospores. Members of this group are commonly called “whitish truffles” (Zambonelli et al. 2000a), in order to distinguish them from the most precious species Tuber magnatum Pico (the Italian white truffle ). Puberulum group s.l. is the most widely distributed group and has the highest species richness within Tuber genus (Bonito et al. 2010a). Its members have been found all over the Northern Hemisphere associated with angiosperm and/or gymnosperm in a wide range of habitats: from the northern boreal forests of Europe and North America to the semiarid environments of Mexico and North Africa. Moreover, unclassified members of the Puberulum group were also found as ectomycorrhizas (ECMs) in the Southern Hemisphere (Bonito et al. 2010b). The Puberulum group s.l. includes commercially valuable species which are becoming increasingly popular in the marketplace. The most noteworthy species is Tuber borchii Vittad. (“bianchetto” truffle) which, actually, is cultivated outside its native areas and the extent of its plantations is increasing around the world. Actually, it is successfully cultivated in New Zealand and Australia, and, more recently, it has also been introduced in the USA (Zambonelli et al. 2015). Tuber gibbosum Harkn. (Oregon spring white truffle) and Tuber oregonense Trappe, Bonito and P. Rawl. (Oregon winter white truffle) are well appreciated in North America, but the efforts to cultivate them are in their infancy (Lefevre 2012). The potential culinary value of all the other whitish truffles has to be investigated yet.
The term “Puberulum” was used for the first time by Knapp (1950) to group together the Tuber species forming ascomata with smooth, papillate or rough surface, soft texture, and globose asci bearing an early-disappearing stalk and reticulate ascospores. Furthermore, the author define a Puberulum group A including nine species ( Tuber puberulum Berk. & Broome, Tuber albidum Pico, Tuber michailowskianum Bucholtz, Tuber rapaeodorum Tul. & C. Tul., T. borchii, Tuber maculatum Vittad., Tuber mougeotii Quél., Tuber asa-foetida Lesp., and Tuber lacunosum Mattir.) with small-meshed (<10 μm) and regularly reticulate ascospores and a Puberulum group B consisting of three species ( Tuber dryophilum Tul. & C. Tul., Tuber foetidum Vittad., and T. magnatum ) with large-meshed (>10 μm) and irregularly reticulate ascospores. Thereafter, taxonomic relationships of the whitish truffle species have been debated for decades by many other European mycologists. Based on the spore dimension and morphology, Gross (1987) identified a Puberulum “cluster” within the white truffle species with small-sized ascomata, which included T. borchii var. sphaerosperma Malençon (=T. puberulum var. c), T. puberulum (=var. b), Tuber murinum R. Hesse (=T. puberulum var. a), Tuber exiguum R. Hesse, and T. borchii. Montecchi and Lazzari (1987) reported a redundant increase of species within the Puberulum group and accepted as valid only T. borchii, T. rapaeodorum, T. dryophylum, T. maculatum, T. puberulum, and T. foetidum. As time passes, the validity of some of these species was questioned, and new European species were included within the Puberulum group such as Tuber oligospermum (Tul. & C. Tul.) Trappe (Riousset et al. 2001) and Tuber cistophilum P. Alvarado, G. Moreno, Manjón, Gelpi & J. Muñoz (Alvarado et al. 2012).
The recent studies on molecular phylogeny of truffles have significantly improved the taxonomy of Puberulum group s.l., unraveling most of the phylogenetic affiliations within it. For example, Jeandroz et al. (2008) demonstrated that T. magnatum is phylogenetically well differentiated from the whitish truffles and belongs to the Aestivum group. On the contrary, many Asiatic and American species have been recently added to the Puberulum group s.l. (Wang et al. 2007, 2013; Jeandroz et al. 2008; Bonito et al. 2010a; Guevara et al. 2013).
Based on recent insight, we can consider as whitish truffles the Tuber species forming fruiting bodies with the following general features (Fig. 7.1):
Ascomata: hypogeous, small (commonly <5 cm in diameter), solid, and firm; globose or subglobose, sometimes lobed; whitish at first becoming pale yellowish to yellow-brown or reddish-brown; surface smooth or finely pubescent, rarely with minute and flat warts (T. foetidum)
Peridium: pseudoparenchymatous or plectenchymatous; yellow or darker toward the surface, otherwise white/hyaline
Dermatocystidia: cylindric or needle with septa when present; hyaline or yellowish
Gleba: whitish at first becoming grayish-brown to yellowish-brown, marbled with branching white veins arising from different points on the peridium
Asci: globose to ovate with 1–4 (−5) spores, thin-walled, sessile or short stipitate
Ascospores: hyaline at first becoming yellow-brown or reddish-brown at maturity, bearing an alveolate reticulum 3–6 μm deep
The large amount of molecular data deposited in the public sequence repositories have represented a great resource to study the relationships among whitish truffle species but their analysis also revealed inconsistencies and contradictions. Indeed, part of the misidentified entries have been generated from the difficulties in identifying the whitish truffle ascomata because only few taxonomic informative traits of peridium and spores are available for species identification. However, most of the conflicting entries are from European species described before the twentieth century because the poor quality of the original descriptions led to disagreement among taxonomists and a different application of the species concepts (Jeandroz et al. 2008; Zambonelli et al. 2012). Then, the poor quality of the oldest holotypes in herbarium collections made often difficult to obtain reference DNA sequences useful to solve these controversies. Considering also that Puberulum group s.l. contains the highest number of insufficiently identified taxa and most of the diversity within Tuber genus (Bonito et al. 2010a), it remains the most controversial group of truffle species.
This chapter aimed to investigate the phylogenetic relationships and the diversity within Puberulum group s.l. based on the recent findings and the screening of the internal transcribed spacer (ITS) rDNA sequences available in GenBank database. We attempted to select an ITS reference sequence and, consequently, to assess the current extent of misidentified entries for each whitish truffle species. Finally, we reported the geographical distribution and intraspecific variability of each member of the Puberulum group s.l. as well as the description of mycorrhizas formed by these fungi.
2 Phylogeny of the Puberulum Group s.l.
Halász et al. (2005) were the first to explore the phylogenetic relationships within Puberulum group , validating the distinction among T. rapaeodorum, T. foetidum, T. maculatum, T. borchii, and T. puberulum. Wang et al. (2007) and Jeandroz et al. (2008) sorted members of this group in four subclades, including new species from Europe, Asia, and North America. Later, Bonito and colleagues (2010a) separated the whitish truffle species in two different clades, Puberulum and Maculatum , accounting for 37 (25 undescribed) and 19 (12 undescribed) species, respectively, all around the world. A third closely related lineage, the Gibbosum clade , was identified by only four species from North America. More recently, this latter phylogenetic reconstruction was also confirmed by the analysis of three different individual loci (28S large subunit rDNA, elongation factor 1-alpha, RNA polymerase II) in addition to the common ITS rDNA-based phylogeny, and it was estimated that the most recent common ancestors of Puberulum, Maculatum, and Gibbosum clades diverged 65, 67, and 27 Mya, respectively (Bonito et al. 2013).
Actually (date of accession 18 January 2015), about 800 ITS1-5.8S-ITS2 rDNA complete sequences (>400 bp) attributable to the Puberulum group s.l. are deposited in GenBank. To retrieve all the sequences (identified and insufficiently identified) belonging to this group of truffles, three reference sequences were selected for each clade (Puberulum, Maculatum, and Gibbosum) and BLAST against GenBank nucleotide database adjusting to 1000 the “max target sequences” parameter. The output sequences of each clade were aligned together by Muscle in MEGA6 (http://www.megasoftware.net/, Tamura et al. 2013), and those of poor quality and short length as well as the redundant entries were removed by the datasets. A provisional neighbor-joining tree was then generated for each of the three dataset for identifying and removing the sequences belonging to other Tuber lineages after Bonito et al. (2010a). We assumed 97 % ITS sequence similarity as threshold for phylotype definition and species approximation. Phylotypes were defined based on a similarity matrix constructed using MEGA6. Sequences with a p-distance < 3 % were grouped into the same phylotype. The 3 % is commonly accepted as global cutoff values for intraspecific variation in fungi (Nilsson et al. 2008; Smith et al. 2007) and in Tuber spp. (Bonito et al. 2013) although it is unable to discriminate cryptic species of whitish truffles (Bonuso et al. 2010; Wang et al. 2013). For some species of the Maculatum clade , we adopted a more stringent threshold for species definition after the work of Guevara et al. (2013) on North American truffles. We defined a reference ITS sequence for each described species of the Puberulum group s.l. according to one of the following criteria (listed in order of priority) (Table 7.1, second column): (1) sequences from holotypes considered by the “ITS RefSeq Target Loci project” (Schoch et al. 2014); (2) sequences from holotypes characterized in various scientific works; and (3) sequences from specimens in which characters are congruent with those reported by Pegler et al. (1993), Astier (1998), Zambonelli et al. (2000b), Montecchi and Sarasini (2000), Riousset et al. (2001), and Ceruti et al. (2003).
The validity of the reference sequences selected for T. borchii and T. maculatum is supported by the analysis carried out by Mello et al. (2000) which amplified some neotypes of “Vittadini original” collections with the species-specific primer pairs TBA–TBB (Mello et al. 1999) and TmacI–TmacII (Amicucci et al. 1998), respectively.
The ITS sequence divergence of each phylotype was measured by p-distance in MEGA6, using either the correctly identified or misidentified sequences as well as the unidentified sequences. Mean and max values of p-distance are reported in Table 7.1.
It has not been possible to assign reference ITS sequences to T. asa and T. sphaerospermum because, in our opinion, their genetic and morphological identity is still controversial. All sequences from GenBank identified as T. asa (1) and T. sphaerospermum (5) clustered within our T. oligospermum phylotype (<3 % diversity).
T. asa was considered synonym of T. oligospermum by Alvarado et al. (2012), but they were regarded as true species by other authors which, instead, considered T. lacunosum synonym of T. asa (Ceruti 1960; Montecchi and Sarasini 2000; Riousset et al. 2001; Ceruti et al. 2003).
Tuber sphaerospermum has been promoted to the rank of species only recently by Roux (2006) but it was firstly identified as T. borchii var. sphaerosperma by Malençon (1973). The original description of its ascomata reported regularly spherical spores and a pseudoparenchymatous peridium. Tuber oligospermum shows the same spore morphology but a plectenchimatous peridium as described by Ceruti et al. (2003) who examined a syntype of the Mattirolo herbarium.
No ITS sequences are available in GenBank for the American truffle species Tuber guzmanii Trappe and Cázares, Tuber irradians Gilkey, and Tuber levissimum Gilkey which were included within the Puberulum group by Bonito et al. (2010a) based on the sequence of their LSU rDNA gene.
3 Unreliability of the ITS Accessions and Unknown Species
Several studies have questioned the taxonomic reliability of the entries in public sequence databases, demonstrating that more than 10 % of the identified ITS sequences from fungi have been incorrectly annotated (Bridge et al. 2003; Nilsson et al. 2006). Despite the efforts recently made by the scientific community to improve the quality and the interpretation of database submissions (Tedersoo et al. 2011; Nilsson et al. 2012), compromised annotations still hamper the correct interpretation of the molecular data of some fungal taxa. Among these, whitish truffles account for a significant number of incorrect annotations.
GenBank ambiguities have been revealed in two different ways with respect to each reference entry: (1) entries with the same ITS sequence but differently named (Table 7.1, column 6) and (2) entries with the same name but with ITS sequence clustering in a different phylotype (Table 7.1, column 8). According to our criteria of analysis, we found 83 ambiguous entries (31 % of total identified sequences belonging to the Puberulum group s.l.) in the first case and 127 ambiguous entries (47 % of total) in the second case. Erroneous annotations were found in 10 out of 38 described whitish truffle species represented by at least one GenBank entry (Table 7.1). The highest number of misidentified entries has been found for T. puberulum, T. maculatum, T. oligospermum, T. rapaeodorum, T. dryophilum, and T. borchii (Table 7.1). More than 75 % of the sequences identified as T. puberulum (31 out of 39), T. oligospermum (28 out of 36), and T. rapaeodorum (22 out of 25) fall into other phylotypes in addition to that of the respective reference sequence (Table 7.1, columns 4 and 8). On the contrary, T. maculatum and T. dryophilum show the highest number of sequences (24 and 23, respectively) erroneously accessioned under other whitish truffle species (Table 7.1, column 6).
The reliability of the recent described Asiatic species Tuber polyspermum (Fan et al. 2011a) and Tuber microsphaerosporum (Fan et al. 2012a) must be verified because they are both represented by a single ITS sequence clustering within the Tuber lijiangense L. Fan and J. Z. Cao phylotype. Likewise, the unique sequence identified as T. murinum (JF261371) is similar to those of the Tuber beyerlei Trappe, Bonito, and Guevara holotype. The validity of T. murinum was debated for a long time, and, recently, it has been ignored by taxonomists (Ceruti et al. 2003).
Also the number of insufficiently identified sequences attributable to described species of the Puberulum group s.l. is remarkable. In fact, 42 % (249 out of 594) of the sequences of these species are mainly from ECMs or soil clones with the highest values registered in T. maculatum (53 out of 90) and Tuber anniae W. Colgan & Trappe (29 out of 46).
Based on the sequences from mycorrhiza, soil clone and unidentified ascomata deposited in GenBank, Bonito et al. (2010a) added 25 and 12 putative undescribed species to the Puberulum and Maculatum clades , respectively. A number of these unknown phylotypes have been recently identified and their ascomata characterized (Guevara et al. 2013). Our analysis found a total of 53 unidentified phylotypes in the Puberulum (43) and Maculatum (10) groups, whereas no unidentified species were revealed for the Gibbosum group. Most of these unidentified phylotypes appeared only recently in GenBank (later than Bonito et al. 2010a), and 18 out of 53 are only represented by one sequence.
4 Phylogeography
Whitish truffles represent the most widely distributed group of species in the world although many of them appear to have a native distribution range restricted to continental or subcontinental scale. Indeed, truffle cultivation, forestry, and nursery trade have contributed to introduce a number of whitish truffle species into novel habitats (Barroetaveña et al. 2005; Hall et al. 2007; Bulman et al. 2010; Wang et al. 2013). However, to date, the number of species shared between continents is still low (six for both Puberulum and Maculatum groups).
4.1 Puberulum Clade
Puberulum clade is the most abundant and ubiquitous clade of whitish truffle species, with members distributed across Europe, Asia, North America, South America, and northern Africa (Fig. 7.2). A new unidentified species belonging to the Puberulum group was also found in Argentina analyzing ITS sequences from ECMs of Nothofagus spp. and Salix humboldtiana Willd. natural stands (Bonito et al. 2013). Based on the sequence metadata, all described species from Asia and America have been found only in China (11 species), the USA ( Tuber californicum Harkn.; Tuber pacificum Trappe, Castellano, and Bushnell; Tuber sphaerosporum Gilkey), or both the USA and Mexico ( Tuber separans Gilkey). However, the number of entries attributable to these species is probably still too low to determine their entire range of distribution. On the contrary, four out of five species are widespread in Europe and have been also found in Iran (T. dryophilum and T. puberulum) and Morocco (T. oligospermum) or are cultivated around the world (T. borchii). According to the current metadata, T. cistophilum is only present in the Iberian Peninsula, and as T. oligospermum, it might be a species confined to the Western Mediterranean areas, with a preference for acid soils (Alvarado et al. 2012). The higher intraspecific diversity within T. oligospermum than T. cistophilum is probably due to the different range of collection sites. Tuber oligospermum has also been found outside the Iberian Peninsula, and its ITS sequences are grouped in two subclades, the first distributed in Morocco, Portugal, and Spain and the second in Sardinia (Italy) and Spain. On the contrary to the latter two species, the phylotype identified by us as T. puberulum almost grows in the northern temperate areas of middle Europe, from France and England to Poland and Czech Republic. To date, no molecular evidence of this species in Italian and Iberian Peninsulas as well as in the south Balkans or Scandinavia has been found.
Neighbor-joining tree (p-distance) of described and undescribed species of Puberulum clade. Accessions without the species name are referred to undescribed Tuber species. The bootstrap consensus tree was inferred from 100 replicates. Branches corresponding to partitions reproduced in less than 50 % bootstrap replicates are collapsed. Evolutionary analyses were conducted in MEGA6. Am America, N-Am North America, SAm South America, EAs East Asia, SAs South Asia, WAs West Asia, Eu Europe, NEu North Europe, SEu South Europe, CEu Central Europe, EEu East Europe
Tuber borchii and T. dryophilum are ubiquitous in Europe and share the same distribution range and, often, also the same habitat (Iotti et al. 2010). Both species grow in cold-temperate to Mediterranean regions, from Iberian to Balkan Peninsulas (Hall et al. 2007). They are commonly found in calcareous sub-alkaline soils but can also colonize host species in acid soils (Peintner et al. 2007; Lancellotti and Franceschini 2013). ITS sequences of T. borchii are also from nonnative countries (the USA, British Columbia, New Zealand) probably due to increased cultivation of this truffle or introduced host plants. Tuber borchii is the whitish truffle mostly represented in GenBank with more than 120 entries, and our analysis revealed highest diversity within Puberulum group s.l. Its ITS variation is distributed between two main subclades, considered as cryptic species by Bonuso et al. (2010). Collections of the first group (clade I of Bonuso’s work) are distributed mainly throughout Italy, whereas the second group (clade II) is ubiquitous in Europe. A significant genetic diversity was also found in T. dryophilum and the analysis of ITS sequence from 44 entries distinguished 6 subclades without any geographic structure. However, most of the diversity was found in Italy which might represent the area of diversification for this species.
Tuber anniae is the species of the Puberulum group with the largest geographical distribution. Its ascomata and ECMs have been mainly found in the coldest region of the Northern Hemisphere, but there is evidence for introduction of this species also in New Zealand (Bulman et al. 2010) and Hawaii (Hynson et al. 2013). Wang et al. (2013) considered T. anniae a species complex composed by three distinct clades not supported by distinct morphological differences of their ascomata. Members of the clade I have been only found in North America, whereas clades II and III represent geographically disjunct phylotypes, which both include sequences from Europe and North America (Wang et al. 2013). Tuber anniae has been recently found also in northern Japan (Hashimoto et al. 2012) to form symbiosis with Betula ermanii Cham., a tree species widespread also in Russian, Korea, and China (Li and Skvortsov 1999). So, this species complex could have a wider distribution range than previously supposed.
With respect to Bonito et al. (2010a), we found 23 additional unidentified phylotypes within Puberulum clade, mostly from East Asia. Two unidentified phylotypes (Tuber sp. 33 and Tuber sp. 29 from Bonito’s work) have been recently described as Tuber pseudosphaerosporum L. Fan (Fan and Yue 2013) and T. lijiangense (Fan et al. 2011b).
4.2 Maculatum Clade
Maculatum clade includes ten described species found exclusively in North America ( Tuber shearii Harkn.; Tuber lauryi Trappe, Bonito & Guevara; Tuber whetstonense J. L. Frank, D. Southw. & Trappe; Tuber walkeri Healy, Bonito & Guevara; T. beyerlei ; Tuber guevarai Bonito & Trappe; Tuber castilloi Guevara, Bonito & Trappe; Tuber miquihuanense Guevara, Bonito & Cázares; Tuber mexiusanum Guevara, Bonito & Cázares, and Tuber linsdalei Gilkey) and four described species from Europe (T. maculatum , Tuber scruposum R. Hesse, T. rapaeodorum , T. foetidum ) rarely found also in Asia (Fig. 7.3). In general, intraspecific ITS diversity of European species is lower than American species.
Neighbor-joining tree (p-distance) of described and undescribed species of Maculatum clade. Accessions without the species name are referred to undescribed Tuber species. The bootstrap consensus tree was inferred from 100 replicates. Branches corresponding to partitions reproduced in less than 50 % bootstrap replicates are collapsed. Evolutionary analyses were conducted in MEGA6. N-Am North America, As Asia, EAs East Asia, SAs South Asia, WAs West Asia, Eu Europe, NEu North Europe, SEu South Europe, EEu East Europe
Within Maculatum group, T. maculatum was the phylotype with the highest number of ITS entries which are grouped in six main subclades. Members of these subclades share a similar geographic range of distribution, from the Netherlands to Iran and from Italy to Finland. A unique sequence from China forms an additional basal subclade which might represent a native Asiatic cryptic species of T. maculatum (GU134516). Our analysis revealed also the introduction of T. maculatum in Canada, the USA, and New Zealand.
Tuber maculatum ascomata can be easily confused with those of T. rapaeodorum and our analysis confirmed the taxonomic difficulties in distinguishing between these species (Table 7.1). According on our criteria for species selection, T. rapaeodorum should be only present in East Europe and South Caspian region.
Tuber scruposum was found in the same areas where T. rapaeodorum grow as well as in central Europe (Italy, Germany, and Austria). Its ITS sequences are grouped in two subclades which do not show any geographic structure. The reference sequence selected for T. scruposum correspond to the Tuber sp. 37 (scruposum 1) phylotype after Bonito et al. (2010a) which is phylogenetically distinct from Tuber sp. 40 (scruposum 2), found both in North America and Europe. ITS diversity between these two phylotypes (>7 %) does not seem supported by evident differences in morphology of their ascomata. In fact, Badalyan et al. (2005) classified as T. scruposum the ascomata of both species collected in an oak-hornbeam forest near Dilijan (Armenia).
With respect to Bonito et al. (2010a), our analysis revealed only one new unidentified phylotype from ECMs collected in a T. magnatum truffle ground located in South Italy (Leonardi et al. 2013). Two unidentified phylotypes (Tuber sp. 39 and Tuber sp. 45 from Bonito’s work) and other four new species have been recently described by Guevara et al. (2013).
4.3 Gibbosum Clade
Gibbosum clade includes only four described species (T. gibbosum , Tuber bellisporum Bonito & Trappe, Tuber castellanoi Bonito & Trappe, and T. oregonense ). All 34 analyzed ITS sequences have been obtained by ascomata or ECMs collected in western North America. Montecchi and Sarasini (2000) and Pomarico et al. (2007) reported T. gibbosum ascomata from Emilia-Romagna (northern Italy) and Basilicata (Southern Italy), respectively, but evidences for these collections are not available in GenBank database. This truffle species was probably introduced in Italy together with Pseudotsuga menziesii (Mirb.) Franco, a nonnative European tree present in both collection sites. Species of the Gibbosum clade are characterized by a low intraspecific ITS variation (Table 7.1), and unlike the other Tuber lineages, they appear to be exclusively associated with Pinaceae hosts (Bonito et al. 2010b; Bonito et al. 2013). Five years after Bonito et al. (2010a), also our analysis did not reveal unidentified phylotypes within Gibbosum clade.
5 Mycorrhizas
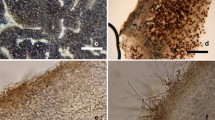
Many species of the Puberulum group s.l. have been proven to form ECMs with a wide range of host plants either in greenhouse or in the field. Mycorrhizas of T. borchii have been synthesized and described on a wide range of host plants: Pinus strobus L. (Scannerini and Palenzona 1967), Pinus pinea L. (Zambonelli et al. 1995), Pinus radiata D. Don (Duñabeitia et al. 1996), Quercus robur L. (Fontana et al. 1992; Boutahir et al. 2013), Quercus pubescens Willd. (Zambonelli et al. 1993), Quercus suber L. (Zambonelli and Branzanti 1989), Tilia platyphyllos Scop. (Granetti et al. 1995; Sisti et al. 1998), Corylus avellana L. (Zambonelli and Branzanti 1984; Fontana et al. 1992; Rauscher et al. 1996), Populus sp. (Fontana and Palenzona 1969), Castanea sativa Miller (Zambonelli and Branzanti 1989), Alnus cordata (Loisel.) Desf. (Zambonelli and Branzanti 1989), Carya illinoinensis (Wangenh.) K. Koch (Benucci et al. 2012), and Arbutus unedo L. (Lancellotti et al. 2014). ECMs of T. puberulum on Picea abies Karst. (Blaschke 1988), T. maculatum on Ostrya carpinifolia Scop. (Zambonelli et al. 1999), T. oligospermum on Quercus cerris L. (Bencivenga et al. 1997), and Q. robur (Boutahir et al. 2013) have also been synthesized. In natural condition whitish truffles have been found in association with a broad host range, including both gymnosperm and angiosperm (Kovács and Jakucs 2006; Iotti et al. 2010; Leonardi et al. 2013). Whitish truffles are also susceptible to orchid colonization (Tešitelová et al. 2012).
ECMs established by the whitish truffle species share a common morphology and only little differences could be detected in some anatomical characters (Fig. 7.1). However, these differences are usually wide even within the same species and partially overlap between species of whitish truffles. Unramified ends are commonly club-shaped, straight, yellow ochre (younger tissues) to brown (older tissues), with a smooth or spiny surface. The mantel is pseudoparenchymatous with roundish to epidermoid cells, sometimes covered by a hyphal network, and the cystidia, when present, are needle-shaped, straight, or slightly bent, hyaline with a blunt tip and one basal septa (sometimes 2). Emanating hyphae are rare, septate, hyaline, without clamps, and not frequently ramified. Rhizomorphs have never been found.
Characters useful to discriminate ECMs of different whitish truffles can be only found in cystidia and mantle cells although the shape of mantle cells can slightly vary depending on the strain and the host plant (Giomaro et al. 2000). Kovács and Jakucs (2006) confirmed this statement analyzing ECMs formed by different species of the Puberulum group s.l. in natural conditions. For example, cystidia of T. oligospermum show a frequent basal inflation which can be used to discriminate ECMs of this species (Bencivenga et al. 1997; Boutahir et al. 2013). Tuber maculatum ECM differs from those of T. borchii for the shorter cystidia (Zambonelli et al. 1999).
6 Conclusions
The Puberulum group s.l. is the phylogenetic group inside the genus Tuber having the greatest genetic and species diversity, not supported by a similar variability in morphology of ascomata, ECM, or mycelia. Most of the whitish truffle species are difficult to differentiate morphologically and the number of cryptic species within this group is probably high. As a consequence, these species are often misidentified or insufficiently identified in GenBank, and, so, caution has to be exercised in interpreting the BLAST results for the molecular identification.
This context led to confusion among researchers who are concerned with the study of these truffles. Moreover, being some whitish truffles of high economic importance, this confusion may have negative effects on truffle market and cultivation. In fact, in many parts of Italy and throughout Europe, the precious species T. borchii is very often confused with other Tuber species in the Puberulum group s.l. such as T. maculatum, T. dryophilum, and T. puberulum which have poor culinary quality (Montecchi and Sarasini 2000). Misidentification of T. borchii ascomata may produce ecological and economic problems when alien or nontarget species are used to inoculate the seedlings for truffle cultivation.
We aim that reference sequences and morphological considerations reported in this chapter will help in research and practical studies on whitish truffles and will be a starting point for developing shared species concepts.
References
Alvarado P, Moreno G, Manjón JL (2012) Comparison between Tuber gennadii and T. oligospermum lineages reveals the existence of the new species T. cistophilum (Tuberaceae, Pezizales). Mycologia 104(4):894–910. doi:10.3852/11-254
Amicucci A, Zambonelli A, Giomaro G, Potenza L, Stocchi V (1998) Identification of ectomycorrhizal fungi of the genus Tuber by species-specific ITS primers. Mol Ecol 7(3):273–277. doi:10.1046/j.1365-294X.1998.00357.x
Astier J (1998) Truffes blanche et noires (Tuberaceae et Terfeziaceae). Louis-Jean, Gap
Badalyan SM, Hovsepyan RA, Iotti M, Zambonelli A (2005) On the presence of truffles in Armenia. Flora Medit 15:683–692
Barroetaveña C, Rajchenberg M, Cázares E (2005) Mycorrhizal fungi in Pinus ponderosa introduced in central Patagonia (Argentina). Nova Hedwigia 80(3–4):453–464. doi:10.1127/0029-5035/2005/0080-0453
Bencivenga M, Tanfulli M, Donnini D (1997) Micorrizazione di Quercus cerris L. con Tuber oligospermum (Tul. et Tul.) Trappe. Micol Ital 26(3):89–93
Benucci GMN, Bonito G, Baciarelli Falini L, Bencivenga M (2012) Mycorrhization of pecan trees (Carya illinoinensis) with commercial truffle species: Tuber aestivum Vittad. and Tuber borchii Vittad. Mycorrhiza 22(5):383–392. doi:10.1007/s00572-011-0413-z
Berkeley MJ, Broome CE (1846) Notices of British hypogaeous fungi. Ann Mag Nat Hist 18:73–82
Blaschke H (1988) Tuber puberulum. In: Agerer R (ed) Colour Atlas of ectomycorrhizae. Einhorn, Schwabisch Gmund, p 22
Bonito GM, Gryganskyi AP, Trappe JM, Vilgalys R (2010a) A global meta-analysis of Tuber ITS rDNA sequences: species diversity, host associations and long-distance dispersal. Mol Ecol 19(22):4994–5008. doi:10.1111/j.1365-294X.2010.04855.x
Bonito G, Trappe JM, Rawlinson P, Vilgalys R (2010b) Improved resolution of major clades within Tuber and taxonomy of species within the Tuber gibbosum complex. Mycologia 102(5):1042–1057. doi:10.3852/09-213
Bonito G, Smith ME, Nowak M, Healy RA, Guevara G, Cazares E, Kinoshita A, Nouhra ER, Dominguez LS, Tedersoo L, Murat C, Wang Y, Moreno BA, Pfister DH, Nara K, Zambonelli A, Trappe JM, Vilgalys R (2013) Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage. PLoS One 8(1), e52765. doi:10.1371/journal.pone.0052765
Bonuso E, Zambonelli A, Bergemann SE, Iotti M, Garbelotto M (2010) Multilocus phylogenetic and coalescent analyses identify two cryptic species in the Italian bianchetto truffle, Tuber borchii Vittad. Conserv Genet 11(4):1453–1466. doi:10.1007/s10592-009-9972-3
Boutahir S, Iotti M, Piattoni F, Zambonelli A (2013) Morphological and molecular characterization of Tuber oligospermum mycorrhizas. Afr J Agric Res 8(29):4081–4087. doi:10.5897/AJAR2013.7354
Bridge PD, Roberts PJ, Spooner BM, Panchal G (2003) On the unreliability of published DNA sequences. New Phytol 160(1):43–48. doi:10.1046/j.1469-8137.2003.00861.x
Bulman SR, Visnovsky SB, Hall IR, Guerin-Laguette A, Wang Y (2010) Molecular and morphological identification of truffle-producing Tuber species in New Zealand. Mycol Prog 9(2):205–214. doi:10.1007/s11557-009-0626-0
Ceruti A (1960) Elaphomycetales et tuberales. In: Bresadola J (ed) Iconographia mycologica, vol 28 (Suppl 2). Trento
Ceruti A, Fontana A, Nosenzo C (2003) Le specie Europee del genere Tuber, una revisione storica. Museo Regionale di Scienze Naturali, Monographie XXXVII. Regione Piemonte, Torino
Chen J, Liu PG (2007) Tuber latisporum sp. nov. and related taxa, based on morphology and DNA sequence data. Mycologia 99(3):475–481. doi:10.3852/mycologia.99.3.475
Colgan W, Trappe JM (1997) NATS tuffle and truffle-like fungi 7: Tuber anniae sp. nov. (Ascomycota). Mycotaxon 64:437–441. doi:10.1007/s11557-012-0862-6
Deng XJ, Liu PG, Liu CY, Wang Y (2013) A new white truffle species, Tuber panzhihuanense from China. Mycol Prog 12(3):557–561. doi:10.1007/s11557-012-0862-6
Duñabeitia MK, Hormilla S, Salcedo I, Peña JI (1996) Ectomycorrhizae synthesized between Pinus radiata and eight fungi associated with Pinus spp. Mycologia 88(6):897–908. doi:10.2307/3761052
Fan L, Cao JZ (2012) Two new species of white truffle from China. Mycotaxon 121:297–304. doi:10.5248/121.297
Fan L, Yue SF (2013) Phylogenetic divergence of three morphologically similar truffles: Tuber sphaerosporum, T. sinosphaerosporum, and T. pseudosphaerosporum sp. nov. Mycotaxon 125:283–288. doi:10.5248/125.283
Fan L, Hou CL, Cao JZ (2011a) Tuber sinoalbidum and T. polyspermum—new species from China. Mycotaxon 118:403–410. doi:10.5248/118.403
Fan L, Cao JZ, Liu Y, Li Y (2011b) Two new species of the genus Tuber from China. Mycotaxon 116:349–354. doi:10.5248/116.349
Fan L, Cao JZ, Li Y (2012a) Tuber microsphaerosporum and Paradoxa sinensis spp. nov. Mycotaxon 120:471–475. doi:10.5248/120.471
Fan L, Hou C, Li Y (2012b) Tuber microverrucosum and T. huizeanum – two new species from China with reticolate ascospores. Mycotaxon 122:161–169. doi:10.5248/122.161
Fan L, Cao JZ, Li Y (2012c) Tuber sinosphaerosporum sp. nov. from China. Mycotaxon 122:347–353. doi:10.5248/122.347
Fontana A, Palenzona M (1969) Sintesi micorrizica di Tuber albidum in coltura pura con Pinus strobus e pioppo euroamericano. Allionia 15:99–104
Fontana A, Ceruti A, Meotto F (1992) Criteri istologici per il riconoscimento delle micorrize di Tuber albidum. Micol Veg Medit 7(1):121–136
Frank JL, Southworth D, Trappe JM (2006) NATS truffle and truffle-like fungi 13: Tuber quercicola and T. whetstonense, new species from Oregon, and T. candidum redescribed. Mycotaxon 95:229–240
Gilkey HM (1916) A revision of the Tuberales of California. Univ Calif Publ Bot 6:275–356
Gilkey HM (1939) Tuberales of North America. Or State Monogr Stud Bot 1:1–63
Gilkey HM (1954) Tuberales. N Am Flora 1:1–36
Giomaro G, Zambonelli A, Cecchini D, Stocchi V, Saffi V (2000) Anatomical and morphological characterization of mycorrhizas of five strains of Tuber borchii Vittad. Mycorrhiza 10(3):107–114. doi:10.1007/s005720000065
Granetti B, Angelini P, Rubini A (1995) Morfologia e struttura delle micorrize di Tuber magnatum Pico e di Tuber borchii Vitt. con Tilia platyphyllos. Micol Ital 24(2):27–34
Gross G (1987) Zu den europäischen Sippen der Gattung Tuber. In: Derbsch H, Schmitt JA (eds) Atlas der Pilze des Saarlandes, Teil 2, Nachweise, Okologie, Vorkommen und Beschreibungen. Derlattinia, Saarbruken, pp 79–99
Guevara G, Bonito G, Trappe JM, Cázares E, Williams G, Healy RA, Schadt C, Vilgalys R (2013) New North American truffles (Tuber spp.) and their ectomycorrhizal associations. Mycologia 105(1):194–209. doi:10.3852/12-087
Halász K, Bratek Z, Szego D, Rudnoy S, Racz I, Lasztity D, Trappe JM (2005) Tests of species concepts of the small, white, European group of Tuber spp based on morphology and rDNA ITS sequences with special reference to Tuber rapaeodorum. Mycol Prog 4(4):281–290. doi:10.1007/s11557-006-0132-6
Hall IR, Brown GT, Zambonelli A (2007) Taming the truffle: the history, lore and science of the ultimate mushroom. Timber Press, Portland
Harkness HW (1899) Californian hypogeous fungi. Proc Calif Acad Sci 3(1):241–292
Hashimoto Y, Fukukawa S, Kunishi A, Suga H, Richard F, Sauve M, Selosse MA (2012) Mycoheterotrophic germination of Pyrola asarifolia dust seeds reveals convergences with germination in orchids. New Phytol 195(3):620–630. doi:10.1111/j.1469-8137.2012.04174.x
He XY, Li HM, Wang Y (2004) Tuber zhongdianense sp. nov. from China. Mycotaxon 90(1):213–216
Hesse R (1891) Die Hypogaeen Deutschlands, Band 1: Die Hymenogastreen. Verlag von Ludw/Hofstetter, Halle
Hynson NA, Merckx VS, Perry BA, Treseder KK (2013) Identities and distributions of the co-invading ectomycorrhizal fungal symbionts of exotic pines in the Hawaiian Islands. Biol Invasions 15(11):2373–2385. doi:10.1007/s10530-013-0458-3
Iotti M, Lancellotti E, Hall I, Zambonelli A (2010) The ectomycorrhizal community in natural Tuber borchii grounds. FEMS Microbiol Ecol 72(2):250–260. doi:10.1111/j.1574-6941.2010.00844.x
Jeandroz S, Murat C, Wang Y, Bonfante P, Le Tacon F (2008) Molecular phylogeny and historical biogeography of the genus Tuber, the ‘true truffles’. J Biogeogr 35(5):815–829. doi:10.1111/j.1365-2699.2007.01851.x
Knapp A (1950) Die Europaischen Hypogaeen Gattungen und ihre Gattungstypen. Schwelz Zeits Pilzk 28:29–42, 101–118, 153–179
Kovács GM, Jakucs E (2006) Morphological and molecular comparison of white truffle ectomycorrhizae. Mycorrhiza 16(8):567–574. doi:10.1007/s00572-006-0071-8
Lancellotti E, Franceschini A (2013) Studies on the ectomycorrhizal community in a declining Quercus suber L. stand. Mycorrhiza 23(7):533–542. doi:10.1007/s00572-013-0493-z
Lancellotti E, Iotti M, Zambonelli A, Franceschini A (2014) Characterization of Tuber borchii and Arbutus unedo mycorrhizas. Mycorrhiza 24(6):481–486. doi:10.1007/s00572-014-0564-9
Lefevre C (2012) Native and cultivated truffles of North America. In: Zambonelli A, Bonito GM (eds) Edible ectomycorrhizal mushrooms, current knowledge and future prospects, vol 34, Soil biology. Springer, Berlin, pp 209–226. doi:10.1007/978-3-642-33823-6_12
Leonardi M, Iotti M, Oddis M, Lalli G, Pacioni G, Leonardi P, Maccherini S, Perini C, Salerni E, Zambonelli A (2013) Assessment of ectomycorrhizal fungal communities in the natural habitats of Tuber magnatum (Ascomycota, Pezizales). Mycorrhiza 23(5):349–358. doi:10.1007/s00572-012-0474-7
Li PC, Skvortsov AK (1999) Betula ermanii. In: Flora of China, vol 4, Betulaceae, p 310. http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200006146
Li SH, Zheng LY, Liu CY, Wang Y, Li L, Zhao YC, Zhang XL, Yang M, Xiong HK, Qing Y, Wang L, Zhou DQ (2014) Two new truffle species, Tuber alboumbilicum and Tuber pseudobrumale from China. Mycol Prog 13:1157–1163. doi:10.1007/s11557-014-1004-0
Malençon G (1973) Champignons hypogés du Nord de l’Afrique. Persoonia 7(2):261–288
Mello A, Garnero L, Bonfante P (1999) Specific PCR-primers as a reliable tool for the detection of white truffle in mycorrhizal roots. New Phytol 141(3):511–516. doi:10.1046/j.1469-8137.1999.00356.x
Mello A, Vizzini A, Longato S, Rollo F, Bonfante P, Trappe JM (2000) Tuber borchii versus Tuber maculatum: neotype studies and DNA analyses. Mycologia 92(2):326–331. doi:10.2307/3761569
Montecchi A, Lazzari G (1987) Un nuovo tartufo di montagna: Tuber regianum sp. nov. Riv Micol 30:3–11
Montecchi A, Sarasini M (2000) Atlante fotografico di funghi ipogei. Associazione Micologica Bresadola, Vicenza
Murrill WA (1920) Another new truffle. Mycologia 12(3):157–158. doi:10.2307/3753258
Nilsson RH, Ryberg M, Kristiansson E, Abarenkov K, Larsson KH, Kõljalg U (2006) Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PLoS One 1(1):e59. doi:10.1371/journal.pone.0000059
Nilsson RH, Kristiansson E, Ryberg M, Hallenberg N (2008) Intraspecific ITS variability in the kingdom fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol Bioinform 4:193–201
Nilsson RH, Tedersoo L, Abarenkov K, Martin Ryberg M, Kristiansson E, Hartmann M, Schoch CL, Nylander JAA, Bergsten J, Porter TM, Jumpponen A, Vaishampayan P, Ovaskainen O, Hallenberg N, Bengtsson-Palme J, Eriksson KM, Larsson KH, Larsson E, Koljalg U (2012) Five simple guidelines for establishing basic authenticity and reliability of newly generated fungal ITS sequences. MycoKeys 4:37–63. doi:10.3897/mycokeys.4.3606
Pegler DN, Spooner BM, Young TWK (1993) British truffles, a revision of British hypogeous fungi. Royal Botanic Gardens, Kew
Peintner U, Iotti M, Klotz P, Bonuso E, Zambonelli A (2007) Soil fungal communities in a Castanea sativa (chestnut) forest producing large quantities of Boletus edulis sensu lato (porcini): where is the mycelium of porcini? Environ Microbiol 9(4):880–889. doi:10.1111/j.1462-2920.2006.01208.x
Pomarico M, Figliuolo G, Rana GL (2007) Tuber spp. biodiversity in one of the southernmost European distribution areas. Biodivers Conserv 16:3447–3461. doi:10.1007/s10531-006-9013-1
Rauscher T, Muller WR, Agerer R, Chevalier G (1996) T. borchii Vitt. + Corylus avellana L. In: Agerer R, Danielson RM, Egli S, Ingleby K, Luoma D, Treu R (eds) Description of ectomycorrhizae, vol 1. Einhorn, Schwabisch-Gmund, pp 173–178
Riousset G, Riousset L, Chevalier G, Bardet MC (2001) Truffes d’Europe et de Chine. INRA, Paris
Roux P (2006) Mille et un champignons. Edition Roux
Scannerini S, Palenzona M (1967) Ricerche sulle ectomicorrize di Pinus strobus in vivaio, III Micorrize di Tuber albidum Pico. Allionia 13:187–194
Schoch CL, Robbertse B, Robert V, Vu D, Cardinali G, Irinyi L, Meyer M, Nilsson RH, Hughes K, Miller AN, Kirk PM, Abarenkov K, Aime MC, Ariyawansa HA, Bidartondo M, Boekhout T, Buyck B, Cai Q, Chen J, Crespo A, Crous PW, Damm U, De Beer ZW, Dentinger BTM, Divakar PK, Dueñas M, Feau N, Fliegerova K, García MA, Ge ZW, Griffith GW, Groenewald JZ, Groenewald M, Grube M, Gryzenhout M, Gueidan C, Guo L, Hambleton S, Hamelin R, Hansen K, Hofstetter V, Hong SB, Houbraken J, Hyde KD, Inderbitzin P, Johnston PR, Karunarathna SC, Kõljalg U, Kovács GM, Kraichak E, Krizsan K, Kurtzman CP, Larsson KH, Leavitt S, Letcher PM, Liimatainen K, Liu JK, Lodge DJ, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, Manamgoda D, Martín MP, Minnis AM, Moncalvo JM, Mulè G, Nakasone KK, Niskanen T, Olariaga I, Papp T, Petkovits T, Pino-Bodas R, Powell MJ, Raja HA, Redecker D, Sarmiento-Ramirez JM, Seifert KA, Shrestha B, Stenroos S, Stielow B, Suh SO, Tanaka K, Tedersoo L, Telleria MT, Udayanga D, Untereiner WA, Uribeondo JD, Subbarao KV, Lgyi CV, Visagie C, Voigt K, Walker DM, Weir BS, Weiß M, Wijayawardene NN, Wingfield MJ, Xu JP, Yang ZL, Zhang N, Zhuang WY, Federhen S (2014). Finding needles in haystacks: linking scientific names, reference specimens and molecular data for fungi. Database 1–21. doi:10.1093/database/bau061
Sisti D, Giomaro G, Zambonelli A, Rossi I, Ceccaroli P, Citterio B, Stocchi V, Benedetti PA (1998) In vitro mycorrhizal synthesis of micropropagated Tilia platyphyllos Scop. plantlets with Tuber borchii Vittad. mycelium in pure culture. Acta Hortic 457:379–387. doi:10.17660/ActaHortic.1998.457.47
Smith ME, Douhan GW, Rizzo DM (2007) Intra-specific and intra-sporocarp ITS variation of ectomycorrhizal fungi as assessed by rDNA sequencing of sporocarps and pooled ectomycorrhizal roots from a Quercus woodland. Mycorrhiza 18(1):15–22. doi:10.1007/s00572-007-0148-z
Su KM, Xiong WP, Wang Y, Li SH, Xie R, Baima D (2013) Tuber bomiense a new truffle species from Tibet, China. Mycotaxon 126:127–132. doi:10.5248/126.127
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. doi:10.1093/molbev/mst197
Tedersoo L, Abarenkov K, Nilsson RH, Schussler A, Grelet GA, Kohout P, Oja J, Bonito GM, Veldre V, Jairus T, Ryberg M, Larsson KH, Kõljalg U (2011) Tidying up international nucleotide sequence databases: ecological, geographical, and sequence quality annotation of ITS sequences of mycorrhizal fungi. PLoS One 6(9):e24940. doi:10.1371/journal.pone.0024940
Tešitelová T, Tešitel J, Jersáková J, Ríhová G, Selosse MA (2012) Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am J Bot 99(6):1020–1032. doi:10.3732/ajb.1100503
Trappe JM (1979) The orders, families and genera of hypogeous Ascomycotina (truffles and their relatives). Mycotaxon 9(1):297–340
Trappe JM, Castellano MA (2000) New sequestrate Ascomycota and Basidiomycota covered by the Northwest Forest Plan. Mycotaxon 75:153–179
Tulasne LR, Tulasne C (1843) Champignons hypogés de la famille des Lycoperdacées. Ann Sci Nat Bot 19:373–381
Tulasne LR, Tulasne C (1845) Fungi nonulli hypogaei, novi v. minus cogniti. G Bot Ital 1(2):55–63
Vittadini C (1831) Monographia Tuberacearum. Ex Typographia Felicis Rusconi, Milano
Wang YJ, Tan ZM, Murat C, Jeandroz S, Le Tacon F (2007) Molecular taxonomy of Chinese truffles belonging to Tuber rufum and Tuber puberulum groups. Fungal Divers 24:301–328
Wang XH, Benucci GMN, Xie XD, BonitoG LM, Liu PG, Shamekh S (2013) Morphological, mycorrhizal and molecular characterization of Finnish truffles belonging to the Tuber anniae species-complex. Fungal Ecol 6(4):269–280. doi:10.1016/j.funeco.2013.03.002
Xu AS (1999) Notes on the genus of Tuber from Tibet. Mycosystema 18(4):361–365
Zambonelli A, Branzanti B (1984) Prove di micorrizzazione del nocciolo con Tuber aestivum e Tuber albidum. Micol Ital 13(1):47–52
Zambonelli A, Branzanti MB (1989) Mycorrhizal synthesis of Tuber albidum Pico with Castanea sativa Mill., Quercus suber L. and Alnus cordata Loisel. Agric Ecosyst Environ 28(1):563–567. doi:10.1016/0167-8809(90)90099-Y
Zambonelli A, Salomoni S, Pisi A (1993) Caratterizzazione anatomo-morfologica delle micorrize di Tuber spp. su Quercus pubescens Willd. Micol Ital 22(3):73–90
Zambonelli A, Salomoni S, Pisi A (1995) Caratterizzazione anatomo-morfologica delle micorrize di Tuber borchii, Tuber aestivum, Tuber mesentericum, Tuber brumale, Tuber melanosporum su Pinus pinea. Micol Ital 24(2):119–137
Zambonelli A, Iotti M, Amicucci A, Pisi A (1999) Caratterizzazione anatomo-morfologica delle micorrize di Tuber maculatum Vittad. su Ostrya carpinifolia Scop. Micol Ital 28(3):29–35
Zambonelli A, Iotti M, Rossi I, Hall I (2000a) Interaction between Tuber borchii and other ectomycorrhizal fungi in a field plantation. Mycol Res 104(6):698–702. doi:10.1017/S0953756299001811
Zambonelli A, Rivetti C, Percurdani R, Ottonello S (2000b) TuberKey: a DELTA-based tool for the description and interactive identification of truffles. Mycotaxon 74:57–76
Zambonelli A, Iotti M, Boutahir S, Lancellotti E, Perini C, Pacioni G (2012) Ectomycorrhizal fungal communities of edible ectomycorrhizal mushrooms. In: Zambonelli A, Bonito GM (eds) Edible ectomycorrhizal mushrooms, current knowledge and future prospects, vol 34, Soil biology. Springer, Berlin, pp 105–124. doi:10.1007/978-3-642-33823-6_7
Zambonelli A, Iotti M, Hall IR (2015) Current status of truffle cultivation: recent results and future perspectives. Micol Ital 44:31–40
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lancellotti, E., Iotti, M., Zambonelli, A., Franceschini, A. (2016). The Puberulum Group Sensu Lato (Whitish Truffles). In: Zambonelli, A., Iotti, M., Murat, C. (eds) True Truffle (Tuber spp.) in the World. Soil Biology, vol 47. Springer, Cham. https://doi.org/10.1007/978-3-319-31436-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-31436-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31434-1
Online ISBN: 978-3-319-31436-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)