Abstract
Quantitative EEG (QEEG) refers to a computational method that utilizes mathematical and analytical algorithms to transform and compress raw electroencephalography (EEG) signals into a graphical data representation (Fig. 12.1). The most common clinical use of QEEG is for seizure detection. However, QEEG applications are widespread and range from applications in psychiatric diseases (such as biomarkers and biofeedback) to applications in other neurological diseases (such as dementia and stroke). This chapter will focus on the use of QEEG for seizure identification in critically ill patients. When QEEG is applied in the intensive care unit (ICU) setting, it is sometimes referred to as digital trend analysis (DTA) or digital trending. This chapter will describe the trends used for QEEG seizure identification, summarize the literature, and provide examples of seizures, artifacts, and interictal patterns on QEEG trends.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Quantitative EEG (QEEG) refers to a computational method that utilizes mathematical and analytical algorithms to transform and compress raw electroencephalography (EEG) signals into a graphical data representation (Fig. 1). The most common clinical use of QEEG is for seizure detection. However, QEEG applications are widespread and range from applications in psychiatric diseases (such as biomarkers and biofeedback) to applications in other neurological diseases (such as dementia and stroke). This chapter will focus on the use of QEEG for seizure identification in critically ill patients. When QEEG is applied in the intensive care unit (ICU) setting, it is sometimes referred to as digital trend analysis (DTA) or digital trending. This chapter will describe the trends used for QEEG seizure identification, summarize the literature, and provide examples of seizures, artifacts, and interictal patterns on QEEG trends.
Sample 30 min QEEG panel and corresponding raw EEG. (a) QEEG panel consisting of the following QEEG tools: rhythmicity spectrogram (displayed for the left and right hemispheres), CDSA (displayed for the left and right hemispheres), aEEG (displayed for the left and right hemispheres), and asymmetry index (displayed as both absolute and relative values). Vertical black arrows denote electrographic seizures. (b–d) Consecutive ictal EEGs (10 s each) corresponding to the first seizure marked on the QEEG panel demonstrating a left central electrographic seizure
Basic Principles
QEEG was initially developed in the 1960s with the development of compressed spectral array (CSA). There are now several types of QEEG trends available for clinical use as part of commercial QEEG software packages. The primary advantage of QEEG is that it allows for a large amount of data to be displayed on a single screen in contrast to only 10–20 s of data with raw EEG. QEEG also simplifies the information that is displayed, in such a way that it may be amenable for interpretation by non-neurophysiologists. One study found that there was no significant difference in the ability of neurophysiologists, EEG technologists, and neuro ICU nurses to detect seizures on QEEG panels alone [1]. This makes QEEG particularly attractive as a potential bedside patient monitor as a component of ICU multimodality monitoring.
QEEG has several putative advantages over raw EEG review. First, it may reduce the time required for data review. Indeed, raw EEG review is quite labor intensive. One study found that QEEG-guided review of the raw EEG was able to shorten the review process time by 78 % [2]. A survey showed that approximately half of neurophysiologists utilize QEEG as part of their ICU continuous EEG (cEEG) protocol [3]. The usage of QEEG will vary between institutions and readers (various trends used, derivations of trends, frequency of review, and amount of data that is reviewed only by QEEG).
Another potential advantage of QEEG in the ICU setting is that it could allow for real-time data transmission to the treatment team. EEG data obtained by conventional raw EEG review by neurophysiologists is always relayed to the ICU team in a post hoc fashion. Real-time review of the raw EEG is very difficult. In a 2014 survey, the majority of neurophysiologists review each record two or more times a day [3]. Therefore, with conventional EEG-only review, up to 12 h may pass with seizures being undetected. This could lead to delays in treatment of seizures in critically ill patients and potentially adverse outcomes. To date, no clinical studies assessing the role of QEEG on outcomes in the ICU environment are available.
Trends Used for QEEG Seizure Detection
Although many QEEG trends are available for use, this chapter will discuss the trends that have been studied for seizure detection in critically ill patients. These include envelope trend (ET), color density spectral array (CDSA), rhythmicity spectrogram, asymmetry index, amplitude-integrated EEG (aEEG), and automated seizure detectors. This section will describe these trends and provide examples of seizure appearance for each trend. The QEEG samples in this chapter for CDSA, rhythmicity spectrogram, and aEEG will be displayed for the left and right hemispheres, as this is the preference at the author’s institution and was also recently validated in a retrospective trial [1]. It is important to note that other QEEG trends and other derivations may be used. Instead of displaying the left and right hemispheres for each trend, the QEEG trend display may be modified to display individual channels separately or by quadrant. Furthermore, the asymmetry index and aEEG examples in this chapter will be displayed as separate trends, but other institutions may choose to display these as overlapping trends. All QEEG panels displayed in this chapter were created from Persyst (Persyst Development Corporation, Prescott, AZ).
Automated Seizure Detection
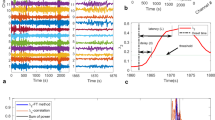
Automated seizure detectors are typically part of QEEG software packages and will vary between manufacturers. The algorithms recognize rhythmic patterns based on waveform morphology, distribution, and evolution over time [4]. Once a certain threshold is reached, the software program assigns a pattern as a seizure. The Persyst 12 automated seizure detector has two types of outputs: a binary output of yes/no based on the detection of discrete electrographic seizure events lasting ≥11 s and a seizure probability curve that displays the probability of each 1 s epoch as being categorized as a seizure (Fig. 2). Of note, most automated seizure detection algorithms (ASDA) are trained on a sample of seizures obtained from various EEGs pooled from the epilepsy monitoring unit (EMU), ICU, and ambulatory EEGs. The complex interictal patterns and sometimes subtle nature of seizures in critically ill patients combined with numerous sources of ICU artifact lead to challenges in successful identification by automated seizure detectors (Fig. 2).
Seizure identification on seizure probability trend and corresponding EEG. (a) Seizure probability trend containing one electrographic seizure (approximate onset marked by the vertical black arrow). The seizure probability trend does identify the seizure, but is not able to discriminate it from numerous non-seizure events. (b) Corresponding rhythmicity spectrogram (displayed for the left and right hemispheres). (c) Ictal EEG corresponding to the time point on the QEEG trends as marked by the vertical blue line. This EEG sample contained abundant artifact (most notably in the T6 electrode), rhythmic delta activity (RDA), and brief rhythmic discharges (BRDs) resulting in poor seizure identification on QEEG
Frequency-Based Trends
Color Density Spectral Array
CDSA is known by several other names: Color spectral array (CSA), fast Fourier transform (FFT) spectrogram, and density spectral array (DSA). CDSA displays a three-dimensional, frequency-based graphical display of the EEG data over time. Time is shown on the x-axis, and the EEG frequency is shown on the y-axis. The various colors represent the power of various frequency bands. The power is the area under the Fourier spectrum curve within a given frequency range (i.e., delta power). In other words, the power is the amplitude (or voltage) of the EEG within a specific frequency range. The power is represented by color. The colors used in the graphical display of the power in the CDSA trend will vary between QEEG software programs. Each program will display a color scale with the CDSA trend. The CDSA trends shown in this chapter were created from Persyst with cooler colors (blue and green) indicating lower power and warmer colors (red, yellow, pink) indicating higher power.
Seizures often consist of an increase in frequency and amplitude and therefore will appear on CDSA trend as a paroxysmal event with increased power. Warmer colors will take the place where cooler colors previously were seen. Additionally, the characteristic seizure evolution in terms of amplitude and frequency can be appreciated on CDSA as an upward arch shape (Fig. 3). Some seizures in critically ill patients consist of little or no increase in amplitude and/or frequency and therefore might be missed on CDSA.
Seizure appearance on the CDSA trend, 0–20 Hz (displayed for the left and right hemispheres) for two different patients. Vertical black arrows denote the approximate onset of electrographic seizures. The upward arch shape of seizures can be appreciated on both patients. (a) Recurrent right hemispheric seizures seen as an increase in power (represented by warmer colors). Note the evolution of power increase (shown by the red and yellow colors). Soon after the onset of the seizure, there is a gradual decrease in frequency, then increase, and then decrease again before cessation. This is superimposed on a diffuse mild increase in power (shown by green and teal colors during seizure activity). (b) A single right hemispheric seizure on the CDSA trend. Aside from a brief increase in high power (denoted by diagonal black arrow) in mid-frequency range, the majority of the seizure consists of highest power (red, pink, and white) in the delta frequency range. This is superimposed on a diffuse mild increase in power (shown by green color)
Rhythmicity Spectrogram
The rhythmicity spectrogram, rhythmic run detection and display, is a proprietary tool developed by Persyst, Inc. An example of a rhythmicity spectrogram is shown in Fig. 4. Like CDSA, the rhythmicity spectrogram is a three-dimensional display. Time is on the x-axis and frequency is on the y-axis (but on a logarithmic scale to accentuate lower frequencies). Although the power is displayed by color-coding (darker blue color indicating more power), it differs from CDSA by only displaying the power in components that have a high degree of rhythmicity, instead of displaying all the power. Seizures will present as areas that are darker in color. The rhythmicity spectrogram is particularly helpful in displaying the evolution of seizures (Fig. 4).
Seizure appearance on the rhythmicity spectrogram, 0–25 Hz (displayed for the left and right hemispheres) for two different patients. Vertical black arrows denote the approximate onset of electrographic seizures. The evolution of the seizure can be appreciated on both patients. (a) Recurrent right hemispheric seizures beginning with an increased power (darker blue coloration) in alpha activity. As the seizure progresses (shown by the red arrow), there is gradual evolution of increased power into lower frequency ranges before cessation. (b) Three generalized seizures (with left hemisphere predominance) beginning with a subtle, increased power in the delta frequency range that gradually increases in power (light blue becoming darker blue). As the seizure progresses, an increase in power is seen in the alpha and beta frequency ranges as well followed by abrupt cessation
Subtle seizures can often be seen only on the rhythmicity spectrogram while not appearing on other trends. However, the rhythmicity spectrogram is prone to highlighting interictal periods and artifact that are easily mistaken for seizures. Examples of these will be discussed later in the chapter.
Asymmetry Index
The asymmetry index compares the difference in power between homologous electrodes (i.e., the difference in power between F3 vs. F4 and O1 vs. O2, etc.). The difference is represented in a graphical display. Typically, there are two graphs that are separate or overlapping: the absolute asymmetry index and the relative asymmetry index (Fig. 5). The absolute asymmetry index (yellow trace) calculates the absolute difference, always displaying a positive score. There is an upward deflection with increasing asymmetry and a downward deflection with decreasing asymmetry. The relative asymmetry index (green trace) is able to show lateralization for the asymmetry. An upward deflection represents more power in the right hemisphere, and a downward deflection represents more power in the left hemisphere. This trend is particularly helpful for focal or lateralized seizures. However, a bilateral or generalized seizure with similar power in both hemispheres will likely not show up well on the asymmetry index.
Example of three left hemispheric seizures on asymmetry index and asymmetry spectrogram (approximate onset marked by vertical black arrows). There is a subtle, upward deflection of the absolute asymmetry index (yellow trace) indicating a period of increased asymmetry. There is a corresponding downward deflection of the relative asymmetry index (green trace) indicating increased power in the left hemisphere. Interictally, there is equal power in the left and right hemispheres, as seen by equal red and blue coloration on the asymmetry spectrogram. The seizures appear on the asymmetry spectrogram as a period of dark blue indicating higher power in the left hemisphere. There is increased power in the right hemisphere after each seizure due to postictal left hemispheric suppression
The asymmetry spectrogram (Fig. 5) also displays similar information regarding the power in homologous electrodes. Colors indicate where more power is present (red = more power in the right hemisphere and blue = more power in the left hemisphere). The degree of asymmetry is represented by the darkness of the color. In addition to seizure detection, the asymmetry index and asymmetry spectrogram are also particularly helpful for ischemia detection.
Amplitude-Based Trends
Envelope Trend
The envelope trend (ET) is a QEEG trend that is based only on amplitude. The raw EEG is divided into 10–20 s epochs. For each epoch, the median amplitude is calculated and plotted over time, creating the ET display. This trend is often displayed separately for the left and right hemispheres, but can be customized to separately display the ET for a specific set of electrodes. By plotting only the median amplitude, the ET has the advantage of being able to filter out short-duration artifacts. Conversely, it may miss very brief seizures due to the fact that the ET is calculated in 10–20 s epochs. Seizures on ET are visualized as an upward deflection in the trace (Fig. 6).
Example of three generalized seizures on envelope trend. The blue trace corresponds to the left hemisphere and the red trace corresponds to the right hemisphere. Vertical black arrows mark seizures. For each seizure, there is a clear, upward deflection in both the red and blue traces. Seizure duration is approximately 5 min
Amplitude-Integrated EEG
The amplitude-integrated EEG (aEEG) trend is another trend calculated only by amplitude. For each data point, the raw EEG is filtered and rectified (all values made positive). The amplitude-integrated EEG (aEEG) trend is displays the minimum and maximum amplitude of the raw EEG signal in a predefined time frame (typically 1–2 s) on a semilogarithmic scale. Seizures appear as an increase in the minimum amplitude, creating an upward arch shape (Fig. 7). There is often a corresponding increase in the maximum amplitude. This trend is also known as a cerebral function monitor (CFM) and has been utilized extensively for seizure detection in neonates. The original CFM display represented EEG data from one raw EEG channel placed over the parietal regions (P3 and P4). To have the ability to detect lateralized abnormalities, it is now common for CFM machines to display two channels of data (C3-P3 and C4-P4). Commercial QEEG software, such as Persyst, has the ability to display aEEG trends by any group of electrodes and is often displayed separately for the left and right hemispheres, incorporating all lateralized electrodes from the standard 10–20 montage.
Example of seizures on the aEEG trend (displayed for the left and right hemispheres) for two different patients. Approximate seizure onset is marked by vertical black arrows. (a) Bilateral seizures are represented by a large, upward deflection in the minimum and maximum amplitudes of the baseline of both traces. The gradual increase in amplitude (evolution) can be appreciated well. (b) Right hemispheric seizures are represented by an increase in the minimum amplitude of the red trace, without a notable change in the maximum amplitude. This subtle seizure appearance on aEEG is more common in critically ill patients than the seizures shown in panel (a)
Data for Quantitative EEG Utilization in Seizure Detection
Sensitivity of Quantitative EEG Used in Isolation for Seizure Detection
The majority of studies on QEEG for seizure detection have been in the pediatric and neonatal population, although there are an increasing number of studies evaluating QEEG in critically ill adults. Beyond just patient’s age, there is significant heterogeneity in these studies. Some utilize QEEG trends obtained from full-montage cEEGs, while others are obtained from limited channel cEEGs. Furthermore, even when a full cEEG montage is used, the QEEG trend studied may be derived from all channels or from a limited number of channels. Although certain QEEG trends are studied more often than others, the type of QEEG trend studied (commercially available vs. a novel QEEG algorithm) often differs between studies. Some studies may employ only one trend while others use more than one. Another potentially confounding variable in QEEG studies is the variability in expertise of QEEG readers and the extent of QEEG training provided. Studies may use neurophysiologists as readers, but they may not be considered “experienced readers” as many neurophysiologists have not had training/experience with QEEG trends. Conversely, many of the studies in the neonatal population utilize neonatologists as readers since they are more likely to be the ones interpreting the bedside CFM. Neonatologists may not have experience in reading raw EEGs, but they might be considered “experienced readers” since some have had several years experience in interpreting CFMs. Furthermore, the manner in which sensitivity and specificity are calculated (scoring based on capturing individual seizures or scoring based on the presence/absence of seizures in patients or epochs) vary between studies. However, in actual clinical practice, knowing the exact number of seizures present may not be necessary, and simply knowing if seizures are present or not may be sufficient to guide therapy.
There are numerous other variables to consider when comparing QEEG studies: if the readers have access to the raw EEG, the overall QEEG record duration, the display timescale of the QEEG, the QEEG dataset (all patients with seizures vs. some with seizures and some without), and the role of the QEEG reader (mark seizures vs. mark area of concern).
Overall, studies in the adult and pediatric population evaluating individual QEEG trends (ET, aEEG, or CDSA) report sensitivities for seizure detection of 44–83 % when interpreted by neurophysiologists [5–10]. Studies evaluating the ability of non-neurophysiologists (pediatric or neurology residents, general neurologists, intensivists, and neonatologists) to interpret single QEEG trends (ET, aEEG, or CDSA) for seizure detection report sensitivities of 41–89 % [5, 6, 11–14]. As mentioned previously, there is a significant variability between studies, making direct comparisons very difficult. This heterogeneity contributes to the wide range in reported sensitivities. Therefore, it remains unclear if one QEEG trend is superior to another for seizure detection.
In clinical practice, the bedside QEEG display often shows more than one QEEG trend, which may improve seizure detection. To address this question, there have been two studies evaluating the combination of two QEEG trends [6, 15] and one study evaluating the combination of a panel of QEEG trends [1]. There was an improved sensitivity (66 %) for neurophysiologists when ET and CDSA trends were combined as compared to individually (sensitivity of 50 % for both ET and CDSA). However, non-experienced readers, neurologists, did not reveal an improvement in sensitivity when ET and CDSA were presented as a combination (50 % sensitivity for ET + CDSA and 50 % for individual trends) [6]. Another study reported a very high sensitivity (93 %) for the detection of the presence of seizures by non-neurophysiologists (one fellow, one neurology resident, and two neuro ICU nurses) when readers evaluated a combination of aEEG and CDSA (two channels each). However, this was not compared to their performance on individual trends. Of note, the derivation of the two-channel aEEG and CDSA trends varied between records as the authors preselected the channels that would best display ictal activity [15].
As mentioned previously, it is common for bedside QEEG displays to be customized to show numerous QEEG trends at once. The sensitivity of a panel of QEEG trends (rhythmicity spectrogram (Persyst Development Corporation, Prescott, AZ), CDSA, asymmetry index, and aEEG) was found to be 87 % for five neurophysiologists, 80 % for seven EEG technologists, and 87 % for five neuro ICU nurses for the detection of the presence of seizures on randomized 1 h epochs [1]. However, this was not compared to the reader’s performance using individual QEEG trends. There was no significant difference between the three groups with regard to sensitivity. This study utilized QEEG trends derived from all lateralized electrodes in a standard 10–20 montage, while most all other QEEG studies for seizure detection (with the exception of one [14]) employed QEEG trends derived from a limited number of electrodes, even if a full 10–20 montage is performed for the cEEG recording. Seizures in critically ill patients have significant variability in appearance ranging from subtle, low-amplitude, focal seizures to obvious, generalized, high-amplitude seizures. This variability in seizure appearance highlights the importance of a panel of QEEG trends. For example, seizures in one patient may appear best on rhythmicity spectrogram, while another patient’s seizures may be best observed on aEEG. Furthermore, a panel of QEEG trends makes it easier to discriminate seizures from artifact.
False-Positive Rate
One important concern regarding the use of QEEG for seizure detection is the rate of false positives. Previous studies utilizing both single and multiple QEEG trends for seizure detection by neurophysiologists and non-neurophysiologists have found the false-positive rate to be between 5 and 39 % [1, 7, 8, 11, 12, 15]. One of these studies found that the most common reason for false-positive seizure diagnosis (18 %) by aEEG was movement artifact [7]. However, it is common for various types of artifact to be mistaken for seizures on QEEG in addition to various interictal patterns (to be further discussed later in the chapter). If QEEG trends were used alone without confirmation of events by interpretation of raw EEG by a neurophysiologist, the result would be unnecessary treatment. Therefore, QEEG should not be used in isolation.
Utility of Combination Raw EEG and QEEG
Although several studies, discussed above, have been performed to evaluate the sensitivity and specificity of isolated review of QEEG trends, many neurophysiologists use QEEG in combination with raw EEG review to assist the review process. A 2014 survey found that 52 % of neurophysiologists utilized QEEG as part of their cEEG protocol [3]. The addition of CDSA linked to raw EEG was found to speed the review process by 78 % with little loss in sensitivity when compared to traditional EEG review without QEEG [2]. In the study group, the reviewer’s primary mode of assessment was CDSA interpretation, but reviewers were able to evaluate short periods of the raw EEG. To review 24 h of data, traditional raw EEG review took an average of 38 min compared with an average of 8 min for CDSA-guided review. The sensitivity for CDSA-guided review was 78 % [2].
Automated Seizure Detection
Automated EEG detection systems were developed 40 years ago. Commercial QEEG software packages often include an automated seizure detection algorithm. Currently, automated seizure detectors are more frequently used to assist with seizure detection in EMUs than in the ICU.
The currently available software for automated seizure detection has either a low sensitivity or a high false-positive rate. Studies have found the sensitivity of these algorithms to range from 33 to 93 % [16], with variability in the algorithms and in the datasets as the likely explanation for the wide range. A recent and very promising study of a novel automated seizure detection algorithm (ICU-ASDA) had a mean sensitivity of 90 % with a false-positive rate of 1.6/24 h when applied to ICU EEG recordings [16]. This was compared against two commercially available automated seizure detection products that resulted in much lower sensitivity (sensitivity of 13 and 10 % with false-positive rates of 1.036/h and 0.013/h).
In a separate study, the Persyst 12 automated seizure detection algorithm (described earlier) was used on ICU EEG samples that contained EEG patterns that typically make identification of seizures problematic (periodic patterns, ictal-appearing artifacts, and normal variants that appear epileptiform). The software detected 76 % of all seizures with a false-positive rate of 0.9/h [4]. As automated seizure detection algorithms continue to improve the discrimination between seizures and artifacts and interictal events, it is likely that these will begin to enter regular clinical practice in the ICU.
Seizure Characteristics and QEEG
Several studies have attempted to identify certain EEG characteristics that affect seizure identification on QEEG. These EEG characteristics fall into two categories: intrinsic seizure characteristics and interictal EEG patterns or artifacts. All studies that have evaluated the relationship between seizure duration and identification on QEEG have found that shorter seizures (typically less than 1–2 min) are more likely to be missed by experienced and non-experienced QEEG readers alike [1, 2, 8, 10, 12, 15]. Additionally, there appears to be a consistent finding that low-amplitude seizures (typically less than 75 μV) are more likely to be missed by QEEG readers [10, 12, 15]. The relationship between other seizure characteristics and seizure identification on QEEG has been inconsistent. Focal or bilateral independent seizures may be missed more often by QEEG readers [1, 12, 15], although others have not found a relationship between seizure spatial extent and seizure identification [8].
It is not entirely clear how the interictal EEG pattern affects seizure identification on QEEG. Two studies have correlated lower QEEG interpretation performance with EEGs that either contain abundant interictal discharges [10] or periodic patterns [4]. However, another report found that neurophysiologists were more likely to correctly identify seizures in the presence of a periodic EEG background [1].
Recognizing Seizures on QEEG
The most challenging aspect of QEEG in critically ill patients is discriminating artifact and interictal patterns from seizures and being able to recognize subtle seizures. The most common reason for false-positive seizure diagnosis by aEEG was movement artifact [7]. As discussed previously, short- and low-amplitude seizures tend to be missed by QEEG readers [1, 2, 8, 10, 12, 15]. Furthermore, various interictal patterns may hinder correct seizure identification. This section will give several examples of QEEGs highlighting these issues.
Artifact Recognition
Many sources of artifact are present in the ICU and are unavoidable. Some sources of artifact (such as bed percussion) are easily differentiated from seizures due to the long duration and invariable appearance of bed percussion artifact on QEEG. Conversely, other artifacts may easily be mistaken for seizures due to a shorter time course and appearing to show evolution (sternal rub and electromyographic (EMG) artifact) (Figs. 8 and 9). Other common sources of QEEG artifact in the ICU include chewing (Fig. 10), alternating current (AC) artifact from various ICU devices, patient disconnection (Fig. 11), and electrode artifact from high-impedance electrodes. These artifacts result in paroxysmal changes on raw EEG and QEEG, and it can be extremely difficult to distinguish seizures from artifact on QEEG. Review of the raw EEG is critical to avoid false-positive results.
Appearance of EMG artifact compared with seizures on QEEG. (a) QEEG panel (rhythmicity spectrogram, CDSA, aEEG, and asymmetry index) displaying a period of EMG artifact (marked by horizontal black arrow). (b) QEEG panel containing one seizure (approximate onset marked by vertical black arrow) occurring later in the recording for the same patient. Note the difference in appearance of artifact and seizure on the rhythmicity spectrogram and CDSA. The appearance on aEEG is strikingly similar. If aEEG were used in isolation, there would be a high likelihood of a false positive. (c) Raw EEG consisting of EMG artifact corresponding to the time point on panel a marked by the vertical blue line. (d) Ictal EEG corresponding to the time point on panel (b) marked by the vertical blue line
Appearance of EMG artifact compared with seizures on a QEEG panel (rhythmicity spectrogram and CDSA, displayed for the left and right hemispheres). The rhythmicity spectrogram and CDSA trends display a period of EMG artifact (marked by horizontal black arrow) and an electrographic seizure (approximate onset marked by vertical black arrow). The appearance of EMG artifact in the alpha and beta frequency ranges on the rhythmicity spectrogram is very common
Appearance of chewing artifact compared with seizures on QEEG. (a) QEEG panel (rhythmicity spectrogram, CDSA, and aEEG for the left and right hemispheres) displaying recurrent periods of chewing artifact (marked by horizontal black arrow). (b) QEEG panel containing one seizure (approximate onset marked by vertical black arrow) occurring later in the recording for the same patient. (c) Raw EEG consisting of chewing artifact corresponding to the time point on panel (a) marked by the vertical blue line. (d) Ictal EEG corresponding to the time point on panel (b) marked by the vertical blue line
Appearance of EEG disconnection on QEEG. (a) QEEG panel (rhythmicity spectrogram, CDSA, aEEG, and asymmetry index). Disconnection is marked by the vertical white arrow. (b) Raw EEG corresponding to the time point on panel (a) marked by the vertical blue line showing the patient’s background EEG pattern. (c) Raw EEG corresponding to the time point on panel (a) marked by the vertical red line after the patient was disconnected from EEG. After disconnection, the QEEG is picking up a large amount of artifact from the environment and from movement as seen by the large deflections on all QEEG trends. After the artifact subsides, the EEG appearance looks similar to electrocerebral inactivity (ECI)
Figures 8, 9, and 10 show examples of QEEG panels that contain both discrete seizures and periods of artifact. Although it may initially be difficult to distinguish artifact from seizures on QEEG, the skill of pattern recognition will improve with continued experience. As with raw EEG, seizure evolution can often be appreciated, especially on rhythmicity spectrogram (Fig. 4). In contrast, artifact often has a sudden onset and offset without displaying evolution. Artifacts in the ICU (especially EMG artifact) tend to appear in the higher-frequency ranges, while it is common for seizures in critically ill patients to be limited to the delta and theta range (Fig. 9). Furthermore, seizure morphology on QEEG tends to be stereotyped, making subsequent seizure identification easier over time. Due to inter-patient seizure variability, it is common for seizures not to be well defined on all QEEG trends. Seizure characteristics will determine varying appearance on different QEEG trends. The author’s institution utilizes a panel of QEEG trends for this reason.
Interictal Patterns
There are numerous rhythmic and periodic EEG patterns encountered in critically ill patients that pose difficulties when interpreting QEEG.
Periodic Patterns
Periodic EEG patterns, such as burst suppression, burst attenuation, lateralized periodic discharges (LPDs), and generalized periodic discharges (GPDs), can appear as paroxysmal events on QEEG under certain circumstances. Short-duration, low-frequency, monotonous periodic patterns will likely not change the QEEG background appearance. However, if the duration of bursts in burst suppression/attenuation patterns is of sufficient duration, a paroxysmal change on QEEG may appear. Similarly, a change in the frequency of LPDs and GPDs to higher-frequency runs of LPDs and GPDs can appear as a paroxysmal event on QEEG especially if this change is rather abrupt. Figure 12 displays an example of a discrete (but subtle) seizure on QEEG contrasted with interictal activity of consisting of LPDs.
Appearance of lateralized periodic discharges (LPDs) compared with seizures on QEEG. (a) QEEG panel (rhythmicity spectrogram, CDSA, aEEG, and asymmetry index). (b) Interictal raw EEG demonstrating continuous left hemispheric LPDs corresponding to the time point on panel (a) marked by the vertical blue line. Although the LPDs occur continuously throughout the patient’s record, there are some periods when the LPDs become more prominent and spread to the right posterior quadrant and result in a very subtle change in the rhythmicity spectrogram (marked by diagonal black arrows). (c) Ictal EEG corresponding to the time point on panel (a) marked by the vertical red line. Although this seizure is subtle, its appearance on QEEG (panel a) can be visualized on the left hemisphere rhythmicity spectrogram as darker blue coloration in the alpha frequency range and by a thinned, arch-like shape on left hemisphere aEEG
Nonperiodic Interictal Patterns
There are certain nonperiodic, episodic, interictal EEG patterns that may make QEEG interpretation difficult. These include brief rhythmic discharges (BRDs), lateralized or generalized rhythmic delta activity (LRDA or GRDA), stimulus-induced rhythmic periodic or ictal discharges (SIRPIDs), and state changes. BRDs, LRDA, GRDA, and SIRPIDs may appear as discrete events on QEEG, depending on characteristics such as duration, amplitude, frequency, and deviation from baseline. Figures 13, 14, and 15 show examples of the appearance of BRDs, RDA, and state changes on QEEG, respectively.
Appearance of a brief rhythmic discharge (BRD) on QEEG. (a) QEEG panel (rhythmicity spectrogram and CDSA, for the left and right hemispheres). Asymmetry index and aEEG are not shown as there was no change in these trends during the BRDs for this patient. During each BRD, there is an increase in power in the lower frequency ranges that appears on rhythmicity spectrogram as intermittent darker blue coloration and on CDSA as intermittent episodes of white/red/yellow coloration. (b) Raw EEG displaying a 5 s long BRD consisting of rhythmic 3 Hz spike and wave discharges corresponding to the time point on panel (a) marked by the vertical blue line
Appearance of frontally predominant generalized rhythmic delta activity (GRDA) on QEEG. (a) QEEG panel (rhythmicity spectrogram and CDSA, for the left and right hemispheres). During each episode of RDA, there is a subtle increase in power in the delta (and to a lesser extent theta) frequency range that appears on rhythmicity spectrogram as intermittent darker blue coloration and on CDSA as intermittent occurrences of white/red/yellow coloration. The periods of RDA are more prominent in the first half of the QEEG panel. (b) Raw EEG demonstrating an example episode of frontally predominant GRDA corresponding to the time point on panel a marked by the vertical blue line
Appearance of patient’s state change and EMG artifact on QEEG. (a) QEEG panel (rhythmicity spectrogram, CDSA, aEEG, and asymmetry index). (b) Raw EEG corresponding to the time point on panel a marked by the vertical blue line while the patient is sleeping. (c) Raw EEG corresponding to the time point on panel a marked by the vertical red line after the patient awakens and is moving. During this period, there is a diffuse change in power bilaterally that is seen on rhythmicity spectrogram and CDSA. On aEEG, there is an increase in the maximum and minimum amplitude. Little difference is seen on asymmetry index
Subtle Seizures
Similar to raw EEG interpretation, subtle seizures can be difficult to identify on QEEG. These include seizures that are short duration, low amplitude, low frequency, slowly evolving, and of limited spatial extent. These seizures are especially difficult to identify on QEEG when the appearance of artifact is more prominent than the seizures themselves (Fig. 16). Furthermore, even though an individual patient’s seizures initially appear easy to detect, treatment with antiepileptic drugs may cause them to become subtle in appearance on QEEG by reducing the spatial extent, duration, frequency, and/or amplitude (Fig. 17).
Appearance of episodic EMG artifact compared with very subtle seizures on QEEG. (a) QEEG panel (rhythmicity spectrogram, CDSA, aEEG, and asymmetry index) displaying intermittent periods of EMG artifact (marked vertical white arrows) and three extremely subtle seizures (marked by blue circles). Due to the periodic, prominent EMG artifact and very subtle nature of seizures, it would be easy to overlook the seizures and mistake the artifact for seizures. (b) Ictal EEG demonstrating the seizure corresponding to the time point on panel (a) marked by the vertical blue line. This seizure is only seen slightly on left hemisphere rhythmicity spectrogram. The seizures do not appear on the other QEEG trends. (c) Raw EEG consisting of EMG artifact corresponding to the time point on panel (a) marked by the vertical red line. This artifact appears prominent on CDSA and aEEG
Appearance of obvious vs. subtle seizures on QEEG. (a) QEEG panel (rhythmicity spectrogram, CDSA, aEEG, and asymmetry index) displaying two seizures in the same patient, separated by approximately 15 min. (b) Ictal EEG demonstrating a focal left central seizure corresponding to the time point on panel (a) marked by the vertical blue line. This seizure is seen predominantly on left hemisphere rhythmicity spectrogram and left hemisphere CDSA. It is difficult to visualize this seizure on aEEG and asymmetry index. (c) Ictal EEG demonstrating a focal left central seizure corresponding to the time point on panel (a) marked by the vertical red line. This seizure, in the same patient, consists of rhythmic sharp waves in the same distribution and frequency, but of lower amplitude. Due to the reduced amplitude, this seizure is less noticeable on left hemisphere rhythmicity spectrogram than the seizure in panel (b) and even less noticeable on the other QEEG trends
Conclusion
There has been an increased clinical utilization of QEEG for detection of seizures in critically ill adult and pediatric patients. The goals of QEEG for seizure detection are to assist in interpretation of large volumes of cEEG data and possibly expedite seizure identification and treatment. Ongoing research efforts are attempting to answer various questions such as which QEEG trends should be used; which personnel can serve as QEEG readers, if automated seizure detectors can be used; and which is the best electrode derivations for QEEG trends.
Although QEEG trends can be used to assist cEEG data interpretation, it cannot be used in isolation. Patient treatment decisions must be made on the basis of raw EEG interpretation by neurophysiologists. As QEEG software continues to improve, it is likely that QEEG will continue to have a growing presence in the ICU for seizure detection.
References
Swisher CB, Shah D, Sinha SR, Husain AM. Baseline EEG pattern on continuous ICU EEG monitoring and incidence of seizures. J Clin Neurophysiol. 2015;32(2):147–51.
Moura LM, Shafi MM, Ng M, et al. Spectrogram screening of adult EEGs is sensitive and efficient. Neurology. 2014;83:56–64.
Gavvala J, Abend N, LaRoche S, et al. Continuous EEG monitoring: a survey of neurophysiologists and neurointensivists. Epilepsia. 2014;55:1864–71.
Sierra-Marcos A, Scheuer ML, Rossetti AO. Seizure detection with automated EEG analysis: A validation study focusing on periodic patterns. Clinical Neurophysiology. 2014 Jun 30.
Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–7.
Akman CI, Micic V, Thompson A, Riviello Jr JJ. Seizure detection using digital trend analysis: factors affecting utility. Epilepsy Res. 2011;93:66–72.
Evans E, Koh S, Lerner J, Sankar R, Garg M. Accuracy of amplitude integrated EEG in a neonatal cohort. Arch Dis Child Fetal Neonatal Ed. 2010;95:F169–73.
Pensirikul AD, Beslow LA, Kessler SK, et al. Density spectral array for seizure identification in critically ill children. J Clin Neurophysiol Off Publication Am Electroencephalogr Soc. 2013;30:371–5.
Shah DK, Mackay MT, Lavery S, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54.
Stewart CP, Otsubo H, Ochi A, Sharma R, Hutchison JS, Hahn CD. Seizure identification in the ICU using quantitative EEG displays. Neurology. 2010;75:1501–8.
Nitzschke R, Muller J, Engelhardt R, Schmidt GN. Single-channel amplitude integrated EEG recording for the identification of epileptic seizures by nonexpert physicians in the adult acute care setting. J Clin Monit Comput. 2011;25:329–37.
Rennie JM, Chorley G, Boylan GB, Pressler R, Nguyen Y, Hooper R. Non-expert use of the cerebral function monitor for neonatal seizure detection. Arch Dis Child Fetal Neonatal Ed. 2004;89:F37–40.
Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–7.
Williamson CA, Wahlster S, Shafi MM, Westover MB. Sensitivity of compressed spectral arrays for detecting seizures in acutely ill adults. Neurocrit Care. 2014;20:32–9.
Dericioglu N, Yetim E, Bas DF, et al. Non-expert use of quantitative EEG displays for seizure identification in the adult neuro-intensive care unit. Epilepsy Res. 2015;109:48–56.
Sackellares JC, Shiau DS, Halford JJ, LaRoche SM, Kelly KM. Quantitative EEG analysis for automated detection of nonconvulsive seizures in intensive care units. Epilepsy Behav E&B. 2011;22 Suppl 1:S69–73.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Swisher, C.B. (2017). QEEG in Seizure Detection. In: Husain, A., Sinha, S. (eds) Continuous EEG Monitoring. Springer, Cham. https://doi.org/10.1007/978-3-319-31230-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-31230-9_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31228-6
Online ISBN: 978-3-319-31230-9
eBook Packages: MedicineMedicine (R0)