Abstract
The pressure response to sodium is heterogeneous among both persons with normal blood pressure and with hypertension. Nevertheless, sodium restriction is typically recommended for everyone with hypertension. As reviewed here, categorizing a person as “salt sensitive” has important prognostic and therapeutic implications. Determination of salt sensitivity is typically accomplished by assessment of the pressure response to administration of an oral or intravenous sodium load. In this chapter, we discuss an alternative way to administer a sodium load through stress exposure. Animal and human studies have demonstrated clinically significant sodium retention during and after stress, which in effect generates positive sodium balance and thus delivers a sodium load. Persons demonstrating this response develop a volume-dependent blood pressure elevation. Similar to findings in salt-sensitive populations, target organ changes have also been associated with impaired sodium handling during stress. Sodium retention in response to stress has been reported as improved or reversed after treatment with antihypertensive medications that block the renin-angiotensin-aldosterone system. Evidence suggests that the variability of the pressure response to dietary sodium intake and to stress should be considered in our strategies to prevent and control hypertension.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Salt sensitivity
- Sodium
- Stress-induced sodium retention
- Pressure natriuresis
- Sympathetic nervous system
- Renin-angiotensin-aldosterone system
- Obesity
- Angiotensin II
Introduction
Although there is a direct relation between sodium intake and blood pressure level in population studies, “sodium sensitivity” is heterogeneous within populations. Typically sodium sensitivity is defined as an elevation of blood pressure with administration of exogenous sodium and, in comparison, a drop in pressure with volume depletion or decreased sodium intake. Less well appreciated is the fact that variations in sodium handling during stress can also demonstrate salt sensitivity. In some persons, exposure to stress leads to sodium retention and blood pressure elevation. In studies of stress-related sodium retention, renal tubular responses control the endogenous response. Indeed, studies of the effects of stress on sodium handling in salt-sensitive people may offer insight into how environmental factors influence the development of hypertension.
Sodium Intake and Blood Pressure
A positive association between sodium intake and blood pressure (BP) levels has been demonstrated in many animal and human studies (He and MacGregor 2006, 2009; Van Vliet and Montani 2008; Denton et al. 1995; Intersalt Cooperative Research Group 1988). A key animal study compared BP in 12 chimpanzees fed a diet that progressively increased its sodium content over several weeks to ten chimps fed their regular, low-sodium diet (Denton et al. 1995). Animals fed the high-salt diet had increasing BP that returned to baseline when the sodium intake returned to normal (Denton et al. 1995). Human population studies have shown that people fed a higher-salt diet have higher BPs than those consuming a modest salt intake (He and MacGregor 2009). The INTERSALT cross-sectional study conducted in the 1980s in 10,000 adults in 32 countries (Intersalt Cooperative Research Group 1988) confirmed this point. The influence of sodium intake on BP was recently verified in an even larger international study of >100,000 adults in 18 countries and with varying income levels (The PURE study) (Mente et al. 2014). In that study, BP increased by 2.11 mmHg systolic and 0.78 mmHg diastolic for each 1 gm increase in sodium intake. The findings in the PURE study correlated with age, the presence of hypertension, and sodium intake (Mente et al. 2014).
Similarly, in children a direct relation between salt intake and BP was appreciated by He et al., who evaluated cross-sectional data on 1,658 children and adolescents, ages 4–18 years, from the National Diet and Nutrition survey conducted in 1997 in the United Kingdom (He et al. 2008). In these free-living children, an increase in salt intake of 1 g (400 mg sodium) per day was associated with an increase in systolic BP and pulse pressure by 0.4 and 0.6 mmHg, respectively. Examination of NHANES (2003–2008) data from the United States also confirmed a positive association between BP and sodium intake (Yang et al. 2012). Analysis of those data on 6,235 US children and adolescents ages 8–18 years demonstrated a progressive rise in systolic BP with increasing sodium intake quartile with accentuation of this association in overweight/obese participants. When children and adolescents in the highest sodium intake quartile were compared to those in the lowest quartile, the odds ratio (OR) for high BP was 1.98 for the overall group but was 3.51 for overweight/obese participants. These results were extended by Rosner et al. who examined additional NHANES surveys to expand the sample size to 11,636 and assessed BP based on values for normal weight children (Rosner et al. 2013). Even after adjusting for obesity, these investigators found an increased risk for elevated BP (prehypertension or hypertension) in children consuming more than 1.5 times the recommended dietary intake for sodium (OR 1.36). This effect was independent of race, age, and gender. While casual BPs have primarily been utilized to assess the relationship between sodium intake and BP, a recent small study conducted in Portuguese children ages 8–9 years utilized ambulatory blood pressure monitoring (ABPM). The investigators found that for each gram of salt intake, daytime systolic pressure increased by 1.00 mmHg in overweight/obese boys only. These results may have been influenced by a greater salt intake in males or possibly earlier onset of puberty in the females (Correia-Costa et al. 2016). Lastly, a relation between sodium and BP in children has also been implied by many studies that have analyzed the ability of salt restriction to reduce BP. He and MacGregor performed a meta-analysis of ten controlled pediatric trials involving 966 participants, ages 8–16 years (He and MacGregor 2006). As the methods used to assess adherence to salt restriction differed between trials, the authors calculated the percent reduction in salt intake for the individual study for analysis. Using this approach, the median reduction in salt intake was 42% with an interquartile range of 7–58%. Pooled results showed significant decreases of −1.17 mmHg for systolic and −1.28 mmHg for diastolic pressures, though seemingly small such changes in blood pressure would be amplified if achieved population wide (He and MacGregor 2006).
Salt-Sensitive Populations
While the studies noted above support a relation between sodium and BP in unselected populations, this tendency is not universal but rather follows a Gaussian curve. This phenomenon was demonstrated by Weinberger et al., who evaluated the BP response to salt loading followed by volume depletion in 378 normotensive and 198 hypertensive persons (Weinberger et al. 1986). Participants were “loaded” with salt by infusion of 2 liters of 0.9% normal saline intravenously over 2 h and also received a high-salt diet during that time. The following day, volume depletion was induced by restricting dietary sodium intake to 10 mEq in conjunction with 40 mg of intravenous furosemide every 6 h for three doses. Salt sensitivity was defined by >10 mmHg difference in BPs obtained at completion of salt loading and at the end of volume depletion. Those participants demonstrating <5 mmHg change in pressure between the two periods (loading versus depletion) were considered salt resistant. Twenty-six percent and 51% of normotensive and hypertensive subjects, respectively, were classified as salt sensitive. A Gaussian distribution of BP response to salt loading and depletion was demonstrated for both hypertensive and normotensive subjects. Similarly, these authors demonstrated a normal distribution of salt sensitivity in normotensive adults fed a modestly salt-restricted diet (<80 mEq of sodium/day) for 3 months (Luft and Weinberger 1997). For both normotensive and hypertensive groups, salt-sensitive participants were more likely to be older than those who were salt resistant (Luft and Weinberger 1997; Weinberger et al. 1986).
Although older age is associated with salt sensitivity, an enhanced BP response to salt intake has been demonstrated in adolescents and young adults. In young adults, ages 18–23 years, Faulkner and Kushner demonstrated salt sensitivity by oral administration of 10 g of sodium chloride daily for 14 days (Falkner and Kushner 1990). Defining salt sensitivity as a >5% rise in mean arterial pressure, 31% were identified as salt sensitive overall, (41% of normotensives and 23% of hypertensives). These results contrast with those of Weinberger et al. above which the frequency of salt sensitivity in hypertensive subjects was twice that of normotensives (Weinberger et al. 1986). Apart from dissimilarities in definitions of salt sensitivity and protocols, these conflicting results may also be related to the aging phenomenon and differences in racial composition of the study populations. Blacks represented 69% of the cohort studied by Falkner and Kushner as compared to 22% of those evaluated by Weinberger et al. Long-term studies in young adults by Falkner and associates demonstrated an association between the BP response to oral sodium loading and change in BP over 5 years (Falkner et al. 1992). Further studies in pediatric and young adult populations have suggested that obesity, hyperinsulinemia, African-American race, family history of hypertension, and low birth weight are risk factors for salt sensitivity (Falkner and Kushner 1990; Falkner et al. 1981, 1992; Simonetti et al. 2008; de Boer et al. 2008; Rocchini et al. 1989).
Mechanisms Generating Salt Sensitivity
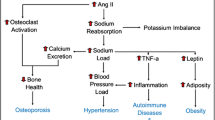
Several different mechanisms can result in the phenotype of salt sensitivity. Less sodium is filtered when there is a decrease in glomerular filtration rate (GFR), which contributes to hypertension in persons with chronic kidney disease. However, this mechanism is not a likely factor for most people with essential hypertension, as GFR is typically normal or near normal. Impaired sodium handling by the renal tubule has been frequently invoked in the pathogenesis of salt sensitivity, but the specific segment(s) and abnormality(ies) enhancing reabsorption of sodium have yet to be established. Investigation of monogenic disorders such as Liddle’s syndrome, apparent mineralocorticoid excess, and pseudohypoaldosteronism type II (see Chap. 7, “Monogenic and Polygenic Contributions to Hypertension”) has led to an appreciation that defects in the functioning of the epithelial sodium channel heighted response to mineralocorticoids in the distal tubule or alteration in activity of WNK proteins increase BP (Fujita 2014). Although monogenic conditions are rare, subtle abnormalities in distal tubule sodium reabsorption may prove to be involved in the pathogenesis of essential hypertension. Additionally, sodium handling in the proximal tubule, a site of action for angiotensin II and the sympathetic nervous system (SNS), may play a role in determining BP levels (Burnier et al. 2006). Using lithium excretion as a marker, Chiolero et al. were able to link failure to reduce proximal tubule sodium reabsorption in response to a high sodium intake with salt sensitivity in animals and humans (Chiolero et al. 2001). Extensive research into the role of various natriuretic and anti-natriuretic systems and possible gene or gene products involved in generating the phenotype of salt sensitivity have been conducted (Elijovich et al. 2016). Whatever the route to the phenotype, salt sensitivity is complex and likely dependent, in most cases, on the interaction of genetic factors, the environment, and physiological conditions.
Is Clinical Determination of Salt Sensitivity Important?
Classification of patients with hypertension as salt sensitive identifies them as being at greater risk for hypertensive target organ changes and cardiovascular morbidity as compared to salt-resistant patients. For example, Bihorac et al. found increased frequency of hypertensive retinopathy, left ventricular hypertrophy, microalbuminuria, and higher serum creatinine in salt-sensitive hypertensive patients as compared to salt-resistant hypertensives (Bihorac et al. 2000). A greater risk for cardiovascular events has also been linked with salt sensitivity (Morimoto et al. 1997). Furthermore, studies support the contention that long-term survival among persons with hypertension is influenced by salt sensitivity. Weinberger analyzed long-term data on normotensive and hypertensive adults who had been previously classified as salt sensitive or salt resistant (Weinberger et al. 2001). Salt-sensitive hypertensives had the poorest survival of all groups. Interestingly, persons who were normotensive but had been found to be salt sensitive demonstrated similar survival to hypertensives over time and had significantly reduced survival compared to normotensive salt-resistant adults. Identification of salt sensitivity may also be helpful in determining those patients for whom strict reduction in salt intake might not be advantageous (Mente et al. 2014; Kotchen et al. 2013). It has been suggested that sodium restriction may be detrimental in some populations such as patients with diabetes mellitus and patients with congestive heart failure on high-dose diuretics (Kotchen et al. 2013). Additionally investigators have raised concerns regarding long-term implications for salt-sensitive people who may have untoward metabolic and hormonal effects such as a reduction in insulin sensitivity and stimulation of the renin-angiotensin-aldosterone system (RAAS) (Kotchen et al. 2013; Graudal et al. 1998).
Additionally, pragmatically, the response to antihypertensive medications can be influenced by salt sensitivity. Weir et al. demonstrated that variation in salt intake influenced the response to an angiotensin-converting enzyme inhibitor (ACEi) versus a calcium channel blocker (Weir et al. 1998). Similar findings have been noted with other medications and non-pharmacologic interventions such as the Dietary Approaches to Stop Hypertension (DASH ) diet and weight loss protocols (Sacks et al. 2001; Weir et al. 2001, 2010; Hoffmann et al. 2008). For example, the DASH diet was most effective in lowering BP when sodium intake was also restricted (Sacks et al. 2001). Thus, failure to consider salt sensitivity may compromise management of patients and may complicate trials of antihypertensive medications whose efficacy may differ based on the presence or absence of salt sensitivity among study participants.
Identification of Salt Sensitivity
The heterogeneity of the response to sodium loading indicates a difference in sodium handling in salt-sensitive versus salt-resistant persons. At a given BP level, salt-sensitive persons demonstrate reduced sodium excretion compared to those who are salt resistant. For example, in a study of young adults, Falkner and Kushner (1990) observed a negative correlation between sodium excretion and change in mean BP after oral sodium loading in salt-sensitive participants (r = −0.28, p < 0.01). Similar findings have been reported by other investigators (Weinberger et al. 1986; Palacios et al. 2004; Rydstedt et al. 1986).
The complexity of protocols that assess salt sensitivity, as described above, and the resultant burden to patients have limited the clinical utility of establishing salt sensitivity (Nichols et al. 2012; Mattes and Falkner 1999). Thus, identification of salt sensitivity is most typically accomplished by demonstrating an increase in BP in response to sodium loading by either the enteral or parenteral routes. Novel approaches for the determination of salt sensitivity are under investigation. In a small study, Gidea et al. found that markers reflective of dopamine and/or angiotensin II activation can be identified in proximal renal tubule cells shed into the urine of patients previously identified as salt sensitive (Gildea et al. 2013). Additionally, analysis of ABPM to characterize an ABPM phenotype that predicts salt sensitivity has been promising (Castiglioni et al. 2013; Bursztyn and Ben-Dov 2013). Castiglioni et al. found that the combination of reduced nocturnal dipping and increased pulse pressure identified salt sensitivity in untreated hypertensive patients with a sensitivity of 74% and specificity of 78% (Castiglioni et al. 2013). However, other investigators have not demonstrated reproducibility (Elijovich et al. 2016); thus, further validation would be required.
Stress and the Demonstration of Salt Sensitivity
The tactics for determination of salt sensitivity mentioned above are based on the ability of sodium loading or volume/sodium restriction to elicit clinically significant changes in BP. A potential alternative method to demonstrate salt sensitivity involves taking advantage of the anti-natriuretic response to sympathetic nervous system (SNS) arousal to identify people who retain rather than excrete sodium during stress, referred to as stress-induced sodium retention or impaired stress-induced pressure natriuresis (Harshfield et al. 2009). The stress-induced sodium retention phenomenon is manifested by one in five Caucasians and one in three African-Americans who retain sodium in response to stress. This response adds a volume component to the resistance-mediated increase in blood pressure during stress, which remains elevated until the volume expansion diminishes and thereby increases the sodium-induced blood pressure load through the kidneys. To study this phenomenon, an increase in BP is induced by exposure to stress tasks, such as a 10 min social stressor interview, a 10 min virtual reality car driving test, or a 10 min competitive video game.
The primary evidence for using a stress-response approach to classifying salt sensitivity comes from two convergent lines of research in the animal literature. One is the investigation by psychologists of the mechanisms through which stress contributes to hypertension via its effects on renal sodium handling. These include the pioneering studies by Friedman (Friedman and Iwai 1976) using a chronic conflict avoidance task in Dahl hypertensive rats and the extensive studies by Koepke (see recent review by Harshfield et al. 2009). The other is the work of the renal physiologist DiBona (1992, 2002, 2003), which defined the mechanisms through which SNS activation of the renal nerves can contribute to the development of hypertension in at-risk animals. Further studies by additional investigators implicate anti-natriuretic actions of stress-induced efferent renal sympathetic activity in the development of hypertension directly and indirectly by SNS stimulation of renin activity with subsequent increase in angiotensin II levels (Veelken et al. 1996; Le Fevre et al. 2003; Wagner et al. 1999). Generation of angiotensin II is presumed due to the observed effects of anti-renin-angiotensin system medications in blocking the stress-induced response pattern (Veelken et al. 1996; Le Fevre et al. 2003; Wagner et al. 1999).
Factors Related to Stress-Induced Sodium Retention in Humans
Surprisingly there have been very few human studies that examined changes in sodium excretion during mental stress. However, we and others have demonstrated stress-induced sodium retention and identified several factors associate with this phenomenon in humans (summarized in Table 1). The initial study by Light in 1983 in young adult Caucasian men reported that sodium retention during mental stress occurred more commonly in those with borderline hypertension or a parental history of hypertension (defined as high risk) as compared to those with a negative family history and normal BP (Light et al. 1983). In a subsequent study, Light and Turner reported that sodium retention was associated with higher cardiac output and stroke volume during stress as compared to sodium excretion during stress (Light and Turner 1992). Reduced natriuresis with stress was noted in blacks and in those with a family history of hypertension. Our group replicated these findings in studies on healthy normotensive African-American adolescents (Harshfield et al. 2002a, b, 2007). After consuming a controlled sodium diet for 3 days prior to testing, African-American youth (ages 15–18 years) underwent a 5 h protocol that included 1 h of stress (Harshfield et al. 2002b). Thirty-two percent of the participants demonstrated impaired pressure natriuresis with sodium retention, leading to a volume-mediated rise in BP (Harshfield et al. 2002b). The other participants had a resistance-mediated rise in their pressure that resulted in natriuresis and a return of the BP to normal (see Fig. 1).
Stress-induced changes in systolic blood pressure and sodium excretion. Stress-induced changes in systolic blood pressure (SBP) shown in panel (a) and sodium excretion (mEq/h) shown in panel (b), based on direction of the stress-induced changes in sodium excretion. Data expressed as least square means. Data compared for baseline, stress condition, and recovery periods (Adapted and used with permission Harshfield et al. (2002b))
It is well recognized that obesity is present in epidemic proportions in children and adolescents. A series of studies has examined the impact of greater adiposity on sodium retention during stress. Barbeau et al. compared sodium handling during stress in lean versus overweight/obese black youth (Barbeau et al. 2003). The overweight/obese group displayed a significantly smaller stress-related increase in sodium excretion, despite a similar increase in BP. A subsequent study by Wilson et al. was performed on a cohort of 127 youths that included both black and white participants (Wilson et al. 2004). Percent body fat independently accounted for 4.6% of the variance of the stress-induced change in sodium excretion and 11.2% of the variance of the level of sodium excretion during stress. Another study of 151 boys and 141 girls reported that body mass index (BMI) was inversely related to sodium excretion during the stress period in boys (Harshfield et al. 2003). The magnitude of the correlation became greater when data from boys with a BMI >25 kg/m2 were analyzed.
The propensity for salt sensitivity in African-Americans and older persons has been well recognized. In concordance with racial differences noted in conventional sodium loading studies, Light and Turner reported that sodium retention during stress occurred more frequently in black adults compared to white adults (Light et al. 1983). Similarly in adolescents, Harshfield et al. found that sodium excretion in response to stress was significantly lower in blacks compared to whites; see Fig. 2 (Harshfield et al. 2002a). The stress-induced increase in urinary sodium excretion was only 2 + 6 mEq/h in African-American adolescents compared to 7 + 10 mEq/h in white adolescents. With regard to the effect of age, recent data from our group has demonstrated that the magnitude of stress-induced sodium retention increases with age (GA Harshfield, CD Hanevold, unpublished, Augusta University and University of Washington).
Racial differences in sodium excretion with stress exposure. Bars signify change in sodium excretion (Delta UNaV) expressed in mEq/h before and after stress with standard deviations (Figure generated from data in Harshfield et al. 2002a)
Sodium retention during stress has been linked to preclinical measures of target organ damage (Harshfield et al. 2009). Specifically, African-American adolescents who retained sodium during stress have a 10% greater albumin excretion rate than those that excrete sodium during stress (Hanevold et al. 2008). Furthermore, sodium retention was associated with cardiac remodeling (Harshfield et al. 2002a), degradation of endothelial function (Maya et al. 2006), and diastolic dysfunction (Kapuku et al. 2003).
Mechanisms Generating Stress-Induced Sodium Retention
The mechanisms underlying stress-induced salt sensitivity in humans have yet to be established. Studies in animals have supported the importance of the RAAS and the SNS in the genesis of impaired sodium handling during stress. Working with Dahl rats, Koepke et al. demonstrated urine sodium retention when sodium loading was followed by behavioral stress (Koepke et al. 1983). Administration of propranolol followed by the same procedure resulted in a higher sodium excretion. Treatment with other beta-blockers characterized by less central nervous system penetration under the same protocol showed impaired sodium excretion. Of note, Light et al. were not able to replicate this effect of beta-blockers in humans (Light 1992). A role for the SNS was also supported by work in Sprague-Dawley rats subjected to air stress (Veelken et al. 1996). In these studies, anti-natriuresis was shown to be associated with renal sympathetic nerve activity and was abolished by renal denervation or by pretreatment with an angiotensin receptor blocker (ARB). A recent study also highlighted the role of RAAS system and the importance of angiotensin II in generating salt sensitive increases BP in response to stress (Loria et al. 2015). Independent from angiotensin II, in this Dahl rat model, salt sensitivity did not significantly impact BP patterns in response to stress.
Drawing on the above animal studies, mechanistic studies in humans have focused on the role of the RAAS (as summarized in Table 2). Treatment for a month with an ACEi improved sodium excretion in Caucasian hypertensive adults as compared to those treated with placebo (Fauvel et al. 1994). Similarly, other investigators have demonstrated that treatment with an ACEi lessened sodium retention (Schneider et al. 2001; Rollnik et al. 1995) in Caucasian adults. We recently expanded these findings to treatment with an ARB in a normotensive African-American population (GA Harshfield, CD Hanevold, DL Stewart, LA Ortiz, V George, SK Mathur, unpublished data, Augusta University and University of Washington). The study demonstrated a change from sodium retention to sodium excretion during stress with pretreatment with an ARB. The BP response to stress was also reduced.
Data on stress-induced sodium retention in people with a family history of hypertension have been conflicting. Two studies reported that stress-induced sodium retention was greater in persons with a positive family history (Light et al. 1983; Harshfield et al. 2002a), while two studies did not find differences between persons with or without a family history of hypertension (Ducher et al. 2002; Schneider et al. 2001). Subsequent investigation in twins suggested racial differences in heritability for sodium excretion during stress, which was greater in blacks (58%) than in whites (42%) (Harshfield et al. 2009). Furthermore, these heritabilities could be attributed to genes that were only expressed under stress. The stress-specific genetic influences were twice as large in blacks (47%) as compared to whites (23%). Approximately 40% of the individual differences in the sodium excretion in response to stress could be explained by genetic factors in both blacks and whites. Additionally, a subsequent genetic study identified the potential significance of the G protein-coupled receptor kinase 4 (GRK4) in sodium handling and hypertension (Zhu et al. 2006). Overall, these studies suggest a role for a genetic contribution to sodium retention. However, the specific genes involved in this complex response pattern (or trait) remain to be established.
Conclusions
Identification of salt sensitivity carries important therapeutic and prognostic implications. Unfortunately, definitions and methodologies utilized to characterize salt sensitivity have varied between studies making comparisons of the findings challenging. From a practical standpoint, there is no reasonable way to identify a salt-sensitive person with administration of a sodium load outside of a research setting. In the office setting, salt sensitivity may be implied if a patient’s BP improves with salt restriction. However, this pragmatic approach is complicated by confounding factors, particularly uncertainty about the reliability of adherence. Demonstration of stress-induced sodium retention is an alternative tactic to conventional sodium loading and could allow for a tailored approach to BP control. However, utility has been limited thus far to the research arena, and clinical applicability requires further study. Furthermore, the mechanisms underlying stress-induced sodium retention need further investigation. Reversal of stress-induced sodium retention with renal denervation in animals and with blocking of the RAAS in humans suggests that interplay between these systems results in sodium retention during stress. Further studies exploring the link between the brain and the kidney are indicated.
Abbreviations
- ABPM:
-
Ambulatory blood pressure monitoring
- ACEi:
-
Angiotensin-converting enzyme inhibitor
- ARB:
-
Angiotensin receptor blocker
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- DASH:
-
Dietary Approaches to Stop Hypertension
- GFR:
-
Glomerular filtration rate
- GRK4:
-
G protein-coupled receptor kinase 4
- OR:
-
Odds ratio
- RAAS:
-
Renin-angiotensin-aldosterone system
- SNS:
-
Sympathetic nervous system
References
Barbeau P, Litaker MS, Harshfield GA (2003) Impaired pressure natriuresis in obese youths. Obes Res 11(6):745–751
Bihorac A, Tezcan H, Özener Ç, Oktay A, Akoglu E (2000) Association between salt sensitivity and target organ damage in essential hypertension. Am J Hypertens 13(8):864–872
Burnier M, Bochud M, Maillard M (2006) Proximal tubular function and salt sensitivity. Curr Hypertens Reps 8(1):8–15
Bursztyn M, Ben-Dov IZ (2013) Sex differences in salt-sensitivity risk approximated from ambulatory blood pressure monitoring and mortality. J Hypertens 31(5):900–905
Castiglioni P, Parati G, Brambilla L, Brambilla V, Gualerzi M, Di Rienzo M, Coruzzi P (2013) A new index of sodium sensitivity risk from arterial blood pressure monitoring during habitual salt intake. Int J Cardiol 168(4):4523–4525
Chiolero A, Würzner G, Burnier M (2001) Renal determinants of the salt sensitivity of blood pressure. Nephrol Dial Transplant 16(3):452–458
Correia-Costa L, Cosme D, Nogueira-Silva L, Morato M, Sousa T, Moura C, Mota C, Guerra A, Albino-Teixeira A, Areias JC, Schaefer F, Lopes C, Afonso AC, Azevedo A (2016) Gender and obesity modify the impact of salt intake on blood pressure in children. Pediatr Nephrol 31(2):279–288
de Boer MP, IJzerman RG, de Jongh RT, Eringa EC, Stehouwer CD, Smulders YM, Serné EH (2008) Birth weight relates to salt sensitivity of blood pressure in healthy adults. Hypertension 51(4):928–932
Denton D, Weisinger R, Mundy NI, Wickings EJ, Dixson A, Moisson P, Pingard AM, Shade R, Carey D, Ardaillou R et al (1995) The effect of increased salt intake on blood pressure of chimpanzees. Nat Med 1(10):1009–1016
DiBona GF (1992) Sympathetic neural control of the kidney in hypertension. Hypertension 19 (1 Suppl):I28–I35. Epub 1992/01/01. PubMed PMID: 1730452
DiBona GF (2002) Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypert 11(2):197–200. Epub 2002/02/22. PubMed PMID: 11856913
DiBona GF (2003) Neural control of the kidney: past, present, and future. Hypertension 41(3 Pt 2):621–624. Epub 2003/03/08
Ducher M, Bertram D, Pozet N, Laville M, Fauvel JP (2002) Stress-induced renal alterations in normotensives offspring of hypertensives and in hypertensives. Am J Hypertens 15(4):346–350
Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL, American Heart Association Professional, Public Education Committee of the Council on Hypertension, Council on Functional Genomics, Translational Biology, Stroke Council (2016) Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 68(3):e7–e46
Falkner B, Kushner H (1990) Effect of chronic sodium loading on cardiovascular response in young blacks and whites. Hypertension 15(1):36–43
Falkner B, Onesti G, Angelakos E (1981) Effect of salt loading on the cardiovascular response to stress in adolescents. Hypertension 3(6 Pt 2):II-195
Falkner B, Hulman S, Kushner H (1992) Hyperinsulinemia and blood pressure sensitivity to sodium in young blacks. J Am Soc Nephrol 3(4):940–946
Fauvel J, Laville M, Bernard N, Hadj-Aissa A, Daoud S, Thibout E, Pozet N, Zech P (1994) Effects of lisinopril on stress-induced peak blood pressure and sodium excretion: a double-blind controlled study. J Cardiovasc Pharmacol 23(2):227–231
Friedman R, Iwai J (1976) Genetic predisposition and stress-induced hypertension. Science 193(4248):161–162
Fujita T (2014) Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol 25(6):1148–1155
Gildea JJ, Lahiff DT, Van Sciver RE, Weiss RS, Shah N, McGrath HE, Schoeffel CD, Jose PA, Carey RM, Felder RA (2013) A linear relationship between the ex-vivo sodium mediated expression of two sodium regulatory pathways as a surrogate marker of salt sensitivity of blood pressure in exfoliated human renal proximal tubule cells: the virtual renal biopsy. Clin Chim Acta; Int J Clin Chem 421:236–242
Graudal NA, Galloe AM, Garred P (1998) Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA 279(17):1383–1391
Hanevold CD, Pollock JS, Harshfield GA (2008) Racial differences in microalbumin excretion in healthy adolescents. Hypertension 51(2):334–338
Harshfield GA, Treiber FA, Davis H, Kapuku GK (2002a) Impaired stress-induced pressure natriuresis is related to left ventricle structure in blacks. Hypertension 39(4):844–847
Harshfield GA, Wilson M, Hanevold C, Kapuku G, Mackey L, Gillis D, Treiber F (2002b) Impaired stress-induced pressure natriuresis increases cardiovascular load in African American youths. Am J Hypertens 15(10):903–906
Harshfield GA, Wilson ME, McLeod K, Hanevold C, Kapuku GK, Mackey L, Gillis D, Edmonds L (2003) Adiposity is related to gender differences in impaired stress-induced pressure natriuresis. Hypertension 42(6):1082–1086
Harshfield GA, Hanevold C, Kapuku GK, Dong Y, Castles ME, Ludwig DA (2007) The association of race and sex to the pressure natriuresis response to stress. Ethn Dis 17(3):498–502
Harshfield GA, Dong Y, Kapuku GK, Zhu H, Hanevold CD (2009) Stress-induced sodium retention and hypertension: a review and hypothesis. Curr Hypertens Rep 11(1):29–34
Harshfield G, Hanevold C, Ortiz L, Nwobi O, Johnson M, Stewart D (2013) Suppression of Ang II inhibits sodium retention during mental stress. Hypertension 62:A634
He FJ, MacGregor GA (2006) Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension 48(5):861–869
He FJ, MacGregor GA (2009) A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens 23(6):363–384
He FJ, Marrero NM, Macgregor GA (2008) Salt and blood pressure in children and adolescents. J Hum Hypertens 22(1):4–11
Hoffmann IS, Alfieri AB, Cubeddu LX (2008) Salt-resistant and salt-sensitive phenotypes determine the sensitivity of blood pressure to weight loss in overweight/obese patients. J Clin Hypertens (Greenwich) 10(5):355–361
Intersalt (1988) Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 297(6644):319–328
Jackson RW, Treiber FA, Harshfield GA, Waller JL, Pollock JS, Pollock DM (2001) Urinary excretion of vasoactive factors are correlated to sodium excretion. Am J Hypertens 14(10):1003–1006
Kapuku G, Harshfield G, Wilson M, Mackey L, Gillis D, Edmunds L, Hartley B, Treiber F (2003) Impaired pressure natriuresis is associated with preclinical markers of abnormal cardiac structure and function. Am J Hypetens 16:211A. (abstract)
Koepke JP, Grignolo A, Light KC, Obrist PA (1983) Central beta adrenoceptor mediation of the antinatriuretic response to behavioral stress in conscious dogs. J Pharmacol Exp Ther 227(1):73–77
Kotchen TA, Cowley AW Jr, Frohlich ED (2013) Salt in health and disease--a delicate balance. N Engl J Med 368(26):2531–2532
Le Fevre ME, Guild S-J, Ramchandra R, Barrett CJ, Malpas SC (2003) Role of angiotensin II in the neural control of renal function. Hypertension 41(3):583–591
Light KC (1992) Differential responses to salt intake-stress interactions. In: Turner JR, Sherwood A, Light KC (eds) Individual differences in cardiovascular response to stress, Perspectives on individual differences, vol 1. Plenum Press, New York, pp 245–263
Light KC, Turner JR (1992) Stress-induced changes in the rate of sodium excretion in healthy black and white men. J Psychosom Res 36(5):497–508
Light KC, Koepke JP, Obrist PA, Willis PW (1983) Psychological stress induces sodium and fluid retention in men at high risk for hypertension. Science 220(4595):429–431
Loria AS, Pollock DM, Pollock JS (2015) Angiotensin II is required to induce exaggerated salt sensitivity in Dahl rats exposed to maternal separation. Physiol Rep 3(5):e12408
Luft FC, Weinberger MH (1997) Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am J Clin Nutr 65(2 Suppl):612S–617S
Mathur S, Pollock D, Pollock J, Harshfield GA (2015) Impact of urinary endothelin-1 on derangements in stress-induced pressure natriuresis. Hypertension 66(Suppl 1):P642
Mattes RD, Falkner B (1999) Salt-sensitivity classification in normotensive adults. Clin Sci 96(5):449–459
Maya E, Harshfield G, Kapuku G (2006) Impaired stress induced pressure natriuresis clusters with reduced endothelial function in African American youth at risk of hypertension. International Society of Hypertension in Blacks, Atlanta, p 50
Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S, Investigators P (2014) Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371(7):601–611
Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T , Kimura G (1997) Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350 (9093):1734–1737. S0140-6736(97)05189-1[pii]
Nichols J, Elijovich F, Laffer CL (2012) Lack of validation of a same-day outpatient protocol for determination of salt sensitivity of blood pressure. Hypertension 59(2):390–394
Palacios C, Wigertz K, Martin BR, Jackman L, Pratt JH, Peacock M, McCabe G, Weaver CM (2004) Sodium retention in black and white female adolescents in response to salt intake. J Clin Endocrinol Metab 89(4):1858–1863
Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M (1989) The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med 321(9):580–585
Rollnik JD, Mills PJ, Dimsdale JE (1995) Characteristics of individuals who excrete versus retain sodium under stress. J Psychosom Res 39(4):499–505
Rosner B, Cook NR, Daniels S, Falkner B (2013) Childhood blood pressure trends and risk factors for high blood pressure: the NHANES experience 1988–2008. Hypertension 62:247–254
Rydstedt LL, Williams GH, Hollenberg NK (1986) Renal and endocrine response to saline infusion in essential hypertension. Hypertension 8(3):217–222
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG (2001) Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. New Engl J Med 344(1):3–10
Schneider MP, Klingbeil AU, Schlaich MP, Langenfeld MR, Veelken R, Schmieder RE (2001) Impaired sodium excretion during mental stress in mild essential hypertension. Hypertension 37(3):923–927
Simonetti GD, Raio L, Surbek D, Nelle M, Frey FJ, Mohaupt MG (2008) Salt sensitivity of children with low birth weight. Hypertension 52(4):625–630
Van Vliet BN , Montani JP (2008) The time course of salt-induced hypertension, and why it matters. Int J Obes (Lond) 32 Suppl 6:S35-S47. ijo2008205 [pii]
Veelken R, Hilgers KF, Stetter A, Siebert HG, Schmieder RE, Mann JF (1996) Nerve-mediated antidiuresis and antinatriuresis after air-jet stress is modulated by angiotensin II. Hypertension 28(5):825–832
Wagner C, Hinder M, Krämer BK, Kurtz A (1999) Role of renal nerves in the stimulation of the renin system by reduced renal arterial pressure. Hypertension 34(5):1101–1105
Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg N (1986) Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 8(Suppl II):II-127–II-134
Weir MR, Chrysant SG, McCarron DA, Canossa-Terris M, Cohen JD, Gunter PA, Lewin AJ, Mennella RF, Kirkegaard LW, Hamilton JH, Weinberger MH, Weder AB (1998) Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin-converting enzyme inhibitor or a calcium channel antagonist in salt-sensitive hypertensives. Hypertension 31(5):1088–1096
Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M (2001) Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37(2):429–432
Weir MR, Smith DH, Neutel JM, Bedigian MP (2001) Valsartan alone or with a diuretic or ACE inhibitor as treatment for African American hypertensives: relation to salt intake. Am J Hypertens 14(7):665–671
Weir MR, Yadao AM, Purkayastha D, Charney AN (2010) Effects of high-and low-sodium diets on ambulatory blood pressure in patients with hypertension receiving aliskiren. J Cardiovasc Pharmacol Ther 15:356–363
Wilson ME, Harshfield GA, Ortiz L, Hanevold C, Kapuka G, Mackey L, Gillis D, Edmonds L, Evans C (2004) Relationship of body composition to stress-induced pressure natriuresis in youth. Am J Hypertens 17(11 Pt 1):1023–1028
Yang Q, Zhang Z, Kuklina EV, Fang J, Ayala C, Hong Y, Loustalot F, Dai S, Gunn JP, Tian N, Cogswell ME, Merritt R (2012) Sodium intake and blood pressure among US children and adolescents. Pediatrics 130(4):611–619
Zhu H, Lu Y, Wang X, Snieder H, Treiber FA, Harshfield GA , Dong Y (2006) The G protein-coupled receptor kinase 4 gene modulates stress-induced sodium excretion in black normotensive adolescents. Pediatr Res 60 (4):440–442. 01.pdr.0000238250.64591.44 [pii]
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG (outside the USA)
About this entry
Cite this entry
Hanevold, C.D., Harshfield, G.A. (2018). Stress and Salt Sensitivity in Childhood Hypertension. In: Flynn, J., Ingelfinger, J., Redwine, K. (eds) Pediatric Hypertension. Springer, Cham. https://doi.org/10.1007/978-3-319-31107-4_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-31107-4_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31106-7
Online ISBN: 978-3-319-31107-4
eBook Packages: MedicineReference Module Medicine