Abstract
Intracranial germ cell tumors (GCTs) are a group of relatively uncommon tumors, comprising less than 1 % of all central nervous system (CNS) tumors, that have histological, genetic, biochemical, diagnostic, and therapeutic similarities to GCTs that occur outside the CNS (Ostrom et al. 2014). In this chapter, we review the epidemiology of intracranial GCTs, the pathologic features of both benign and malignant GCTs, and their molecular and cytogenetic characteristics. We discuss the clinical features of intracranial GCTs and the role of imaging and laboratory investigations in diagnosis. In broaching the controversy surrounding diagnostic biopsy, we delineate the arguments for and against mandatory biopsy prior to treatment. We review recent changes in practice with de-escalation in radiotherapy and chemotherapy treatment approaches. Lastly, we discuss risk stratification to intensify treatment in patients with intracranial GCTs that have a poor prognosis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
Intracranial germ cell tumors (GCTs) are a group of relatively uncommon tumors, comprising less than 1 % of all central nervous system (CNS) tumors, that have histological, genetic, biochemical, diagnostic, and therapeutic similarities to GCTs that occur outside the CNS (Ostrom et al. 2014). They share extraembryonic origins in the fetal yolk sac that account for their numerous similarities, while subsequent migratory paths in early fetal development are responsible for differences in location. There are two distinct histological groups within the larger group of intracranial GCTs: germinomas and nongerminomatous GCTs (NGGCTs). Germinomas are more common, accounting for 50–75 % of the total number of intracranial GCT (Al-Hussaini et al. 2009; Jooma and Kendall 1983; Oi and Matsumoto 1992). NGGCTs represent one third of intracranial GCTs and consist of embryonal carcinoma, endodermal sinus (yolk sac) tumor, choriocarcinoma, teratoma, and GCTs of mixed cellular origin. Jennings et al. found that germinomas accounted for 65 % of intracranial GCTs, followed by teratomas (18 %), endodermal sinus tumors (7 %), embryonal carcinomas (5 %), and choriocarcinomas (5 %) (Jennings et al. 1985b). Other studies show a higher incidence of mixed tumors ranging from 21 % to 32 % (Matsutani et al. 1997; Salzman et al. 1997). As classified by the WHO, although most of these tumors are malignant, some are defined as benign (Table 6.1) (Louis et al. 2007).
Intracranial GCTs most commonly arise from the pineal or suprasellar region – deep locations that have reduced the likelihood of gross total resection. For this reason, radiation alone, frequently encompassing a large treatment volume, was considered the preferred treatment standard. Over the last several decades, effective chemotherapy in combination with improved neurosurgical procedures and radiation techniques has resulted in dramatic improvements in survival. However, in children, the morbidity caused by radiation therapy, particularly with craniospinal irradiation (CSI), has led to de-escalation in volume and dose of radiotherapy while preserving high cure rates for patients with only focal disease (Shirato et al. 1997; Choi et al. 1998; Matsutani et al. 1998; Aoyama et al. 2002; Jensen et al. 2010).
In this chapter, we review the epidemiology of intracranial GCTs, the pathologic features of both benign and malignant GCTs, and their molecular and cytogenetic characteristics. We discuss the clinical features of intracranial GCTs and the role of imaging and laboratory investigations in diagnosis. In broaching the controversy surrounding diagnostic biopsy, we delineate the arguments for and against mandatory biopsy prior to treatment. We review recent changes in practice with de-escalation in radiotherapy and chemotherapy treatment approaches. Lastly, we discuss risk stratification to intensify treatment in patients with intracranial GCTs that have a poor prognosis.
6.2 Epidemiology
6.2.1 Location
Intracranial GCTs account for less than 4 % of pediatric brain tumors in North America, although slightly more common in Japan with an incidence of greater than 10 % among pediatric brain tumors. Most intracranial GCTs originate near the third ventricle, extending from the suprasellar cistern to the pineal gland. Pineal region GCTs outnumber those in the suprasellar region by a ratio of 2:1, but in 5–10 % of cases, the tumor is found in both regions (Jennings et al. 1985b). Whether this is due to synchronous bifocal disease or metastatic tumor, the spread remains unknown. Intracranial GCTs occur less commonly in other midline locations such as basal ganglia, thalamus, and ventricles, particularly the fourth ventricle. Intracranial GCTs have also been reported in the cerebellum (Nakase et al. 1994), brainstem (Nakajima et al. 2000; Madden et al. 2009; Hao et al. 2013), and optic nerves (Iizuka et al. 1996). By GCT subtype, germinomas are more frequent in the suprasellar region and in females, while NGGCTs are more common in the pineal region and in males.
6.2.2 Age, Sex, and Geographic Variation
In Western countries, intracranial GCTs account for 0.4–3.4 % of all intracranial tumors, whereas in Japan and Taiwan, intracranial GCTs are more common and account for 2.1–11.1 % of brain tumors (Jellinger 1973; Jennings et al. 1985b; Hoffman et al. 1991; Lin et al. 1997). This phenomenon is also seen in testicular GCTs for which the incidence in Japan is far greater than that seen in the United States (Packer et al. 2000). Most intracranial GCTs occur in adolescents and young adults (80–90 %), with peak incidence occurring at 10–14 years of age. However, these lesions can be seen in newborns as well as in older adults. In particular, NGGCTs preferentially arise in younger children, whereas germinomas are most common in teenagers (Jennings et al. 1985b; Rosenblum et al. 2007).
Intracranial GCTs are not distributed equally by gender. In the United States, between 1986 and 1995, incidence rates were 2.3 per million for males and 0.9 per million for females, representing a male predominance of 2.5:1. When examined by histology, NGGCTs demonstrate a male/female ratio of 3.2:1, while germinomas reveal a male:female ratio of only 1.8:1 (Jennings et al. 1985b). In females, 75 % of intracranial GCTs develop in the suprasellar region, whereas in males 70 % are found in the pineal area. The reason for these gender differences is unclear. Between the 1970s and the 1990s, the incidence of intracranial GCTs increased in the United States from 0.6 per million between 1975 and 1979 to 1.9 per million between 1990 and 1995 (Bernstein et al. 1999).
6.3 Pathology
6.3.1 Etiology
GCTs can be divided into extragonadal tumors and gonadal tumors, the latter encompassing half of all such tumors. Among extragonadal sites, half are sacrococcygeal and 40 % arise intracranially. Rare sites of extragonadal GCTs include midline regions such as the retroperitoneal and nasopharynx. Their sites of origin notwithstanding the features of GCTs, whether by light microscopy, electron microscopy, or enzyme or immunohistochemical assays, are identical (Jennings et al. 1985b; Felix and Becker 1990).
The pathogenesis of intracranial GCTs remains elusive. Although gonadotropins have been implicated in the pathogenesis of gonadal GCTs, such evidence for intracranial GCTs is lacking. One hypothesis is that GCTs arise most commonly near centers of gonadotropin regulation because such regions serve as sanctuary sites for undifferentiated germ cells (Jennings et al. 1985b). An additional role for the pineal gland in the neuroendocrine regulation of neoplastic growth has also been suggested (Lapin and Ebels 1981).
The etiology of intracranial GCTs is thought to be mismigration of primordial germ cells during embryonic development, followed by malignant transformation. According to the “germ cell theory,” primordial germ cells normally develop from the extraembryonic yolk sac endoderm and migrate to the gonadal folds. Germinomas as well as embryonal carcinomas can develop by further differentiation and malignant transformation of the original primordial germ cells. Embryonal carcinomas are composed of pluripotent cells that develop into endodermal sinus tumors, choriocarcinomas, or teratomas depending on the developmental pathway the cells undertake (Teilum 1976). Others have suggested that primordial germ cells can differentiate to yield either embryonal carcinomas or teratomas by differentiation through embryonic pathways or endodermal sinus tumors or choriocarcinomas by extraembryonic pathways (Takei and Pearl 1981).
The “germ cell theory” is supported by the fact that interaction of the c-kit receptor with its ligand, steel factor (SLF), mediates the migration of primordial germ cells. Lack of c-kit in animal models prevents germ cell migration. The gradient of SLF found from the yolk sac to the gonadal ridge is thought to guide the migration of primordial germ cells, and extragonadal GCTs are thought to arise from such mismigration. The proto-oncogene c-kit encodes a cell-surface receptor that carries an intrinsic tyrosine kinase activity in its cytoplasmic portion. The interaction of Kit with SLF leads to receptor dimerization, kinase activation, and tyrosine phosphorylation of specific cytoplasmic proteins. Mutations in Kit and SLF that result in a defective signaling pathway leading to infertility have been identified (Loveland and Schlatt 1997; Cushing et al. 2002).
An alternative theory, the “embryonic cell theory,” suggests that a pluripotent embryonic cell escapes normal developmental signals and gives rise to GCTs. This theory may be supported by the finding of altered Wnt pathway signaling components including distinct expression levels of E-cadherin and beta-catenin in various GCTs (Honecker et al. 2004; Snow et al. 2009). A third hypothesis contends that germinoma is the only neoplasm arising from germ cells, and other GCTs arise from misfolding and misplacement of embryonic cells into the lateral mesoderm early in embryogenesis, leading to the entrapment of these cells into a variety of different brain regions (Sano et al. 1989). And finally, a more recent hypothesis argues that neural stem cells, because of their pluripotent potential in vitro, may be the initiating cell for intracranial germ cell lesions (Hoei-Hansen et al. 2006; Tan and Scotting 2013).
6.3.2 Classification
The current World Health Organization (WHO) classification of GCTs is based on histology and tumor markers such as alpha-fetoprotein (AFP) and beta-human chorionic gonadotropin (β-HCG) that have become important in diagnosis as well as prognosis (Louis et al. 2007). As mentioned above, different GCTs may represent the malignant forms of distinct stages of normal embryonic development. For example, primordial germ cells result in germinomas, embryonic differentiation gives rise to teratomas and embryonal carcinomas, and extraembryonic derivatives of the yolk sac and trophoblast give rise to endodermal sinus tumors and choriocarcinomas, respectively. Intracranial GCTs can also be classified based on tumor markers found in serum or cerebrospinal fluid (CSF), which can influence diagnoses and prognoses of patients with intracranial GCTs. Typically, germinomas are nonsecreting tumors, whereas NGGCTs usually secrete AFP and/or β-HCG. Germinomas are associated with better prognoses than NGGCTs.
6.3.3 Histopathology
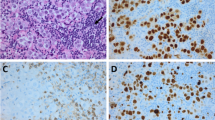
Intracranial germinomas are histologically identical to dysgerminomas of the ovary and seminomas of the testis (Beeley et al. 1973). Microscopically, they are large monomorphic cells with abundant clear cytoplasm, arranged in nests separated by bands of connective tissue. The differential diagnosis includes lymphoma and endodermal sinus tumor. Germinomas can be identified by either positive placental alkaline phosphatase (PLAP) staining or positive OCT4 staining, with the latter being increasingly adopted as superior, whereas endodermal sinus tumors stain positive for AFP (Hattab et al. 2005).
Among NGGCTs, teratomas are designated as mature or immature, based on the absence or presence of differentiated tissues. Mature teratomas contain mature tissues from all three embryonic layers (ectoderm, mesoderm, and endoderm). Immature teratomas are distinguished from mature teratomas by the presence of immature tissues, usually neuroepithelium. Embryonal carcinomas arise from pluripotent embryonic cells and are characterized by large cells with large nuclei and nucleoli with varying amounts of central necrosis. Embryonal carcinomas can produce both AFP and β-HCG. Unlike other GCTs, CD30 (Ki-1 antigen) immunohistochemical staining is positive in embryonal carcinomas. Endodermal sinus tumors arise from differentiated extraembryonic tissue, which usually occur as part of mixed GCTs, and produce AFP. Choriocarcinomas arise from placental trophoblastic tissue, which also generally occur as part of mixed GCTs, and are characterized by the presence of syncytiotrophoblasts that secrete β-HCG (Felix and Becker 1990; Hawkins 1990; Cushing et al. 2002).
6.3.4 Molecular Biology and Cytogenetics
Multiple complex karyotypes have been reported for intracranial GCTs including loss of chromosomes 4, 9p, 11, 13, and 17p as well as gain of chromosomes 8q, 21, and 1q. In addition, whereas isochromosome 12p seems to be important in the development of testicular tumors, its role in intracranial germ cell tumors is unclear. Multiple early studies observed low incidence in extragonadal tumors (de Bruin et al. 1994; Yu et al. 1995; Lemos et al. 1998), but isochromosome 12p has been found at more modest levels of 25 % in a more recent series (Sukov et al. 2010 A study of comparative genomic hybridization to analyze pineal region GCTs reported various abnormalities including gains on 12p (40 %), 8q (27 %), and 1q (20 %), as well as losses on 13q (47 %), 18q (33 %), 9q, and 11q (20 % each). The authors also noted different cytogenetic abnormalities based on histology. For example, the most common chromosomal changes in germinomas were −13q and −18q (38 % each), whereas in mixed teratomas, germinomas frequent abnormalities included +8q (100 %), +12p (75 %), −13q (75 %), and −9q (50 %) (Rickert et al. 2000). A recent series confirmed high-frequency (46 %) 12p polysomy (Sukov et al. 2010). Okada et al. examined 25 intracranial GCTs and found an increased number of X chromosomes in 23/25 cases and noted hypomethylation of the additional X chromosome in 81 % of the tumors. Only 20 % of cases had increased copy number of 12p and 12 % had loss of 13q. They concluded that along with the increased incidence of intracranial GCTs in males as well as predisposition in patients with Klinefelter syndrome, sex chromosome aberrations might have an important role in the development of GCTs (Okada et al. 2002).
In addition to cytogenetic changes, some of the genes that may be important in the development of GCTs have been defined. Alterations in the mdm-2 gene, often amplified in sarcomas, have been implicated in tumorigenesis of some testicular and intracranial GCTs. The mdm-2 is a negative regulator of the p53 tumor suppressor gene product and is, in turn, induced by p53. Iwato et al. searched for p53 mutations and mdm-2 amplifications in intracranial GCTs and found mdm-2 amplifications in 19 % of intracranial GCTs. Theoretically, increases in mdm-2 protein level would antagonize p53 function (Iwato et al. 2000b).
Iwato et al. examined the INK4a/ARF locus for alterations in intracranial GCTs and found alterations in 71 % of 21 tumors. The INK4a/ARF genes are tumor suppressor genes, and the INK4a protein inhibits cyclin-dependent kinases and decreases phosphorylation of the retinoblastoma protein, resulting in cell cycle arrest. The ARF protein interacts with mdm-2 and stimulates the latter’s degradation. Interestingly, alterations in INK4a/ARF were more common in germinomas (90 %) than in NGGCTs (55 %) (Iwato et al. 2000a).
A recent next-generation sequencing analysis of 62 intracranial GCT demonstrated frequent (53 %) novel somatic mutations in the KIT/RAS signaling pathway including KIT, KRAS, NRAS, CBL, and AKT1 (Wang et al. 2014). KIT mutations and overexpression were principally observed in germinomas, predominantly clustered in exons 17 and 11. Copy number gains were noted in AKT1 at 14q32.33 in 19 % of patients, the majority of which had wild-type KIT, KRAS, and NRAS. Loss-of-function mutations and loss of heterozygosity were noted in the tumor suppressors BCORL1 and CBL, respectively.
6.4 Clinical Features: Signs and Symptoms
Presenting symptoms of pineal region tumors are directly related to tumor location. Pineal region tumors usually present with symptoms of eye-movement disorders or symptoms caused by increased intracranial pressure due to obstructive hydrocephalus. Headache, nausea, and vomiting are the most common symptoms, seen in 56–93 % of patients. Blurred vision and somnolence are seen in 20–54 % of patients, while ataxia, seizures, and behavioral disturbances are seen in 10–28 % of patients (Saitoh et al. 1991; Drummond and Rosenfeld 1999; Steinbok and Cochrane 2001). Involvement of adjacent midbrain structures can result in visual disturbances, such as Parinaud’s syndrome, which are seen in 25–50 % of pineal region GCTs. Parinaud’s syndrome is an impairment of upward gaze in combination with dilated pupils that are nonreactive to light (pseudo-Argyll Robertson pupils), but responsive to accommodation. Upward gaze may in addition elicit rhythmic convergence of the eyes followed by retraction nystagmus of the eyes into the orbits. Eyelid retraction and conjugate downward gaze in the primary position (sun-setting sign) may be observed (Jennings et al. 1985b).
On examination, papilledema is present in about half of the patients. In patients with pineal region GCTs, approximately 80 % present with symptoms of increased intracranial pressure, whereas less than 10 % of patients with suprasellar GCTs present with increased intracranial pressure. Endocrinopathies such as diabetes insipidus or precocious puberty occur in patients with intracranial GCTs and account for approximately 6–12 % of presenting symptoms. In fact, patients with suprasellar GCTs most commonly present with endocrinopathies such as diabetes insipidus and manifestations of anterior pituitary dysfunction such as growth failure. These symptoms were seen in 87 % of patients versus only 8 % of patients with pineal region GCT (Jooma and Kendall 1983; Edwards et al. 1988; Hoffman et al. 1991; Saitoh et al. 1991; Kang et al. 1998; Steinbok and Cochrane 2001). Intracranial GCTs may infiltrate adjacent structures such as the hypothalamus (11 %) and third ventricle (22 %) or disseminate throughout the CSF (10 %). For endodermal sinus tumors and choriocarcinomas, dissemination is more common, and third ventricular involvement is present in over 40 % of cases. Extracranial spread to the lungs and bones has also been reported in approximately 3 % of patients (Gay et al. 1985; Jennings et al. 1985a, b).
6.5 Diagnosis and Staging
Operative morbidity and mortality prior to the 1980s were high and impeded histological diagnoses of many intracranial GCTs. Therefore, radiodiagnostic trials of 20 Gy historically functioned as surrogates for a histological diagnosis of germinoma, since these tumors were characteristically radioresponsive. Poor response to 20 Gy indicated an alternate diagnosis such as NGGCT or glioma. A robust response to 20 Gy suggested a diagnosis of germinoma, and treatment was continued to 50 Gy for definitive treatment. In light of the advances in neurosurgical techniques as well as the ability to differentially treat with chemotherapy, surgical biopsy in the modern era is generally much safer and usually recommended prior to treatment. However, some controversy remains whether biopsy is indicated for these tumors, and this decision determines the management plan.
Regardless of the ultimate histology, all GCTs are staged in a similar manner using magnetic resonance imaging (MRI) scans of the brain and spine in addition to CSF examination. Local lesions are categorized as M0. M1 disease is defined by microscopic dissemination in the CSF, while M2/M3 disease shows disseminated macroscopic lesions in the spinal region or cranial subarachnoid space visible on imaging.
6.5.1 Laboratory Investigations
AFP is normally expressed during embryonic development. It is the earliest serum-binding protein in the fetus, which reaches peak concentration at 12–14 weeks of gestation and then gradually falls to reach adult levels of 10 ng/dL at 1–2 years of age. As AFP levels decline during fetal development, albumin becomes the predominant binding protein. The presence of AFP (>25 ng/mL) indicates that there are malignant components in the tumor consisting of yolk sac elements or embryonal carcinoma. The half-life of AFP is 5–7 days and is a useful marker to follow, with one caveat: due to the variable rates of AFP levels in infants, AFP levels are less informative in this very young age group. Of note is the phenomenon of increasing AFP levels due to chemotherapy-induced tumor lysis and not necessarily due to disease progression. β-HCG is produced by syncytiotrophoblasts during pregnancy to maintain the corpus luteum, and minute amounts are found in normal adults. Pathologic elevations of β-HCG (>50 IU/L) are found when there is a clonal disorder of syncytiotrophoblasts, such as in choriocarcinoma, or when syncytiotrophoblastic giant cells are found in germinomas or embryonal carcinomas. Therefore, when an elevation of one of these tumor markers is present, it is highly suggestive of GCT.
Embryonal carcinomas secrete both AFP and β-HCG, while endodermal sinus tumors secrete only AFP, and choriocarcinomas secrete only β-HCG. However, in as many as 30 % of GCTs, more than one histological subtype is found (Matsutani et al. 1997). The most useful laboratory values for the diagnosis of GCTs are elevations of AFP and/or β-HCG in serum or CSF. It is important to sample both serum and CSF, as serum levels can be normal in the presence of elevated CSF levels and vice versa. If present, the protein levels can serve as useful tumor markers since they decrease as tumor burden decreases. GCTs that have elevations of these tumor markers show worse prognosis when matched with patients with identical histological diagnoses, but normal marker levels (Itoyama et al. 1995; Nishizaki et al. 2001). AFP can be used as a tumor marker in endodermal sinus tumors, and β-HCG is useful in choriocarcinoma.
Another helpful tumor marker is PLAP, which is a fetal isoenzyme of alkaline phosphatase, and is almost always elevated in germinomas (Cushing et al. 2002). Therefore, one controversial option in patients with elevated PLAP, but normal β-HCG and AFP, would be to assume the diagnosis is germinoma and treat accordingly (Steinbok 2001) (Table 6.2). However, PLAP is not readily available as a test in many institutions, and such empiric diagnoses are extraordinarily rare. Another marker for germinoma that has been employed is c-kit (CD117), and its soluble isoform, s-kit. Elevations of s-kit were found in the CSF of patients with germinoma and mixed GCT and may correlate with patients’ clinical courses. Moreover, the level of s-kit was remarkably higher in patients with tumor dissemination, such that s-kit has been a useful tumor marker (Miyanohara et al. 2002; Kamakura et al. 2006). In addition, immunohistochemical staining for OCT4, an 18-kDa POU-domain transcription factor encoded by the POU5F1 gene, has been shown to be a highly specific and sensitive test for germinomas, preferred over PLAP (Hattab et al. 2005). Recently, the novel stem-cell marker SALL4, a zinc-finger transcription factor upstream of OCT4, has been shown a highly sensitive and specific diagnostic marker for intracranial GCT expressed in germinomas, yolk sac tumors, and embryonal carcinomas (Mei et al. 2009). Glypican 3 (GPC3) immunostaining may also have diagnostic utility for detection of yolk sac tumors (Zynger et al. 2008).
6.5.2 Diagnostic Imaging
Like other brain tumors, computed tomography (CT) and MRI are the most common modalities used to diagnose intracranial GCTs. Of historic interest only, pineal region tumors can be detected on plain skull films by the presence of calcifications. MRI is the study of choice, although CT has an advantage over MRI in identifying calcifications. The identification of calcification in the pineal gland in a child younger than 6 years old is an indication for an MRI, even when no mass is apparent on CT (Zimmerman and Bilaniuk 1982; Steinbok 2001).
Findings on CT or MRI are almost never sufficient for the diagnosis of GCTs (Awa et al. 2014). Germinomas are usually diffusely enhancing on CT and MRI, whereas NGGCTs are more likely to be heterogeneous in part due to hemorrhage. A tumor in the suprasellar region in association with a pineal tumor is usually a GCT, most likely a germinoma (Fig. 6.1). Larger germinomas can have a heterogeneous appearance and fill the third ventricle (Fig. 6.2). A bifocal location is not guaranteed to be a germinoma as GCTs with mixed elements can also appear in two locations (Fig. 6.3). A recent or old hemorrhage seen in the tumor suggests an NGGCT, particularly common with choriocarcinoma (Fig. 6.4). Intracranial teratomas tend to be well circumscribed and have large cysts and calcifications within the tumor, which can be helpful in distinguishing them from germinomas (Fig. 6.5). Immature teratomas tend to have fewer cysts and calcifications and may secrete tumor markers (Fujimaki et al. 1994).
A sagittal T1-weighted image following contrast shows an unusual case of a mixed germ cell tumor containing both germinoma and teratoma located in the suprasellar, sellar, and pineal regions. The sellar component was biopsied through a transsphenoidal approach. The area of low signal intensity within the sella represents a fat patch to prevent postoperative CSF leak
An axial CT image from a teenage boy who presented with headaches and a change in his mental status. A large partially hemorrhagic tumor is seen extending into the lateral ventricles. An endoscopic biopsy was consistent with choriocarcinoma. β-HCG as measured in the CSF was 88,767 IU/L (normal: <1.5). Hydrocephalus is present, and an external ventricular catheter has been inserted into the anterior portion of the ventricle
A sagittal T1-weighted image following contrast (left) and a T2-weighted image (right) demonstrate the typical appearance of a mixed GCT, in this case a teratoma with germinoma. The tumor is heterogeneous with robust enhancement of the solid component although there are also multiple cysts within the tumor
Molecular imaging approaches including positron emission tomography (PET) have been evaluated with initial reports of (11)C-methionine demonstrating greater diagnostic and treatment planning utility over (18)F-fluorodeoxyglucose (Okochi et al. 2014); however, PET approaches are not currently widely employed in diagnosis of intracranial GCT.
In addition to GCT, the differential diagnosis of a pineal lesion includes pineoblastoma, trilateral retinoblastoma in a patient with bilateral retinoblastoma, pineocytoma, glioma, meningioma, lymphoma, or a benign lesion such as a cyst. Benign cysts can generally be distinguished from malignant cystic neoplasms by the lack of enhancement or a very thin rim of enhancement surrounding a hypointense center (Steinbok 2001).
6.5.3 Obtaining Tissue Diagnosis
There is geographic variation in management strategies. In 1992, Oi and Matsumoto noted that the majority (84 %) of Japanese neurosurgeons were comfortable using a radiodiagnostic trial of 20 Gy in lieu of histological confirmation of a germinoma. In contrast, the majority (78 %) in Western countries recommended histological diagnosis as the initial management of pineal region tumors. This discrepancy may be due to the fact that pineal region tumors are much more common in Japan, and the incidence of germinomas in particular is higher (Oi and Matsumoto 1992). A follow-up study showed that by 1998, radical resection of the tumor was recommended as the initial procedure by only 22 % of Japanese neurosurgeons, while 39 % recommended biopsy, and 39 % recommended radiation therapy. The authors suggested tissue diagnosis by ventriculoscopic or stereotactic approach as the most appropriate initial step for the treatment planning of pineal region tumors (Oi et al. 1998).
Because histology influences the choice of treatment and is coincident with improved surgical techniques, the need for obtaining tumor histology is now recognized (Aydin et al. 1992; Sawamura et al. 1997). Although a definitive conclusion cannot be reached, a prudent strategy would be to utilize a safe and minimally invasive technique to obtain tissue for histological analysis. Such techniques would include endoscopic biopsy at the time of third ventriculostomy or aqueductoplasty or stereotactic biopsy. A review of 370 cases of stereotactic biopsies in France reported only 1.3 % mortality and 1 % major morbidity rates (Regis et al. 1996). Despite these relatively low rates, most surgeons are more comfortable with open biopsy in this region due to the close proximity of deep cerebral veins. One of the advantages of open biopsy is that sampling error can be minimized by taking several biopsies. This is particularly important since mixed GCTs are commonly encountered. Advances in surgical techniques allow open procedures to access the pineal region without major morbidity.
As results from intracranial GCT studies have emerged, stratification into “intermediate”- and “high”-risk prognostic groups has refined the role of biopsy. Specifically, serum and/or CSF β-HCG levels greater than 1000 IU/L and AFP levels greater than 1000 ng/mL nearly uniformly indicate the diagnosis of pure or predominant choriocarcinoma and yolk sac tumor, respectively (Matsutani et al. 1997; Kretschmar et al. 2007). Such conspicuous marker elevations may obviate the need for biopsy since they denote pure and/or predominant malignant elements and thus should be treated within the highest risk group. Current studies have further relaxed the need for biopsy in the presence of even lower serum or CSF levels of β-HCG or AFP >50 IU/L.
6.6 Treatment
6.6.1 Role of Surgery
Prior to 1970, surgery resulted in 25–70 % morbidity and mortality rates and led to radiotherapy becoming the treatment of choice with a modern 10-year overall survival, at least for germinomas, in the order of 80–100 %. In Japan, the standard of care was administration of a radiodiagnostic trial of 20 Gy followed by definitive doses of radiation if 20 Gy induced a tumor response (Handa and Yamashita 1981). Conventional radiotherapy for CNS germinomas involved 30 Gy of CSI followed by a boost to the primary disease site to a total dose of 50 Gy. The main role of surgery at that time was for treatment of hydrocephalus by placement of ventriculoperitoneal shunts, which resulted in peritoneal metastases at times (Brandes et al. 2000). Currently, other than as a diagnostic tool, surgery has no proven role in the treatment of intracranial germinomas due to the high operative morbidity and excellent outcomes with chemoradiotherapy (Pollack 2012). Several studies have shown no benefit to radical surgical resection in overall survival for germinoma patients (Sawamura et al. 1997).
For NGGCTs, surgery plays an important role along with other treatment modalities. In a study by Weiner et al., radical resection in addition to chemotherapy improved prognosis for patients with intracranial NGGCT. They recommended delayed surgical resection for patients who have normalized tumor markers with persistent radiographic abnormalities after three cycles of initial chemotherapy in order to avoid unnecessary radiation or further chemotherapy (Balmaceda et al. 1996; Weiner et al. 2002). For mature teratomas, it is generally accepted that gross total surgical resection is sufficient for cure. For immature teratomas, adjuvant chemoradiotherapy should be used if tumor markers are present because it is assumed that malignant germ cell elements are present. Even following gross total resection of nonsecreting immature teratomas, there is still a risk of relapse without additional adjuvant therapy (Sawamura et al. 1998b). Endoscopic approaches may more effectively access these midline suprasellar lesions (Somma et al. 2014; Tseng et al. 2012).
Second-look surgery, in particular, was proposed by some authors (Friedman et al. 2001; Weiner et al. 2002). A retrospective review of 126 patients enrolled on the First and Second International Central Nervous System Germ Cell Tumor Studies for patients with newly diagnosed CNS GCTs sought to establish the role of delayed surgical resection in patients who exhibit less than complete radiographic response despite declining tumor markers after initial chemotherapy. Indeed, after at least three cycles of chemotherapy, ten patients underwent delayed surgical resection due to residual radiographic abnormalities in the setting of declining or completely normalized serum and CSF levels of β-HCG and AFP. Second-look surgery revealed three mature teratomas, two immature teratomas, and five cases of necrosis or scar tissue alone. At an average follow-up time of 36.9 months (range 3–96 months), only three of the ten patients had experienced tumor recurrence. Three of the four patients with NGGCTs whose tumor markers had not completely normalized ultimately developed tumor dissemination/progression and required radiation therapy even though pathology at second-look surgery showed only teratoma or necrosis/scar tissue. In contrast, three of four patients with NGGCTs whose tumor marker levels had completely normalized did not progress and did not require radiation therapy. The authors concluded that delayed surgical resection was indicated in patients with GCTs who have residual radiographic abnormalities and normalized tumor markers following chemotherapy (Weiner et al. 2002). The current experimental schema for localized GCT, ACNS1123, recommends second-look surgery following induction chemotherapy for less than complete response with normalization of markers (COG ACNS 1123 2012).
6.6.2 Chemotherapy
Chemotherapy was incorporated into the treatment of intracranial GCTs after agents known to have activity against testicular GCTs were shown to cross the blood–brain barrier (Ginsberg et al. 1981; Brandes et al. 2000). As single agents, actinomycin D, vinblastine, bleomycin, doxorubicin, cisplatin, carboplatin, etoposide, ifosfamide, and cyclophosphamide are active against GCTs, and combinations of these agents are the basis for treatment regimens. The most common combinations are PEB (cisplatin, etoposide, and bleomycin), PVB (cisplatin, vinblastine, and bleomycin), and JEB (carboplatin with etoposide and bleomycin) (Hawkins et al. 1986; Pinkerton et al. 1990; Cushing et al. 2002; Einhorn and Donohue 2002). In addition, ifosfamide has been found to be the third most active agent against GCTs, following cisplatin and etoposide, and was investigated as salvage therapy in patients with refractory disease (Nichols 1996). The recent Children’s Oncology Group NGGCT study, ACNS0122, utilized a regimen of carboplatin and etoposide alternating with ifosfamide and etoposide that demonstrated safety and efficacy.
However, efforts to omit radiation and treat intracranial GCTs with chemotherapy alone have been less promising. Yoshida et al. saw a response rate of 80–85 % in patients treated with a combination regimen of cisplatin and etoposide, but survival rates at 2 years were disappointing at 88 % in patients with germinomas and 48 % in patients with NGGCTs. Baranzelli et al. reported that of 13 AFP- and β-HCG-secreting GCTs treated by chemotherapy and surgery alone, 12 recurred. Approximately 50 % of the patients with tumor recurrence experienced remission following salvage radiation therapy. Because of the need for salvage radiation therapy, the authors concluded that focal radiation therapy should be part of the treatment of these tumors.
Finally, Balmaceda et al. enrolled 45 patients with germinomas and 26 with NGGCTs in a clinical study and treated them with four cycles of carboplatin, etoposide, and bleomycin. Those with a complete response defined by imaging studies received two additional cycles, and those with less than a complete response received two additional cycles intensified by cyclophosphamide. Overall, 78 % of patients achieved a complete response with chemotherapy only. However, of the 54 patients surviving at 2 years, 32 (59 %) received irradiation. The 2-year survival was 84 % for patients with germinoma and 62 % for those with NGGCT. Thus, it appears that chemotherapy alone does not provide comparable cure rates when compared to combined modality treatment (Yoshida et al. 1993; Balmaceda et al. 1996; Baranzelli et al. 1998). Of particular pertinence to NGGCTs, results from the First International CNS GCT Study Group indicated that approximately one third of NGGCT patients who initially had a complete response to chemotherapy had recurrent disease. And most importantly, unlike recurrent germinoma patients, these NGGCT patients were not amenable to salvage with radiation therapy (Balmaceda et al. 1996).
Due to their differing prognoses, more recent studies have made an effort to exclusively enroll and evaluate specific GCT subtypes in order to assess the efficacy of chemotherapy-only regimens. Kellie et al., looking exclusively at germinomas, confirmed the aforementioned generally disappointing results with chemotherapy (Kellie et al. 2004a). Here 19 patients were enrolled and treated with two courses of cisplatin, etoposide, cyclophosphamide, and bleomycin. If a complete response was achieved, patients then completed two courses of carboplatin, etoposide, and bleomycin. If complete response was not achieved, patients still received the above second regimen and, following a complete response, an additional cycle of both regimens. However, if, even after this intensive treatment, residual disease was still present, patients underwent second-look surgery and/or irradiation. Five-year event-free survival (EFS) was 47 % and 5-year overall survival was 68 %. Thus, because of the higher cure rates achieved with radiation therapy, there is no currently established role for chemotherapy-only regimens in the treatment of germinomas.
For NGGCTs, radiation alone produces 5-year survival rates of only 30–40 %. Excellent response rates to chemotherapy have shifted the standard treatment of NGGCT to combined modality therapy consisting of chemotherapy and radiation. However, a recent study by Kellie et al. suggested promising results for chemotherapy-only regimens in the treatment of NGGCT. In this study, 20 NGGCT patients were enrolled and received two courses of cisplatin, etoposide, cyclophosphamide, and bleomycin (Regimen A). Patients who had a complete response subsequently received two cycles of carboplatin, etoposide, and bleomycin (Regimen B). Those with less than a complete response to the initial regimen still received two cycles of Regimen B. If a complete response was then achieved, they received two additional cycles – one of Regimen A and one of Regimen B. If a complete response was absent, patients who did not respond to either Regimen A or B were taken off protocol for surgery and/or irradiation. These results improved upon historical controls, with a 5-year overall survival of 75 % and a reduction in deaths from chemotherapy-related toxicities (Kellie et al. 2004b).
6.6.3 Radiation Therapy
Published reports corroborate poorer prognoses for patients with NGGCT compared to those with germinoma. For NGGCTs, radiation alone produces 5-year survival rates of only 30–40 % with high rates of early relapse (Fuller et al. 1994; Matsutani et al. J Neurosurg 1997). These dismal results for radiation-only therapies, coupled with the success of chemotherapy in improving overall survival, have shifted the standard of care for NGGCTs toward multimodality approaches. However, open questions still remain, regarding radiation dose and volume.
There is little literature addressing the appropriate radiation field for localized NGGCT, with only small patient numbers. In a study by the French Society of Pediatric Oncology, chemotherapy and focal radiation resulted in five relapses among 24 patients with localized NGGCTs (Bouffet et al. 1999). A similar regimen in a separate study resulted in 3 of 18 patients experiencing disease recurrence, 2 with isolated spinal relapses (Robertson et al. 1997).
Additional studies support the opinion that “intermediate”-risk NGGCT patients (see Sect. 6.5.3 earlier for delineation of “intermediate”- and “high”-risk groups), particularly those with complete responses, do not require CSI. The Japanese cooperative group reported an excellent 5-year survival rate of 89 % for “intermediate”-risk patients who were treated with five cycles of chemotherapy, 30 Gy to whole ventricular fields, and 54 Gy total dose to the primary tumor volume (Matsutani 2008). The International Society of Pediatric Oncology treated patients with localized NGGCTs with four cycles of cisplatin, etoposide, and ifosfamide, followed by focal radiation to a total dose of 54 Gy. Progression-free survival was 67 %, although nearly half of the 34 patients with residual disease faced a recurrence even after chemotherapy (Calaminus and Patte 2005).
Because of their relative radioresistance, recommendations for the multimodality approach to NGGCT have emerged from recent studies. Within that context, for patients with NGGCT and complete responses to chemotherapy, Buckner recommended 54 Gy limited-field irradiation and 30 Gy CSI, if the spinal axis is involved; in patients with partial response, 59.4 Gy limited field was recommended with 36 Gy CSI, if spinal involvement is evident (Buckner et al. 1999).
Our recommendation for NGGCTs again depends on the stage at diagnosis. For disseminated disease (M1-M3), we recommend CSI to a dose of 24–36 Gy depending on variables that include patient age, tumor response, and disease bulk, with focal boosts to macroscopic disease to a dose of 54 Gy. For M0 lesions, because adjuvant or neoadjuvant chemotherapy is now often included in the treatment regimen, we frequently limit radiation volumes. For those with “intermediate”-risk disease and a complete response to induction chemotherapy, we recommend 30 Gy to a whole ventricular field and 54 Gy total dose to a focal radiation field. A consensus atlas is available for definition of whole ventricular field that includes the third, fourth, and lateral ventricles, along with the prepontine cistern (Mailhot et al. ASTRO 2013). For those with “intermediate”-risk with less than a complete response and for “high”-risk patients, we recommend 36 Gy to the craniospinal axis and 54 Gy to the primary tumor volume.
Among intracranial GCTs, germinoma represents the most common and prognostically favorable subgroup. The roles of surgery, chemotherapy, and radiation are constantly evolving. Unlike surgery, radiation remains a critical component of the treatment of intracranial germinomas. In fact, the gold standard against which all new approaches must be measured remains radiotherapy alone. ANCS0232 attempted to compare neoadjuvant chemotherapy followed by radiotherapy to radiotherapy alone, but failed to accrue. A series from Mayo Clinic with long-term follow-up demonstrated freedom from progression of 80 % at 10 years for patients receiving neoadjuvant platinum-based chemotherapy with complete response followed by reduced-field radiotherapy, although cautioned against local field only RT due to a freedom from progression rate of 44 % in this subgroup vs 100 % for those treated with extended fields (Jensen et al. 2010). A historical series from UCSF likewise demonstrated improved outcomes with whole ventricular radiotherapy over focal radiation irrespective of chemotherapy (Schoenfeld et al. 2014). For M1–M3 disease, volumes involving the entirety of the craniospinal axis with focal boosts to macroscopic disease remain standard of care. Current evidence substantiates omitting CSI from the treatment of localized germinoma. Spinal failure rates of <10 % in the absence of CSI, reported in most contemporary series, do not justify routine incorporation of CSI into treatment strategies for localized germinoma. Indeed, a recent review of the data regarding volumes and doses for M0 germinoma concluded that, because the rate of spinal relapse is similar, regardless of whether CSI or whole brain irradiation is used, prophylactic whole brain and CSI are contraindicated (Rogers et al. 2005). The late effects of CSI are increasingly recognized as particularly debilitating in the pediatric population and may include neurocognitive, endocrine, visual, and auditory pathway impairments, as well as secondary malignant neoplasms, such that reduced volume radiotherapy is largely recommended for localized disease (Broniscer et al. 2004; Fossati et al. 2009). The current standard “involved field” volume includes the whole ventricle to 24 Gy followed by a boost to a total dose of 45 Gy to the tumor bed and any gross residual tumor that may be present, at a dose of 1.5 Gy per fraction. Dose de-escalation is noted on current combined chemoradiation protocols below. Some have gone still further in questioning the value of whole ventricular radiation, although whole ventricular fields are employed in current protocols.
Modern radiation techniques have demonstrated greater dose sparing of nontarget cerebral hemispheric tissue. As compared to whole ventricular irradiation with 3-D conventional radiotherapy techniques, IMRT has the ability to reduce uninvolved tissue treatment volumes at both high- and low-dose levels, with a small absolute increase in dose to peripheral body volumes (Chen 2010). Moreover, further dose sparing may be afforded by proton radiation. A series of 22 patients receiving proton radiotherapy for localized CNS germinomas demonstrated excellent survival outcomes (LC 100 % with median follow-up of 28 months) and increased normal tissue sparing as compared with IMRT plans generated in parallel (MacDonald et al. 2011). Other dosimetric studies comparing proton and photon-based approaches have shown excellent target coverage with improved dose sparing of temporal and hippocampal regions for both scattered and spot scanning proton delivery techniques as compared to IMRT in treatment of intracranial germ cell tumors (Park 2015). Other highly conformal radiotherapy techniques such as stereotactic radiosurgery have been effectively employed as a limited-field tumor boost following whole ventricular radiation (Endo et al. 2005).
Although most investigators now omit whole brain radiation, several studies have reported higher recurrence rates when only the tumor volume is treated (Uematsu et al. 1992; Shibamoto et al. 1994b; Wolden et al. 1995; Brandes et al. 2000; Rogers et al. 2005). Specifically, tumor recurrences following radiation fields confined to the primary tumor volume usually occur within the adjacent brain parenchyma and ventricles. Therefore, investigators have recommended initial inclusion of the entire ventricular field followed by a boost to the primary tumor to a total dose of 45 Gy for tumors less than 4 cm in size and 20 Gy for spinal prophylaxis in case of positive cytology (Uematsu et al. 1992; Shibamoto et al. 1994a). Shibamoto et al. suggested modulating the radiotherapy dose according to tumor diameter with 40 Gy for tumors up to 2.5 cm, 45 Gy for tumors between 2.5cm and 4 cm, and 50 Gy for tumors over 4 cm (Shibamoto et al. 1994b). Dissemination to the hypothalamus, third ventricle, or spinal cord identifies a high-risk group that warrants consideration of CSI with systemic chemotherapy (Jennings et al. 1985b).
We recommend whole ventricular irradiation, followed by a boost to the primary tumor for localized germinoma, when using radiation alone. Some question the rationale of whole ventricular irradiation, given the continuity of CSF space throughout the entire CNS. We argue that the natural history of germinomas is characterized by multifocality and intracranial relapses at foci separate from the primary tumor with lesser propensity for diffuse CNS involvement (Eom et al. 2008). These features distinguish germinomas from other CNS malignancies, such as medulloblastoma, that require CSI for cure. Whether more generous local radiation fields sterilize occult multifocal disease or target direct ventricular invasion, there appears to be a role for whole ventricular irradiation in the treatment of localized germinoma. In addition, the literature supports a dose of ≥45 Gy to the primary tumor for germinomas treated with radiation alone.
6.6.4 Combined Modality Treatment
In an effort to increase cure rates and limit toxicities associated with radiation, investigators have formulated multimodal regimens for the treatment of these lesions (Allen et al. 1987). Calaminus et al. reported promising results for patients with secreting intracranial GCTs given four courses PEI, cisplatin (20 mg/m2 day 1–5), VP-16 (100 mg/m2 day 1–3), and ifosfamide (1.5 g/m2 day 1–5) and resection, if feasible, of the residual tumor, followed by radiation consisting of 30 Gy CSI and an additional 24 Gy boost to the primary site. EFS was 81 % with 11 months follow-up, representing a significant improvement from previous studies (Calaminus et al. 1997). Robertson et al. reported improved outcome for intracranial NGGCTs after a treatment plan of initial radical surgical resection followed by three to four cycles of adjuvant chemotherapy with cisplatin (100 mg/m2/cycle) and VP-16 (500 mg/m2/cycle), followed by radiotherapy and finally four additional cycles of postradiation chemotherapy. Four-year actuarial EFS and overall survival rates were 67 % and 74 %, respectively (Robertson et al. 1997). It remains to be seen whether further intensification using myeloablative chemotherapy with autologous stem-cell rescue has a role in patients with poor-prognosis GCTs or relapsed GCTs.
A retrospective analysis of 41 patients concluded that treatment for NGGCTs should be tailored to histological subtype. For patients in the intermediate-risk group, which included those with germinoma with syncytiotrophoblastic giant cells, immature teratoma, teratoma with malignant transformation, and mixed tumors composed of germinoma or teratoma, there was a significant difference in overall survival for patients who had combined chemotherapy, radiation, and surgery (84 %) compared to those who had only radiation and surgery (44 %) (Ogawa et al. 2003). In this instance, chemotherapy consisted of various carboplatin or cisplatin combinations. However, in the poor-prognosis group (choriocarcinoma, yolk sac tumor, embryonal carcinoma, and mixed tumors), those who had incomplete resection, chemotherapy, and radiation had an abysmal 5-year survival rate of 8 %. For those with complete macroscopic resection, survival was more favorable, arguing for a possible role for surgery in NGGCT patients with these particular subtypes.
In the SIOP CNS GCT-96 trial (Calaminus et al. 2013), 135 patients with localized disease received four cycles of neoadjuvant chemotherapy consisting of cisplatin, ifosfamide, and etoposide followed by involved field radiotherapy to 54 Gy. At a median follow-up of 39 months, the progression free survival (PFS) for the patients was 69 ± 5 %. Relapses were predominantly local.
The recent Children’s Oncology Group NGGCT study, ACNS0122, demonstrated 2-year PFS and OS of 84 ± 4 % and 93 ± 3 %, respectively, with a regimen of 6 cycles of induction chemotherapy followed by 36 Gy CSI and involved field boost to 54 Gy (Goldman et al. 2015). Seventy-nine of the 104 patients enrolled on ACNS0122 were noted to have localized tumors. CR and PR rates following induction chemotherapy were 31.65 % and 21.5 %, respectively. Of the 18 patients who underwent second-look surgery after induction therapy, 8 were found to have had only mature teratomas, residual scar, or fibrosis on pathology. Three-year EFS rates were 92 %, 94.1 %, and 85.7 % for these three groups, respectively (p=ns).
Sawamura et al. have also recommended further risk stratification of patients into three categories. They have categorized pure germinoma and mature teratoma as the good-prognosis group, with the poor-prognosis group including embryonal carcinoma, yolk sac tumor, choriocarcinoma, and mixed GCT containing any embryonal carcinoma, yolk sac, or choriocarcinoma elements. The intermediate-prognosis group includes germinoma with elevated (β-HCG, immature teratoma, extensive/multifocal germinoma, and mixed GCT containing only germinoma with teratoma elements (Sawamura et al. 1998a). Further therapeutic studies incorporating this type of risk stratification are warranted to determine if prognosis can be improved in the poor-prognosis group while minimizing therapy-induced physical or cognitive sequelae.
Historically, intracranial germinomas have been treated with radiation alone, producing excellent cure rates. The significant long-term toxicity of radiation, particularly in children, has prompted investigations into alternative treatments that minimize the dose and volume of irradiation. However, these alternative approaches must preserve the high cure rates established with radiation alone. Combined modality approaches in which chemotherapy precedes radiation have gained credence and are now considered a standard alternative to radiation alone. The current GCT protocol, ACNS1123, indicates dose de-escalation to 18 Gy whole ventricular irradiation followed by 12 Gy boost to the primary disease site for patients evidencing a complete response with normalization of markers following four cycles of induction cisplatin/etoposide. For patients with partial response, 24 Gy whole ventricular irradiation followed by 12 Gy boost is indicated.
Several series have reported excellent clinical outcome with preirradiation chemotherapy followed by focal irradiation. Buckner et al. and Sawamura et al. reported 100 % survival with median follow-up times of 51 months and 24 months, respectively (Sawamura et al. 1998c; Buckner et al. 1999). Buckner et al. treated nine patients with germinomas and eight with mixed GCTs. Treatment consisted of etoposide (100 mg/m2/day) plus cisplatin (20 mg/m2/day) daily for 5 days every 3 weeks for four cycles, followed by radiation therapy. They recommend that germinoma patients with complete responses after standard chemotherapy receive 30 Gy to a limited field with the addition of 20 Gy CSI for disseminated disease. For patients with partial responses, doses of 54 Gy to a limited field were recommended with 30 Gy CSI for disseminated disease.
Another study reported excellent results for patients treated with chemoradiation. Patients with pure germinomas were treated with EP (etoposide, 100 mg/m2, and cisplatin, 20 mg/m2) given for 5 days every 4 weeks for four cycles, and patients with other pathologic types were treated with ICE (ifosfamide, 900 mg/m2; cisplatin, 20 mg/m2; and etoposide, 60 mg/m2) for five consecutive days every 4 weeks for up to six cycles depending on chemoresponsiveness, extent of surgical resection, and tumor marker levels. At 5 years, the overall survival rate was 100 %, and relapse-free survival rates were 90 % for germinoma patients and 44 % for patients with β-HCG-secreting germinomas, which represent a mixed GCT with β-HCG-secreting syncytiotrophoblastic giant cells. This is strong evidence that treatment should be directed at the most malignant element. The 5-year overall survival rates were 93 % for non-β-HCG-secreting germinomas and 75 % for β-HCG-secreting germinomas (Aoyama et al. 2002). The authors recommend that following EP chemotherapy, dose and volume be reduced to 24 Gy in 12 fractions for non-β-HCG-secreting germinomas, but higher radiation doses should be maintained for β-HCG-secreting germinomas (Aoyama et al. 2002). Most recently, the volume of radiation in the setting of chemoradiation for germinomas has been addressed by Eom et al. They reviewed 81 patients treated for histologically confirmed intracranial germinomas with either radiation alone or chemoradiation. Of 42 patients who received chemotherapy followed by focal radiation, 4 relapsed, 1 in the primary tumor bed, 2 in the ventricles outside the radiation fields, and 1 in the spinal epidural space. This contrasts with no relapses among 39 patients who were treated with radiation alone consisting of craniospinal fields followed by a focal boost. Thus, recurrence-free survival at 5 years was 100 % in the radiation alone arm and 88.1 % in the chemoradiation arm, a difference that was statistically significant. The authors concluded that chemotherapy cannot prevent subependymal spread that is very effectively controlled by radiation, and they argued that whole ventricular radiation fields are appropriate following induction chemotherapy (Eom et al. 2008).
In the most current NGGCT protocol, patients who demonstrate complete or partial response with normalization of markers following six cycles of induction chemotherapy with alternating carboplatin/etoposide and ifosfamide/etoposide receive 30.6 Gy whole ventricular radiotherapy followed by 23.4 Gy boost to the primary site (COG ACNS1123 2012).
6.6.5 Treatment of Recurrent Disease
As with most malignancies, relapse poses a formidable problem. Imber et al. reported high rates of effective salvage treatment in a series of 24 patients with NGGCT reflected in a 10-year OS of 88 % despite a 5-year progression-free survival of <50 % after initial multimodal therapy (Imber et al. 2015). For patients who relapse with intracranial GCTs, salvage therapy using the same chemotherapy regimen followed by radiotherapy has been effective (Sawamura et al. 1998a). Kobayashi et al. used combinations of cisplatin and etoposide in four cases of recurrent intracranial GCT (three malignant teratomas and one germinoma) and saw a response rate of 100 % (Kobayashi et al. 1989). Aoyama et al. successfully treated recurrent germinomas with further chemotherapy and reirradiation (Aoyama et al. 2002). However, improving prognosis for NGGCTs remains a concern. Encouraging results from a study using high-dose chemotherapy (200 mg/m2 cisplatin, 1250 mg/m2 etoposide, and 150 mg/m2 ACNU) with autologous stem-cell rescue in six patients with high-risk, intracranial NGGCT showed 100 % survival at 1–7-year follow-up (Tada et al. 1999). Although trials using high-dose chemotherapy with stem-cell rescue show promise in relapsed extracranial GCTs, it remains to be seen whether myeloablative consolidation therapy has a role in the treatment of intracranial GCTs.
6.6.6 Future Trials
The Children’s Oncology Group has been actively investigating treatment approaches for intracranial GCTs. The first trial has focused exclusively on NGGCTs, attempting to improve overall and progression-free survival by using a neoadjuvant 3-drug combination consisting of carboplatin, VP-16, and ifosfamide, followed by CSI with involved field boost. For those patients that have persistently positive markers, residual tumor, or unresectable disease, even after induction chemotherapy, myeloablative chemotherapy followed by stem-cell rescue is to be attempted before CSI.
For germinomas, a Phase III trial has stratified patients according to extent of disease (M0 for local, M+ for multifocal, and modified M+ for assumed occult multifocal) and then randomized them into one of two treatment arms: Regimen A consists exclusively of radiotherapy; M0 and modified M+ patients receive ventricular radiation with a focal boost, while those with disseminated disease receive CSI. In Regimen B, patients with focal disease that experience a favorable response to induction chemotherapy receive reduced-dose and volume-involved field radiotherapy. Those with disseminated disease who respond well to induction chemotherapy receive a reduction in CSI and boost doses. Those with modified M+ disease who respond well receive reductions in ventricular and boost doses. Final results of these two studies are pending. Such response-directed fields and doses of radiation have gained favor.
Future trials for intracranial NGGCTs will likely further stratify patients into “intermediate”- and “high”-risk groups in an effort to identify those patients who do not require as aggressive a regimen as is currently administered. Such a group of “intermediate”-risk tumors will include those with immature teratoma, mixed GCT with predominantly germinoma or teratoma components, and histologically confirmed NGGCTs with β-HCG <1,000 IU/L or AFP <1,000 ng/mL. Although the current NGGCT Children’s Oncology Group protocol dictates CSI for all such patients, emerging evidence indicates that less toxic approaches will not compromise clinical outcome in this group of patients. Thus, in future trials, M0 “intermediate”-risk NGGCT patients will likely receive chemotherapy followed by response-based radiation consisting of a whole ventricular field and a boost to the primary tumor region.
6.7 Outcome
The outcome for patients with pure intracranial germinoma is significantly better than the outcome for those with NGGCT. Cure rates above 90 % with radiation alone establish radiation as the benchmark against which combined modality therapy must be compared. Comparable outcomes are achieved in patients with intracranial teratoma. Prognosis for NGGCTs other than teratoma is worse than that for germinomas, and historically the 5-year survival rates for these tumors have been between 20 % and 49 % (Jennings et al. 1985b; Schild et al. 1996; Matsutani et al. 1997; Drummond and Rosenfeld 1999; Jaing et al. 2002). However, it is clear that combined modality therapy has improved dramatically on the poor historic survival rates of patients with NGGCTs. The roles of surgical resection and high-dose chemotherapy with stem-cell rescue for patients with NGGCTs are currently under investigation.
Early studies identified “intermediate”- and “high”-risk groups among NGGCT patients. More than a decade ago, Matsutani et al. found 27 % survival rates at 3 years for those with pure malignant GCTs (choriocarcinoma, endodermal sinus tumor, and embryonal carcinoma), compared with 70 % or greater survival rates for patients with mixed germinoma and teratoma, and mixed teratoma or germinoma with some pure malignant elements (Matsutani et al. 1997). In contrast, mixed tumors with predominantly pure malignant elements had less than 10 % survival at 3 years.
In addition to histology, a key prognostic factor for NGGCTs is tumor marker elevation. In particular, serum and/or CSF β-HCG or AFP levels greater than 1,000 IU/L or 1,000 ng/mL, respectively, portent significantly worse outcome (Matsutani et al. 1997; Kellie et al. 2004b; Kretschmar et al. 2007). For example, the Second International CNS Germ Cell Study Group reported that from 20 patients with NGGCTs treated with chemotherapy alone, 4 of 9 patients with serum and/or CSF β-HCG or AFP levels greater than 1,000 IU/L or ng/mL, respectively, died of disease progression, whereas only 1 death occurred among 11 patients without such marker elevations (Kellie et al. 2004b). Lesion size greater than 4 cm may also be associated with poorer outcome (Huo et al. 2015).
Given higher cure rates for patients with intracranial GCTs, long-term toxicities are clearly evident. Sawamura et al. reported a variety of late adverse effects of therapy including stroke, secondary malignancy, and cognitive, endocrinologic, auditory, and visual dysfunctions. Of 85 patients, 58 required hormone replacement therapy and 26 showed poor performance status (Sawamura et al. 1998a). Young patients are at an increased risk of physical as well as neuropsychologic deficits. As expected, patients who received less than 55 Gy showed higher Karnofsky performance scores (Ono et al. 1994).
Sands et al. reported on quality of life and neuropsychologic functioning in patients enrolled in the First International CNS Germ Cell Tumor Study. Patients who received CNS radiation therapy had worse physical health, but similar psychosocial health. Patients with germinomas significantly outperformed those with NGGCTs on all neuropsychologic measures, and younger patients were at increased risk for psychosocial and physical problems as well as neuropsychologic deficits (Sands et al. 2001). In the study by Aoyama et al., using chemotherapy followed by low-dose, involved field radiotherapy, the authors noted no remarkable deterioration in quality of life or neurocognitive function (Aoyama et al. 2002).
Combination chemoradiotherapy regimens with risk stratification and dose adjustments will likely decrease the long-term side effects of therapy while improving the prognosis for those with the high-risk intracranial GCTs. A study of nine children with germinomas, all of whom received radiotherapy and five of whom received neoadjuvant chemotherapy, confirmed the relative safety of limited-field and reduced-dose radiotherapy when supplemented with chemotherapy (Strojan et al. 2006). Another retrospective study examined data from 19 patients, 14 of whom received various chemotherapies in addition to radiation, and the authors found adverse effects to be relatively limited (Osuka et al. 2007).
While chemoradiation is the reigning paradigm for the treatment of both germinoma and NGGCT, new approaches hold promise as well. Osada et al. provide a case report of dendritic cell-based immunotherapy in a patient with relapsed, intracranial GCT who had significant tumor shrinkage as well as decrease in tumor markers after four infusions of peripheral blood dendritic cells followed by monocyte-derived dendritic cells (Osada et al. 2001).
6. Conclusions
In this chapter, we have reviewed the epidemiology, pathology, clinical features, diagnosis, and treatment of intracranial GCTs. The use of tumor markers, improved imaging technologies, and safer biopsy techniques has made the diagnosis of intracranial GCTs relatively straightforward. Mixed GCTs remain a diagnostic challenge. The outcomes of patients with GCTs have paralleled the success that has been achieved with other types of pediatric cancers, with the advances in combinatorial regimens and intensification of treatments. As with other pediatric malignancies, the challenge is to distinguish the patients who require more intensive therapy from those needing standard treatment. Risk-stratified treatment protocols individualized to a patient’s tumor profile are ongoing with promising initial results. Technological advances in the field of diagnostic radiology, neurosurgery, and radiation oncology have improved efficacy of diagnosis and treatment while decreasing the long-term sequelae of therapies. As the molecular profiles of these rare tumors are further revealed, it is hoped that novel, targeted, and less toxic therapeutics will be increasingly available.
References
Al-Hussaini M, Sultan I, Abuirmileh N, Jaradat I, Qaddoumi I (2009) Pineal gland tumors: experience from the SEER database. J Neurooncol 94(3):351–358. doi:10.1007/s11060-009-9881-9
Allen JC, Kim JH, Packer RJ (1987) Neoadjuvant chemotherapy for newly diagnosed germ-cell tumors of the central nervous system. J Neurosurg 67:65–70
Aoyama H, Shirato H, Ikeda J, Fujieda K, Miyasaka K, Sawamura Y (2002) Induction chemotherapy followed by low dose involved-field radiotherapy for intracranial germ cell tumors. J Clin Oncol 20:857–865
Awa R, Campos F, Arita K, Sugiyama K, Tominaga A, Kurisu K et al (2014) Neuroimaging diagnosis of pineal region tumors-quest for pathognomonic finding of germinoma. Neuroradiology 56(7):525–534. doi:10.1007/s00234-014-1369-4
Aydin F, Ghatak NR, Radie-Keane K, Kinard J, Land SD (1992) The short-term effect of low-dose radiation on intracranial germinoma. A pathologic study. Cancer 69:2322–2326
Balmaceda C, Heller G, Rosenblum M, Diez B, Villablanca JG, Kellie S, Maher P, Vlamis V, Walker RW, Leibel S, Finlay JL (1996) Chemotherapy without irradiation–a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol 14:2908–2915
Baranzelli MC, Patte C, Bouffet E, Portas M, Mechinaud-Lacroix F, Sariban E, Roche H, Kalifa C (1998) An attempt to treat pediatric intracranial alphaFP and betaHCG secreting germ cell tumors with chemotherapy alone. SFOP experience with 18 cases. Societe Francaise d’Oncologie Pediatrique. J Neurooncol 37:229–239
Beeley JM, Daly JJ, Timperley WR, Warner J (1973) Ectopic pinealoma: an unusual clinical presentation and a histochemical comparison with a seminoma of the testis. J Neurol Neurosurg Psychiatry 36:864–873
Bernstein L, Smith MA, Liu L, Deapen D, Friedman DL (1999) Germ cell, trophoblastic and other gonadal neoplasms. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamara T, Young JL, Bunin GR (eds) Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. National Cancer Institute SEER Program, Bethesda
Bouffet E, Baranzelli MC, Patte C, Portas M, Edan C, Chastagner P, Mechinaud-Lacroix F, Kalifa C (1999) Combined treatment modality for intracranial germinomas: results of a multicentre SFOP experience. Societe Francaise d’Oncologie Pediatrique. Br J Cancer 79:1199–1204
Brandes AA, Pasetto LM, Monfardini S (2000) The treatment of cranial germ cell tumours. Cancer Treat Rev 26:233–242
Broniscer A, Ke W, Fuller CE, Wu J, Gajjar A, Kun LE (2004) Second neoplasms in pediatric patients with primary central nervous system tumors. Cancer, 100:2246–2252
Buckner JC, Peethambaram PP, Smithson WA, Groover RV, Schomberg PJ, Kimmel DW, Raffel C, O’Fallon JR, Neglia J, Shaw EG (1999) Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol 17:933–940
Calaminus G, Patte C (2005) Germ cell tumors in children and adolescents. In: Agarwal BR, Perilongo G, Rogers P, Strahl Endorf C, Eden OB (eds) International society of paediatric oncology (SIOP) education book. International Society of Paediatric Oncology, Vancouver, pp 109–116
Calaminus G, Andreussi L, Garre ML, Kortmann RD, Schober R, Gobel U (1997) Secreting germ cell tumors of the central nervous system (CNS). First results of the cooperative German/Italian pilot study (CNS sGCT). Klin Padiatr 209:222–227
Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garrè ML, Patte C, Ricardi U, Saran F, Frappaz D (2013) SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma,comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 15(6):788–796
Chen MJ, Santos Ada S, Sakuraba RK, Lopes CP, Gonçalves VD, Weltman E, Ferrigno R, Cruz JC (2010) Intensity-modulated and 3D-conformal radiotherapy for whole-ventricular rradiation as compared with conventional whole-brain irradiation in the management of localized central nervous system germ cell tumors. Int J Radiat Oncol Biol Phys 76(2):608–614
Choi JU, Kim DS, Chung SS, Kim TS (1998) Treatment of germ cell tumors in the pineal region. Childs Nerv Syst 14:41–48
Cushing B, Perlman EJ, Marina NM, Castleberry RP (2002) Germ cell tumors. In: Pizzo PA, Poplack DG (eds) Principles and practice of pediatric oncology. Lippincott Williams & Wilkins, Philadelphia, pp 1091–1113
de Bruin TW, Slater RM, Defferrari R, Geurts van Kessel A, Suijkerbuijk RF, Jansen G, de Jong B, Oosterhuis JW (1994) Isochromosome 12p-positive pineal germ cell tumor. Cancer Res 54:1542–1544
Drummond KJ, Rosenfeld JV (1999) Pineal region tumours in childhood. A 30-year experience. Childs Nerv Syst 15:119–126, discussion 127
Edwards MS, Hudgins RJ, Wilson CB, Levin VA, Wara WM (1988) Pineal region tumors in children. J Neurosurg 68:689–697
Einhorn LH, Donohue J (2002) Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. J Urol 167:928–932, discussion 933
Endo H, Kumabe T, Jokura H, Tominaga T (2005) Stereotactic radiosurgery followed by whole ventricular irradiation for primary intracranial germinoma of the pineal region. Minim Invasive Neurosurg 48(3):186–190
Eom KY, Kim IH, Park CI, Kim HJ, Kim JH, Kim K, Kim SK, Wang KC, Cho BG, Jung HW, Heo DS, Kang HJ, Shin HY, Ahn HS (2008) Upfront chemotherapy and involved-field radiotherapy results in more relapses than extended radiotherapy for intracranial germinomas: modification in radiotherapy volume might be needed. Int J Radiat Oncol Biol Phys 71:667–671
Felix I, Becker LE (1990) Intracranial germ cell tumors in children: an immunohistochemical and electron microscopic study. Pediatr Neurosurg 16:156–162
Fossati P, Ricardi U, Orecchia R (2009) Pediatric medulloblastoma: toxicity of current treatment and potential role of proton therapy. Cancer Treat Rev 35(1):79–96
Friedman JA, Lynch JJ, Buckner JC, Scheithauer BW, Raffel C (2001) Management of malignant pineal germ cell tumors with residual mature teratoma. Neurosurgery 48:518–522, discussion 522–513
Fujimaki T, Matsutani M, Funada N, Kirino T, Takakura K, Nakamura O, Tamura A, Sano K (1994) CT and MRI features of intracranial germ cell tumors. J Neurooncol 19:217–226
Fuller BG, Kapp DS, Cox R (1994) Radiation therapy of pineal region tumors: 25 new cases and a review of 208 previously reported cases. Int J Radiat Oncol Biol Phys 28:229–245
Gay JC, Janco RL, Lukens JN (1985) Systemic metastases in primary intracranial germinoma. Case report and literature review. Cancer 55:2688–2690
Ginsberg S, Kirshner J, Reich S, Panasci L, Finkelstein T, Fandrich S, Fitzpatrick A, Shechtman L, Comis R (1981) Systemic chemotherapy for a primary germ cell tumor of the brain: a pharmacokinetic study. Cancer Treat Rep 65:477–483
Goldman S, Bouffet E, Fisher PG, Allen JC, Robertson PL, Chuba PJ, Donahue B, Kretschmar CS, Zhou T, Buxton AB, Pollack IF (2015) Phase II Trial Assessing the Ability of Neoadjuvant Chemotherapy With or Without Second-Look Surgery to Eliminate Measurable Disease for Nongerminomatous Germ Cell Tumors: A Children’s Oncology Group Study. J Clin Oncol 1;33(22):2464–2471
Handa H, Yamashita J (1981) Current treatment of pineal tumors (author’s transl). Neurol Med Chir 21:147–154
Hao S, Li D, Feng J, Wang L, Wu Z, Zhang J, Zhang L (2013) Primary medulla oblongata germinomas: two case reports and review of the literature. World J Surg Oncol 11(1):274. doi:10.1186/1477-7819-11-274
Hattab EM, Tu PH, Wilson JD, Cheng L (2005) OCT4 immunohistochemistry is superior to placental alkaline phosphatase (PLAP) in the diagnosis of central nervous system germinoma. Am J Surg Pathol 29:368–371
Hawkins EP (1990) Pathology of germ cell tumors in children. Crit Rev Oncol Hematol 10:165–179
Hawkins EP, Finegold MJ, Hawkins HK, Krischer JP, Starling KA, Weinberg A (1986) Nongerminomatous malignant germ cell tumors in children. A review of 89 cases from the Pediatric Oncology Group, 1971–1984. Cancer 58:2579–2584
Hoei-Hansen CE, Sehested A, Juhler M, Lau Y-FC, Skakkebaek NE, Laursen H, Rajpert-de Meyts E (2006) New evidence for the origin of intracranial germ cell tumours from primordial germ cells: expression of pluripotency and cell differentiation markers. J Pathol 209(1):25–33. doi:10.1002/path.1948
Hoffman HJ, Otsubo H, Hendrick EB, Humphreys RP, Drake JM, Becker LE, Greenberg M, Jenkin D (1991) Intracranial germ-cell tumors in children. J Neurosurg 74:545–551
Honecker F, Kersemaekers A-MF, Molier M, Van Weeren PC, Stoop H, De Krijger RR et al (2004) Involvement of E-cadherin and beta-catenin in germ cell tumours and in normal male fetal germ cell development. J Pathol 204(2):167–174. doi:10.1002/path.1614
Huo L, Wang X, Allen PK, Wang L, Liao Y, Han Z, Shen L, Tu Q, Zhong M, Zhuang Y, Li J, Hong J (2015) Predictors of long-term survival following postoperative radiochemotherapy for pathologically confirmed suprasellar germ cell tumors. Mol Clin Oncol 3(2):430–434
Iizuka H, Nojima T, Kadoya S (1996) Germinoma of the optic nerve: case report. Noshuyo Byori 13:95–98
Imber B, Braunstein S, Banerjee A, Tihan T, Bollen A, Haas-Kogan D, Mueller S (2015) Intracranial nongerminomatous germ cell tumors: treatment strategies and outcomes. Neuro Oncol 17(supp 3)
Itoyama Y, Kochi M, Kuratsu J, Takamura S, Kitano I, Marubayashi T, Uemura S, Ushio Y (1995) Treatment of intracranial nongerminomatous malignant germ cell tumors producing alpha-fetoprotein. Neurosurgery 36:459–464, discussion 464–456
Iwato M, Tachibana O, Tohma Y, Arakawa Y, Nitta H, Hasegawa M, Yamashita J, Hayashi Y (2000a) Alterations of the INK4a/ARF locus in human intracranial germ cell tumors. Cancer Res 60:2113–2115
Iwato M, Tachibana O, Tohma Y, Nitta H, Hayashi Y, Yamashita J (2000b) Molecular analysis for p53 and mdm2 in intracranial germ cell tumors. Acta Neuropathol 99:21–25
Jaing TH, Wang HS, Hung IJ, Tseng CK, Yang CP, Hung PC, Lui TN (2002) Intracranial germ cell tumors: a retrospective study of 44 children. Pediatr Neurol 26:369–373
Jellinger K (1973) Primary intracranial germ cell tumours. Acta Neuropathol 25:291–306
Jennings CD, Powell DE, Walsh JW, Mortara RH (1985a) Suprasellar germ cell tumor with extracranial metastases. Neurosurgery 16:9–12
Jennings MT, Gelman R, Hochberg F (1985b) Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 63:155–167
Jensen AW, Laack NN, Buckner JC, Schomberg PJ, Wetmore CJ, Brown PD (2010) Long-term follow-up of dose-adapted and reduced-field radiotherapy with or without chemotherapy for central nervous system germinoma. Int J Radiat Oncol Biol Phys 77(5):1449–1456. doi:10.1016/j.ijrobp.2009.06.077
Jooma R, Kendall BE (1983) Diagnosis and management of pineal tumors. J Neurosurg 58:654–665
Kamakura Y, Hasegawa M, Minamoto T, Yamashita J, Fujisawa H (2006) C-kit gene mutation: common and widely distributed in intracranial germinomas. J Neurosurg 104(3 Suppl):173–180. doi:10.3171/ped.2006.104.3.173
Kang JK, Jeun SS, Hong YK, Park CK, Son BC, Lee IW, Kim MC (1998) Experience with pineal region tumors. Childs Nerv Syst 14:63–68
Kellie SJ, Boyce H, Dunkel IJ, Diez B, Rosenblum M, Brualdi L, Finlay JL (2004a) Intensive cisplatin and cyclophosphamide-based chemotherapy without radiotherapy for intracranial germinomas: failure of a primary chemotherapy approach. Pediatr Blood Cancer 43:126–133
Kellie SJ, Boyce H, Dunkel IJ, Diez B, Rosenblum M, Brualdi L, Finlay JL (2004b) Primary chemotherapy for intracranial nongerminomatous germ cell tumors: results of the second international CNS germ cell study group protocol. J Clin Oncol 22:846–853
Kobayashi T, Yoshida J, Ishiyama J, Noda S, Kito A, Kida Y (1989) Combination chemotherapy with cisplatin and etoposide for malignant intracranial germ-cell tumors. An experimental and clinical study. J Neurosurg 70:676–681
Kretschmar C, Kleinberg L, Greenberg M, Burger P, Holmes E, Wharam M (2007) Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer 48:285–291
Lapin V, Ebels I (1981) The role of the pineal gland in neuroendocrine control mechanisms of neoplastic growth. J Neural Transm 50:275–282
Lemos JA, Barbieri-Neto J, Casartelli C (1998) Primary intracranial germ cell tumors without an isochromosome 12p. Cancer Genet Cytogenet 100:124–128
Lin IJ, Shu SG, Chu HY, Chi CS (1997) Primary intracranial germ-cell tumor in children. Zhonghua Yi Xue Za Zhi 60:259–264
Loveland KL, Schlatt S (1997) Stem cell factor and c-kit in the mammalian testis: lessons originating from Mother Nature’s gene knockouts. J Endocrinol 153:337–344
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
MacDonald SM, Trofimov A, Safai S, Adams J, Fullerton B, Ebb D, Tarbell NJ, Yock TI (2011) Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int J Radiat Oncol Biol Phys 79(1):121–129
Madden J, Foreman NK, Liu AK (2009) Germ cell tumors of the brainstem: report on two cases with pulmonary complications and a review of the literature. J Neurooncol 93(3):405–408. doi:10.1007/s11060-008-9780-5
Mailhot Vega RB, Kim J, Bussiere M, Hattangadi J, Hollander A, Michalski J, Tarbell NJ, Yock T, MacDonald SM (2013) Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer 119:4299–4307
Matsutani M (2008) Treatment of intracranial germ cell tumors: the second phase II study of Japanese GCT Study Group. In: 13th International Symposium on Pediatric Neuro-Oncology. Chicago. p 420
Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T (1997) Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 86:446–455
Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O (1998) Combined treatment with chemotherapy and radiation therapy for intracranial germ cell tumors. Childs Nerv Syst 14:59–62
Mei K(1), Liu A, Allan RW, Wang P, Lane Z, Abel TW, Wei L, Cheng H, Guo S, Peng Y, Rakheja D, Wang M, Ma J, Rodriguez MM, Li J, Cao D (2009) Diagnostic utility of SALL4 in primary germ cell tumors of the central nervous system: a study of 77 cases. Mod Pathol 22(12):1628–1636
Miyanohara O, Takeshima H, Kaji M, Hirano H, Sawamura Y, Kochi M, Kuratsu J (2002) Diagnostic significance of soluble c-kit in the cerebrospinal fluid of patients with germ cell tumors. J Neurosurg 97:177–183
Nakajima H, Iwai Y, Yamanaka K, Yasui T, Kishi H (2000) Primary intracranial germinoma in the medulla oblongata. Surg Neurol 53:448–451
Nakase H, Ohnishi H, Touho H, Karasawa J, Tsunoda S (1994) Cerebellar primary germ-cell tumor in a young boy. Brain Dev 16:396–398
Nichols CR (1996) Ifosfamide in the treatment of germ cell tumors. Semin Oncol 23:65–73
Nishizaki T, Kajiwara K, Adachi N, Tsuha M, Nakayama H, Ohshita N, Ikeda N, Ito H, Suzuki M (2001) Detection of craniospinal dissemination of intracranial germ cell tumours based on serum and cerebrospinal fluid levels of tumour markers. J Clin Neurosci 8:27–30
Ogawa K, Toita T, Nakamura K, Uno T, Onishi H, Itami J, Shikama N, Saeki N, Yoshii Y, Murayama S (2003) Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: a multiinstitutional retrospective analysis of 41 patients. Cancer 98:369–376
Oi S, Matsumoto S (1992) Controversy pertaining to therapeutic modalities for tumors of the pineal region: a worldwide survey of different patient populations. Childs Nerv Syst 8:332–336
Oi S, Matsuzawa K, Choi JU, Kim DS, Kang JK, Cho BK (1998) Identical characteristics of the patient populations with pineal region tumors in Japan and in Korea and therapeutic modalities. Childs Nerv Syst 14:36–40
Okada Y, Nishikawa R, Matsutani M, Louis DN (2002) Hypomethylated X chromosome gain and rare isochromosome 12p in diverse intracranial germ cell tumors. J Neuropathol Exp Neurol 61:531–538
Okochi Y, Nihashi T, Fujii M, Kato K, Okada Y, Ando Y, Maesawa S, Takebayashi S, Wakabayashi T, Naganawa S (2014) Clinical use of (11)C-methionine and (18)F-FDG-PET for germinoma in central nervous system. Ann Nucl Med 28(2):94–102. doi:10.1007/s12149-013-0787-4
Ono N, Kakegawa T, Zama A, Nakamura M, Inoue HK, Tamura M, Wakao T, Uki J, Takeda F, Kurihara H et al (1994) Factors affecting functional prognosis in survivors of primary central nervous system germinal tumors. Surg Neurol 41:9–15
Osada T, Fujimaki T, Takamizawa M, Tsuno NH, Kirino T, Shibata Y (2001) Dendritic cells activate antitumor immunity for malignant intracranial germ cell tumor: a case report. Jpn J Clin Oncol 31:403–406
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J et al (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 4):iv1–iv63. doi:10.1093/neuonc/nou223
Osuka S, Tsuboi K, Takano S, Ishikawa E, Matsushita A, Tokuuye K, Akine Y, Matsumura A (2007) Long-term outcome of patients with intracranial germinoma. J Neurooncol 83:71–79
Packer RJ, Cohen BH, Cooney K (2000) Intracranial germ cell tumors. Oncologist 5:312–320
Park J, Park Y, Lee SU, Kim T, Choi Y-K, Kim J-Y (2015) Differential dosimetric benefit of proton beam therapy over intensity modulated radiotherapy for a variety of targets in patients with intracranial germ cell tumors Radiation Oncology 10:135
Pinkerton CR, Broadbent V, Horwich A, Levitt J, McElwain TJ, Meller ST, Mott M, Oakhill A, Pritchard J (1990) ‘JEB’–a carboplatin based regimen for malignant germ cell tumours in children. Br J Cancer 62:257–262
Pollack IF (2012) Surgical options for pineal region tumors. World Neurosurg 77(2):302–303. doi:10.1016/j.wneu.2011.06.020
Regis J, Bouillot P, Rouby-Volot F, Figarella-Branger D, Dufour H, Peragut JC (1996) Pineal region tumors and the role of stereotactic biopsy: review of the mortality, morbidity, and diagnostic rates in 370 cases. Neurosurgery 39:907–912, discussion 912–904
Rickert CH, Simon R, Bergmann M, Dockhorn-Dworniczak B, Paulus W (2000) Comparative genomic hybridization in pineal germ cell tumors. J Neuropathol Exp Neurol 59:815–821
Robertson PL, DaRosso RC, Allen JC (1997) Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol 32:71–80
Rogers SJ, Mosleh-Shirazi MA, Saran FH (2005) Radiotherapy of localised intracranial germinoma: time to sever historical ties? Lancet Oncol 6:509–519
Rosenblum MK, Nakazato Y, Matsutani M (2007) Germ cell tumors. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) World Health Organization classification of tumours. Pathology and genetics of tumours of the central nervous system. IARC Press, Lyon, pp 197–204
Saitoh M, Tamaki N, Kokunai T, Matsumoto S (1991) Clinico-biological behavior of germ-cell tumors. Childs Nerv Syst 7:246–250
Salzman KL, Rojiani AM, Buatti J, Quisling RG, Marcus RB Jr, Maria BL, Mickle JP, Kedar A (1997) Primary intracranial germ cell tumors: clinicopathologic review of 32 cases. Pediatr Pathol Lab Med 17:713–727
Sands SA, Kellie SJ, Davidow AL, Diez B, Villablanca J, Weiner HL, Pietanza MC, Balmaceda C, Finlay JL (2001) Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: from the First International CNS Germ-Cell Tumor Study. Neuro Oncol 3:174–183
Sano K, Matsutani M, Seto T (1989) So-called intracranial germ cell tumours: personal experiences and a theory of their pathogenesis. Neurol Res 11:118–126
Sawamura Y, de Tribolet N, Ishii N, Abe H (1997) Management of primary intracranial germinomas: diagnostic surgery or radical resection? J Neurosurg 87:262–266
Sawamura Y, Ikeda J, Shirato H, Tada M, Abe H (1998a) Germ cell tumours of the central nervous system: treatment consideration based on 111 cases and their long-term clinical outcomes. Eur J Cancer 34:104–110
Sawamura Y, Kato T, Ikeda J, Murata J, Tada M, Shirato H (1998b) Teratomas of the central nervous system: treatment considerations based on 34 cases. J Neurosurg 89:728–737
Sawamura Y, Shirato H, Ikeda J, Tada M, Ishii N, Kato T, Abe H, Fujieda K (1998c) Induction chemotherapy followed by reduced-volume radiation therapy for newly diagnosed central nervous system germinoma. J Neurosurg 88:66–72
Schild SE, Haddock MG, Scheithauer BW, Marks LB, Norman MG, Burger PC, Wong WW, Lyons MK, Schomberg PJ (1996) Nongerminomatous germ cell tumors of the brain. Int J Radiat Oncol Biol Phys 36:557–563
Schoenfeld A, Haas-Kogan DA, Molinaro A, Banerjee A, Nicolaides T, Tihan T,Bollen AW, Gupta N, Mueller S (2014) Pure germinomas of the central nervous system: treatment strategies and outcomes. J Neurooncol 120(3):643–649
Shibamoto Y, Oda Y, Yamashita J, Takahashi M, Kikuchi H, Abe M (1994a) The role of cerebrospinal fluid cytology in radiotherapy planning for intracranial germinoma. Int J Radiat Oncol Biol Phys 29:1089–1094
Shibamoto Y, Takahashi M, Abe M (1994b) Reduction of the radiation dose for intracranial germinoma: a prospective study. Br J Cancer 70:984–989
Shirato H, Nishio M, Sawamura Y, Myohjin M, Kitahara T, Nishioka T, Mizutani Y, Abe H, Miyasaka K (1997) Analysis of long-term treatment of intracranial germinoma. Int J Radiat Oncol Biol Phys 37:511–515
Snow GE, Kasper AC, Busch AM, Schwarz E, Ewings KE, Bee T, Spinella MJ, Dmitrovsky E, Freemantle SJ (2009) Wnt pathway reprogramming during human embryonal carcinoma differentiation and potential for therapeutic targeting. BMC Cancer 9:383. doi:10.1186/1471-2407-9-383
Somma AD, Bronzoni C, Guadagno E, Solari D, Dell’aversana GO, De Caro BS, Cappabianca P (2014) The “extended” endoscopic endonasal approach for the removal of a mixed intrasuprasellar germinoma: technical case report. Surg Neurol Int 5:14. doi:10.4103/2152-7806.126043
Steinbok P (2001) Management of malignant pineal germ cell tumors with residual mature teratoma. Neurosurgery 49:1271
Steinbok P, Cochrane DD (2001) Pineal region and intracranial germ cell tumors. In: McLone DG (ed) Pediatric neurosurgery: surgery of the developing nervous system. W.B. Saunders, Philadelphia, pp 711–724
Strojan P, Zadravec LZ, Anzic J, Korenjak R, Jereb B (2006) The role of radiotherapy in the treatment of childhood intracranial germinoma: long-term survival and late effects. Pediatr Blood Cancer 47:77–82
Sukov WR, Cheville JC, Giannini C, Carlson AW, Shearer BM, Sinnwell JP, Ketterling RP (2010) Isochromosome 12p and polysomy 12 in primary central nervous system germ cell tumors: frequency and association with clinicopathologic features. Hum Pathol 41(2):232–238. doi:10.1016/j.humpath.2009.07.017
Tada T, Takizawa T, Nakazato F, Kobayashi S, Koike K, Oguchi M, Ishii E, Amano Y (1999) Treatment of intracranial nongerminomatous germ-cell tumor by high-dose chemotherapy and autologous stem-cell rescue. J Neurooncol 44:71–76
Takei Y, Pearl GS (1981) Ultrastructural study of intracranial yolk sac tumor: with special reference to the oncologic phylogeny of germ cell tumors. Cancer 48:2038–2046
Tan C, Scotting PJ (2013) Stem cell research points the way to the cell of origin for intracranial germ cell tumours. J Pathol 229(1):4–11. doi:10.1002/path.4098
Teilum G (1976) Special tumors of ovary and testis and related extragonadal lesions: comparative pathology and histological identification. J.B. Lippincott, Philadelphia
Tseng KY, Ma HI, Liu WH, Tang CT (2012) Endoscopic supracerebellar infratentorial retropineal approach for tumor resection. World Neurosurg 77(2):399.E1–E4. doi:10.1016/j.wneu.2011.05.035
Uematsu Y, Tsuura Y, Miyamoto K, Itakura T, Hayashi S, Komai N (1992) The recurrence of primary intracranial germinomas. Special reference to germinoma with STGC (syncytiotrophoblastic giant cell). J Neurooncol 13:247–256
Wang L, Yamaguchi S, Burstein MD, Terashima K, Chang K, Ng H-K et al (2014) Novel somatic and germ line mutations in intracranial germ cell tumours. Nature 511(7508):241–245. doi:10.1038/nature13296
Weiner HL, Lichtenbaum RA, Wisoff JH, Snow RB, Souweidane MM, Bruce JN, Finlay JL (2002) Delayed surgical resection of central nervous system germ cell tumors. Neurosurgery 50:727–733, discussion 733–724
Wolden SL, Wara WM, Larson DA, Prados MD, Edwards MS, Sneed PK (1995) Radiation therapy for primary intracranial germ-cell tumors. Int J Radiat Oncol Biol Phys 32:943–949
Yoshida J, Sugita K, Kobayashi T, Takakura K, Shitara N, Matsutani M, Tanaka R, Nagai H, Yamada H, Yamashita J et al (1993) Prognosis of intracranial germ cell tumours: effectiveness of chemotherapy with cisplatin and etoposide (CDDP and VP-16). Acta Neurochir 120:111–117
Yu IT, Griffin CA, Phillips PC, Strauss LC, Perlman EJ (1995) Numerical sex chromosomal abnormalities in pineal teratomas by cytogenetic analysis and fluorescence in situ hybridization. Lab Invest 72:419–423
Zimmerman RA, Bilaniuk LT (1982) Age-related incidence of pineal calcification detected by computed tomography. Radiology 142:659–662
Zynger DL, Everton MJ, Dimov ND, Chou PM, Yang XJ (2008) Expression of glypican 3 in ovarian and extragonadal germ cell tumors. Am J Clin Pathol 130(2):224–230. doi:10.1309/8DN7DQRDFB4QNH3N
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing
About this chapter
Cite this chapter
Braunstein, S., McBride, S.M., Haas-Kogan, D.A. (2017). Intracranial Germ Cell Tumors. In: Gupta, N., Banerjee, A., Haas-Kogan, D. (eds) Pediatric CNS Tumors. Pediatric Oncology. Springer, Cham. https://doi.org/10.1007/978-3-319-30789-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-30789-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30787-9
Online ISBN: 978-3-319-30789-3
eBook Packages: MedicineMedicine (R0)