Abstract
The complex method of chemical treatment and diffusive chrome-plating is discussed and investigational diffusive layers are picked up by this method.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The chemical heat treatment of surfaces of machine parts is an effective method for generating appropriate surface-hardened layers [1]. To change these characteristics, another technological effect can be applied simultaneously or sequentially with chemical–thermal treatment [2, 3]. This provides a comprehensive treatment of hardened surface layers' required parameters.
Objective: To develop a new method of surface hardening, providing the required quality characteristics of machine parts and tools.
2 Materials and Methods
To harden a surface or restore machine parts, a complex method of chemical treatment and plating diffusion is offered [4–8]. It is prepared first by deposition on the surface of renewable details of Ni-Co-P chemical coating in defined aqueous solution formulations and adopted by diffusion chromium plating modes. As a result of recovery on the workpiece, a surface diffusion layer is formed. Its structure (depending on the mode of the applied method) consists of several zones, which are working with the external composite zone, which reaches 250 μm. During the recovery process, universal equipment available in the workplace is used in the complex method.
Chemical treatment (Table 12.1) is applied to the surface details of the preliminary machining, and is followed by cleaned, degreased, chemical deposition in an aqueous solution of a particular recipe. Due to the increased chemical deposition load the process lasts 45 min. The obtained result is a Ni-Co-P amorphous-type chemical coating with thickness of 8–12 μm.
Chemical–thermal treatment—diffusion plating is carried out at a temperature of 1050 °C. The detail is placed in a retort with a powder mixture of ferrochrome, aluminum oxide, and ammonium chloride and a consumable sealed gate. To form the desired diffusion layer structure, isothermal aging at 700 or 800 °C with a duration of 1 or 1.5 h is used.
Compared with the traditional chrome diffusion, which is made up of hardened layers of thickness 15–30 μm, consisting of chromium carbides Cr23C6 and Cr7C3, after a complex method of chemical treatment and recovery the chrome diffusion layer is formed by diffusion (Fig. 12.1), which for medium and high carbon steels contains four zones: a composite outer zone consisting of columnar chromium carbide matrix Cr solid solution in α-Fe thickness of 100–250 μm and integrated microhardness of 11–15 GPa; an area solid solution of chromium in αFe 10–50 μm thickness and microhardness of 4.5 GPa; an eutectoid zone thickness of 10–30 μm and microhardness 4 GPa; and a carbonless zone thickness of 100–180 μm and 1.4–1.6 GPa microhardness for the ferritic component and core parts. Composite structure zones can significantly increase the relaxation life because of the accumulation in the course of internal microstresses in the soft phase—solid solution of chromium in αFe, at a time when the main burden will perceive a solid phase—grain columnar chromium carbide high hardness (up to 18 GPa).
3 Discussion
Particular attention to the diffusion layers is obtained on a complex method of steel. With the implementation of regimes the complex method of chemical treatment and plating diffusion of 5-h diffusion in chrome and 1050 °C 1-h isothermal holding at 700 °C we get the steel reinforced layer, which consists of four main zones (Fig. 12.2). The outdoor composite zone 1 (thickness 100 μm), consists of packages transkrystal micrograin chromium carbides. At the same time, there are grain carbide inclusions, which are mainly located at the physical surface (closer to the source of chromium).
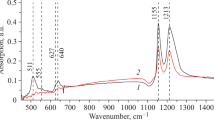
Phase analysis conducted on these samples showed the presence of chromium carbides Cr7C3 here and αFe (Table 12.1).
Schedule diffusion element distribution indicates the presence of a large number of Ni-Co-P in the zone 2 solid solution of chromium in αFe (Fig. 12.3), indicating active diffusion processes. Peak bursts of chromium content and, accordingly, a sharp drop in the concentration of other elements in these areas indicate the presence of colonies formed in the carbide composite zone.
Microhardness integrated composite samples of zone 1 are equal to 11 GPa (Fig. 12.4). Here you can see colonies of solid carbide micrograins. There micrograin carbide forms colonies in the source material.
After a comprehensive restoration parts made of steel with 0.45 % C with chemical coating, 7-h diffusion chrome (at 1050 °C), and isothermal holding hour (with 800 °C) the morphology develops a diffusion layer composite zone structure (Fig. 12.5).
The composite layer 1, a thickness of 250 μm, is a typical developed network stretched to the physical surface of carbide grains, which are placed in a matrix of a solid solution of chromium in αFe. On the border of zone 2, these grains fused into a continuous strand of carbides. It is interesting that the carbide grains do not completely permeate zone 1 and 30–50 μm do not reach the physical surface. The integrated composite microhardness zone is 12 GPa (Fig. 12.6).
Schedule distribution diffusion elements (Fig. 12.6) confirm that the surface area of a solid solution of chromium in αFe, in which the diffusion element concentration stabilizes, and peaks (above 50 %) of the concentrations of chromium (under falling concentration in these areas of other elements) by depth location carbide grains (Table 12.2). The zone 2 homogeneous solid solution of chromium in αFe content is characterized by high values of Ni (10 %) and Co (up 3 %). Thickness zone 2 is on average 25–40 μm. Obviously, the nickel pushes carbon from the subsurface zone, and the formation of elongated carbide grains is observed, which are located on the border zones 1 and 2 toward the physical surface (Table 12.3).
The presence of the complex method of chemical treatment and the effect of the liquid metal phase, which occurs as a result of this, and isothermal holding allows a reinforcing ply to develop at a fairly great depth. The composite zone 1 layer, which in detail friction pairs is working, reaches 250 μm, providing an increased service life.
Phase analysis was performed on this sample twice through large unidentified peaks, indicating the complex state of the presence of a stress-hardened layer,. But there is definitely the availability of a large number of Cr7C3 and αFe.
The diffusion layer on the steel with 1.0 % C (Fig. 12.8), obtained after a complex method of implementation mode recovery is 7 h diffusion plating (with 1050 °C), the previous hour isothermal holding (at 800 °C) with chemical treatment, characterized by a high volume content of chromium carbide grains in composite zone 1. These grains are elongated to the physical surface shape, and on the border with the zone 2 forming solid carbide with chromium carbide grains, fused together. There is a visually observed difference between these types of grains, according to the elongated grain carbide—a Cr23C6, and carbide fused together—Cr7C3 (Table 12.4). The integrated composite microhardness zone 1 reaches 15 GPa (Fig. 12.9).
Some graphics division diffusion elements (Fig. 12.10), which is the depth that reflects zone 1, shows a fairly even content diffusion of elements with small vibrations, and only in the end zone; at the location of a solid carbide colony an increase in chromium content (50 %) was noticed, and accordingly a decrease in concentrations of other elements. Content diffusion elements in zone 1 are relatively high and there is stable zone thickness (excluding solid carbide locations near the boundaries of zone 2). Homogeneous zone 2 Cr in solid solution Feα is characterized by unstable thickness, and in places very close to zone 1 and/or zone 3. This model has a maximum content of Ni and Co in zone 2 (about 18 % Ni and Co 3 %).
After the restoration of the complex by chemical processing and diffusion plating on steel with 1.0 % C in us, just as the steel with 0.45 % C, characterized traced all areas, including eutectoid zone 3. This difference appears to HCS. Phase analysis, carried out twice showed that this layer presented chromium carbides Cr7C3, Cr23C6, and α- and γ-iron.
Chart distribution diffusion elements (Ni, Co, P), shown in Figs. 12.3, 12.6, and 12.10, placed their increased concentration between the grains of chromium carbides. For example, Ni, which despite increased strength and ductility simultaneously increases, the material is observed to increase it to 10 %. Thus, the carbide grains such as “wrapped” plastic material (Ni), which is well able to relax internal stresses that occur when the parts are working, which increases the life of the parts, constitute the refurbished complex method of chemical processing and diffusion plating.
4 Conclusion
Implementation of the complex chemical processing method and diffusion plating to restore the machine parts enables gets diffusion layers that are different in structure, thickness, and hardness.
The optimal method for recovering chemical processing machine parts is made of structural steel with 0.3–0.6 % C. On manufactured or remanufactured parts that are made of steel 45, diffusion layers, the composite thickness of the outer zone reaches 250 μm, and the integral microhardness of 12 GPa. The phase composition composite zone consists of chromium carbides Cr23C6, Cr7C3, and α-Fe (002).
The complex method of chemical treatment and plating diffusion can restore parts made of high carbon steel. The diffusion layer has a composite zone of highly integrated microhardness (about 15 GPa) and thickness to 200 μm with tightly spaced carbide grains. The phase composition composite zone consists of chromium carbides Cr23C6, Cr7C3, and αFe (002).
Parts that are made of steel with 0.3–0.6 % C operating under dynamic loads should recover in modes that provide 5-h diffusion of chromium hour isothermal holding at 700 °C. This enables restored diffusion layers, the outer composite area of which consists of fine whiskers of chromium carbides in a Cr matrix solid solution in αFe about 100 μm thick and integral microhardness to 11 GPa. The phase composition of such layers are chromium carbides Cr7C3, αFe (110), and αFe (002).
References
Lampman S (1991) Introduction to surface hardening of steels, heat treating. In: ASM handbook, vol 4. ASM International, Novelty, OH, pp 259–267
Kulka M, Pertek A (2003) Characterization of complex (B + C + N) diffusion layers formed on chromium and nickel-based low-carbon steel. Appl Surf Sci 218(1–4):114–123
Murali M, Sambathkumar M, Saravanan MSS (2014) Micro structural and mechanical properties of AA 7075/Tio2 in situ composites. Univers J Mater Sci 2(3):49–53
Stetsko A (2013) Technological support resource of manufactured and remanufactured parts, Monograph, Lviv, p 240. ISBN 978-966-2739-29-9
UA 110046, C23C 8/70, C23C 10/32, 10 Nov 2015
UA 109285, C23C 10/32, C23C 22/62, C23C 22/05, C23C 10/02, 10 Aug 2015
UA 109283, C23C 10/32, C23C 22/62, C23C 22/05, C23C 10/02, 10 Aug 2015
UA 108895, C23C 22/62, C23C 10/18, C23C 10/32, C23C 10/38, C23C 10/40, 25 Jun 2015
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Stetsko, A. (2016). Composite Coatings Formed by Complex Methods of Surface Hardening. In: Fesenko, O., Yatsenko, L. (eds) Nanophysics, Nanophotonics, Surface Studies, and Applications. Springer Proceedings in Physics, vol 183. Springer, Cham. https://doi.org/10.1007/978-3-319-30737-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-30737-4_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30736-7
Online ISBN: 978-3-319-30737-4
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)