Abstract
Tracheobronchopathia osteochondroplastica (TO) is a rare disorder of unknown etiology, characterized by the development of multiple cartilaginous and bony nodules in the submucosal layer of central airways. The clinical presentation is non-specific with chronic cough, sputum production, intermittent hemoptysis, and breathlessness. In many patients, diagnosis is made incidentally on computed tomography (CT) or bronchoscopy performed for unrelated indications. Occasionally, patients present with acute respiratory distress, hypoxemia, and unanticipated difficulty during intubation. Although chest CT is the most useful imaging modality, the diagnosis can be missed in the early stages of disease. Bronchoscopy is the gold standard for diagnosis. The presence of hard nodules projecting into the lumen from anterior and lateral walls with sparing of posterior wall provides an instant diagnosis during bronchoscopy. Whether biopsy is needed for definitive diagnosis is a debatable matter. The majority of patients follow a benign clinical course. No treatment is needed in asymptomatic or minimally symptomatic patients. Adequate long-term control of symptoms can be achieved with laser photoresection and mechanical debulking in symptomatic patients with advanced central airway obstruction. Surgery may be needed in some patients if the interventional bronchoscopic procedures are not feasible. TO should feature in the differential diagnosis of chronic and persistent cough, hemoptysis, and treatment-resistant asthma.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tracheobronchopathia osteochondroplastica
- Cough
- Central airway obstruction
- Bronchoscopy
- Tracheobronchial amyloidosis
Introduction
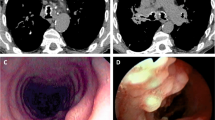
Tracheobronchopathia osteochondroplastica (TO) is an uncommon disorder characterized by the development of multiple cartilaginous and bony nodules in the submucosal layer of central airways [1–3]. The nodules project into the lumen from the anterior and the lateral walls of trachea and bronchi (Fig. 7.1) causing a variety of respiratory symptoms. Typically, the posterior membranous wall of central airways is not involved with the disease process. The underlying cause of this disorder remains unknown. The clinical presentation depends on the size and distribution of the nodules. In mild cases, patients may remain asymptomatic. On the other extreme, large and confluent cartilaginous or bony nodules can cause severe airway obstruction, atelectasis, and life-threatening difficulties during intubation. In this chapter, we discuss the clinical features, diagnosis, and treatment of this rare but interesting clinical entity.
Bronchoscopic findings in mild (a) and severe (b) tracheobronchopathia osteochondroplastica. Note extensive nodules arising from anterior and lateral wall of trachea with sparing of posterior membrane. Reprinted from [3]. With permission from Springer Science+Business Media
Historical Background
The original description of TO comes from Samuel Wilks in 1857 who reported several ossified deposits in larynx, trachea, and bronchi upon autopsy in a 38-year-old patient who died of tuberculosis [4]. In 1863, Virchow [5] proposed that cartilaginous and bony nodules in TO arise from ecchondrosis and exostosis of normal tracheobronchial cartilage. Von Schoretter is said to have seen the TO lesions for the first time in a living patient using a laryngeal mirror in 1896 [6]. The credit of first description of bronchoscopic findings of TO goes to Killian in 1897 [7]. In 1910, Aschoff-Frieburg [8] coined the term tracheopathia osteochondroplastica and proposed that these nodules arise from metaplasia of tracheal and bronchial elastic connective tissue.
Incidence
The exact incidence of TO is unknown, but by most accounts, it is exceedingly uncommon. A detailed review of the literature by Dalgaard in 1947 found 90 cases [9], and another literature review by Martin in 1974 revealed only 245 reported cases of TO [10]. Except for a series of thirty cases by Harma and Suurkari in 1977 [11] and another series of 41 cases by GERM“O”P group in 2001 [12], the majority of reports have involved a single or a handful of cases. Still, there is a possibility that TO is more common than reported because many cases go unnoticed due to the absence of any significant symptoms. Before advent of bronchoscopy, the majority of cases were detected on autopsy. In these cases, TO was most often an incidental finding, unrelated to the underlying cause of death. In one such series, findings consistent with TO were found in 2 out of 800 (0.25 %) autopsies [13]. Widespread use of chest computed tomography (CT) and flexible bronchoscopy has increasingly allowed antemortem detection of TO in live patients. The incidence of TO is reported to vary from 0.02 to 0.77 % of bronchoscopies performed for unrelated indications [14–21].
Pathology
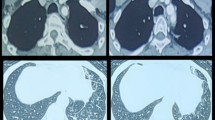
Table 7.1 summarizes the pathologic findings of TO. Several autopsy studies provide a detailed description of pathological findings in TO [13, 22]. Most commonly, the disease process involves the lower two-third of trachea. Involvement of the main-stem and lobar bronchi is also common. Very seldom, segmental bronchi may also be involved. Isolated involvement of main-stem bronchi without involvement of trachea has been described in some cases. The involvement of larynx in TO is uncommon but has been reported by several authors [22, 23]. On gross examination, the tracheal wall is thickened and the nodules are visible over the mucosal surface of anterior and lateral wall sparing the posterior membranous wall. Many of the nodules have stony hard consistency. Microscopic examination of the trachea shows bony and cartilaginous nodules within the submucosa and lamina propria of trachea and bronchi (Fig. 7.2). The nodules vary in size and shape. In some cases, there are only a few small nodules [24], while in other cases, the nodules are too numerous to count, becoming confluent in certain areas. Partial ossification of cartilaginous nodules is a common finding [22]. Interestingly, many but not all nodules show an anatomical continuity with the perichondrium of the cartilaginous ring. Some bony nodules contain marrow space with fatty tissue and a small amount of hematopoietic cells. The overlying epithelium frequently demonstrates squamous metaplasia. The inflammatory reaction is conspicuously minimal or absent.
Histological findings in tracheobronchopathia osteochondroplastica, illustrating submucosal calcification, ossification, and cartilage formation. Reprinted from [3]. With permission from Springer Science+Business Media
Small biopsies obtained during flexible bronchoscopy may reveal many of the typical pathological findings described above, but it is not possible to retrieve a diagnostic specimen in every instance. It is also unrealistic to expect a classical pathological description of TO even when adequate bronchoscopic biopsies are obtained. This is well illustrated in a series of 41 patients, in which histopathology of bronchoscopic biopsies was diagnostic in 28 of 40 (70 %) of patients [12]. Bony nodules in submucosa was the most common finding, seen in 23 (58 %) of patients. Other findings included cartilaginous nodules in 15 (38 %), calcification in 8 (20 %), and squamous metaplasia in 19 (48 %) patients.
Etiology and Pathogenesis
The events that lead to development of TO are currently unknown. For more than a century, the debate has centered around two different views to explain how cartilaginous and bony nodules develop within the submucosa of central airways in TO. According to one of these theories advanced by Virchow [5], the new formations in this disorder represent ecchondrosis or exostosis arising from tracheal cartilage. A direct anatomical continuity between these lesions and the perichondrium of tracheal cartilage on serial sections, especially in early stages of the disease process, lends some support to this view [13, 24]. However, not every nodule is connected to the tracheal cartilage. The presence of isolated bony and cartilaginous islands can be explained by thinning, stretching, and eventual separation as the nodules elongates after their origin from the perichondrium of tracheal cartilage [25]. Aschoff [8] in 1910 proposed an alternative theory, according to which the cartilaginous and bony nodules arise from metaplasia of elastic connective tissue normally present in the submucosal layer of trachea. According to Dalgaard [9], the undifferentiated connective tissue cells found in close relation to submucosal elastic fibers are the parent cells from which TO lesions originate. Additional support for Aschoff’s proposal also comes from the frequent presence of elastic fibers in close relationship to the nodules on detailed pathological examination [22]. However, sparing of posterior tracheal membrane, which is rich in elastic fibers, cannot be explained on the basis of this theory.
Even more mysterious are the cellular mechanisms that eventually lead to the formation of cartilaginous and bony nodules in TO. It is not difficult to imagine that chondrogenesis and osteogenesis in such an unusual site would involve a complex interplay between several cells, growth factors, and regulatory pathways. In this context, there is some indication that bone morphogenic protein (BMP) and transforming growth factor beta-1 (TGF-β-1) are involved in pathogenesis of TO. Bone morphogenic proteins belong to TGF-β superfamily and are known to play a critical role in embryogenesis and tissue homeostasis. An important property of BMP is its ability to promote differentiation of mesenchymal stem cells into osteoprogenitor cells [26]. There is also evidence for role of BMP in the development of cartilaginous tissue [27]. Apart from promoting formation of cartilage and bone, BMP also plays important role in mineralization and formation of hematopoietic marrow. In this regard, BMP is shown to have an active role not only where the normal bones are found in the body but also at ectopic sites outside the skeleton [28].
Many biological actions of TGF-β and BMP converge as they share similar cellular receptors and intracellular transduction pathways [29]. TGF-β stimulates the production of extracellular matrix proteins by chondrocytes [30] and is shown to induce formation of bone [31]. In this regard, TGF-β seems to complement BMP through different phases of bone formation [32]. Since the key pathological features of TO include formation of cartilage and bone at ectopic site in the tracheal submucosal layer, it is not unreasonable to speculate that BMP and TGF-β may have some role in pathogenesis of this disorder.
This possibility was addressed in one study in which immunohistochemical methods were used to identify the presence of bone morphogenic protein-2 (BMP-2) and TGF-β-1 in 2 autopsy cases of TO [33]. Significant amount of BMP-2 activity was located in the mesenchymal cells around the osteocartilaginous nodules. BMP-2 immunoreactivity was also located in the chondroblasts, the new cartilage, and the immature matrix of the cartilage. Although a significant BMP-2 activity was noted in the newly formed nodules, no significant BMP-2 was found in the adjacent normal tracheal cartilage. TGF-β-1 was detected in the chondrocytes and osteocytes of the submucosal nodules. TGF-β-1 activity was also found in bronchial epithelial cells. Based on these findings, the authors speculated that BMP-2 and TGF-β-1 have synergistic role in promoting formation of osteocartilaginous nodules in TO.
What initiates the development of TO lesions has not been identified. Except for a single report of TO in a mother and daughter [34], there are no other reports of familial occurrence or genetic predisposition. No other factor has been conclusively linked to the development of TO, although many possibilities have been raised, as discussed below.
Concurrent finding of amyloidosis in certain cases has led some investigators to suggest that TO may be a late stage of primary tracheobronchial amyloidosis [35–39]. Some support to this view comes from a series of 32 patients with tracheobronchial amyloidosis in which 7 (22 %) cases also had bronchoscopic and biopsy evidence of TO lesions [40]. Similarly, in a series of 41 patients with TO, 16 biopsy specimens specifically were examined for amyloidosis with Congo red stain and 2 (13 %) biopsies disclosed the presence of amyloidosis [12]. While coexistence of such rare entities in some patients justifiably provide a reason to associate TO and amyloidosis, there are several points against this proposal. First, the presence of amyloidosis has been reported only a small fraction of all reported cases of TO. We find no cases of tracheobronchial amyloidosis among additional 97 patients from 7 case series of TO [11, 16–20, 41]. Similarly, except for one report [40], no patients with tracheobronchial amyloidosis has been reported to have a simultaneous diagnosis of TO in a number of case series [42–45]. Further, posterior membrane of trachea is frequently involved with tracheobronchial amyloidosis where as it is characteristically spared in TO. Taken together, there is no persuasive evidence to establish an etiologic link between TO and tracheobronchial amyloidosis.
In contrast, the association between ozena and TO appears more than coincidental. Ozena is a chronic nasal disease characterized by the progressive atrophic rhinitis, atrophy of underlying bone and turbinates, thick mucopurulent nasal secretions that form crusts that emit foul odor [46]. The characteristic pathological changes in ozena include chronic inflammation, squamous metaplasia, ciliary destruction, bone destruction, and formation of crusts. Klebsiella ozaenae is frequently isolated from nasal secretions, but it is still unsettled whether or not it is the underlying cause of the problem.
Frequent clinical association and similarity in the histological appearance of nasal and the tracheal mucosa has led many investigators to speculate that ozena increases the future risk of developing TO [47–49]. Several recent case series appear to support this view. In a series of 18 patients from Scandinavia, ozena or recurrent maxillary sinusitis was reported in 6 (33 %) of patients with TO [20]. Although no data were provided, TO was stated to be more severe when two conditions were present simultaneously. Symptoms of atrophic rhinitis, sinusitis, or pharyngitis were reported in 29 % of patients in another series of 41 patients with TO [12]. K. ozaenae was isolated from bronchial secretions in 8 (20 %) of these patients. In another report, atrophic rhinitis was detected in 2 (20 %) patients in a series of 10 patients with TO from Iran [18]. The most convincing association between these two entities comes from a series of 30 patients from Finland in which 23 patients had atrophic rhinitis and 4 (13 %) patients had K. ozaenae on culture of tracheal secretions [11]. Interestingly, 6 of these patients revealed purulent and crusty secretions over the tracheal mucosa reminiscent of atrophic rhinitis and ozena. The authors used the term tracheozaena to describe these findings. These findings do raise a possibility that K. ozaenae is somehow involved in pathogenesis of TO. In an experimental study, exposure to K. ozaenae led to the development of an amorphous material causing irregular clumping of nasal mucosal cilia. The damaging effect of the bacteria on ciliary function in the nose was proposed as a possible cause for the development of atrophic rhinitis and ozena [50]. It can be argued that similar events take place in tracheal mucosa of TO patients with K. ozaenae infection.
Although these reports make a convincing case, any association between ozena and TO cannot be taken as an evidence of cause and effect. It can be argued that a primary defect in mucosal defenses due to squamous metaplasia and loss of cilia could itself favor colonization and proliferation of K. ozaenae and other bacteria in the trachea. Thus, tracheal and bronchial infections with K. ozaenae may represent a complication and not the cause of TO. Regardless, these observations do provide a strong rationale for the physicians to diligently look for TO when atrophic rhinitis is detected and perform a thorough nasal and upper airway examination when TO is diagnosed.
No other consistent causative factor has been identified. Tracheal cultures have isolated Mycobacterium avium-intercellulare [51, 52], M. gordanae, and M. tuberculosis [12] in several reported cases, but there is no reason to believe that these infections are in any way related to pathogenesis of TO. Same can be said about a report of tracheal botyromycosis in a patient with underlying TO [53]. There is a single report of selective IgA deficiency with frequent sino-pulmonary infections in a patient with TO [54], but systematic investigations have revealed no abnormality in immunoglobulin or complement levels and cell mediated immunity [11]. No underlying endocrine problem has been found in patients with TO. Calcium and phosphate levels are usually within normal range. Occurrence of dermatomyositis, multiple myeloma, and other systemic disorders in some reports is coincidental [12]. A case of TO in a patient with underlying silicosis has been reported, but there is no reason to postulate a link between these two entities [55]. Report of ectopic calcification of falx cerebri in a 31-year-old patient is interesting but does not appear related to TO [56]. Finally, persuasive case for cause and effect relationship cannot be made on the basis of infrequent and isolated reports of non-Hodgkin lymphoma [57] and lung cancer [17, 58] in patients with TO.
Clinical Features
The clinical features of TO are highly variable. In early and mild disease, patients are asymptomatic and the diagnosis is established on the basis of incidental radiologic or bronchoscopic findings. On the other extreme are cases in which extensive involvement of air passages seriously compromised the airway lumen and caused rapidly progressive respiratory failure needing urgent interventions [59, 60]. The majority of diagnosed cases fall somewhere in-between these two extremes. The age distribution is highly variable. The diagnosis is usually made in fifth through seventh decade of life. However, many patients have been diagnosed in the second through fourth decade of life [11, 12, 18, 41]. In fact, in pediatric literature, the disease has been described in patients as young as 5 and 9 years of age [61, 62]. Quite possibly, the disease progresses slowly and remains asymptomatic for several years before the clinical symptoms surface in later years of life. Even so, it can be seen that in many reported cases, non-specific respiratory symptoms were ignored for several years before the correct diagnosis was made. There is no difference in distribution of cases according to gender or ethnicity. Smoking does not seem to increase the risk of TO. No environmental exposure or genetic predisposition is consistently identified in patients with TO.
Table 7.2 lists the presenting features and symptoms of TO. There are no pathognomonic signs and symptoms. Many patients are entirely asymptomatic, and it is not unusual to have diagnosis made incidentally on thoracic imaging or flexible bronchoscopy [11, 12].
Chronic cough is the most common presenting symptom experienced by 50–70 % of patients [12, 18, 41]. Some patients report chronic cough for several years or decades before correct diagnosis is made [19, 63]. While cough is usually associated with other pulmonary symptoms such as hemoptysis, dyspnea, and chest discomfort, in some instances chronic cough is the sole manifestation of the disease [12]. Cough is usually dry but up to one-third of patients report significant sputum production. Purulent sputum production is due to superimposed bacterial infection and is easily mistaken for acute bronchitis, chronic obstructive pulmonary disease (COPD) exacerbation, or bronchiectasis.
Dyspnea is reported by 20–90 % of patients with TO. The severity of dyspnea depends on degree of airway involvement [12, 18–20, 41]. Although dyspnea is usually mild in majority of cases, 11 of 41 (27 %) of patients in one series had rapidly progressive breathlessness [12]. Sometimes, emergent endotracheal intubation or tracheostomy is needed in TO patients presenting with stridor and acute respiratory failure.
Hemoptysis is the third most common symptom, reported by 20–60 % of patients [12, 18, 20, 41]. Intermittent mild hemoptysis in these patients is usually secondary to ulceration of mucosa overlying a prominent bony or cartilaginous nodule. Hemoptysis is usually self-limiting and is of limited clinical relevance. However, this is one symptom for which there is low threshold for performing bronchoscopy, thus providing opportunity to detect an otherwise unsuspected diagnosis. Massive hemoptysis is unusual and should suggest an alternative diagnosis.
The nasal and sinus symptoms are reported by at least one-third of patients [12, 20]. Symptoms of atrophic rhinitis or ozena are common, as discussed in the previous sections. Many patients experience recurrent episodes of acute sinusitis. Hoarseness and dysphonia are reported by up to 20 % of patients [12, 19]. Persistent hoarseness is uncommon but is seen when larynx and subglottis are involved with the disease process [23, 41, 64]. Laryngeal cancer should be excluded in such cases with careful direct laryngeal examination. Difficulty in swallowing has also been reported in some patients with TO.
There are several reports of recurrent lower respiratory tract infections, fever and pneumonia as presenting symptoms of TO [41, 65–67]. In one study, bronchial cultures were positive in 61 % of patients with TO [12]. The most common organisms isolated are K. ozaenae, Pseudomonas aeruginosa, and Staphylococcus aureus. Quite possibly, a defect in local mucosal defense due to squamous metaplasia and impaired mucociliary clearance is the root cause of bacterial colonization, which in turn increases the risk of recurrent pneumonia in these patients.
Extensive endobronchial obstruction has also caused lobar atelectasis and recurrent pneumonia in some patients with TO. For example, Meyer and associates described a patient suffering from right middle lobe atelectasis for 6 years which was later proven to be due to severe and diffuse involvement of the middle lobe bronchus with TO [68]. Similarly, Hodges and Israel have reported two patients with TO who presented with right middle lobe collapse. In each case, the opening of right middle lobe bronchus was more than 90 % occluded with TO lesions [69]. In both of these reports, although right middle lobe bronchus was most severely affected, typical TO nodules were also seen throughout the trachea and bronchi. Lobar atelectasis as the sole manifestation of TO is unusual but is reported. In one case, the patient developed right upper lobe atelectasis due to complete occlusion of the corresponding bronchus with a hard mass lesion later proven to be TO on pathological examination on surgical specimen [70]. In second case, an isolated TO lesion caused complete occlusion of sub-segmental (right 3b bronchus) resulting in post-obstructive pneumonia [71]. The most unusual feature in these cases was complete absence of TO lesions elsewhere in the tracheobronchial tree.
Several reports have also drawn attention to unexpected difficulties during planned endobronchial intubation for general anesthesia in TO [72, 73]. Grating sensation has been felt during attempts at passage of endotracheal tube into the trachea [74]. In some cases, the failure to pass endotracheal tube through the trachea has forced the providers to cancel the planned surgery [75, 76]. In one reported case, single-lung ventilation failed because nodular outgrowths projecting into airway lumen prohibited sufficient occlusion of right main-stem bronchus by the balloon blocker [77]. Laryngeal mask airway has been successfully used in one patient with failed prior intubation who required general anesthesia for an intra-abdominal surgery [78].
Patients with failed prior intubation in above reports showed narrowing of trachea on a subsequent bronchoscopy. Interestingly, none of these patients had any prior clinical symptoms on preoperative evaluation. Evidently, there are patients who never develop clinically significant symptoms despite severe involvement of central airways with TO.
Non-specific signs such as wheezing, rhonchi, and crackles can be appreciated in 30–60 % of TO patients, but these findings are not helpful in suspecting correct underlying diagnosis. Stridor and tachypnea are sometimes observed in patients with severe tracheal narrowing and impending respiratory failure [59].
Laboratory Investigations
Routine laboratory tests including calcium and phosphate levels are normal in TO [11, 67]. Serum immunoglobulin and complement levels are also normal. No serological tests or systemic endocrine abnormalities have been identified. Based on current data, extensive laboratory testing is not indicated in suspected or confirmed patients with TO.
Cultures of respiratory secretions are indicated in patients who present with lower respiratory tract infection. Identification of offending organism may help clinicians to choose most appropriate antibiotic therapy. Microbiologic evaluation in asymptomatic patients is unlikely to have any meaningful value.
Imaging
Chest radiograph has a limited value in diagnosis of TO. In isolated cases, chest radiographs have been reported to exhibit diffuse and irregular narrowing of airways, and scalloping or thickening of tracheal wall, best appreciated on lateral films [79–81]. Tracheal calcification is rarely seen on plain films. In one series, chest radiograph from 38 TO patients showed narrowing of trachea in 8 (21 %), calcification in 4 (11 %), atelectasis in 4 (11 %), and pneumonia in 10 (26 %) of patients [12].
In actual clinical practice, chest radiographs are rarely helpful since the findings on chest radiograph are usually subtle and are easily overlooked. It is also not uncommon to have normal chest radiograph despite a significant involvement of central airways with the disease process [19, 41]. Non-specific findings on chest radiography are common in TO but are insufficient to persuade clinician to consider TO as a diagnostic possibility [16, 20].
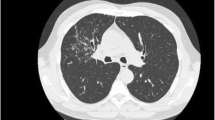
Chest CT scan is more helpful than chest radiograph and is the imaging modality of choice. The most common CT finding is the presence of multiple calcified and non-calcified nodules arising from inner anterior and lateral walls of trachea and projecting into the lumen without involving the posterior wall [82–85] (Fig. 7.3). The nodules vary from 3 to 8 mm in diameter. Thickening of tracheal wall is another commonly reported finding. In one series, CT was performed in 31 TO patients [12]. 74 % of patients had submucosal nodules, 61 % had submucosal calcification, and 10 % had tracheal stenosis. Even though CT is the most accurate imaging modality to determine the extent and distribution of the lesions, diagnosis can be overlooked in early stages and especially in patients with minimal involvement [18].
Chest CT showing irregular calcified nodules involving anterior and lateral walls of trachea (arrows) with sparing of posterior tracheal wall (arrowheads). Reprinted from [107]. With permission from Springer Science+Business Media
In some reports, repeated CTs have been performed presumably to assess future course of disease, but value of this practice is uncertain [86]. Follow-up CTs in one case series showed progression of lesions in 2 of 20 (10 %) of patients [12]. We do not recommend serial CTs in these patients as it would unnecessarily expose these patients to diagnostic radiation and, in the absence of new symptoms, would not provide any useful clinical information.
Findings on magnetic resonance imaging (MRI) have also been reported in one patient. These included diffuse irregular mural thickening of the trachea with the absence of contrast enhancement, sparing of the posterior wall, and low-signal dots on T1- and T2-weighted spin echo, suggestive of calcification within the tracheobronchial walls [87]. However, MRI does not provide any unique perspective about the disease process and is less sensitive than CT in detecting submucosal calcification. Therefore, there is no reason to perform MRI in patients with suspected TO.
Pulmonary Function Tests
Pulmonary function tests can be normal in early stages but may disclose obstructive ventilator defect when airway lumen is significantly compromised. In a series of 41 patients, pulmonary function tests were performed in 28 patients [12]. Airflow obstruction was detected in 11 (39 %), and restrictive defect was found in 5 (18 %) patients. Peak flows were reduced in 9 of 14 (64 %) patients. Spirometry was normal in 43 % of patients. In another series, 7 of 8 patients had obstructive ventilator defect and one remaining patient had a combined obstructive and restrictive defect [19]. Obstructive defect has also been reported by others [17, 20, 41]. Longitudinal assessments of pulmonary functions in one study showed no deterioration of spirometric parameters over a mean follow-up period of 4.2 years [88]. Reversibility after bronchodilator administration is sometimes reported [17, 19], but airway hyper-responsiveness on formal methacholine challenge test is not found [88]. Flow volume loop showed expiratory plateau in 3 of 16 (19 %) and inspiratory plateau in 2 of 16 (13 %) patients in one series [12]. Others have reported similar findings in a small number of patients [41]. Flow volume loop has been suggested to be a useful parameter to follow in TO [89], but data on its ability to detect progression of disease are rather limited.
Bronchoscopy
Bronchoscopy is the gold standard for diagnosis of TO. The most characteristic finding is the presence of multiple bony or cartilaginous nodules arising from the anterior and lateral wall of the airways and sparing the posterior tracheal membrane [1–3] (Fig. 7.1). The nodules measure 1–10 mm in diameter. Larger and confluent nodules can be seen to project into the airway lumen causing a variable degree of airway obstruction. The distribution of nodules is said to be “scattered” when few nodules are present with areas of normal mucosa between them, “diffuse” when numerous nodules are covering entire airway surface without intervening normal mucosa and “confluent” when the nodules are seen to coalesce and fuse together [12]. Sometimes, the bronchoscopic findings of this disorder have been compared to cobble stone appearance, rock garden, and stalactite cave. The overlying mucosal membrane may appear normal, but thinning of mucosa [41], ulceration, and hemorrhagic changes [12] can be appreciated in some patients. The presence of excessive amounts of serous, mucoid, or purulent secretions in the central airways is not unusual. Although the disease process is said to be most profuse in the distal two-third of the trachea [19], nodules are usually widely distributed and can involve nearly every part of central airways [12]. Several cases with involvement of larynx, subglottis, and proximal trachea can be found in the published literature [12, 23–41, 90]. The rigidity of airway wall limits the dynamic movement of airways with respiration seen in normal subjects. In heavily involved areas, it may be difficult to advance the bronchoscope through the narrow air passages. It is ill-advised to force the bronchoscope through such areas. In one instance, bronchoscope was reported to get stuck requiring considerable force to retrieve the scope out of the air passages [31]. Difficulty may also be encountered during passage of rigid bronchoscope. A grating sound sometimes heard as rigid instrument is advanced through the airways containing large bony projections. Similar grinding of flexible scope against the hard nodules has also been described in some reports [12].
Bony hard consistency makes it difficult to obtain diagnostic biopsies using standard bronchoscopic techniques. The biopsy forceps tends to slip and slide away from the lesion, and small mucosal fragments are all that can be retrieved in many cases. Repeat bronchoscopy may be needed after failure to obtain diagnostic specimen on the first attempt. For instance, in one series, endobronchial biopsies were obtained in 28 of 40 (70 %) of patients [12]. Diagnostic specimen was obtained on first bronchoscopy in 22 of 40 (55 %) patients. Remaining 18 of 40 (45 %) patients required additional bronchoscopy to obtain diagnostic tissue. Many patients required repeated bronchoscopies before the histological diagnosis could be secured. Larger biopsy forceps via rigid bronchoscope may have better success in obtaining diagnostic biopsy specimens.
In one case, mucosa was reported to have a smooth and continuous green appearance on autofluoresecence bronchoscopy, as expected in healthy tracheobronchial lining [91]. In another interesting case report, probe-based confocal laser endomicroscopy (pCLE) over the airway nodules showed a mottled and brightly autofluorescing submucosa and not the regular and cross-hatched healthy basement membrane as seen in normal subjects [92]. Future studies are indicated to address the predictive value of autofluorescence bronchoscopy and optical biopsy techniques in diagnosis of TO.
Atypical presentation of TO in some cases may not allow immediate diagnosis to be made on the basis of visual inspection, thus posing a considerable diagnostic challenge. For example, in one reported case, bronchoscopy in a 20-year-old patient showed a 3.2-cm endoluminal mass attached to the anterior wall of trachea causing near-total occlusion of the lumen [93]. No other submucosal nodules were identified either proximal or distal to the tracheal mass. Histological examination of the lesion obtained after primary surgical resection of the lesion revealed findings consistent with TO. In another atypical case, a patient presenting with chronic and mild hemoptysis showed three discrete localized and vascular appearing growths involving right middle, left upper, and left lower lobe openings, later proven to be TO on histological examination. There were no usual TO findings elsewhere on bronchoscopic examination in this case either [94]. We have already alluded to reports of TO presenting with isolated mass lesions blocking lobar or segmental bronchi causing lobar or segmental atelectasis [70, 71]. In the absence of typical bronchoscopic findings in such atypical cases, biopsy evidence is needed to establish the diagnosis. Fortunately, such cases are exceptions rather than the rule. Overwhelming majority of patients has classical bronchoscopic appearance which is sufficient for instant diagnosis in the bronchoscopy room.
Curiously, in one case, considerable difficulty was experienced in performing transbronchial needle aspiration in a TO patients with unrelated mediastinal lymph node enlargement [95]. The difficulty in the insertion of needle through the airway wall was presumably due to the presence of calcified nodules in the submucosal layer of the tracheal wall.
Diagnosis
There are no pathognomonic signs or symptoms of TO. It is therefore not surprising that diagnosis in pre-bronchoscopy era was exclusively made on autopsy. As clinical presentation is non-specific in a vast majority of patients, the most critical prerequisite for diagnosing TO is high index of suspicion that can only come from awareness of the disease entity. Bronchoscopy is most likely to provide diagnosis in early stages since radiologic findings as well as abnormalities on pulmonary function testing may be subtle or absent. In advanced stages, chest CT can provide useful clues to the underlying diagnosis, but diagnostic confirmation still requires direct inspection of tracheobronchial tree with bronchoscopy.
There is a debate whether histological evidence is essential for diagnosis of TO. To some extent, this debate is fueled by technically difficulties in obtaining representative biopsies during flexible bronchoscopy. According to one view, characteristic bronchoscopic appearance is sufficient and histological evidence is not necessary for diagnosing TO [2]. However, there are others who disagree and suggest that histological confirmation is essential in order to exclude other conditions such as tracheobronchial amyloidosis that can be mistaken for TO, especially by inexperienced operators [3, 15]. We share the latter view and perform bronchoscopic biopsies as much as possible to secure a firm histological diagnosis if characteristic endobronchial features are not present.
Delay in diagnosis of TO is very common. Failure to include TO in differential diagnosis and lack of familiarity with the disease entity are the main causes of delays in diagnosis. According to some accounts, TO is among the most common conditions that are identified on bronchoscopy performed for chronic and refractory cough. For example, in one study, bronchoscopy was performed in 25 patients with chronic and persistent cough [96]. The underlying cause of cough could be identified with bronchoscopy in 7 (28 %) of patients. TO was diagnosed in 2 of these patients. Similar findings were reported in another study in which 82 bronchoscopies were performed for the evaluation of chronic and unexplained cough [97]. Diagnosis was established on bronchoscopy in 9 (11 %) of 82 patients. TO was the leading diagnosis, found in 7 (8.5 %) of the bronchoscopies. In majority of these cases, the involvement of airways with the disease process was relatively mild, not sufficient to cause significant airway obstruction or abnormal CT findings.
Asthma is the most common incorrect diagnoses made in patients with TO [41]. Many such patients have received anti-asthma therapy for several years before correct diagnosis is identified [98, 99]. In order to avoid this pitfall, it is a sound practice to question the validity of asthma diagnosis when the clinical features are atypical, response to therapy is inadequate, and methacholine challenge test is negative. It is useful to remember asthma mimics such as TO under such circumstances. Flexible bronchoscopy is appropriate for further evaluation of many of these patients.
Calcification of tracheal and bronchial cartilage, which is seen most often in older women, is sometimes mistaken for TO. Calcium deposit is seen in anterior and lateral wall without involvement of the posterior wall due to the absence of cartilage in this location. However, the inner lining of trachea is thin and smooth in age-related calcification of tracheal cartilage in contrast to thickened and nodular appearance in TO. On direct inspection, tracheal and bronchial mucosa is normal in patients with age-related calcification of cartilage, whereas it shows typical nodular changes as described above. In most cases, the distinction between age-related calcification of tracheal cartilage and TO can be made on the basis of radiological findings, and bronchoscopy is not needed for this purpose.
Primary tracheobronchial amyloidosis should be excluded in every patient suspected to have TO. CT findings of tracheobronchial amyloidosis include concentric, smooth, or nodular thickening of submucosa with or without calcification [42]. Bronchoscopy may also reveal diffuse and nodular changes in tracheal and bronchial mucosal lining. One distinguishing feature is frequent involvement of posterior tracheal wall in amyloidosis, which is characteristically uninvolved in TO. Positive staining of endobronchial biopsy specimens with Congo red stain in tracheobronchial amyloidosis is also helpful in differential diagnosis.
Patients with relapsing polychondritis can develop thickening of anterior and lateral wall of trachea due to inflammation and calcification of the tracheal cartilage with sparing of the posterior tracheal wall [100]. However, the inner wall of trachea appears smooth on CT, and the mucus membrane does not show nodular changes on bronchoscopic examination in relapsing polychondritis, which is very different from nodular appearance seen in TO. Further, it is common to detect tracheobronchomalacia on CT and bronchoscopy in relapsing polychondritis [101], whereas the airway wall shows a characteristic rigidity in TO. The presence of extra-pulmonary manifestations such as joint pain, uveitis, and inflammation of cartilage at other sites in relapsing polychondritis is also helpful in differential diagnosis.
Endobronchial tuberculosis, sarcoidosis, and tracheobronchial papillomatosis sometimes also feature in the differential diagnosis, but these conditions are easily differentiated from TO on the basis of bronchoscopic appearance, cultures, and histological examination of the biopsy material. Bony hard submucosal nodules are not found on bronchoscopy in these conditions.
Treatment
There is no medical therapy that can remove the existing lesions, prevent formation of new nodules, or prevent future progression of the disease. Fortunately, most patients have minimal or no symptoms and do not require any therapy. Humidification, mucolytics agents, inhaled bronchodilators, and antibiotic therapy are indicated for management of recurrent pulmonary infections [2, 3]. The results of sputum cultures may help with the selection of most appropriate antibiotics. Oral or inhaled corticosteroids have no established role and must be avoided as much as possible. It is not uncommon for several TO patients to have received inhaled and oral corticosteroids for several years for presumed treatment-resistant asthma. All efforts must be made to discontinue corticosteroids in such patients.
Interventional bronchoscopic procedures are needed in a minority of patients who develop severe symptoms due to advanced central airway obstruction. The most common approach is to perform laser photoresection and mechanical debulking of the airways using rigid bronchoscope and large biopsy forceps [18, 41, 59, 102]. Adequate removal of nodules may not be feasible with flexible bronchoscope. Application of laser usually does not vaporize the calcified nodules but makes it easier to extract the lesions with the biopsy forceps. Temporary placement of silicone stent has been needed in some cases [59]. Metallic stents should not be used. In one reported case, bronchoscopic cryotherapy was used to control low-grade chronic hemoptysis [41]. With proper patient selection, successful application of the interventional bronchoscopy procedures has yielded excellent long-term results in severe and symptomatic TO [103]. Unfortunately, in the absence of specific guidelines, the selection of patient for interventional therapy is mostly driven by personal experience and local practices. This is illustrated well by comparing the treatment modalities in two different series of TO patients. In one series of 41 patients, the majority of patients received only symptomatic treatment [12]. Only one patient received laser treatment and one patient required tracheostomy for management of respiratory symptoms. In contrast, in a series from Iran, all 10 patients received laser photoresection, 5 patients underwent core-out procedure with rigid bronchoscope and one patient had airway stent placement [18].
Utmost restraint must be exercised in selecting appropriate patients for interventional bronchoscopy. The fundamental purpose of these procedures is to restore the airway lumen and to relieve symptoms. In the absence of symptoms, interventional bronchoscopic procedures cannot be expected to provide any meaningful benefit to the patients. Interventional bronchoscopy procedures are complicated and expensive and are not without complications. Such procedures must be offered only after a thorough risk–benefit analysis.
Bronchoscopic therapies may not be feasible in some patients who have severe disease and need relief from troubling symptoms [31]. Surgical treatment may be needed in some of these patients. In one report, 4 such patients with severe disease not suitable for interventional bronchoscopic procedures underwent linear tracheoplasty operation [104]. No major complications were encountered. Excellent long-term results were achieved in all patients undergoing the surgery. Respiratory obstruction was relieved, and patients were able to resume normal daily activities. Modified slide tracheoplasty has provided successful long-term outcome in another patient with severe involvement of proximal half of trachea with the disease process [105]. Surgical excision and end-to-end anastomosis of a section of trachea have also been performed in one case with limited but high-grade involvement of central airways [60]. Thus, surgery is a viable option for those who have severe symptoms that cannot be relieved with less invasive options. However, such operations are complicated and must only be performed by an experienced surgeon in an advanced medical facility.
Anesthesia should be carefully planned in patients with known or suspected TO. It is ill-advised to force the ETT if resistance is met as it can cause trauma, bleeding, and inflammatory edema resulting in further airway compromise.
Prognosis
The majority of TO patients follows a benign clinical course. Usually, patients continue to experience mild and non-specific respiratory symptoms after the diagnosis. Clinical course is often punctuated by superimposed lower respiratory tract infection and bacterial pneumonia. Several reported patients have shown no clinical deterioration for as long as 20 years after the diagnosis [63]. Very occasionally, patients have been reported to develop rapidly progressive symptoms and acute respiratory distress [106].
In one series, a repeat bronchoscopy after initial diagnosis was performed in 18 patients [12]. The disease burden remained stable in 10 (55 %) patients. Progression was noted in 8 (45 %) patients, but it was clinically significant only in 3 (17 %) of patients. No regression of lesions was observed.
It must again be stressed that no form of therapy is known to alter the natural history of TO. Therefore, the fundamental purpose of bronchoscopic procedures and surgery is relief of distressing respiratory symptoms. There is no reason to pursue such therapies in asymptomatic or minimally symptomatic patients.
Summary
TO is an uncommon and benign disease characterized by the development of multiple cartilaginous and bony nodules in the anterior and lateral walls of central airways without involving the posterior wall. The underlying cause of TO is still unknown. Most commonly, the disease is diagnosed in 5th to 7th decade of life. The clinical presentation is non-specific with chronic cough, sputum production, intermittent hemoptysis, and breathlessness. In many instances, the diagnosis is discovered as an incidental finding on CT or bronchoscopy performed for unrelated indications. A few patients do develop severe airway compromised and present with severe respiratory distress, hypoxemia, and stridor. Chest CT is the most useful imaging technique, but early disease can easily be missed on CT. Bronchoscopy is the gold standard for diagnosis. The presence of hard nodules arising from anterior and lateral wall of central airways provides strong indication of diagnosis instantly in the bronchoscopy suite. Whether biopsy is essential for diagnosis is a debatable matter. No treatment is needed in asymptomatic or minimally symptomatic patients. Excellent long-term results have been achieved with laser photoresection and mechanical debulking in symptomatic patients with advanced central airway obstruction. Surgery may be needed in some patients who are not suitable for interventional bronchoscopic procedures. However, the majority of patients follows a benign clinical course and requires no specific therapy.
References
Prakash U. What is tracheobronchopathia osteochondroplastica? J Bronchol. 2001;8:75–7.
Prakash U. Tracheobronchopathia osteochondroplastica. Semin Respir Crit Care Med. 2002;23:167–75.
Abu-Hijleh M, Lee D, Braman SS. Tracheobronchopathia osteochondroplastica: a rare large airway disorder. Lung. 2008;186:353–9.
Wilks S. Ossific deposits on the larynx, trachea and bronchi. Trans Pathol Soc Lond. 1857;8:88.
Virchow R. Die krankhaften Geschwulste. Berlin: Hirschwald; 1863. p. 442–3.
Muckleston HS. On so called multiple osteoma of tracheal mucus membrane. Laryngoscope. 1909;19:881–93.
Killian G. Ein unter Schwebelaryngoskopie entferter grosser subglottischer Tumor. Laryngol Gesellsch. 1914;17:7.
Aschoff-Frieburg L. Ueber tracheopathia osteoplastica. Verh Dtsch Pathol. 1910;14:125–7.
Dalgaard JB. Tracheopathia chondro-osteoplastica. A case elucidating the problems concerning development and ossification of elastic cartilage. Acta Pathol Microbiol Scand. 1947;24:118–34.
Martin CJ. Tracheobronchopathia osteochondroplastica. Arch Otolaryngol. 1974;100:290–3.
Harma RA, Suukari S. Tracheopathia chondro-osteoplastica. A clinical study of thirty cases. Acta Otolaryngol. 1997;84:118–23.
Leske V, Lazor R, Coetmeur D, Crestani B, Chatte G, Cordier J-F. Tracheobronchopathia osteochondroplastica. A study of 41 patients. Medicine. 2001;80:378–90.
Pounder DJ, Pieterse AS. Tracheopathia osteoplastica: report of four cases. Pathology. 1982;14:429–33.
van Nierop MA, Wagennar SS, van den Bosch JM, Westermann CJ. Tracheobronchopathia osteochondroplastica. Report of four cases. Eur J Respir Dis. 1983;64:129–33.
Parez-Rodriguez E, Nunez N, Alvarado C, et al. Diagnosis of tracheopathia osteochondroplastica. Chest. 1990;97:763.
Briones-Gomez A, Cases-Viedma E, Cordero-Rodriguez PJ, Pietro-Rodriguez M, Sanchis-Aldas JL. Tracheopathia osteoplastica. Series of six cases. J Bronchol. 2000;7:301–5.
Bioque JC, Feu N, Rubio JM, et al. Tracheobronchopathia osteochonderoplastica. Clinical study and follow-up in nine cases. J Bronchol. 2001;8:78–83.
Jabbardarjani HR, Radpey B, Kharabian S, Masjedi MR. Tracheobronchopathia osteochondroplastica: presentation of ten cases and review of literature. Lung. 2008;186:293–7.
Lundgren R, Stjernberg NL. Tracheobronchopathia osteochondroplastica. A clinical bronchoscopic and spirometric study. Chest. 1981;80:706–9.
Vilkman S, Keistinen T. Tracheobronchopathia osteochondroplastica. Report of a young man with severe disease and retrospective review of 18 cases. Respiration. 1995;62:151–4.
Jindal S, Nath A, Neyaz Z, Jaiswal S. Tracheobronchopathia osteochondroplastica. A rare or an overlooked entity. Radiol Case. 2013;7:16–25.
Way SPB. Tracheopathia osteoplastica. J Clin Pathol. 1967;20:814–20.
Paaske PB, Tang E. Tracheopathia osteoplastica in the larynx. J Laryngol Otol. 1985;99:305–10.
Pounder DJ, Pieterse AS. Tracheopathia osteoplastica. A study of minimal lesion. J Pathol. 1982;138:235–9.
Young R, Sandstrom R, Mark G. Tracheopathia osteochondroplastica: clinical, radiologic and pathologic correlation. J Thoracic Cardiovasc Surg. 1980;79:537–41.
Miyazono K, Kamiya Y, Morikawa M. Bone morphogenic protein receptors and signal transduction. J Biochem. 2010;147:35–51.
Tsumaki N, Yoshikawa H. The role of bone morphogenetic proteins in endochondral bone formation. Cytokines Growth Factor Rev. 2005;16:279–85.
Shi S, de Gorter JJ, Hoogaars WMH, ’t Hoen PAC, Ten Dijeke P. Overactive bone morphogenic protein signaling in heterotopic ossification and Duchene muscular dystrophy. Cells Mol Life Sci. 2013;70:407–23.
Miyazoneo K, Kusanagi K, Inoue H. Divergence and convergence of TGF-β/BMP signaling. J Cell Physiol. 2001;187:265–7.
Robey PG, Young MF, Flander KC, et al. Osteoblasts synthesize and respond to transforming growth factor type beta (TGF-β) in vitro. J Cell Biol. 1987;105:457–63.
Bonewald LF, Dallas SL. Role of active and latent transforming growth factor-beta in bone formation. J Cell Biochem. 1994;55:350–7.
Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–88.
Tajima K, Yamakawa M, Katagiri T, Sasaki H. Immunohistochemical detection of bone morphogenetic protein-2 and transforming growth factor beta-1 in tracheopathia osteochondroplastica. Virchow Arch. 1997;431:359–63.
Prakash UB, McCullough AE, Edell ES, Nienhuis DM. Tracheopathia osteoplastica: familial occurrence. Mayo Clin Proc. 1989;64:1091–6.
Sakula A. Tracheobronchopathia osteoplastica. Its relationship to primary tracheobronchial amyloidosis. Thorax. 1968;23:105–10.
Jones AW, Chatterji AN. Primary tracheobronchial amyloidosis with tracheobronchopathia osteoplastica. Br J Dis Chest. 1977;71:268–72.
Alroy GG, Lichtig C, Kaftori JK. Tracheopathia osteoplastica: end stage of primary lung amyloidosis. Chest. 1972;61:465–8.
Kirbas G, Dagli CE, Tanrikulu AC, et al. Unusual combination of tracheobronchopathia osteochondroplastica and AA amyloidosis. Yonsei Med J. 2009;50:721–4.
Phillips MJ. Tracheopathia osteoplastica. Proc Roy Soc Med. 1976;69:18–9.
Piazza C, Cavaliere S, Foccoli P, Toninelli C, Bolzoni A, Peretti G. Endoscopic management of laryngo-tracheobronchial amyloidosis: a series of 32 patients. Eur Arch Otorhinolaryngol. 2003;260:349–54.
Nienhuis DM, Prakash UBS, Edell ES. Tracheobronchopathia osteochondroplastica. Ann Otol Rhinol Laryngol. 1990;99:689–94.
O’Regan A, Fenlon HM, Beamis JF, Steele MP, Skinner M, Berk JL. Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine. 2000;79:69–79.
Diaz-Jimenez JP, Rodriguez A, Ballarin JIM, Castro MJ, Argemi TM, Manresa F. Diffuse tracheobronchial amyloidosis. J Bronchol. 1999;6:13–7.
Capizzi SA, Betancourt E, Prakash UBS. Tracheobronchial amyloidosis. Mayo Clin Proc. 2000;75:1148–52.
Hui AN, Koss MN, Hochholzer L, et al. Amyloidosis presenting in the lower respiratory tract: clinicopathologic, radiologic, immunohistochemical, and histochemical studies on 48 cases. Arch Pathol Lab Med. 1986;110:212–8.
Shehata MA. Atrophic rhinitis. Am J Orolaryngol. 1996;17:81–6.
Vaheri E, Vaheri E. Tracheopathia osteoplastica. Acta Otolaryngol. 1967;64:251–5.
Jepsen O, Sorensen H. Tracheopathia osteoplastica and ozaena. Acta Otolaryngol. 1960;51:79–83.
Clee MD, Anderson JM, Johnston RN. Clinical aspects of tracheobronchopathia osteochondroplastica. Br J Dis Chest. 1983;77:308–14.
Ferguson J, McCaffrey TV, Kern EB, Martin WJ. Effect of Klebsiella ozaenae on ciliary activity in vitro: implication in the pathogenesis of atrophic rhinitis. Otolaryngol Head Neck Surg. 1990;102:207–11.
Al-Ajam MR, Al-Khasawneh KR, Galli WP. Tracheobronchopathia osteochondroplastica. J Bronchol Intervent Pulmonol. 2010;17:149–51.
Baugnee PE, Delaunois LM. Mycobacterium avium-intercellulare associated with tracheobronchopathia osteochondroplastica. Eur Respir J. 1995;8:180–2.
Shih J-Y, Hsueh P-R, Chang Y-L, et al. Tracheal botryomycosis in a patient with tracheopathia osteochondroplastica. Thorax. 1998;53:73–6.
Dincer HE, Dunitz JM. Tracheobronchopathia osteochondroplastica and selective IgA deficiency. J Bronchol Intervent Pulmonol. 2012;19:54–6.
Pinheiro GA, Antao VCS, Muller NL. Tracheobronchopathia osteochondroplastica in a patient with silicosis. CT, bronchoscopy and pathology findings. J Comput Assist Tomogr. 2004;28:801–3.
Rizzo S. Tracheobronchopathia osteochondroplastica associated with calcification of falx cerebri and rhinobronchial syndrome with nasal polyposis. J Bronchol. 1998;5:128–31.
Karlikaya C, Yuksel M, Kilicli S, Candan L. Tracheobronchopathia osteochondroplastica. Respirology. 2000;5:377–80.
Erelel M, Yakar F, Bingol ZK, Yakar A. Tracheopathia osteochondroplastica. Two unusual cases. J Bronchol Intervent Pulmonol. 2010;17:241–3.
Datau H, Musani AI. Treatment of severe tracheobronchopathia osteochondroplastica. J Bronchol. 2004;11:182–5.
Khan AM, Shim C, Simmons N, et al. Tracheobronchopathia osteochondroplastica. A rare cause of tracheal stenosis—TPO stenosis. J Thorac Cardiovasc Surg. 2006;132:714–6.
Sant’Anna CC, Pires-de-Mello P, Morgado Mde F, March Mde F. Tracheobronchopathia osteochondroplastica in a 5 year old girl. Indian Pediatr. 2012;49:985–6.
Simsek PO, Ozcelik U, Demirkazik F, et al. Tracheobronchopathia osteochondroplastica in a 9-year old girl. Pediatr Pulmonol. 2006;41:95–7.
Huang C-C, Kuo C-C. Chronic cough: tracheobronchopathia osteochondroplastica. CMAJ. 2010;182:E859.
Pinto JA, da Silva LC, Perfeito DJP, Soares JDS. Osteochondroplastic tracheobronchopathy—report of 2 cases and bibliographic review. Braz J Otolaryngol. 2010;76:789–93.
Brend N, Harrison AC, Hartnett BJS, Lawson J, Marlin GE. Tracheobronchopathia osteochondroplastica. Aust NZ J Med. 1979;9:188–92.
Los H, Schramel FMNH, van der Harten JJ, Golding RP, Postmus PE. An unusual cause of recurrent fever. Eur Respir J. 1997;10:504–7.
Castella J, Puzo C, Cornudella R, Curell R, Tarres J. Tracheobronchopathia osteochondroplastica. Respiration. 1981;42:129–34.
Meyer CN, Dossing M, Broholm H. Tracheobronchopathia osteochondroplastica. Respir Med. 1997;91:499–502.
Hodges MK, Israel E. Tracheobronchopathia osteochondroplastica presenting as right middle lobe collapse. Diagnosis by bronchoscopy and computerized tomography. Chest. 1988;94:842–4.
Doshi H, Thankachen R, Philip MA, Kurien S, Shukla V, Korula RJ. Tracheobronchopathia osteochondroplastica presenting as an isolated nodule in the right upper lobe bronchus with upper lobr collapse. J Thorac Cardiovasc Surg. 2005;130:901–2.
Shigematsu Y, Sugio K, Yasuda M, et al. Tracheobronchopathia osteochondroplastica occurring in a subsegmental bronchus and causing obstructive pneumonia. Ann Thorac Surg. 2005;80:1936–8.
Smith DC, Pillai R, Gillbe CE. Tracheopathia osteochondroplastica. A cause of unexpected difficulty in tracheal intubation. Anesthesia. 1987;42:536–8.
Coetmeur D, Bovyn G, Leroux P, Niel-Duriez M. Tracheobronchopathia osteochondroplastica presenting at the time of a difficult intubation. Respir Med. 1997;91:496–8.
Gurunathan U. Tracheobronchopathia osteochondroplastica: a rare cause of difficult intubation. Br J Anesthesia. 2010;104:787–8.
Tadjeddein A, Khorgami Z, Akhlaghi H. Tracheobronchopathia osteochondroplastica: a cause of difficult intubation. Ann Thorac Surg. 2006;81:1480–2.
Warner MA, Chestnut DH, Thompson G, Bottcher M, Tobert D, Nofftz M. Tracheobronchopathia osteochondroplastica and difficult intubation: case report and perioperative recommendations for anesthesiologists. J Clin Anesthesia. 2013;25:659–61.
Martens PR, Van den Brande FG. Failure of one-lung ventilation because of tracheopathia osteoplastica during a heartport procedure with EZ blocker. J Cardiothorac Vasc Anesth. 2010;26:e35.
Ishii H, Fugihara H, Ataka T, et al. Successful use of laryngeal mask airway for a patient with tracheal stenosis with tracheobronchopathia osteochondroplastica. Anesth Analg. 2002;95:781–2.
Howland WJ Jr, Good CA. Radiographic features of tracheopathia osteochondroplastica. Radiology. 1958;71:847–50.
Secrest PG, Kendig TA, Beland AJ. Tracheobronchopathia osteochondroplastica. JAMA. 1964;36:815–8.
Ozmen I, Kongar NA, Oruc K, Kiziltas S, Yilmaz A, Calisir HC. Tracheobronchopathia osteochondroplastica. An unusual presentation. J Bronchol Interent Pulmonol. 2010;17:80–3.
Zack JR, Rozenshtein A. Tracheobronchopathia osteochondroplastica: report of three cases. J Comput Assist Tomogr. 2002;26:33–6.
Mariotta S, Pallone S, Pedicelli G, Bisetti A. Spiral CT and endoscopic findings in a case of tracheobrochopathia osteochondroplastica. J Comput Assist Tomogr. 1997;21:418–20.
White BD, Kong A, Khoo E, Southcott AM. Computerized tomography diagnosis of tracheobronchopathia osteochondroplastica. Autralas Radiol. 2005;49:319–21.
Restrepo S, Pandit M, Villami MA, Rojas IC, Perez JM, Gascue A. Tracheobronchopathia osteochondroplastica: helical CT findings in 4 cases. J Thorac Imaging. 2004;19:112–6.
Al-Busaidi N, Dhuloya D, Habibullah Z. Tracheobronchopathia osteochondroplastica. Case report and literature review. SQU Med J 2012;12:109–12.
Hantous-Zannad S, Sebai L, Zidi A, et al. Tracheobronchopathia osteochondroplastica presenting as a respiratory insufficiency: diagnosis by bronchoscopy and MRI. Eur J Radiol 2003;113–6.
Tukiainen H, Torkko M, Terho EO. Lung function in patients with tracheobronchopathia osteochondroplastica. Eur Respir J. 1988;1:632–5.
Bergeron D, Cormier Y, Desmeules M. Tracheobronchopathia osteochondroplastica. Am Rev Respir Dis. 1976;114:803–6.
Smid L, Lavrencak B, Zargi M. Laryngo-tracheo-bronchopathia chondro-osteoplastica. J Laryngol Otol. 1992;106:845–8.
Cumbo-Nacheli G, Gildea TR. Autofluorescence pattern in tracheobronchopathia osteochondroplastica. J Bronchol Intervent Pulmonol. 2010;17:368–9.
Newton R, Kemp S, Zoumot Z, Yang G-Z, Darzi A, Shah PL. An unusual case of hemoptysis. Thorax. 2010;65:309.
Raess PW, Cowan SW, Haas AR, et al. Tracheopathia osteochondroplastica presenting as a single dominant tracheal mass. Ann Diag Pathol. 2011;15:431–5.
Riker DR, Campagna AC, Beamis JF. Tracheobronchopathia presenting as hemoptysis associated with vascular endobronchial tumors. J Bronchol. 2007;14:212–4.
Rial MB, Fernandez-Villar A, Fernandez VL, et al. Tracheobronchopathia osteochondroplastica: cause of difficult transbronchial needle aspiration. J Bronchol. 2008;15:287–9.
Sen RL, Walsh TE. Fiberoptic bronchoscopy for refractory cough. Chest. 1991;99:33–5.
Decalmer S, Woodcock A, Greaves M, Howe M, Smith J. Airway abnormalities at flexible bronchoscopy in patients with chronic cough. Eur Respir J. 2007;30:1138–42.
Hayes D. Tracheopathia osteoplastica misdiagnosed as asthma. J Asthma. 2007;253–5.
Park SS, Shin DH, Lee DH, Jeon SC, Lee JH, Lee JD. Tracheopathia osteoplastica simulating asthmatic symptoms. Diagnosis by bronchoscopy and computerized tomography. Respiration. 1995;62:43–5.
Lee KS, Ernst A, Trentham D, Lunn W, Feller-Kopman DJ, Boiselle P. Prevalence of functional airway abnormalities in relapsing polychondritis. Radiology. 2006;240:565–73.
Ernst A, Rafeq S, Boiselle P, et al. Relapsing polychondritis and airway involvement. Chest. 2009;135:1024–30.
Gleich LL, Rebeiz EE, Pancratov MM, Shapshay SM. The holmium YAG laser assisted otolaryngologic procedures. Arch Otolayrngol Head Neck Surg. 1995;121:1162–6.
Tibesar RJ, Edell ES. Tracheopathia osteoplastica: effective long term management. Otolaryngol Head Neck Surg. 2003;129:303–4.
Grillo HC, Wright CD. Airway obstruction owing to tracheopathia osteoplastica: treatment by linear tracheoplasty. Ann Thorac Surg. 2005;79:1676–81.
Kutlu CA, Yeginsu A, Ozalp T, Baran R. Modified slide tracheoplasty for the management of tracheobronchopathia osteochondroplastica. Eur J Cardiothorac Surg. 2002;21:140–2.
Molloy AR, McMohan JN. Rapid progression of tracheal stenosis associated with tracheopathia osteo-chondroplastica. Intensive Care Med. 1988;15:60–2.
Acar T. Computed tomography finding of tracheobronchial system disease: a pictorial essay. Jpn J Radiol. 2015;33(2):51–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jain, P., Mehta, A.C. (2016). Tracheobronchopathia Osteochondroplastica. In: Mehta, A., Jain, P., Gildea, T. (eds) Diseases of the Central Airways. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-29830-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-29830-6_7
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-29828-3
Online ISBN: 978-3-319-29830-6
eBook Packages: MedicineMedicine (R0)