Abstract
The skeleton develops from a densely packed, avascular mesenchyme, called the skeletal blastema. Chondrogenesis from this mesenchyme requires a balance between negative and positive maturational factors during initial chondrocyte proliferation and differentiation, as well as during postnatal chondrocyte development and homeostasis. Accurate regulation of this developmental program is crucial for the ultimate size of skeletal elements, as premature or delayed maturation often results in their severe shortening. One essential group of regulators of chondrogenesis comprises members of the Hedgehog (Hh) morphogen family. Hh’s act as long-range morphogens during chondrocyte development and endochondral ossification. Mutations in Hh effectors, receptors, and co-receptors, as well as in ciliary proteins that act as modulators of Hh reception, result in skeletal and craniofacial deformities. In addition to their essential roles in chondrogenesis, both Sonic Hh and Indian Hh family members serve as crucial regulators of endochondral ossification, a process in which calcified hypertrophic cartilage is resorbed and replaced by bone. Finally, dysregulated Hh signaling contributes to cartilage and bone pathologies in the adult. This chapter summarizes the current understanding of Hh production and signaling in chondrocytes in development and disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Growth Plate
- Primary Cilium
- Endochondral Ossification
- Chondrocyte Hypertrophy
- Chondrocyte Differentiation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Hedgehog Functions in Chondrocyte Biology
Two distinct processes form vertebrate skeletons during development: intramembranous and endochondral ossification. Intramembranous bones are directly formed by specialized, terminally differentiated mesenchymal stem cells called osteoblasts. Osteoblasts synthesize densely cross-linked collagen and specialized proteins, including osteocalcin and osteopontin, to form the basis of the cranial vault, some facial bones, and parts of the mandible and clavicle. Endochondral ossification that forms the rest of the skeleton during development, in contrast, relies on the replacement of a cartilaginous template with bone (Yoshida et al. 2004). As a first step in this process, the cartilaginous template (or cartilage anlage) generated from mesenchymal progenitors expresses collagens I, III, and V as a result of mesenchymal cell condensation and chondroprogenitor cell differentiation, called chondrogenesis (Goldring et al. 2006). Fibroblast growth factor 8 and Sonic hedgehog (Shh) (Kmita et al. 2005) are two essential modulators of cell proliferation within this cartilage template (Hall and Miyake 2000), and bone morphogenetic protein (BMP; most BMPs are transforming growth factor beta family members) signaling contributes to the formation of precartilaginous condensations and subsequent chondrocyte differentiation (Yoon et al. 2005). The BMP antagonist, Noggin, further permits precartilage cell differentiation into chondrocytes (Yoon and Lyons 2004; Pizette and Niswander 2000). This process is marked by the expression of cartilage-specific collagens II, IX, and XI. The proliferation of these cells requires Indian hedgehog (Ihh) signaling in parallel with BMP function or BMP signaling acting as a modulator of Ihh function (Minina et al. 2001; Vortkamp 2001). Finally, cells undergo terminal differentiation, or chondrocyte hypertrophy, and apoptosis in the intervening interzone. During chondrocyte hypertrophy, there is a notable increase in cell size, up to 20-fold of its initial resting size. The hypertrophic zone is further characterized by the expression of collagen X and alkaline phosphatase and the subsequent calcification of the matrix (St-Jacques et al. 1999). This process involves matrix remodeling by matrix metalloprotease (MMP)-9, MMP-13, and MMP-14 and vascularization mediated by vascular endothelial growth factor (VEGF) activity. In this process, the hypertrophic cartilage is finally replaced by bone, except for resting chondrocytes embedded in a dense extracellular matrix (ECM) lacking blood vessels, nerves, or lymphatics at the ends of (opposing) bones (called articular cartilage).
A similar sequence of chondrocyte proliferation and differentiation occurs in the postnatal growth plate, leading to rapid growth of the skeleton (Onyekwelu et al. 2009). At birth, the articular cartilage of many joints in humans and mice is still indistinguishable from the epiphyseal growth plate. Soon after birth, however, a secondary ossification center appears within the epiphyseal cartilage, dividing it into the future metaphyseal growth plate proximally and the articular surface distally (for further details, see Chap. 4). Ihh that is still produced in the metaphyseal growth plate directly and indirectly induces parathyroid hormone-related peptide (PTHrP) expression in periarticular resting zone chondrocytes (Karaplis et al. 1994; Lanske et al. 1996; Vortkamp et al. 1996; Chung et al. 2001; Kronenberg 2006) (Fig. 9.1). PTHrP, in turn, induces continued proliferation and inhibits the progression to maturation in proliferating and prehypertrophic chondrocytes. This process, in turn, maintains the length of chondrocyte columns and thus the architecture of the epiphyseal growth plate. In addition, Ihh acts independently of PTHrP on the periarticular chondrocytes and regulates the differentiation of columnar chondrocytes within the proliferative zone (Kobayashi et al. 2005). In postnatal joints, PTHrP and Ihh remain expressed in a zone-specific manner (Onyekwelu et al. 2009), potentially regulating mineralization by chondrocytes at the osteochondral interface of the immature joint (Jiang et al. 2008). Here, Ihh expression is particularly strong in chondrocytes at the articular surface, indicating a role in resisting chondrocyte hypertrophy, mineralization, and/or ossification.
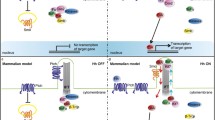
Overview of bone development and Hh signaling. Left: Ihh and PTHrP/PTHR regulate chondrocyte proliferation and differentiation during endochondral bone formation. PTHrP is synthesized by resting chondrocytes and perichondrial cells. Secreted PTHrP diffuses toward the prehypertrophic zone, where it binds to and activates its receptor, PTHR. PTHR activity maintains chondrocyte proliferation (+) and delays chondrocyte differentiation (−) into prehypertrophic and hypertrophic chondrocytes. After chondrocytes stop proliferating at the transition from a proliferating into a hypertrophic phenotype, they start to synthesize Ihh, which indirectly increases the synthesis of PTHrP. Ihh and PTHrP thus participate in a negative feedback loop that regulates the proliferation rate of growth plate chondrocytes. Besides increasing PTHrP synthesis, Ihh also stimulates the proliferation of chondrocytes and directly inhibits their terminal differentiation. PTHrP parathyroid hormone-related protein, Ihh Indian Hedgehog, PTHR parathyroid hormone receptor. Right: The Ihh ligand in producing cells undergoes a series of autoprocessing/lipidation reactions that result in its secretion in multimeric, dual-lipidated form and its firm tethering to the cell surface. All Hh family members are then released from the cell surface via the activity of the 12-span transmembrane protein Dispatched (Disp). Ihh binding to the receptor Patched (Ptc) on the receiving cell releases the 7-pass transmembrane protein Smo from constitutive inhibition, allowing for Smoothened (Smo) translocation to the primary cilium. This activates glioblastoma (Gli)2/Gli3 transcription factors (Gli2/3A) and inhibits the generation of Gli3 repressors (Gli3R). In the presence of Hh, the co-receptors Boc, Cdo, and Gas1 assist in the release of Smo from Ptc inhibition and thereby contribute to Hh pathway activity
Local chondrocytes in the postnatal skeleton are also influenced through endocrine hormones (particularly thyroid hormone and estrogen). Ihh signaling is further known to induce BMP-4, a mitogenic factor, in a PTHrP-independent manner. Notably, this induction depends on mechanical stimulation (Wu et al. 2001). Cyclic mechanical stress induces Ihh expression in chondrocytes; gadolinium, an inhibitor of stretch-activated channels, inhibits Ihh induction. This suggests that the Ihh gene is mechanoresponsive because of the involvement of primary cilia in Hh signaling (discussed in more detail below). Finally, functional interactions between Ihh and the Wingless/Wnt pathways regulate cartilage growth plate control and joint segmentation (Spater et al. 2006). Loss of activity of Wnt9a (a secreted signaling molecule) transiently downregulates Ihh expression and reduces Ihh signaling activity in prehypertrophic chondrocytes; in vivo chromatin immunoprecipitation revealed a direct interaction between the β-catenin/lymphoid enhancer-binding factor 1 (constituting part of the Wnt receptor/signal transduction complex) and the Ihh promoter. Another report demonstrated that Ihh, Wnt5b, and Wnt11 control chondrogenesis in parallel pathways (Church et al. 2002) and that Ihh can cause parallel inhibition of Lrp (Wnt co-receptor) and Sfrp (Wnt antagonist) in chondrocytes (Choi et al. 2012). The conclusion that Wnt5a signaling in the prehypertrophic zone of the cartilage growth plate may be increased, however, is not supported because of the unchanged Wnt5a levels in Ihh mutant mice in vivo (St-Jacques et al. 1999; Long et al. 2001).
9.2 Hedgehog Morphogen Production and Reception
Vertebrates produce three structurally and functionally closely related Hh’s (Sonic Hh (Shh), Indian Hh (Ihh), and Desert Hh). Of these, the function of Shh has been best characterized (reviewed by McMahon et al. 2003), including its role in the development of the head process and in the development of limbs: limb budding, anterior/posterior patterning of the limb skeleton, and specification of vertebrate digit identities (Capdevila and Johnson 2000). Shh expressed in the forebrain also mediates the development of the mid- and upper face, the frontonasal process, and the maxillary processes (Byrnes et al. 2009). Dysregulation of the Shh pathway therefore results in a wide and complex array of skeletal and craniofacial defects, including syndactyly, holoprosencephaly, hypotelorism, cleft palate, and cyclopia (Belloni et al. 1996; Chiang et al. 1996). Desert Hh is expressed in peripheral nerves and in male gonads (Bitgood and McMahon 1995), suggesting a functional role restricted to these tissues. The third vertebrate Hh family member is Ihh. Both Ihh and Shh functions have been studied in cartilage and bone patterning throughout the axial, appendicular, and facial skeletons (Hammerschmidt et al. 1997; Capdevila and Johnson 2000; Chai and Maxson 2006), as well as in calvarial ossification and suture morphogenesis (Pan et al. 2013). Ihh is mainly produced by post-mitotic prehypertrophic chondrocytes adjacent to the proliferative zone that express the parathyroid hormone (PTH)/PTHrP receptor (PTHR) and stimulate the proliferation of chondrocytes at the growth plate and later in development. Ihh further regulates chondrocyte hypertrophic and osteoblast differentiation, either directly or via PTHrP (Nakamura et al. 1997; Mak et al. 2008; Vortkamp et al. 1996) (Fig. 9.1). In the latter system, a negative feedback loop between Ihh and PTHrP regulates the rate of chondrocyte differentiation: Ihh produced by prehypertrophic chondrocytes induces PRTrP expression, which prevents further differentiation of chondrocytes expressing PTHR. Ihh knockout mice show appositional chondrocyte differentiation and loss of PTHrP and either die during mid-gestation because of yolk sac defects or die at birth because of rib cage deformities and respiratory failure (Byrd et al. 2002). Chondrocyte-specific (Col2α1Cre;Ihh d /Ihh d) mice also die at birth, showing delayed chondrocyte hypertrophy, reduced calvarial bone size and ossification, abnormal mineralization of axial and appendicular bones, and widened cranial sutures (Razzaque et al. 2005). These findings demonstrate that chondrocyte-derived Ihh not only is responsible for the regulation of the endochondral skeleton by regulating both chondrocyte proliferation and differentiation, but it is also essential for osteoblast differentiation. Ihh expression in chondrocytes depends on the runt-related transcription factors (Runx)2 and Runx3 (Yoshida et al. 2004). Runx2−/− mice die after birth and completely lack bone formation due to absence of osteoblast differentiation and delayed chondrocyte maturation (Komori et al. 1997; Otto et al. 1997), and Runx3−/− mice show mildly reduced chondrocyte maturation. Runx2/3 compound mutant mice completely lack Ihh expression (Yoshida et al. 2004).
All Hh homologs undergo the same three-step conserved maturation pathway in producing cells (Fig. 9.1). Production of the active Hh protein begins with autocleavage of a HhNC precursor protein into a N-terminal (HhN) signaling domain and the HhC autoprocessing domain. This cleavage reaction is linked to the covalent attachment of a cholesterol moiety to the carbonyl of the C-terminal HhN glycine residue (Porter et al. 1996a, b; Cohen 2003). In a second step, Hh acyltransferase attaches a palmitoyl group to the NH2-terminal cysteine of the Hh signaling domain (Pepinsky et al. 1998). The dually lipidated molecule constitutes the active morphogen (Jacob and Lum 2007; Taylor et al. 2001). Upon secretion to the cell surface, lipidated Hh’s multimerize prior to their release (Dierker et al. 2009a) and transport to cells expressing the Hh receptor Patched (Ptc) (Panakova et al. 2005; Zeng et al. 2001). The paradoxical situation is that a membrane-tethered molecule serves as a long-range morphogen; this requires specific mechanisms for its release and transport. The 12-pass transmembrane protein Dispatched (Disp) is essential for the release of lipid-modified Hh’s (Burke et al. 1999; Caspary et al. 2002; Kawakami et al. 2002). Disp is therefore critical for full signaling within the chondrocyte target field in developing bones and consequently for the establishment of a normal skeletal growth plate (Tsiairis and McMahon 2008). The exact mechanism of Disp-dependent release of lipidated Hh, however, is not yet resolved. Other suggested players in Hh transport include Hh micelle formation by unknown mechanisms (Zeng et al. 2001), Hh transport together with lipoprotein particles (Panakova et al. 2005; Eugster et al. 2007), Hh transport on filopodia (called cytonemes) (Bischoff et al. 2013; Roy et al. 2011), Hh association with the soluble glycoprotein Scube2 (Creanga et al. 2012; Hollway et al. 2006; Johnson et al. 2012; Kawakami et al. 2005; Tukachinsky et al. 2012; Woods and Talbot 2005), or simple diffusion of solubilized Hh after its proteolytic processing from the cell surface (called shedding) (Dierker et al. 2009b; Ohlig et al. 2011; Ohlig et al. 2012). Notably, cytonemes have not been reported on chondrocytes, making this transport mechanism unlikely. Moreover, the very dense extracellular matrix (ECM) of the developing skeleton makes most of these suggested mechanisms—in particular Hh transport on filopodia and transport via large lipoprotein particles or exosomes—hard to envision. It has been firmly established, however, that Hh long-range function depends on the expression of heparan sulfate proteoglycans (HSPGs). Again, the underlying mechanism of HSPG-mediated Hh transport is not clearly defined, but it is assumed that these versatile molecules somehow aid Hh transport by “facilitated diffusion” or Hh stabilization against degradation (Lin 2004; Muller et al. 2013).
In contrast to the components that act to release Hh, Hh signaling components in receiving cells have been studied in more detail (Cohen 2003; Robbins et al. 2012; Ingham and McMahon 2001). Hh proteins induce signaling on receiving cells by direct binding to the Hh receptor Ptc, a 12-pass transmembrane protein (Fuse et al. 1999). The amount of Hh available for Ptc binding is regulated by other Hh-binding proteins, such as Hh-interacting protein (Chuang and McMahon 1999) and growth arrest-specific protein 1 (Gas1) (Evangelista et al. 2006; Lee et al. 2001). Furthermore, the Interference Hh protein family (Ihog in Drosophila and CDO and BOC in humans) (Wilson and Chuang 2006; Kavran et al. 2010) and HSPGs (Bornemann et al. 2004; Beckett et al. 2008) modulate Hh signaling. Hh binding to Ptc (together with Hh binding to Boc/Cdo and Gas1 (Allen et al. 2011)) induces internalization of the receptor/ligand complex and relieves Ptc-mediated catalytic inhibition of the seven-pass transmembrane protein Smoothened (Smo) (Taipale et al. 2002). Active Smo then transduces the Hh signal to the cytoplasm, resulting in processing and activation of the glioblastoma (Gli) family of transcription factors (Gli1–Gli3) (Hatsell and Frost 2007). Gli1, in contrast to Gli2 and Gli3, lacks an amino-terminal repressor domain and thus represents a constitutive activator of the Hh pathway (Hatsell and Frost 2007; Hynes et al. 1997; Karlstrom et al. 2003). Yet, in mouse development, Gli1 is not essential since Gli1−/− mutants survive from birth to adulthood with a normal phenotype (Park et al. 2000). In contrast, Gli2 and Gli3 are required for mouse development and carry an N-terminal repressor domain in addition to the C-terminal activator domain and thus can act as both activators and repressors (Sasaki et al. 1999; Ruiz i Altaba 1999). Their bifunctionality is determined by the presence of Hh signaling: the absolute concentration of Hh ligands specifically induces defined Gli transcription factor activation, resulting in Hh concentration-dependent activation of target genes (Ogden et al. 2004; Harfe et al. 2004). In the absence of Hh signaling, Gli3 is complexed with suppressor of fused (SuFu), which leads to Gli3 phosphorylation by several kinases. This targets Gli3 for proteolytic processing into the truncated repressor form (Gli3R) that locates to the nucleus and inhibits transcription of target genes (Persson et al. 2002). Upon Smo activation in the presence of Hh signaling, however, SuFu is sequestered away from Gli3, proteolytic processing is inhibited, and full-length Gli3 (Gli3A) induces target gene transcription. Gli2 can likewise be converted into a repressor by proteolytic processing in the absence of Hh signaling and is activated by high levels of Hh. However, Gli2 C-terminal processing is less effective than that of Gli3. Therefore, Gli2 mostly remains transcriptionally active even at low levels of Hh signaling in vivo (Fuccillo et al. 2006).
Gli-regulated Hh-dependent target genes include Wnts, BMP, and the Ptc receptor itself. Importantly, upregulation of Ptc in response to Hh signaling constitutes a negative feedback loop by increasing the relative amount of free Ptc on the cell surface, which in turn inhibits Smo activity and signaling. In addition, Ptc directly reduces Hh levels in the ECM by ligand internalization upon binding (Jeong and McMahon 2005). In chondrocytes, another direct consequence of Ihh signaling is the Wnt5A-dependent, yet PTHrP-independent, degradation of Nkx3.2 proteins that are normally expressed in chondrocyte precursor cells and in early-stage chondrocytes (Choi et al. 2012). In these cells, Nkx3.2 proteins enhance chondrocyte differentiation and survival while inhibiting chondrocyte hypertrophy and apoptosis.
9.3 Primary Cilia in Hedgehog Perception
Primary cilia are involved in the regulation of Hh signal transduction, although the precise mechanisms are not fully elucidated (Tran et al. 2008). A primary cilium consists of a singular, immotile organelle, which is present on most cells, including chondrocytes, during interphase (Scherft and Daems 1967). Currently, it is thought that primary cilia provide an environment that facilitates interactions between different Hh pathway components (Ruat et al. 2012), such as Ptc, Smo, and Gli proteins that require ciliary transport in order to activate Hh-dependent gene expression (Keady et al. 2012). Upon Hh binding to Ptc and following Smo stimulation, Smo is translocated to the cilium and subsequently interacts with Gli’s, leading to their activation. Gli’s then move down the cilium to enter the nucleus and transduce the Hh signal (Huangfu and Anderson 2005, 2006).
For these reasons, the targeted inactivation of intraflagellar transport (IFT) proteins, such as components of the kinesin-like protein motor complex and retrograde dynein motors, has been found to affect Hh signal transduction (Ruat et al. 2012). Conditional inactivation of the Kif3a subunit of the kinesin-2 intraflagellar transport motor in mesenchymal skeletal progenitor cells, for example, resulted in severe patterning defects in the craniofacial area, the formation of a split sternum, and the development of polydactyly, deformities reminiscent of those described in mice with deregulated Hh signaling (Koyama et al. 2007).
In Kif3a-deficient mesenchymal tissues, both the repressor function of Gli3 and the activation of the Shh transcriptional targets Ptc and Gli1 are compromised (Kolpakova-Hart et al. 2007). This is consistent with the finding that Gli signaling depends on Kif3a function (Haycraft et al. 2005; Huangfu and Anderson 2005). Kif7, which plays a role in the turnover of Sufu and the exclusion of Sufu-Gli complexes from the primary cilium, regulates the activity of Gli transcription factors through both Sufu-dependent and Sufu-independent mechanisms (Hsu et al. 2011). Mutations in the IFT protein DynC2H1 cause short-rib polydactyly syndrome, a lethal autosomal recessive condition that features cerebral and skeletal abnormalities, including appendicular malformations (Dagoneau et al. 2009; El Hokayem et al. 2012; Merrill et al. 2009). Finally, partial mutation of intraflagellar transport 80 (IFT 80) in humans causes Jeune asphyxiating thoracic dystrophy and short-rib polydactyly syndrome. IFT80 is mainly expressed in growth plate chondrocytes, and IFT80 knockdown impairs chondrocyte cilia formation and chondrogenic differentiation in mouse bone marrow-derived stromal cells by downregulating Hh signaling (Wang et al. 2013). In addition to merely acting as a location for Hh signaling regulation, the primary cilium also plays a role in mechanosensitive Hh signaling in adult articular chondrocytes (Thompson et al. 2014). Mechanical strain promotes Ihh expression and Hh pathway activation; cilia disassembly due to high-magnitude strain prevents this process. However, in comparisons of Ihh- and Kif3a-deficient mice, chondrogenesis differs significantly, indicating that Ihh actions may not solely depend on molecular association of Hh reception components with cilia (Koyama et al. 2007; Kolpakova-Hart et al. 2007).
9.4 Hedgehog Functions in Chondrocyte Pathobiology
Osteoarthritis (OA) is linked to the irreversible degeneration of articular cartilage in adult joints, often due to initial injury. In this disease, articular cartilage chondrocytes undergo phenotypic and gene expression changes that resemble their end-stage differentiation in the growth plate during skeletal development, suggesting that Ihh and the Ihh/PTHrP axis continue to play a role in OA. Indeed, Ihh expression is upregulated in human OA cartilage, and this upregulation correlates with OA progression and changes in chondrocyte morphology. Consistent with this observation, transgenic mice with induced Ihh expression exhibit increased chondrocyte hypertrophy and cartilage damage resembling human OA. In these mice, higher levels of Hh signaling in chondrocytes caused a more severe osteoarthritic phenotype (Lin et al. 2009). Two other genetic studies in mice confirmed this finding, showing that conditional deletion of Ihh in chondrocytes attenuates OA progression (Zhou et al. 2014a, b). Only mild OA changes were observed in Ihh-deficient mice, while control mice displayed significantly more cartilage damage. OA markers such as collagen X and MMP-13 were decreased in Ihh-deficient mice, and the activity of cathepsins and MMPs in knee joints of animals with deletion of Ihh was decreased. Consistent with this finding, PTHrP inhibits mineralization in articular cartilage that is associated with OA (Terkeltaub et al. 1998), and histone deacetylase four was suggested to have chondroprotective properties by inhibiting the Ihh transcription factor Runx2 (Cao et al. 2014). Therefore, the PTHrP/Ihh axis continues to participate in the maintenance of articular cartilage, and dysregulation of this system likely contributes to the pathogenesis of OA.
References
Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, Krauss RS, McMahon AP (2011) Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell 20(6):775–787. doi:10.1016/j.devcel.2011.04.018
Beckett K, Franch-Marro X, Vincent JP (2008) Glypican-mediated endocytosis of hedgehog has opposite effects in flies and mice. Trends Cell Biol 18(8):360–363. doi:S0962-8924(08)00164-5 [pii] 10.1016/j.tcb.2008.06.001
Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HH, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW (1996) Identification of sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet 14(3):353–356
Bischoff M, Gradilla AC, Seijo I, Andres G, Rodriguez-Navas C, Gonzalez-Mendez L, Guerrero I (2013) Cytonemes are required for the establishment of a normal hedgehog morphogen gradient in drosophila epithelia. Nat Cell Biol 15(11):1269–1281. doi:10.1038/ncb2856
Bitgood MJ, McMahon AP (1995) Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172(1):126–138. doi:S0012-1606(85)70010-3 [pii] 10.1006/dbio.1995.0010
Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R (2004) Abrogation of heparan sulfate synthesis in drosophila disrupts the wingless, hedgehog and decapentaplegic signaling pathways. Development 131(9):1927–1938
Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K (1999) Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99(7):803–815
Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L (2002) Hedgehog is required for murine yolk sac angiogenesis. Development 129(2):361–372
Byrnes AM, Racacho L, Grimsey A, Hudgins L, Kwan AC, Sangalli M, Kidd A, Yaron Y, Lau YL, Nikkel SM, Bulman DE (2009) Brachydactyly a-1 mutations restricted to the central region of the N-terminal active fragment of Indian hedgehog. Eur J Hum Genet EJHG 17(9):1112–1120. doi:10.1038/ejhg.2009.18
Cao K, Wei L, Zhang Z, Guo L, Zhang C, Li Y, Sun C, Sun X, Wang S, Li P, Wei X (2014) Decreased histone deacetylase 4 is associated with human osteoarthritis cartilage degeneration by releasing histone deacetylase 4 inhibition of runt-related transcription factor-2 and increasing osteoarthritis-related genes: a novel mechanism of human osteoarthritis cartilage degeneration. Arthritis Res Ther 16(6):491. doi:10.1186/s13075-014-0491-3
Capdevila J, Johnson RL (2000) Hedgehog signaling in vertebrate and invertebrate limb patterning. Cell Mol Life Sci 57(12):1682–1694
Caspary T, Garcia-Garcia MJ, Huangfu D, Eggenschwiler JT, Wyler MR, Rakeman AS, Alcorn HL, Anderson KV (2002) Mouse dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr Biol 12(18):1628–1632
Chai Y, Maxson RE Jr (2006) Recent advances in craniofacial morphogenesis. Dev Dyn 235(9):2353–2375. doi:10.1002/dvdy.20833
Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA (1996) Cyclopia and defective axial patterning in mice lacking sonic hedgehog gene function. Nature 383(6599):407–413
Choi SW, Jeong DU, Kim JA, Lee B, Joeng KS, Long F, Kim DW (2012) Indian hedgehog signalling triggers Nkx3.2 protein degradation during chondrocyte maturation. Biochem J 443(3):789–798. doi:10.1042/BJ20112062
Chuang PT, McMahon AP (1999) Vertebrate hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature 397(6720):617–621. doi:10.1038/17611
Chung UI, Schipani E, McMahon AP, Kronenberg HM (2001) Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest 107(3):295–304. doi:10.1172/JCI11706
Church V, Nohno T, Linker C, Marcelle C, Francis-West P (2002) Wnt regulation of chondrocyte differentiation. J Cell Sci 115(Pt 24):4809–4818
Cohen MM Jr (2003) The hedgehog signaling network. Am J Med Genet A 123(1):5–28
Creanga A, Glenn TD, Mann RK, Saunders AM, Talbot WS, Beachy PA (2012) Scube/You activity mediates release of dually lipid-modified hedgehog signal in soluble form. Genes Dev 26(12):1312–1325. doi:gad.191866.112 [pii] 10.1101/gad.191866.112
Dagoneau N, Goulet M, Genevieve D, Sznajer Y, Martinovic J, Smithson S, Huber C, Baujat G, Flori E, Tecco L, Cavalcanti D, Delezoide AL, Serre V, Le Merrer M, Munnich A, Cormier-Daire V (2009) DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am J Hum Genet 84(5):706–711. doi:10.1016/j.ajhg.2009.04.016
Dierker T, Dreier R, Migone M, Hamer S, Grobe K (2009a) Heparan sulfate and transglutaminase activity is required for the formation of covalently cross-linked hedgehog oligomers. J Biol Chem 284(47):32562–32571. doi:M109.044867 [pii] 10.1074/jbc.M109.044867
Dierker T, Dreier R, Petersen A, Bordych C, Grobe K (2009b) Heparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cells. J Biol Chem 284(12):8013–8022
El Hokayem J, Huber C, Couve A, Aziza J, Baujat G, Bouvier R, Cavalcanti DP, Collins FA, Cordier MP, Delezoide AL, Gonzales M, Johnson D, Le Merrer M, Levy-Mozziconacci A, Loget P, Martin-Coignard D, Martinovic J, Mortier GR, Perez MJ, Roume J, Scarano G, Munnich A, Cormier-Daire V (2012) NEK1 and DYNC2H1 are both involved in short rib polydactyly Majewski type but not in Beemer Langer cases. J Med Genet 49(4):227–233. doi:10.1136/jmedgenet-2011-100717
Eugster C, Panakova D, Mahmoud A, Eaton S (2007) Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev Cell 13(1):57–71
Evangelista M, Tian H, de Sauvage FJ (2006) The hedgehog signaling pathway in cancer. Clin Cancer Res 12(20 Pt 1):5924–5928. doi:12/20/5924 [pii] 10.1158/1078-0432.CCR-06-1736
Fuccillo M, Joyner AL, Fishell G (2006) Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci 7(10):772–783. doi:10.1038/nrn1990
Fuse N, Maiti T, Wang B, Porter JA, Hall TM, Leahy DJ, Beachy PA (1999) Sonic hedgehog protein signals not as a hydrolytic enzyme but as an apparent ligand for patched. Proc Natl Acad Sci U S A 96(20):10992–10999
Goldring MB, Tsuchimochi K, Ijiri K (2006) The control of chondrogenesis. J Cell Biochem 97(1):33–44. doi:10.1002/jcb.20652
Hall BK, Miyake T (2000) All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22(2):138–147. doi:10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4
Hammerschmidt M, Brook A, McMahon AP (1997) The world according to hedgehog. Trends Genet 13(1):14–21
Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ (2004) Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118(4):517–528
Hatsell S, Frost AR (2007) Hedgehog signaling in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 12(2–3):163–173. doi:10.1007/s10911-007-9048-2
Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK (2005) Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1(4):e53. doi:10.1371/journal.pgen.0010053
Hollway GE, Maule J, Gautier P, Evans TM, Keenan DG, Lohs C, Fischer D, Wicking C, Currie PD (2006) Scube2 mediates hedgehog signalling in the zebrafish embryo. Dev Biol 294(1):104–118. doi:S0012-1606(06)00130-8 [pii] 10.1016/j.ydbio.2006.02.032
Hsu SH, Zhang X, Yu C, Li ZJ, Wunder JS, Hui CC, Alman BA (2011) Kif7 promotes hedgehog signaling in growth plate chondrocytes by restricting the inhibitory function of sufu. Development 138(17):3791–3801. doi:10.1242/dev.069492
Huangfu D, Anderson KV (2005) Cilia and hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A 102(32):11325–11330. doi:10.1073/pnas.0505328102
Huangfu D, Anderson KV (2006) Signaling from Smo to Ci/Gli: conservation and divergence of hedgehog pathways from drosophila to vertebrates. Development 133(1):3–14. doi:10.1242/dev.02169
Hynes M, Stone DM, Dowd M, Pitts-Meek S, Goddard A, Gurney A, Rosenthal A (1997) Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron 19(1):15–26
Ingham PW, McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15(23):3059–3087
Jacob L, Lum L (2007) Deconstructing the hedgehog pathway in development and disease. Science 318(5847):66–68
Jeong J, McMahon AP (2005) Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development 132(1):143–154. doi:10.1242/dev.01566
Jiang J, Leong NL, Mung JC, Hidaka C, Lu HH (2008) Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthritis Cartilage 16(1):70–82. doi:10.1016/j.joca.2007.05.014
Johnson JL, Hall TE, Dyson JM, Sonntag C, Ayers K, Berger S, Gautier P, Mitchell C, Hollway GE, Currie PD (2012) Scube activity is necessary for hedgehog signal transduction in vivo. Dev Biol 368(2):193–202. doi:S0012-1606(12)00254-0 [pii] 10.1016/j.ydbio.2012.05.007
Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC (1994) Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev 8(3):277–289
Karlstrom RO, Tyurina OV, Kawakami A, Nishioka N, Talbot WS, Sasaki H, Schier AF (2003) Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development 130(8):1549–1564
Kavran JM, Ward MD, Oladosu OO, Mulepati S, Leahy DJ (2010) All mammalian hedgehog proteins interact with cell adhesion molecule, down-regulated by oncogenes (CDO) and brother of CDO (BOC) in a conserved manner. J Biol Chem 285(32):24584–24590. doi:M110.131680 [pii] 10.1074/jbc.M110.131680
Kawakami A, Nojima Y, Toyoda A, Takahoko M, Satoh M, Tanaka H, Wada H, Masai I, Terasaki H, Sakaki Y, Takeda H, Okamoto H (2005) The zebrafish-secreted matrix protein you/scube2 is implicated in long-range regulation of hedgehog signaling. Curr Biol 15(5):480–488. doi:S0960982205001570 [pii] 10.1016/j.cub.2005.02.018
Kawakami T, Kawcak T, Li YJ, Zhang W, Hu Y, Chuang PT (2002) Mouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signaling. Development 129(24):5753–5765
Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ (2012) IFT25 links the signal-dependent movement of hedgehog components to intraflagellar transport. Dev Cell 22(5):940–951. doi:10.1016/j.devcel.2012.04.009
Kmita M, Tarchini B, Zakany J, Logan M, Tabin CJ, Duboule D (2005) Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature 435(7045):1113–1116. doi:10.1038/nature03648
Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, Kronenberg HM (2005) Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest 115(7):1734–1742. doi:10.1172/JCI24397
Kolpakova-Hart E, Jinnin M, Hou B, Fukai N, Olsen BR (2007) Kinesin-2 controls development and patterning of the vertebrate skeleton by hedgehog- and Gli3-dependent mechanisms. Dev Biol 309(2):273–284. doi:10.1016/j.ydbio.2007.07.018
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89(5):755–764
Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra R, Pacifici M (2007) Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development 134(11):2159–2169. doi:10.1242/dev.001586
Kronenberg HM (2006) PTHrP and skeletal development. Ann N Y Acad Sci 1068:1–13. doi:10.1196/annals.1346.002
Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LHK, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM (1996) PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273:663–666
Lee CS, Buttitta L, Fan CM (2001) Evidence that the WNT-inducible growth arrest-specific gene 1 encodes an antagonist of sonic hedgehog signaling in the somite. Proc Natl Acad Sci U S A 98(20):11347–11352. doi:10.1073/pnas.201418298 98/20/11347 [pii]
Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, Hsu C, Ali SA, Alman BA (2009) Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med 15(12):1421–1425. doi:nm.2055 [pii] 10.1038/nm.2055
Lin X (2004) Functions of heparan sulfate proteoglycans in cell signaling during development. Development 131(24):6009–6021
Long F, Zhang XM, Karp S, Yang Y, McMahon AP (2001) Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 128(24):5099–5108
Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y (2008) Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 135(11):1947–1956. doi:dev.018044 [pii] 10.1242/dev.018044
McMahon AP, Ingham PW, Tabin CJ (2003) Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 53:1–114
Merrill AE, Merriman B, Farrington-Rock C, Camacho N, Sebald ET, Funari VA, Schibler MJ, Firestein MH, Cohn ZA, Priore MA, Thompson AK, Rimoin DL, Nelson SF, Cohn DH, Krakow D (2009) Ciliary abnormalities due to defects in the retrograde transport protein DYNC2H1 in short-rib polydactyly syndrome. Am J Hum Genet 84(4):542–549. doi:10.1016/j.ajhg.2009.03.015
Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, Vortkamp A (2001) BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128(22):4523–4534
Muller P, Rogers KW, Yu SR, Brand M, Schier AF (2013) Morphogen transport. Development 140(8):1621–1638. doi:10.1242/dev.083519
Nakamura T, Aikawa T, Iwamoto-Enomoto M, Iwamoto M, Higuchi Y, Pacifici M, Kinto N, Yamaguchi A, Noji S, Kurisu K, Matsuya T (1997) Induction of osteogenic differentiation by hedgehog proteins. Biochem Biophys Res Commun 237(2):465–469
Ogden SK, Ascano M Jr, Stegman MA, Robbins DJ (2004) Regulation of hedgehog signaling: a complex story. Biochem Pharmacol 67(5):805–814
Ohlig S, Farshi P, Pickhinke U, van den Boom J, Hoing S, Jakuschev S, Hoffmann D, Dreier R, Scholer HR, Dierker T, Bordych C, Grobe K (2011) Sonic hedgehog shedding results in functional activation of the solubilized protein. Dev Cell 20(6):764–774. doi:S1534-5807(11)00202-4 [pii] 10.1016/j.devcel.2011.05.010
Ohlig S, Pickhinke U, Sirko S, Bandari S, Hoffmann D, Dreier R, Farshi P, Gotz M, Grobe K (2012) An emerging role of sonic hedgehog shedding as a modulator of heparan sulfate interactions. J Biol Chem 287(52):43708–43719. doi:M112.356667 [pii] 10.1074/jbc.M112.356667
Onyekwelu I, Goldring MB, Hidaka C (2009) Chondrogenesis, joint formation, and articular cartilage regeneration. J Cell Biochem 107(3):383–392. doi:10.1002/jcb.22149
Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ (1997) Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89(5):765–771
Pan A, Chang L, Nguyen A, James AW (2013) A review of hedgehog signaling in cranial bone development. Front Physiol 4:61. doi:10.3389/fphys.2013.00061
Panakova D, Sprong H, Marois E, Thiele C, Eaton S (2005) Lipoprotein particles are required for hedgehog and wingless signalling. Nature 435(7038):58–65
Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL (2000) Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127(8):1593–1605
Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A (1998) Identification of a palmitic acid-modified form of human sonic hedgehog. J Biol Chem 273(22):14037–14045
Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, Ericson J, Briscoe J (2002) Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev 16(22):2865–2878. doi:10.1101/gad.243402
Pizette S, Niswander L (2000) BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol 219(2):237–249. doi:10.1006/dbio.2000.9610
Porter JA, Ekker SC, Park WJ, von Kessler DP, Young KE, Chen CH, Ma Y, Woods AS, Cotter RJ, Koonin EV, Beachy PA (1996a) Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 86(1):21–34
Porter JA, Young KE, Beachy PA (1996b) Cholesterol modification of hedgehog signaling proteins in animal development. Science 274(5285):255–259
Razzaque MS, Soegiarto DW, Chang D, Long F, Lanske B (2005) Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol 207(4):453–461. doi:10.1002/path.1870
Robbins DJ, Fei DL, Riobo NA (2012) The hedgehog signal transduction network. Sci Signal 5(246):re6, doi:scisignal.2002906 [pii] 10.1126/scisignal.2002906
Roy S, Hsiung F, Kornberg TB (2011) Specificity of drosophila cytonemes for distinct signaling pathways. Science 332(6027):354–358. doi:10.1126/science.1198949
Ruat M, Roudaut H, Ferent J, Traiffort E (2012) Hedgehog trafficking, cilia and brain functions. Differentiation 83(2):S97–S104. doi:10.1016/j.diff.2011.11.011
Ruiz i Altaba A (1999) Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development 126(14):3205–3216
Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H (1999) Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126(17):3915–3924
Scherft JP, Daems WT (1967) Single cilia in chondrocytes. J Ultrastruct Res 19(5):546–555
Spater D, Hill TP, O’Sullivan RJ, Gruber M, Conner DA, Hartmann C (2006) Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 133(15):3039–3049. doi:10.1242/dev.02471
St-Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13(16):2072–2086
Taipale J, Cooper MK, Maiti T, Beachy PA (2002) Patched acts catalytically to suppress the activity of smoothened. Nature 418(6900):892–897
Taylor FR, Wen D, Garber EA, Carmillo AN, Baker DP, Arduini RM, Williams KP, Weinreb PH, Rayhorn P, Hronowski X, Whitty A, Day ES, Boriack-Sjodin A, Shapiro RI, Galdes A, Pepinsky RB (2001) Enhanced potency of human sonic hedgehog by hydrophobic modification. Biochemistry 40(14):4359–4371. doi:bi002487u [pii]
Terkeltaub R, Lotz M, Johnson K, Deng D, Hashimoto S, Goldring MB, Burton D, Deftos LJ (1998) Parathyroid hormone-related proteins is abundant in osteoarthritic cartilage, and the parathyroid hormone-related protein 1-173 isoform is selectively induced by transforming growth factor beta in articular chondrocytes and suppresses generation of extracellular inorganic pyrophosphate. Arthritis Rheum 41(12):2152–2164. doi:10.1002/1529-0131(199812)41:12<2152::AID-ART10>3.0.CO;2-X
Thompson CL, Chapple JP, Knight MM (2014) Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthritis Cartilage 22(3):490–498. doi:10.1016/j.joca.2013.12.016
Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR (2008) THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet 40(4):403–410. doi:10.1038/ng.105
Tsiairis CD, McMahon AP (2008) Disp1 regulates growth of mammalian long bones through the control of Ihh distribution. Dev Biol 317(2):480–485. doi:S0012-1606(08)00152-8 [pii] 10.1016/j.ydbio.2008.02.039
Tukachinsky H, Kuzmickas RP, Jao CY, Liu J, Salic A (2012) Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep 2(2):308–320. doi:S2211-1247(12)00220-3 [pii] 10.1016/j.celrep.2012.07.010
Vortkamp A (2001) Interaction of growth factors regulating chondrocyte differentiation in the developing embryo. Osteoarthritis Cartilage 9(Suppl A):S109–S117
Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ (1996) Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273:613–622
Wang C, Yuan X, Yang S (2013) IFT80 is essential for chondrocyte differentiation by regulating hedgehog and Wnt signaling pathways. Exp Cell Res 319(5):623–632. doi:10.1016/j.yexcr.2012.12.028
Wilson CW, Chuang PT (2006) New “hogs” in hedgehog transport and signal reception. Cell 125(3):435–438. doi:10.1016/j.cell.2006.04.016
Woods IG, Talbot WS (2005) The you gene encodes an EGF-CUB protein essential for hedgehog signaling in zebrafish. PLoS Biol 3(3):e66. doi:10.1371/journal.pbio.0030066
Wu Q, Zhang Y, Chen Q (2001) Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem 276(38):35290–35296. doi:10.1074/jbc.M101055200
Yoon BS, Lyons KM (2004) Multiple functions of BMPs in chondrogenesis. J Cell Biochem 93(1):93–103. doi:10.1002/jcb.20211
Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM (2005) Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci U S A 102(14):5062–5067. doi:10.1073/pnas.0500031102
Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y, Komori T (2004) Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18(8):952–963. doi:10.1101/gad.1174704
Zeng X, Goetz JA, Suber LM, Scott WJ Jr, Schreiner CM, Robbins DJ (2001) A freely diffusible form of sonic hedgehog mediates long-range signalling. Nature 411(6838):716–720
Zhou J, Chen Q, Lanske B, Fleming BC, Terek R, Wei X, Zhang G, Wang S, Li K, Wei L (2014a) Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res Ther 16(1):R11. doi:10.1186/ar4437
Zhou J, Wei X, Wei L (2014b) Indian hedgehog, a critical modulator in osteoarthritis, could be a potential therapeutic target for attenuating cartilage degeneration disease. Connect Tissue Res 55(4):257–261. doi:10.3109/03008207.2014.925885
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Grobe, K. (2016). Hedgehog Signaling in Chondrocytes. In: Grässel, S., Aszódi, A. (eds) Cartilage. Springer, Cham. https://doi.org/10.1007/978-3-319-29568-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-29568-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29566-4
Online ISBN: 978-3-319-29568-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)