Abstract
Digital breast tomosynthesis (DBT) was proved to be feasible at the end of the 1990s, when digital mammography systems were going to be launched into the market (Niklason et al. 1997). It was already known that 2D mammography, despite the advent of new detectors, is inherently limited because of the superimposition of both normal and pathological structures when a transmission X-ray image is acquired. In fact, in mammography the 3D breast structure is projected onto the detector plane perpendicular to the X-ray source, and the multiple tissues and structures appear overlapped in the projection image. This has two effects on radiologists’ capability of detecting subtle lesions in mammography images: on one side, malignant lesions might be masked by the presence of overlapped glandular tissue, producing false negatives; on the other side, the superimposition of normal tissues might determine false positives. The lowering of sensitivity and specificity in conventional mammography caused by tissue superimposition is often called “anatomical” or “structure” noise, i.e., something which is an obstacle for radiologists to correctly interpret image contents (Niklason et al. 1997; Burgess et al. 2001). The adverse effects of the anatomical noise can be reduced by introducing tomographic imaging methods, like tomosynthesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Digital Breast Tomosynthesis
- Flat Panel Detector
- Digital Mammography System
- Tomosynthesis Image
- Digital Breast Tomosynthesis Image
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1.1 Physical Principle of Tomosynthesis
Digital breast tomosynthesis (DBT) was proved to be feasible at the end of the 1990s, when digital mammography systems were going to be launched into the market (Niklason et al. 1997). It was already known that 2D mammography, despite the advent of new detectors, is inherently limited because of the superimposition of both normal and pathological structures when a transmission X-ray image is acquired. In fact, in mammography the 3D breast structure is projected onto the detector plane perpendicular to the X-ray source, and the multiple tissues and structures appear overlapped in the projection image. This has two effects on radiologists’ capability of detecting subtle lesions in mammography images: on one side, malignant lesions might be masked by the presence of overlapped glandular tissue, producing false negatives; on the other side, the superimposition of normal tissues might determine false positives. The lowering of sensitivity and specificity in conventional mammography caused by tissue superimposition is often called “anatomical” or “structure” noise, i.e., something which is an obstacle for radiologists to correctly interpret image contents (Niklason et al. 1997; Burgess et al. 2001). The adverse effects of the anatomical noise can be reduced by introducing tomographic imaging methods, like tomosynthesis.
DBT is a quasi-3D imaging technique, which reconstructs tomographic images of the breast from a series of low-dose projection images acquired by a digital detector while the X-ray tube rotates within a limited arc (Park et al. 2007). This can be relatively easily obtained from a standard digital mammography platform where the gantry is allowed to move around an axis located above the breast support, while the digital detector remains stationary during the acquisition of the low-dose projection. Breast positioning in tomosynthesis is the same as used for conventional digital mammography, with the breast compressed on the breast support to obtain different views (typically cranio-caudal, CC and mediolateral oblique, MLO) (Yaffe et al. 2014).
A schematic view of acquisition and reconstruction in digital breast tomosynthesis is depicted in Fig. 1.1a, b (Reiser et al. 2014).
Tomosynthesis acquisition (a) and reconstruction (b). Only three projections are represented to illustrate the principle, while in real systems the number of projections ranges between 9 and 25 (From Reiser et al. 2014)
The two objects (red and blue) within the breast are located along the z-axis at different depths, A and B. In DBT multiple projection images at low dose are acquired, with the X-ray source at different angles. The two objects appear overlapped in the central projection, but they are separated in the angled projections, with a shift proportional to the tube angle (Fig. 1.1a). The acquisition scheme is simplified for three projections, but in real DBT systems, the number of projection ranges between 9 and 25, depending on the system. In Fig. 1.1b the reconstruction process is illustrated, showing the result of a shift-and-add (SAA) algorithm. Multiple planes corresponding to different depths in the breast are reconstructed by adding the contribution of all the acquired low-dose projection images. For each reconstructed plane, the algorithm permits to have in focus only the structures belonging to that plane, while any other structure located in different planes is blurred. The reconstruction process, blurring everything comes from an out-of-focus plane, reduces the anatomical noise and favorites easier lesion detection (Reiser et al. 2014).
Clinical tomosynthesis images (stack of tomographic planes) appear very similar to standard 2D mammography, but lesion detectability is strongly improved in the lesion in-focus plane.
In the following, the details of acquisition and reconstruction will be explored, to better figure out the contribution of each physical/technological factor to DBT images.
1.2 Acquisition in Tomosynthesis
There are five digital breast tomosynthesis systems currently available in the European market, three of them received the FDA approval and are available also in the United States. All those systems can perform both standard mammography and tomosynthesis.
In Table 1.1 is reported a summary of the main physical parameters illustrating the differences across manufacturers for each DBT solution (Sechopoulos 2013a, part I).
1.2.1 Anode/Filter Material and Technique Factors
It can be noticed from Table 1.1 that the DBT systems mostly use tungsten (W) anode with silver (Ag) or rhodium (Rh) or aluminum (Al) filter, in order to obtain more penetrating X-ray beams than those used for 2D mammography. The only exception is GE, adopting mainly the Rh/Rh in DBT acquisition. Kilovoltage is usually higher than values used for mammography acquisition. The reason why in DBT X-ray beams with higher photon energy are used versus mammography is that the inherent contrast of the series if low-dose projections is not very important (as it was in mammography) because the benefit of tomosynthesis derives from its capability of improving lesion conspicuity by reducing the anatomical noise (Yaffe et al. 2014).
1.2.2 Detector Type and In-plane Resolution
All the commercial systems mount a flat panel detector (FPD), either with a scintillator (cesium iodide) or a photoconductor (selenium) to convert X-rays in a different type of signal. In the GE system, the X-ray photons entering the panel interact with the cesium iodide (CsI) layer, and are converted in light photons, which constitute the signal collected by the flat panel. In all the other systems, the X-ray photons create electric charges in the selenium layer, and a strong electric field drags those charges to the FPD. Each system uses the same flat panel for both mammography and tomosynthesis acquisition.
The original pixel size of the FPD used in mammography is 50 μm for Fuji, 70 μm for Hologic, 85 μm for IMS and Siemens, and 100 μm for GE. In tomosynthesis, GE, IMS, and Siemens use the same pixel size, Hologic opts for 2 × 2 pixel rebinning with an effective pixel size of 140 μm, and Fuji proposes a hexagonal pixel with the opportunity of modulating the effective pixel size from 50 μm up to 150 μm.
As the detector pixel size is either the same or comparable with that used for mammography acquisition, the in-plane resolution (x-y) of tomosynthesis images (tomographic planes) is high, very close to the resolution of mammography images.
1.2.3 Tube Motion
The tube motion around the breast can be either continuous or step-and-shoot. The continuous motion is the same used by computer tomography (CT) systems: the X-ray tube moves continuously along the arc, and at given positions, an X-ray pulse is emitted and a low-dose projection acquired. Its major advantage is the acquisition speed, provided that the detector readout is fast enough, while a disadvantage of the continuous motion is the focal spot blur during tube travel. In the step-and-shoot approach, the X-ray tube stops at each position before acquiring each low-dose projection. This avoids the focal spot blur but requires some extra seconds for the overall scan.
For both continuous and step-and-shoot motions, knowledge of the precise angular position each exposure has been performed is necessary, and this information, recorded in the image header, is used by the algorithm to recombine the projection images and reconstruct the breast volume.
Three out of five commercial DBT systems use the continuous tube motion (Fuji, Hologic, Siemens), the remaining two systems (GE and IMS) adopt the step-and-shoot approach.
1.2.4 Acquisition Geometry
All the DBT systems currently available work in partial isocentric geometry, with the detector stationary during acquisition and the X-ray source moving in an arc around the compressed breast. Only the Hologic system tilts the image detector to follow the X-ray tube.
An alternative would be offered by full isocentric geometry, in which detector and X-ray source move synchronously around the imaged object. This is the acquisition geometry of CT.
1.2.5 Sweep Angle and Number of Projections
The two parameters that characterize tomosynthesis systems are the sweep angle (or scan angle), i.e., the whole arc traveled by the gantry from first to last projection acquisition, and the number of projections (Roth et al. 2014). As reported in Table 1.1, commercial systems are very different in this respect. The sweep angle ranges from a minimum of 15° (±7.5°) for the Hologic equipment and a maximum of 50° (±25°) for the Siemens system. The Fuji product provides two alternative settings, one called “standard mode” equivalent to the Hologic solution and a second one called “high-resolution mode,” with a wider scan angle (40°). The GE system works with an intermediate arc of 25° (±12.5°). The number of low-dose projection ranges from a minimum of 9 (GE) to a maximum of 25 (Siemens).
In general, small scan angles are better for “in-plane resolution” and small objects like microcalcifications are better depicted, while wide angles improve the “out-of-plane resolution” (or z-resolution), and this is preferable for large objects, like masses, whose representation is not limited to an individual in-focus plane. However, the general principle of maximizing the angular range in tomosynthesis is not applicable because of the stationarity of the detector determining a reduction of the effective field of view (FOV) when projections are taken at wide angles. Moreover, due to the constraint applied by manufacturers to keep the radiation dose for a DBT series not much higher than the dose level for a standard mammography view, the use of a wide sweep angle means an increase of the number of projections and the need to decrease the exposure per projection, which should be limited by the presence of quantum noise.
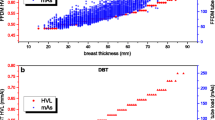
DBT systems mostly distribute radiation dose uniformly along the scan angle, i.e., the same product current x time (mAs) is used for each projection. The only exception is the IMS solution, which uses about 50 % of the total dose for the central projection (to obtain a quasi-standard mammogram), while the remaining dose is variably distributed along the scan angle, with angled projections obtained with a variable angular sampling.
1.2.6 Scatter Radiation
Scatter radiation is known to degrade image quality in standard 2D mammography. For this reason, the use of antiscatter grids has been introduced. Grids are constituted of lead septs oriented in the cathode-anode direction and focused toward the X-ray source; the role of the lead septs is to absorb scattered photons and leave unchanged the trajectory of primary photons. However, the grid usage has also determined the need of increasing radiation dose because the grid lines absorb preferably but not exclusively scattered photons. Despite this side effect on radiation dose, grids are systematically used in mammography because they strongly improve image quality, especially for thick breasts.
In tomosynthesis scatter radiation is still present, but most manufacturers chose to not use an antiscatter grid for multiple reasons: the first is that conventional grids cannot be used as they are out of focus for any projection acquired with the X-ray source at a different angle than 0° and the second is that scatter radiation mainly degrades image contrast, but, as stated for the technique factors, the strong point of tomosynthesis is its capability in reducing the anatomical noise, and consequently, the contrast of the projection images becomes a secondary factor. Finally, it should be noticed that the will of keeping radiation dose per DBT projection as low as possible means that such dose level must be set to produce a sufficient signal-to-noise ratio in the projection image; also the scattered photons contribute to the signal-to-noise ratio and, as such, may have some positive effects on DBT quality.
The only manufacturer who still uses a grid in tomosynthesis is GE that developed a special grid with the lead septa oriented parallel to the chest wall in order to capture the scattered photons while maintaining grid focus during acquisition. As for standard mammography, the benefit of grid use is more evident for thick breasts, for which the amount of scattered radiation is more significant.
1.3 Reconstruction in Tomosynthesis
Reconstruction of volumetric information from a limited number of low-dose projections acquired within a limited angle is a challenge. In fact, the amount of tomographic information available from acquisition is only partial, and most information necessary for a volumetric reconstruction is missing.
1.3.1 Reconstruction Algorithms
Reconstruction algorithms used in tomosynthesis are similar to those used in CT, with some difficulties due to the limited geometry, as mentioned above. There are two main categories, the very classical filtered back-projection (FBP) and the more recent iterative techniques (Sechopoulos 2013b, part II).
Back-projection (BP) just reverses the projection process and realigns structures spatially, obtaining multiple tomographic planes where signal coming from structure belonging to each plane are reinforced (in focus) while any other out-of-plane structure is blurred. Unfortunately, images reconstructed from pure back-projection are unavoidably blurred, and filtration is required to remove blurring (FBP). This is the reference algorithm used in conventional CT, and its major benefit was to be fast, even in the early days of CT when computers were not as powerful as today. Filtered back-projection is performed in the frequency space, also called Fourier domain. Figure 1.2 compares the information available for reconstruction when a full CT acquisition is performed (left: full angular coverage, many projections) to the limited information available when a DBT acquisition is performed (right, limited angle, limited number of projections). Because of the very poor geometrical sampling of tomosynthesis acquisition, the spatial resolution in the direction perpendicular to the detector plane (z-direction) is very limited (Wu et al. 2003).
The iterative techniques include both algebraic and statistical algorithms. They take advantage from the improved performance of computing systems. The iterative reconstruction, currently implemented also in computed tomography, shows particular benefits in tomosynthesis because they can face better than FBP the limitations on image quality caused by the geometrical limitations. Iterative algorithms produce tomographic images with good sharpness and high signal-to-noise ratio. Moreover, they are effective in reducing the reconstruction artifacts caused by the spatial undersampling of tomosynthesis acquisition (Wu et al. 2004).
1.3.2 Reconstructed “Objects”
Three different types of “objects” are reconstructed in tomosynthesis:
-
1.
Tomographic planes (mostly called “slices”)
-
2.
Slabs
-
3.
2D synthetic views
As tomosynthesis is often considered a “small angle CT,” the CT language has been adopted for tomosynthesis. The main reconstructed object is a stack of tomographic planes, i.e., a set of images reproducing the content of planes at different depths in the breast obtained by reconstructing the breast volume and sampling the tomographic planes with a certain sampling interval. Tomosynthesis review is usually performed by scrolling the tomographic planes in the depth direction manually or through a cine-loop mode.
The term “slice” used to indicate a CT-reconstructed image representing the content of a body section cannot be applied with the same meaning in tomosynthesis. In fact, in CT acquisition the detector array is oriented in the z-direction of the patient, and a parameter called “slice thickness” is set before the CT acquisition starts; it determines the thickness of the body section represented by each reconstructed image and thereby the resolution in z-direction. In other words, a reconstructed image in CT includes information coming from a section of the patient and, as such, has a thickness. Tomosynthesis setup is different: the image detector is planar, and the tomographic scan acquires a certain number of projection images; the information in z-direction is not directly acquired but is derived from the volumetric reconstruction. The algorithm produces a set of planes parallel to the detector plane, spaced by 0.5–1.0 mm one to each other. In other words, tomosynthesis images are not slices (plane thickness is zero), but planes and what is usually reported as “slice thickness” is actually the distance between adjacent planes. Distance between planes is 1 mm for four out of five systems considered in Table 1.1. Only GE system uses a sampling interval of 0.5 mm. The sampling interval, together with the breast compressed thickness, determines the number of reconstructed planes. A 5 cm breast reconstructed by DBT at 1 mm includes a stack of 50 images. As reconstructed planes have the same weight (in bits) of standard mammography views, the overall weight of a DBT exam is definitely higher. For the 5 cm breast considered above, the standard four views (two CCs plus two MLOs) of a bilateral mammography can weigh between 60 and 200 MB, while four views of a bilateral tomosynthesis weigh from 3 GB up to 10 GB.
Other types of images which can be reconstructed from tomosynthesis are “slabs”, i.e., “thick slices” obtained by adding together a certain number of tomographic planes. Slabs have thickness, typically 1 cm or more, and are particularly useful to detect microcalcification clusters. In fact, microcalcifications usually grouped in clusters in 2D mammography may be sparse along the depth in the tomographic reconstruction and lose their cluster aspect. Furthermore, slabs allow a quick review of the breast volume, before getting into the details of the tomographic planes if some type of potentially pathological feature is detected. The reviewing workflow is one of the open questions of tomosynthesis, especially when “high-rate” environment are counted, as breast cancer screening, and slabs seem a way to dramatically reduce the radiologists’ interpretation time.
Finally, the last “reconstructed objects” derived from tomosynthesis are the “synthetic 2D mammograms.” It is a pseudo-mammography, conceptually obtainable by collapsing the breast reconstructed volume onto a plane. Compared to standard 2D mammography views, synthetic 2D views are obtained at zero radiation dose. Moreover, as synthetic views come from DBT reconstructions, they include the major benefit of tomosynthesis, i.e., the reduction of the anatomical noise, increasing lesion conspicuity.
1.4 Radiation Dose
Radiation dose in tomosynthesis is evaluated by the mean (or average) glandular dose, MGD, the same parameter used in mammography. It is calculated applying conversion factors computed by Monte Carlo technique to the entrance-measured dose. Compared to mammography MGD, the calculation of MGD with tomosynthesis includes an angular dependence.
In general, the radiation dose level for the acquisition of a tomosynthesis series (associated to a breast view) is expected to be higher than the dose level used to obtain a standard digital mammogram (Svahn et al. 2015). In fact, despite the dose per projection is kept low, in tomosynthesis multiple projection images (between 9 and 25 with current systems) are necessary to permit the volumetric reconstruction, and this unavoidably leads to a dose increase. Most of data come from studies using prototype systems, for which radiation dose for one DBT view was often set “equivalent” to the dose used for 2-view mammography. There are very few results available from the literature about tomosynthesis radiation dose for commercial systems. Depending on the system, its number of projections, X-ray tube, automatic exposure control design, the ratio between the dose per breast tomosynthesis view (CC, MLO, etc.), and the dose per mammography view are usually between 1 and 2 (Feng and Sechopoulos 2012).
However, the reason why there is a certain concern about the increased exposure to radiation dose due to tomosynthesis is associated to the clinical protocol mostly used in screening trials with tomosynthesis. Such protocol includes standard mammography in two views and tomosynthesis in two views, and almost all the results published show the improved clinical performance of the combination of mammography and tomosynthesis compared to mammography alone. With this protocol, also assuming that manufacturers will optimize systems to make dose in DBT equal to dose in mammography, radiation dose would be systematically doubled because of the double examination, and this is questionable in the light of radiation protection, especially in screening population.
The introduction of synthetic 2D mammograms and the positive results obtained indicate that in the next future synthetic mammography obtained from tomosynthesis will replace standard mammography, which will become unnecessary. In other words, the concern about the increased dose level by tomosynthesis can be significantly reappraised if the perspective is to acquire tomosynthesis alone and derive synthetic mammography, at zero dose, from the reconstruction process (Skaane et al. 2014).
References
Burgess AE, Jacobson FL, Judy PF (2001) Human observer detection experiments with mammograms and power-law noise. Med Phys 28:419–437
Feng SS, Sechopoulos I (2012) Clinical digital breast tomosynthesis system: dosimetric characterization. Radiology 263:35–42
Niklason LT, Christian BT, Niklason LE, Kopans DB, Castleberry DE, Opsahl-Ong BH, Landberg CE, Slanetz PJ, Giardino AA, Moore R, Albagli D, DeJule MC, Fitzgerald PF, Fobare DF, Giambattista BW, Kwasnick RF, Liu J, Lubowski SJ, Possin GE, Richotte JF, Wei CY, Wirth RF (1997) Digital tomosynthesis in breast imaging. Radiology 205:399–406
Park JM, Franken EA Jr, Garg M, Fajardo LL, Niklason LT (2007) Breast tomosynthesis: present considerations and future applications. Radiographics 27(Suppl 1):S231–S240
Reiser I, Sechopoulos I (2014) A review of digital breast tomosynthesis. Med Phys Int 2:57–66
Roth RG, Maidment AD, Weinstein SP, Roth SO, Conant EF (2014) Digital breast tomosynthesis: lessons learned from early clinical implementation. Radiographics 34:E89–E102
Sechopoulos I (2013a) A review of breast tomosynthesis. Part I. The image acquisition process. Med Phys 40:014301
Sechopoulos I (2013b) A review of breast tomosynthesis. Part II. Image reconstruction, processing and analysis, and advanced applications. Med Phys 40:014302
Skaane P, Bandos AI, Eben EB, Jebsen IN, Krager M, Haakenaasen U, Ekseth U, Izadi M, Hofvind S, Gullien R (2014) Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology 271:655–663
Svahn TM, Houssami N, Sechopoulos I, Mattsson S (2015) Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 24:93–99
Wu T, Stewart A, Stanton M, McCauley T, Phillips W, Kopans DB, Moore RH, Eberhard JW, Opsahl-Ong B, Niklason L, Williams MB (2003) Tomographic mammography using a limited number of low-dose cone-beam projection images. Med Phys 30:365–380
Wu T, Moore RH, Rafferty EA, Kopans DB (2004) A comparison of reconstruction algorithms for breast tomosynthesis. Med Phys 31:2636–2647
Yaffe MJ, Mainprize JG (2014) Digital tomosynthesis: technique. Radiol Clin North Am 52:489–497
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gennaro, G. (2016). Physics and Radiation Dose of Digital Breast Tomosynthesis. In: Tagliafico, A., Houssami, N., Calabrese, M. (eds) Digital Breast Tomosynthesis. Springer, Cham. https://doi.org/10.1007/978-3-319-28631-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-28631-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28629-7
Online ISBN: 978-3-319-28631-0
eBook Packages: MedicineMedicine (R0)