Abstract
The term gluten-related disorders (GRD) denotes a spectrum of diverse immune-mediated diseases triggered by the ingestion of gluten (protein found in wheat, barley and rye). Coeliac disease (CD) or gluten-sensitive enteropathy is the most recognised and studied entity within GRD. Extraintestinal manifestations are gaining recognition and are increasingly the subject of further studies as they may hold the key to unravelling the pathophysiology of GRD. Such manifestations include skin involvement in the form of dermatitis herpetiformis (DH) and neurological dysfunction (e.g. gluten ataxia and gluten neuropathy). Furthermore, the recent concept of extraintestinal manifestations without enteropathy (termed non-coeliac gluten sensitivity (NCGS)) has become accepted as part of the same spectrum. In this chapter, we review the neurological manifestations in GRD and discuss recent advances in diagnosis and possible pathophysiological mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Coeliac disease
- Gluten sensitivity
- Gluten ataxia
- Gluten neuropathy
- Neurological manifestations
- Transglutaminase antibodies
- Immune pathogenesis
- Gluten-sensitive enteropathy
Introduction
Coeliac disease (CD) was first described by the Greek doctor Aretaeus the Cappadocian, in 100 AD. Long time after, Samuel Gee published his lecture [37] “on the coeliac affection” in which he described the classic presentation of the disease in children. The aetiological agent remained obscure until the observations of Willem Dicke, a Dutch paediatrician, in 1953 of “the presence in wheat, of a factor having a deleterious effect in cases of coeliac disease” [30]. The introduction of endoscopy and small bowel biopsy in the 1950s confirmed the bowel as the principal organ involved [104]. Such biopsies demonstrated for the first time the typical histological abnormalities that now define gluten-sensitive enteropathy: villous atrophy, crypt hyperplasia and increased intraepithelial lymphocytes.
The first evidence of gluten sensitivity-related extraintestinal manifestations became apparent in 1963 when a group of dermatologists published the interesting observation that dermatitis herpetiformis, an itchy vesicular rash, was in fact a form of gluten-related dermatopathy sharing the same small bowel pathology, but less prominent or even no gastrointestinal symptoms [95]. The only reason why small bowel biopsies were done in this group of patients was the observation of persistently low albumin suggestive of protein loss from the gut.
A small number of case reports of patients with presumed CD and neurological manifestations [33, 105, 133] were published prior to the discovery of the aetiological agent and the introduction of small bowel biopsy. Such reports need to be treated with caution given that a diagnosis of CD in those patients was speculative.
The first comprehensive case series of neurological manifestations in the context of histologically confirmed CD was published in 1966 [24]. This detailed clinical and pathology work described the range of neurological manifestations seen in 16 patients with established CD. Of interest was the fact that all patients had gait ataxia and some had peripheral neuropathy as well. The assumption was that such manifestations were nutritional. Indeed all of these patients were grossly malnourished and cachectic. Post-mortem data, however, demonstrated an inflammatory process primarily affecting the cerebellum, but also involving other parts of the central and peripheral nervous systems, a finding that was in favour of an immune-mediated pathogenesis.
Single and multiple case reports of patients with established CD who then developed neurological dysfunction continued to be published [8, 9, 13, 21, 22, 29, 34, 64, 76, 79, 81, 83, 87, 98, 100, 126, 132].
Key findings from these reports were that ataxia and neuropathy were the commonest manifestations always seen in the context of established CD and almost always attributed to nutritional deficiencies. Some reports reported improvement of the neurological problems with adherence to a GFD whilst others did not. None of these reports however documented the strictness of adherence to the gluten-free diet by regular serological testing.
Thirty years after the first comprehensive case series on neurological manifestations of CD saw the publication of a study [41] approaching the subject purely from a neurological perspective. This study investigated the prevalence of serological markers of gluten sensitivity (at the time, IgG and IgA antigliadin antibodies) in patients presenting with neurological dysfunction. The results demonstrated significantly higher prevalence of antigliadin antibodies (AGA) in the neurology group of patients with unclear diagnosis when compared to healthy blood donors and patients with a clear neurological diagnosis. Based on duodenal biopsies, the study showed that the prevalence of CD was 16 times higher than the prevalence of CD in the healthy population. This study sparked an interest for both neurologists and gastroenterologists in a possible link between sensitivity to gluten and neurological disease.
Epidemiology of Neurological Manifestations
There are now several epidemiological studies from Europe and America and a few from other continents demonstrating that the prevalence of CD in healthy individuals is on the increase [17]. Thus, the prevalence of CD in the healthy population has been shown to be at least 1 % [115]. There are no accurate figures of the prevalence of the neurological manifestations of gluten sensitivity in the general population. Some studies have concentrated on the prevalence of neurological dysfunction amongst patients with established CD. Figures of between 10 % and 22.5 % have been published [12, 65]. Such figures are unlikely to be accurate because they are retrospective, derived solely from gastrointestinal clinics and concentrated exclusively on patients with the classic (i.e. gastrointestinal) CD presentation. Some of these studies also included neurological diseases that are highly unlikely to be gluten related (e.g. carpal tunnel syndrome, idiopathic Parkinson’s disease).
Some estimates of prevalence can be made from patient populations attending specialist clinics although caution must be exercised in extrapolating these as they are inevitably affected by referral bias. For example, data collected from the Sheffield dedicated CD clinic (the biggest in the UK) and from the dedicated gluten sensitivity/neurology clinic (the only one in the UK) suggested that for every 7 patients presenting to the gastroenterologist who are then diagnosed with CD, 2 patients present to the neurologist with neurological dysfunction leading to the diagnosis of CD [55]. This ratio is likely to be an underestimate because it does not take into account those patients with neurological manifestations due to sensitivity to gluten that do not have enteropathy (NCGS). In fact, approximately two thirds of patients presenting with neurological dysfunction do not have enteropathy on duodenal biopsy. The authors believe that the prevalence of neurological dysfunction even in patients with CD presenting to gastroenterologists is likely to be much higher to what has been published if patients undergo rigorous neurological workup including MR spectroscopy of the cerebellum. Preliminary results from a prospective study using patients with newly diagnosed CD presenting to a gastroenterologist demonstrated that up to 50 % of such patients have abnormal MR spectroscopy (low NAA/Cr ratios) of the cerebellum [59, 60]. One study in patients with established CD has shown such abnormalities in up to 80 % of patients [58], whilst another study has shown that the prevalence of peripheral neuropathy in this group of patients was 23 % [92]. The above figures are based on patients with CD. The frequency of neurological dysfunction in patients with NCGS is not known. Judging by the fact that two thirds of the cohort of patients seen and assessed in a dedicated gluten/neurology clinic, Sheffield, UK, have NCGS, it is likely that the prevalence of neurological cases with NCGS is even higher than those with CD.
Diagnosis of the Spectrum of Gluten-Related Diseases
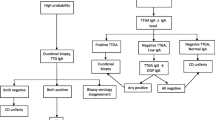
The diagnosis of CD in the hands of an experienced gastroenterologist and gastrointestinal histopathologist can be relatively straightforward. CD is after all defined by the presence of an enteropathy (triad of villus atrophy, crypt hyperplasia and increased intraepithelial lymphocytes), usually a reliable gold standard. It is now, however, accepted that the presence of enteropathy is not a prerequisite for the diagnosis of GRD particularly for those patients where neurological or other extraintestinal manifestations are the presenting feature. Furthermore, the triad of the small bowel mucosal changes mentioned above are merely one part of the small bowel histological spectrum (Marsh classification) that ranges from a normal mucosa to a pre-lymphomatous state [94]. Given that the bowel histology in some cases (as per Marsh classification) can be normal, trying to define GRD using solely the bowel biopsies becomes problematic. Whilst serological testing has enhanced the ability to identify patients with enteropathy, none of these tests are 100 % specific or sensitive. For example, endomysial antibody (EMA) and anti-transglutaminase-2 (TG2) IgA antibody detection are specific for the presence of an enteropathy. These markers are frequently negative in patients with neurological or other extraintestinal manifestations who do not have an enteropathy [55].
The majority of patients presenting with neurological manifestations have no gastrointestinal symptoms [55]. Even patients with CD may not have gastrointestinal symptoms. In patients without overt gastrointestinal involvement (enteropathy), serum antibodies to TG2 may be absent [78]. Patients with extraintestinal manifestations typically have antibodies primarily reacting with different TG isozymes, TG3 in DH and TG6 in patients with gluten ataxia [52, 53, 116]. Reaction of such antibodies with TG2 that takes the form of IgA deposits against TG2 in the intestinal mucosa occurs prior to overt changes in small intestinal morphology and sometimes even before the antibodies are detectable in serum [84]. Such antibody deposits seem to be present in patients with neurological and other extraintestinal manifestations as well and may therefore be diagnostically useful [48]. However, this test is not readily available and requires experience in its interpretation. In practice for suspected neurological manifestations, it is best to perform serological tests for both IgA and IgG antibodies to TG2 (and if available anti-TG6 and anti-TG3) and IgG and IgA antibodies to gliadin. Endomysium antibodies are very specific for the detection of enteropathy, but they detect the same antigen (transglutaminase 2) and have thus largely been replaced by TG2 antibody testing. Any differences between the 2 tests however are likely to be related to the different methodologies used (ELISA for TG2 vs immunofluorescence for the detection of EMA). The diagnosis of NCGS remains problematic by the absence of any biological markers. At the moment, such diagnosis is based on symptomatic improvement after the introduction of a GFD and recurrence of symptoms on reintroduction of gluten in the diet. Antigliadin antibodies of the IgG type can be present in up to 25 % of patients with NCGS attending gastroenterology clinics, and such patients may also have increased intraepithelial lymphocytes [130].
CD has a strong genetic predisposition whereby ~40 % of the genetic load comes from MHC class II association [67]. In Caucasian populations, more than 90 % of CD patients carry the HLA DQ2, with the remaining having the HLA DQ8. A small number of CD patients do not belong into either of these groups, but these have been shown to carry just one chain of the DQ2 heterodimer [71]. HLA genetic testing is therefore another useful tool, particularly as unlike other serological tests it is not dependent on an immunological trigger. However, the HLA DQ genotype can be used only as a test of exclusion of CD as the risk genotype DQ2 is common in Caucasian and Asian populations and many carriers will never develop GRD. Furthermore, patients with NCGS may not have the HLA DQ2 or DQ8. Several genome-wide association studies over the past decade have identified many non-HLA loci that each contribute a small amount of risk for coeliac disease [90]. Most of these additional genes are involved in immune functions, and in fact, several risk loci are shared with other autoimmune conditions including ankylosing spondylitis, rheumatoid arthritis, type 1 diabetes and psoriasis. A recent study showed that including non-HLA variants in addition to HLA in the test for coeliac disease risk improves the accuracy of disease prediction [112]. A bias for loci conferring risk for specific manifestations of GRD remains to be thoroughly investigated.
The Spectrum of Gluten-Related Neurological Manifestations
Gluten Ataxia
Gluten ataxia (GA) is defined as idiopathic sporadic ataxia with positive serological markers for sensitivity to gluten [47]. The original definition was based on the serological tests available at the time (antigliadin IgG and IgA antibodies). In a series of 1000 patients with progressive ataxia evaluated over a period of 20 years in Sheffield, UK, GA had a prevalence of 15 % amongst all ataxias but as high as 41 % amongst idiopathic sporadic ataxias. Using the same AGA assay, the prevalence of positive AGA in genetically confirmed ataxias was 14/110 (13 %) and in healthy volunteers 149/1200 (12 %). A number of studies looking at the prevalence of antigliadin antibodies in ataxias have been published [1, 2, 6, 14–16, 23, 41, 46, 47, 69, 91, 106]. The variations in prevalence may relate to geographical differences in the prevalence of CD, referral bias, variability in the AGA assays used, patient selection (some studies included as idiopathic sporadic ataxia patients with cerebellar variant of multisystem atrophy), small number of patients studied and no controls. The common theme in the majority of these studies is the consistently high prevalence of AGA antibodies in sporadic ataxias when compared to healthy controls.
GA usually presents with pure cerebellar ataxia or rarely ataxia in combination with myoclonus (see below), palatal tremor [52, 53], opsoclonus [26] or, rarely, chorea [109]. GA is usually of insidious onset with a mean age at onset of 53 years. Rarely the ataxia can be rapidly progressive mimicking paraneoplastic cerebellar degeneration. Gaze-evoked nystagmus and other ocular signs of cerebellar dysfunction are common (80 % of cases). All patients have gait ataxia and the majority have limb ataxia. Less than 10 % of patients with GA will have any gastrointestinal symptoms but 40 % will have evidence of enteropathy on biopsy.
Serological diagnosis still relies on the presence of IgG and/or IgA antigliadin antibodies, but more specific biomarkers have been identified. TG6 antibodies have been found to be present in 73 % of patients with idiopathic sporadic ataxia with positive AGA [52, 53]. Furthermore, 32 % of patients with idiopathic sporadic ataxia negative for other serological markers of sensitivity to gluten were found to be positive for TG6 [54, 60]. This may suggest that the prevalence of gluten ataxia may even be higher than previously thought.
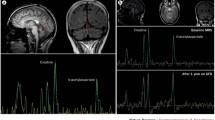
Patients with GA usually have evidence of cerebellar atrophy on MR imaging with particular predilection for the cerebellar vermis. MR spectroscopy of the vermis is abnormal in all patients with GA (low N-acetyl aspartate/creatine ratio) with less prominent changes in the cerebellar hemispheres. Even in patients with GA without cerebellar atrophy, MR spectroscopy is abnormal. MR spectroscopy is a useful monitoring tool. Patients who adhere to strict gluten-free diet often have evidence of improvement of the NAA/Cr ratio within the vermis after a year on the diet.
The response to treatment with a gluten-free diet depends on the duration of the ataxia prior to the diagnosis of sensitivity to gluten. Loss of Purkinje cells in the cerebellum, the end result of prolonged gluten exposure in patients with GA, is irreversible; therefore, prompt treatment is more likely to result in improvement or stabilisation of the ataxia. Whilst the benefits of a gluten-free diet in the treatment of patients with CD and DH have long been established, there are very few studies, mainly case reports, of the effect of gluten-free diet on the ataxia. Most of these reports primarily concern patients with established CD who then develop ataxia [7, 61, 106, 107]. These reports suggest overall favourable responsiveness to a gluten-free diet. A small, uncontrolled study and another case study looked at the use of intravenous immunoglobulins in the treatment of patients with GA with and without enteropathy ([14, 15, 114]). All patients improved. In all of these reports, strict adherence to the gluten-free diet was assumed and no serological evidence was provided. The best marker of strict adherence to a gluten-free diet is serological evidence of elimination of gluten-related antibodies. Only one systematic study of the effect of gluten-free diet on a cohort of patients presenting with ataxia, with or without an enteropathy, has been published [46, 47]. This study also reported serological evidence of elimination of the antigliadin antibodies as a confirmation of strict adherence to the diet. Forty-three patients with gluten ataxia were enrolled. Twenty-six adhered strictly to the gluten-free diet, had serological evidence of elimination of antibodies and comprised the treatment group. Fourteen patients refused the diet and comprised the control group. Patient and control groups were matched at baseline for all variables (age, duration of ataxia, etc.). There was no significant difference in the baseline performance for each ataxia test between the two groups. There was significant improvement in performance in test scores and in the subjective global clinical impression scale in the treatment group when compared to the control group. The improvement was apparent even after excluding patients with an enteropathy. The study concluded that gluten-free diet is an effective treatment for GA.
The current recommendation is that patients presenting with idiopathic progressive cerebellar ataxia should be screened for sensitivity to gluten using antigliadin IgG and IgA, anti-TG2, anti-TG6 and endomysium antibodies [59, 60]. Patients positive for any of these antibodies with no alternative cause for their ataxia should be offered a strict gluten-free diet with regular follow-up to ensure that the antibodies are eliminated (usually takes 6 to 12 months). Stabilisation or even improvement of the ataxia at 1 year would be a strong indicator that the patient suffers from gluten ataxia. The commonest reason for lack of response is compliance with the diet. If patients on strict gluten-free diet continue to progress, with or without elimination of antibodies, the use of immunosuppressive medication (mycophenolate) should be considered. Such cases are rare.
Myoclonic Ataxia and Refractory Coeliac Disease
In 1986, Lu and colleagues published two cases with action myoclonus, ataxia and CD who in addition had epilepsy [87]. The authors provided electrophysiological evidence for the cortical origin of the myoclonus. Similar findings of action and stimulus-sensitive cortical myoclonus were subsequently reported in another patient [126]. This patient had cortical reflex and action myoclonus resembling epilepsia partialis continua, with constant arrhythmic myoclonic activity in the right hypothenar muscles. Electrophysiology confirmed the cortical origin of the myoclonus.
A case series was published in 1995 reporting 4 patients with myoclonus and ataxia with electrophysiological evidence of stimulus-sensitive myoclonus of cortical origin [8]. Pathology showed atrophy of the cerebellar hemispheres with Purkinje cell loss. CD was diagnosed in all four, preceding the onset of the neurological manifestations by years. Such patients unlike those with gluten ataxia appear to be poorly responsive to gluten-free diet and follow a progressive course. The largest series published so far reported 9 patients (6 male, 3 female) with ataxia and asymmetrical irregular jerking [117]. The jerking affected one or more limbs and sometimes face, and it was often stimulus sensitive. All patients later developed more widespread jerking. Six patients had a history of Jacksonian march and five had at least one secondarily generalised seizure. Electrophysiology showed evidence of cortical myoclonus. Four had a phenotype of epilepsia partialis continua. There was clinical, imaging and/or pathological evidence of cerebellar involvement in all cases. Eight patients adhered to a strict gluten-free diet with elimination of gluten-related antibodies, despite which there was still evidence of enteropathy in all thus suggestive of refractory coeliac disease. One patient only just started the diet, and 2 died from enteropathy-associated lymphoma. Five patients were treated with mycophenolate and one in addition with rituximab and IV immunoglobulins. Whilst their ataxia and enteropathy improved, the myoclonus remained the most disabling feature of their illness. This was the first report to highlight the strong association of this unusual phenotype with refractory CD and in 2 of the cases enteropathy-associated lymphoma.
Gluten Neuropathy
Up to 23 % of patients with established CD on gluten-free diet have neurophysiological evidence of a peripheral neuropathy [92]. A large population-based study of over 84,000 patients with CD in Sweden found that CD was associated with polyneuropathy with a hazard ratio of 3.4 [88]. In a UK-based study, 34 % of patients with otherwise idiopathic sporadic sensorimotor axonal length-dependent neuropathy were found to have circulating AGA [48–50]. Using anti-TG2 antibody, an Italian study also found 21 % of patients with peripheral neuropathy to be positive [96]. Finally, in a tertiary referral centre in the USA, retrospective evaluation of patients with neuropathy showed the prevalence of CD to be between 2.5 and 8 % as compared to 1 % in the healthy population [19].
Gluten neuropathy is defined as otherwise idiopathic sporadic neuropathy with serological evidence of sensitivity to gluten. The commonest types are symmetrical sensorimotor axonal length-dependent peripheral neuropathy and sensory ganglionopathy [56]. Other types of neuropathies have also been reported including asymmetrical neuropathy [20, 42, 77], small fibre neuropathy [11] and rarely pure motor neuropathy [42] or autonomic neuropathy [38]. Gluten neuropathy is slowly progressive with a mean age at onset of the neuropathy being 55 years (range 24 to 77) and a mean duration of 9 years (range 1 to 33). A third of the patients will have evidence of enteropathy on biopsy, but the presence or absence of an enteropathy does not influence the effect of a gluten-free diet [48–50].
Limited pathological data available from post-mortem examinations and nerve biopsies are consistent with an inflammatory aetiology (perivascular lymphocytic infiltration). Gluten-free diet has been shown to be beneficial in single and multiple case reports. The only systematic, controlled study of the effect of a gluten-free diet on 35 patients with gluten neuropathy, with regular serological monitoring of the adherence to the gluten-free diet, found significant improvement in the treated compared with the control group after 1 year on gluten-free diet [48–50]. There was significant increase in the sural sensory action potential, the predefined primary endpoint, in the treatment group as well as subjective improvement of the neuropathic symptoms. Subgroup analysis showed that the capacity for recovery is less when the neuropathy is severe.
Sensory ganglionopathy (sometimes also called neuronopathy) is an asymmetric form of pure sensory neuropathy where the pathology is within the dorsal root ganglia. It can be a paraneoplastic syndrome or related to Sjogren’s syndrome. It can also be seen in some inherited neurological illnesses such as Friedreich’s ataxia and mitochondrial diseases (POLG-1). Sensitivity to gluten has proven to be the commonest cause of sensory ganglionopathy (ref). In such cases, there is evidence of inflammatory infiltrates within the dorsal root ganglia. The disease progresses slowly if untreated, but strict adherence to a gluten-free diet may result in stabilisation or even improvement of the neuropathy irrespective of the presence of enteropathy [56].
Headache and Gluten Sensitivity (Gluten Encephalopathy)
Headache is a common feature in patients with GRD. The association was first reported in 2001 based in a series of 10 patients with GRD and headache who in addition had CNS white matter abnormalities on MRI scan [44]. The term “gluten encephalopathy” was used to describe them. The headaches are usually episodic and intractable. They can mimic migraines but do not respond to the usual migraine medication. They characteristically resolve with the introduction of a gluten-free diet. Some patients report a very strong association with ingestion of gluten. The white matter abnormalities are not always present but can be diffuse or focal. They do not always resolve following a gluten-free diet. The diet simply arrests progression of these changes, but the white matter changes can be progressive if the patient does not adhere to a strict gluten-free diet. Their distribution is more suggestive of a vascular rather than demyelinating aetiology. In a prospective study of patients, newly diagnosed with CD frequency of intractable headaches was 44 % [59, 60].
In patients with migraine, there is an overrepresentation of CD with a prevalence of 4.4 % vs 0.4 % in the control population [36]. Using PET brain imaging, a study on regional cerebral perfusion demonstrated that 73 % of patients with CD not on a gluten-free diet had at least one hypoperfused brain region as compared to 7 % in healthy controls and in patients with CD on a gluten-free diet [3]. Another study investigated the prevalence of white matter abnormalities in children with CD and found that 20 % of patients had such abnormalities [80].
Over the last 20 years, we have encountered 100 patients with gluten encephalopathy, a figure that includes the initial 10 patients reported in the 2001 series. Gluten encephalopathy does not always occur in isolation, and such patients will often have additional neurological features such as ataxia. A study from the Mayo clinic emphasised the significant cognitive deficits encountered in 13 such patients [66]. By comparison to gluten ataxia and gluten neuropathy, there is a higher prevalence of enteropathy in patients with gluten encephalopathy (59/100), but the age at onset is the same. The observed improvement of the headaches and arrest of progression in the MRI brain abnormalities suggest a causal link with gluten ingestion [119]. Gluten encephalopathy represents a spectrum of clinical presentations with episodic headaches responsive to a gluten-free diet at one end, to severe debilitating headaches sometimes associated with focal neurological deficits. MRI findings range from normal to extensive white matter abnormalities.
Epilepsy
A link between epilepsy and CD was proposed as far back as 1978 [18, 25, 35]. Whilst studies examining the prevalence of CD amongst patients with epilepsy have suggested a prevalence of 1.2–2.3 %, others failed to demonstrate an increased prevalence [111]. A more recent large (28,885 subjects with CD) population-based cohort study showed that patients with CD were at an increased risk of future epilepsy (HR = 1.42). The absolute risk of future epilepsy in patients with CD was 92/100,000 person-years which equates to an excess risk of 27/100,000 person-years [89]. Most studies on the subject suffer from the same methodological problem of treating epilepsy as a homogeneous disorder. The only study that attempted to look at the prevalence of GRD in well-characterised subgroups of patients with epilepsy found a significant association between AGA and temporal lobe epilepsy with hippocampal sclerosis [102]. Of interest are some case reports on patients with CD and epilepsy whose epilepsy improves following the introduction of gluten-free diet [62, 97].
There is a particular form of focal epilepsy associated with occipital calcifications that appears to have a strong link with CD [39]. This entity is common in Italy but rare in other countries. It tends to affect young patients (mean age 16 years), and in the majority, the seizures are resistant to antiepileptic drugs. The pathogenesis of the cerebral calcifications remains unclear. An autopsy study showed that these depositions consisted of both calcium and silica and microscopically were found in three main types: psammoma-like bodies without any identifiable relationship to cells, vessels or other structures; small granular deposition along small vessels; and focal scanty areas of calcium within neurons [127]. As most of the reported cases are from Italy, Spain and Argentina, it has been hypothesised that the syndrome of coeliac disease, epilepsy and cerebral calcifications is “a genetic, non-inherited, ethnically and geographically restricted syndrome associated with environmental factors” [40]. A case study of a 4-year-old boy with refractory epilepsy, occipital calcifications and coeliac disease reported positive antibody binding to neurons and glia using indirect immunofluorescence. High levels of TG6 antibodies were found in the patient’s serum. After the introduction of gluten-free diet, the child became seizure-free [74].
Myopathy
This is a relatively rare neurological manifestation of GRD, first described by Henriksson et al. [63]. This study from Sweden reported that out of 76 patients with suspected polymyositis investigated at a neuromuscular unit, 17 had a history of gastrointestinal symptoms with evidence of malabsorption. Fourteen of these fulfilled the diagnostic criteria for polymyositis and of those 5 were diagnosed with CD. A more recent study from Spain [118] demonstrated the prevalence of AGA antibodies amongst patients with inflammatory myopathies to be 31 %. This was accompanied by a higher prevalence of CD within the same population when compared to healthy controls.
A case series of 19 patients are based on what we have encountered in the gluten neurology clinic, Sheffield, UK, over the last 20 years. Thirteen of these patients have been reported previously [51]. Enteropathy was identified following duodenal biopsy in 11 of these patients. The mean age at onset of the myopathic symptoms was 54 years. Ten patients had predominantly proximal weakness, 6 patients had both proximal and distal weakness and 4 patients had primarily distal weakness. Two patients had ataxia and neuropathy, and one patient had just neuropathy in addition to the myopathy. Serum creatine kinase (CK) level ranged between normal and 4380 IU/L at presentation (normal, 25–190 IU/L). Inflammatory myopathy was the commonest finding on neuropathological examination. Six patients received immunosuppressive treatment in addition to starting a gluten-free diet, whereas the others went on a gluten-free diet only. In the majority of those patients who did not receive immunosuppressive treatment, there was clinical improvement of the myopathy with gluten-free diet, suggesting that the myopathy was aetiologically linked to the GRD. One patient developed a profound myopathy after inadvertently eating rye flour. He made a full recovery by re-establishing a strict gluten-free diet. Two patients had histological evidence of inclusion body myositis. It is interesting to note that inclusion body myositis shares the same HLA genetic predisposition with CD. One patient was known to have CD already when he developed the myopathy. He was on gluten-free diet already with negative serology for CD. Muscle biopsy showed an inflammatory myopathy, and repeat duodenal biopsy showed a flat mucosa. Further immunohistological examination of the biopsy did not suggest refractory CD. The patient admitted the occasional dietary indiscretion. He underwent further dietary review and has been started on steroids with some clinical improvement.
Myelopathy
Clinical evidence of a myelopathy in the absence of vitamin B12 and other deficiencies (particularly copper) can be a rare manifestation of CD. It is usually associated with normal imaging of the spinal cord although cases of transverse myelitis like MR appearances have been encountered in our cohort of patients. The neurological presentation often coincides with the diagnosis of CD. There have been some case reports of patients with neuromyelitis optica and sensitivity to gluten who have antibodies to aquaporin-4 [72, 73]. Such patients clearly have abnormal MRI of the spinal cord, but the diagnosis of CD was only made at the time of their neurological presentation. Neuromyelitis optica and CD share the same HLA genetic susceptibility (HLA DQ2). There is very limited data on the effect of the diet on the likelihood of relapse of the disease particularly given the fact that most patients with neuromyelitis optica end up on long-term immunosuppressive medication.
Stiff-Man Syndrome
Stiff-man syndrome (SMS) is a rare autoimmune disease characterised by axial stiffness, painful spasms and positivity for anti-GAD. It has a strong association with other autoimmune diseases (e.g. IDDM, hypothyroidism). We have found a high prevalence of gluten-related antibodies in patients with this condition over and above that expected from an association of 2 autoimmune diseases. The relapsing remitting nature of the condition makes a study of any responsiveness to gluten-free diet difficult. There is however evidence of reduction of the anti-GAD antibody titre following the introduction of a gluten-free diet suggesting that the diet may be beneficial in treating the condition [57]. This finding also supports the concept of prevention of autoimmunity in patients with GRD if the gluten-free diet is introduced early enough [129].
The concept of hyperexcitability of the central nervous system in the context of CD is of interest. We have already discussed above the entity of cortical myoclonus and refractory CD and the association with SPS. We have encountered patients with other hyperexcitable CNS disorders such as progressive encephalomyelitis with rigidity and spasms and patients with startle myoclonus who also have CD. A recent study from Italy has demonstrated that a group of 20 patients with newly diagnosed CD (no neurological complaints) had significantly shorter cortical silent period, reduced intracortical inhibition and enhanced intracortical facilitation by comparison to 20 age-matched healthy controls. The authors concluded that a pattern of cortical excitability was found in patients with CD and that immune system dysregulation may be responsible for this [108].
Pathogenesis
Post-mortem data from patients with gluten ataxia demonstrate patchy loss of Purkinje cells throughout the cerebellar cortex, a rather end-stage non-specific finding in many cerebellar disorders. However, findings supporting an immune-mediated pathogenesis include diffuse infiltration mainly of T-lymphocytes within the cerebellar white matter as well as marked perivascular cuffing with inflammatory cells [43]. The peripheral nervous system also shows sparse lymphocytic infiltrates with perivascular cuffing being observed in sural nerve biopsy of patients with gluten neuropathy [48–50], in dorsal root ganglia in patients with sensory neuronopathy [55–57] and in patients with gluten myopathy [51]. GRD patients produce an immune response to gluten involving both the innate and adaptive arm of the immune system [71, 75]. Antibodies to gliadin are part of this response, and their systemic levels appear to mirror the immune reaction triggered by gluten in the intestine including their reduction in response to a clinical improvement of the intestinal mucosa. There is cross-reactivity of these antibodies with antigenic epitopes on Purkinje cells. Purkinje cells of both human and rat origin are recognised from serum of patients with GA and patients with CD but no neurological symptoms [45]. This reactivity can also be seen using polyclonal AGA and the reactivity eliminated by absorption with crude gliadin. When using sera from patients with GA, there is evidence of additional antibodies targeting Purkinje cell epitopes since elimination of AGA alone is not sufficient to eliminate such reactivity. There is evidence that the additional antibodies that may be causing such reactivity are antibodies against one or more transglutaminase isoenzymes (TG2, TG3, TG6) [10].
TG2 belongs to a family of enzymes that covalently cross-link or modify proteins through transamidation, deamidation or esterification of a peptide-bound glutamine residue [5]. Notably, the deamidation reaction may occur in preference over the transamidation reaction, even under conditions that should favour amine incorporation, and this appears to be substrate sequence context-dependent [122]. Gluten proteins (from wheat, barley and rye), the immunological trigger of GRD, are glutamine-rich donor substrates amenable to deamidation. Deamidation of gluten peptides enhances binding with disease-relevant HLAs and thereby enhances presentation, leading to the development of gluten-specific Th1-like CD4+ T cells [71, 128]. The resulting inflammatory cytokine environment drives TG2 expression through direct transcriptional regulation [5, 101], thereby further increasing the production of the immunological trigger. Therefore, activation of TG2 and deamidation of gluten peptides appears to be central to disease development and is now well understood at a molecular level [71]. In genetically predisposed individuals, this is at the centre of a destructive chronic inflammatory reaction manifesting as aphthous stomatitis in the oral cavity or villous atrophy in the upper small intestine at sites where the gluten load through food ingestion is high.
Besides the strong gluten-specific T cell response, one of the hallmarks of GRD is a robust IgA autoantibody response to TG2 or TG2 and further TG isozymes [31, 52, 53, 116]. Assessment of serum anti-TG2 antibodies has become an important tool in CD diagnosis, and new ESPGHAN guidelines enable their use as a surrogate marker of disease [68]. However, events leading to the formation of autoantibodies against TG2 or other TG isozymes are less clear. The recent characterisation of an unusual and overwhelming immature plasma cell response in the small intestine goes some way to explain the strict association of gluten-related disorders with autoantibodies to TGs [28]. Notably, intestinal deposits of IgA antibodies targeting TG2 are present at all stages of CD, including early developing CD [78] as well as late-stage refractory CD [113] where patients may be seronegative. With regard to B cell activation and differentiation, the hapten carrier model proposed by Sollid [121], although not formally demonstrated in vivo, appears to hold true, whereby gluten-specific T cells can provide help to TG-specific B cells. This unusual scenario is enabled by the ability of TGs to form stable complexes with gliadin peptides [123] leading to uptake and ultimately presentation of MHC-gliadin complexes by B cells expressing TG-specific IgD. Recent in vitro studies confirmed that this is indeed possible [28]. Given the relative lack of somatic hypermutation of the antibody repertoire present in adult patients that should have undergone extensive affinity maturation [28, 70], questions remain as to the mechanism by which B cell maturation takes place, and this could involve an extrafollicular pathway [99]. Plasmablasts re-enter the lamina propria via the circulation and form the IgA-secreting plasma cell niche. It is important to keep in mind that B cells have roles beyond antibody production, most notably as highly effective antigen presenting cells for T cell responses. Therefore, B cells may drive clonal expansion of gluten-specific T cells which in turn may support development of B cells specific for TGs as well as deamidated gluten peptides and thereby create an amplification loop. This potentially puts B cells at the centre stage of GRD pathogenesis.
Questions also remain as to the contribution of these autoantibodies to organ-specific deficits. Anti-TG2 antibodies have been shown to be deposited in the small bowel mucosa of patients with GRD and may contribute to the formation of the lesion. Furthermore, such deposits have been found at extraintestinal sites, such as the muscle and liver [85]. Widespread deposition of IgA antibodies has also been found around brain vessels in GA [48–50]. The deposition was most pronounced in the cerebellum, pons and medulla. This finding suggests that such autoantibodies could play a role in the pathogenesis of the whole spectrum of manifestations seen in GRD and that effector functions of antibodies could contribute to tissue damage. IgM antibodies are present in GRD patients and may activate the complement cascade and promote inflammation.
Variations in the specificity of antibodies produced in individual patients could explain the wide spectrum of manifestations. Whilst TG2 has been shown to be the autoantigen in CD [31], the epidermal transglutaminase TG3 has been shown to be the autoantigen in DH [116]. Antibodies against TG6, a primarily brain-expressed transglutaminase [126], have been shown to be present in patients with GA [52, 53]. Similar to anti-TG2 and anti-deamidated gluten peptide antibodies, the production of these anti-TG3 and anti-TG6 antibodies in DH and GA patients, respectively, is gluten-dependent which substantiates the link to a gluten-specific T cell population [59, 60, 116]. In GA and DH, IgA deposits of TG6 and TG3 respectively seem to accumulate in the periphery of blood vessels at sites where in health the respective proteins are absent. Recent data on DH suggests that the deposits originate from immune complexes forming locally as a consequence of enhanced vascular leaking and that TG3 although potentially present in health may normally be rapidly cleared [134]. Furthermore, TG3 within immune complexes retains enzymatic activity and through cross-linking to fibrinogen and cell surface receptors drives innate immune cell activity [124]. Importantly, the demonstration that circulation-derived anti-TG3 antibodies can induce a dermatitis herpetiformis-like pathology in human skin-grafted SCID mice emphasises the central role antibodies play in disease establishment in different organ systems [134]. By inference, this suggests that adaptive immune cell development likely occurs in the gut and is not driven by trafficking of gut-derived T cells to other organ systems. It is likely that vasculature-centred inflammation is also at the heart of GA. Indeed perivascular cuffing with lymphocytes is a common finding in brain tissue from patients with GA but is also seen in peripheral nerve and muscle in patients with gluten neuropathy or myopathy [55–57]). However, it is unclear at present how immune complexes develop and to what extent a compromised blood-brain barrier is a prerequisite to disease development. In most sera reactive with more than one TG isoenzyme, distinct antibody populations are responsible for such reactivity rather than this being a result of cross-reactivity with different TG isozymes [52, 53]. The absence of cross-reactivity was recently substantiated in an analysis of clonal antibodies constituting the antibody repertoire in CD [70]. This makes shared epitopes less likely to be the cause for B cell development to other TGs and points to the possibility that TG isozymes other than TG2 can be the primary antigen in GRD. All 3 TG isozymes (TG2, TG3, TG6) for which autoantibodies have been described can form thioester-linked complexes with gluten peptides which are thought to be responsible for the B cell response to TG isozymes [123]. This implicates the shared activity of these enzymes rather than their sequence similarity in stimulation of antibody production and explains the exquisite specificity of the antibody response to TG family members. Whilst antibodies targeting other autoantigens have been reported, the development of such antibodies is much more sporadic amongst the GRD patient population [32].
IgA deposition in brain vessels and the pathological finding of perivascular cuffing with inflammatory cells may indicate that vasculature-centred inflammation may compromise the blood-brain barrier, allowing exposure of the CNS to pathogenic antibodies and therefore be the trigger of nervous system involvement. Indeed, TG2 is expressed by smooth muscle and endothelial cells in non-inflamed brain and is an abundant component of the choroid plexus extracellular matrix [4], and autoantibody binding could initiate an inflammatory response. However, expression of anti-TG2 antibodies in mice by themselves did not precipitate CD-like lesions in the small intestine or overt systemic manifestations akin of GRD [27], and no antibody deposition in brain vessels was reported. This may relate to the fact that it involves a specific subset of anti-TG2 antibodies that was not represented by the analysed clonal antibodies. It could also suggest that development of antibodies targeting antigens other than TG2 may be a critical step in the precipitation of specific extraintestinal manifestations as illustrated by anti-TG3 antibodies in DH [134]. It is also possible that additional factors other than the autoantibodies themselves play a role. These may either affect vascular permeability, blood-brain barrier integrity or antigen availability. With regard to the latter, TG2 and other TGs adopt a number of vastly different conformations dependent on biological context [110], and the recognition of TG2 by antibodies is conformation dependent [70, 120], or binding sites of TG2 may be masked in situ by other interaction partners as recently documented [70]. One might speculate that an unrelated infection or other insult that causes local inflammation may in the presence of circulation-derived autoantibodies bring about pathogenic immune complexes at the blood-brain barrier. This hypothesis is consistent with experimental evidence showing that antibody-mediated neuronal damage in mice harbouring pathogenic antibodies does only occur upon compromise of the blood-brain barrier [82]. Furthermore, brain areas affected in experimental animals, and therefore induced functional deficits, differed dependent on the mechanism underlying the breach of the blood-brain barrier [82]. It appears therefore that regionally specific vascular permeability leads to localised neuronal damage. Similarly, localised exposure to pathogenic antibodies may explain why patients with cerebellar ataxia or stiff person syndrome present with similar dysfunctions affecting preferentially the cerebellum or spinal cord, respectively.
It could be argued that development and deposition of antibodies is an epiphenomenon rather than being pathogenic. One method to demonstrate the pathological effect of an antibody is the passive transfer of the disease through antibody injection into a naïve animal. Whilst for only very few antibody-mediated diseases such experimental evidence exists, IgG fractions of patients with anti-GAD ataxia and stiff-man syndrome have been shown to compromise motor function and impair learning in rodents, an effect possibly ascribed to antibodies against GAD [93]. A common problem in such studies is to be able to demonstrate whether it is these specific antibodies or other autoantibodies in the IgG-fraction of patient sera that cause neuronal damage. Using a mouse model, we have recently shown that serum from GA patients and clonal monovalent anti-TG immunoglobulins derived using phage display cause ataxia when injected intraventricularly in mice [10]. The fact that not only Ig fractions but also monospecific scFv’s mediate functional deficits shows that there is no requirement for complement activation or for the engagement of Fc receptors on Fc-receptor-bearing cells in the brain. These data therefore provide evidence that anti-TG immunoglobulins (derived from patients) compromise neuronal function in selected areas of the brain once exposed to the CNS and suggest that this involves an immune system independent mode of action.
A bias of the immune response towards TG6 in GRD patients presenting with neurological deficits [52, 53, 59, 60] implicates neuronal TG6 in pathogenesis, at least of GA but possibly also other neurological problems. Further support for this notion comes from the identification of mutations in the gene encoding TG6 in families with autosomal dominant ataxia [88, 131]. This form of spinocerebellar ataxia is now referred to as SCA35. Clinical features associated with TGM6 mutations are those of late-onset cerebellar ataxia, slow progression of gait and limb ataxia, hyperreflexia and cerebellar degeneration but with no cognitive impairment, autonomic and peripheral nerve involvement or epilepsy [86, 131]. This is in keeping with the presentation in patients with immune-mediated cerebellar ataxia (GA) and provides strong evidence for an essential function of TG6 in the CNS. TG6 is, however, expressed by other cells including various epithelia [125] and one of the TGM6 mutations also associated with acute myeloid leukaemia [103]. Functional data on the physiological role of TG6 protein are sparse at present. We have begun to characterise the enzyme biochemically and analyse the gene expression pattern during development, which identified a complex system with splice variants that are differentially expressed and presumably functionally distinct [125]. Using molecular modelling and biochemical assays, we have shown that TG6 is regulated by GTP and Ca2+ similar to TG2 and adopts compact or extended conformations with transamidation activity, respectively [125]. The expression of TG6 during CNS development demonstrated an association with neurogenesis, and this was further confirmed by in vitro differentiation of neuronal precursor cells [125]. All single nucleotide exchanges reported to date lead to alteration of amino acid residues that are strictly conserved in TG6 amongst different species. Based on structural modelling and biochemical analysis [125], we hypothesise that the biological significance of TGM6 mutations lies in the impairment of regulation of transamidation activity. This implicates TG6 in an extracellular function that is critical to neuronal differentiation and survival. However, how cross-linking or modification of extracellular proteins contributes to neuronal survival remains to be identified. Autoantibody binding may sequester TG6 or block its activity and thereby act as a competitive inhibitor of enzyme function.
Conclusions
GRD include immune-mediated diseases triggered by ingestion of gluten proteins. Whilst coeliac disease has been the most comprehensively studied of all GRD, to fully understand the immunological aftermath from gluten ingestion, there is a need to further study extraintestinal manifestations. In addition, there is a need for the early identification of those patients that are specifically at risk of irreversible complications (e.g. gluten ataxia). To that effect, new diagnostic tools are now becoming available (e.g. antibodies against TG6) which may make a more reliable identification of those patients with neurological manifestations a reality. Up to 40 % of patients presenting to the gastroenterologist who are ultimately diagnosed with CD also have antibodies against TG6 in addition to antibodies against TG2. This subgroup of patients with classic CD presentation may well be the ones susceptible to the development of neurological dysfunction if they continue to consume gluten, although this remains to be shown in longitudinal studies. The presence of gastrointestinal symptoms, however, offers a major potential advantage to this group, as it substantially increases their chances of being diagnosed and treated early, whereas the diagnosis of those patients presenting purely with extraintestinal manifestations may be more difficult.
References
Abele M, Bürk K, Schöls L, et al. The aetiology of sporadic adult-onset ataxia. Brain. 2002;125:961–8.
Abele M, Schols L, Schwartz S, et al. Prevalence of antigliadin antibodies in ataxia patients. Neurology. 2003;60:1674–5.
Addolorato G, Di Giuda D, De Rossi G, et al. Regional cerebral hypoperfusion in patients with celiac disease. Am J Med. 2004;116:312–7.
Aeschlimann D, Paulsson M. Cross-linking of laminin-nidogen complexes by tissue transglutaminase. A novel mechanism for basement membrane stabilization. J Biol Chem. 1991;266(23):15308–17.
Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res. 2000;41(1):1–27.
Anheim M, Degos B, Echaniz-Laguna A, et al. Ataxia associated with gluten sensitivity, myth or reality? Rev Neurol. 2006;162:214–21.
Beversdorf D, Moses P, Reeves A, et al. A man with weight loss, ataxia, and confusion for 3 months. Lancet. 1996;347:448.
Bhatia KP, Brown P, Gregory R, et al. Progressive myoclonic ataxia associated with celiac disease. Brain. 1995;18:1087–93.
Binder H, Solitaire G, Spiro H. Neuromuscular disease in patients with steatorrhoea. Gut. 1967;8:605–11.
Boscolo S, Lorenzon A, Sblattero D, et al. Anti Transglutaminase antibodies cause ataxia in mice. PLoS One. 2010;5(3), e9698. doi:10.1371/journal.pone.0009698.
Brannagan TH, Hays AP, Chin SS, et al. Small-fiber neuropathy/neuronopathy associated with celiac disease: skin biopsy findings. Arch Neurol. 2005;62:1574–8.
Briani C, Zara G, Alaedini A, et al. Neurological complications of coeliac disease and autoimmune mechanisms: a prospective study. J Neuroimmunol. 2008;195:171–5.
Bundey S. Adult celiac disease and neuropathy. Lancet. 1967;1:851–2.
Bürk K, Bösch S, Müller CA, et al. Sporadic cerebellar ataxia associated with gluten sensitivity. Brain. 2001;124:1013–9.
Bürk K, Melms A, Schulz JB, et al. Effectiveness of intravenous immunoglobulin therapy in cerebellar ataxia associated with gluten sensitivity. Ann Neurol. 2001;50:827–8.
Bushara KO, Goebel SU, Shill H, et al. Gluten sensitivity in sporadic and hereditary ataxia. Ann Neurol. 2001;49:540–3.
Catassi C, Gatti S, Fasano A. The new epidemiology of celiac disease. J Paed Gastroenterol Nutr. 2014;59:S7–9.
Chapman RWG, Laidlow JM, Colin-Jones D, et al. Increased prevalence of epilepsy in coeliac disease. BMJ. 1978;2:250–1.
Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology. 2003;60:1581–5.
Chin RL, Tseng VG, Green PHR, et al. Multifocal axonal polyneuropathy in celiac disease. Neurology. 2006;66:1923–5.
Coers C, Telerman-Toppet N, Cremer M. Regressive vacuolar myopathy in steatorrhea. Arch Neurol. 1971;24:217–27.
Collin P, Pirttila T, Nurmikko T, et al. Celiac disease, brain atrophy and dementia. Neurology. 1991;41:372–5.
Combarros O, Infante J, Lopez-Hoyos M, et al. Celiac disease and idiopathic cerebellar ataxia. Neurology. 2000;54:2346.
Cooke WT, Thomas-Smith W. Neurological disorders associated with adult coeliac disease. Brain. 1966;89:683–722.
Cronin CC, Jackson LM, Feighery C, et al. Coeliac disease and epilepsy. QJM. 1998;91:303–8.
Deconinck N, Scaillon M, Segers V, et al. Opsoclonus-myoclonus associated with celiac disease. Pediatr Neurol. 2006;34:312–4.
Di Niro R, Sblattero D, Florian F, et al. Anti-idiotypic response in mice expressing human autoantibodies. Mol Immunol. 2008;45(6):1782–91.
Di Niro R, Mesin L, Zheng NY, et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med. 2012;18(3):441–5.
Dick DJ, Abraham D, Falkous G, et al. Cerebellar ataxia in coeliac disease- no evidence of a humoral aetiology. Postgrad Med J. 1995;71:186.
Dicke WK, Weijers HA, Van De Kamer JH. Coeliac disease II. The presence in wheat of a factor having a deleterious effect in cases of coeliac disease. Acta Paediatr. 1953;42:34–42.
Dietrich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nature Med. 1997;3:797–801.
Dieterich W, Esslinger B, Trapp D, et al. Cross linking to tissue transglutaminase and collagen favours gliadin toxicity in coeliac disease. Gut. 2006;55(4):478–84.
Elders C. Tropical sprue and pernicious anaemia, aetiology and treatment. Lancet. 1925;1:75–7.
Finelli P, McEntee W, Ambler M, et al. Adult celiac disease presenting as cerebellar syndrome. Neurology. 1980;30:245–9.
Fois A, Vascotto M, Di Bartolo RM, et al. Celiac disease and epilepsy in pediatric patients. Childs Nerv Syst. 1994;10:450–4.
Gabrielli M, Cremonini F, Fiore G, et al. Association between migraine and celiac disease: results from a preliminary case-control and therapeutic study. Am J Gastroenterol. 2003;98:625–9.
Gee S. On the coeliac affection. St Bartholomew’s Hosp Rep. 1888;24:17–20.
Gibbons CH, Freeman R. Autonomic neuropathy and celiac disease. J Neurol Neurosurg Psychiatry. 2005;76:579–81.
Gobbi G, Bouquet F, Greco L, et al. Coeliac disease, epilepsy and cerebral calcifications. Lancet. 1992;340:439–43.
Gobbi G. Coeliac disease, epilepsy and cerebral calcifications. Brain Dev. 2005;27:189–200.
Hadjivassiliou M, Gibson A, Davies-Jones GAB, et al. Is cryptic gluten sensitivity an important cause of neurological illness? Lancet. 1996;347:369–71.
Hadjivassiliou M, Chattopadhyay AK, Davies-Jones GAB, et al. Neuromuscular disorder as a presenting feature of celiac disease. J Neurol Neurosurg Psychiatry. 1997;63:770–5.
Hadjivassiliou M, Grunewald RA, Chattopadhyay AK, et al. Clinical, radiological, neurophysiological and neuropathological characteristics of gluten ataxia. Lancet. 1998;352:1582–5.
Hadjivassiliou M, Grünewald RAG, Lawden M, et al. Headache and CNS white matter abnormalities associated with gluten sensitivity. Neurology 2001;56:385–8.
Hadjivassiliou M, Boscolo S, Davies-Jones GAB, et al. The humoral response in the pathogenesis of gluten ataxia. Neurology. 2002;58:1221–6.
Hadjivassiliou M, Grünewald RA, Sharrack B, et al. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003;126:685–91.
Hadjivassiliou M, Davies-Jones GAB, Sanders DS, et al. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry. 2003;74(9):1221–4.
Hadjivassiliou M, Maki M, Sanders DS, et al. Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology. 2006;66:373–7.
Hadjivassiliou M, Grunewald RA, Kandler RH, et al. Neuropathy associated with gluten sensitivity. J Neurol Neurosurg Psychiatry. 2006;77:1262–6.
Hadjivassiliou M, Kandler RH, Chattopadhyay AK, et al. Dietary treatment of gluten neuropathy. Muscle Nerve. 2006;34:762–6.
Hadjivassiliou M, Chattopadhyay AK, Grünewald RA, et al. Myopathy associated with gluten sensitivity. Muscle Nerve. 2007;35:443–50.
Hadjivassiliou M, Aeschlimann P, Strigun A, et al. Autoantibodies in gluten ataxia recognise a novel neuronal transglutaminase. Ann Neurol. 2008;64:332–43.
Hadjivassiliou M, Sanders DS, Woodroofe N, et al. Gluten ataxia. Cerebellum. 2008;7:494–8.
Hadjivassiliou M, Aeschlimann P, Sanders DS, et al. Antibodies against TG6 as the only serological marker of gluten ataxia. Proceedings of the 13th international coeliac disease symposium. Amsterdam; 2009. April 09.1:75.
Hadjivassiliou M, Sanders DS, Grunewald RA, et al. Gluten sensitivity: from gut to brain. Lancet Neurol. 2010;9:318–30.
Hadjivassiliou M, Rao DS, Wharton SB, et al. Sensory ganglionopathy due to gluten sensitivity. Neurology. 2010;75:1003–8.
Hadjivassiliou M, Aeschlimann D, Grunewald RA, et al. GAD antibody associated neurological illness and its relationship to gluten sensitivity. Acta Neurol Scand. 2010. doi:10.1111/J.1600-0404.2010.01356.x.
Hadjivassiliou M, Sanders DS, Hoggard N. Magnetic resonance imaging and spectroscopy of the cerebellum in patients with celiac disease and minor neurological complains. Proceedings of the 14th international coeliac disease symposium 2011, Oslo, June 2011. p. 30.
Hadjivassiliou M, Hoggard N, Currie S, Aeschlimann P, Sanders DS, Aeschlimann DP. Neurological dysfunction in patients with newly diagnosed coeliac disease: a large prospective study. Proceedings of the 15th international celiac disease symposium, Chicago; 2013. p. A124.
Hadjivassiliou M, Aeschlimann P, Sanders DS, Maki M, Kaukinen K, Grunewlad RA, Bandmann O, Woodroofe N, Haddock G, Aeschlimann DP. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology. 2013;80:1–6.
Hahn JS, Sum JM, Bass D, et al. Celiac disease presenting as gait disturbance and ataxia in infancy. J Child Neurol. 1998;13:351–3.
Harper E, Moses H, Lagrange A. Occult celiac disease presenting as epilepsy and MRI changes that responded to gluten-free diet. Neurology. 2007;68:533.
Henriksson KG, Hallert C, Norrby K, et al. Polymyositis and adult celiac disease. Acta Neurol Scand. 1982;65:301–19.
Hermaszewski RA, Rigby S, Dalgleish AG. Coeliac disease presenting with cerebellar degeneration. Postgrad Med J. 1991;67:1023–4.
Holmes GKT. Neurological and psychiatric complications in coeliac disease. In: Gobbi G, Anderman F, Naccarato S, Banchini G, editors. Epilepsy and other neurological disorders in coeliac disease. London: John Libbey; 1997.
Hu WT, Murray JA, Greenway MC, et al. Cognitive impairment and celiac disease. Arch Neurol. 2006;63:1440–6.
Hunt KA. Newly identified genetic risk variants for coeliac disease related immune response. Nat Genet. 2008;40:395–402.
Husby S, Koletzko S, Korponay-Szabó IR, et al. ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136–60.
Ihara M, Makino F, Sawada H, et al. Gluten sensitivity in Japanese patients with adult-onset cerebellar ataxia. Intern Med. 2006;45:135–40.
Iversen R, Di Niro R, Stamnaes J, Lundin KE, Wilson PC, Sollid LM. Transglutaminase 2-specific autoantibodies in celiac disease target clustered, N-terminal epitopes not displayed on the surface of cells. J Immunol. 2013;190(12):5981–91.
Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol. 2009;9(12):858–70.
Jacob S, Zarei M, Kenton A, et al. Gluten sensitivity and neuromyelitis optica: two case reports. J Neurol Neurosurg Psychiatry. 2005;76:1028–30.
Jarius S, Jacob S, Waters P, et al. Neuromyelitis optica in patients with Gluten sensitivity associated with antibodies to aquaporin-4. J Neurol Neurosurg Psychiatry. 2008;79:1084.
Johnson AM, Dale RC, Wienholt L, Hadjivassiliou M, Aeschlimann D, Lawson JA. Coelaic disease, epilepsy, and cerebral calcifications: association with TG6 autoantibodies. Develop Med Child Neurol. 2012. doi:10.1111/j.469-8749.2012.04369.x.
Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209(13):2395–408.
Kaplan JG, Pack D, Horoupian D. Distal axonopathy associated with chronic gluten enteropathy: a treatable disorder. Neurology. 1988;38:642–5.
Kelkar P, Ross M, Murray J. Mononeuropathy multiplex associated with celiac disease. Muscle Nerve. 1996;19:234–6.
Kaukinen K, Peräaho M, Collin P, et al. Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scand J Gastroenterol. 2005;40(5):564–72.
Kepes JJ, Chou SM, Price LW. Progressive multifocal leukoencephalopathy with 10-year survival in a patient with nontropical sprue. Neurology. 1975;25:1006–12.
Kieslich M, Errazuriz G, Rosselt HG, et al. Brain white matter lesions in Celiac disease: a prospective study in diet treated patients. Paediatrics. 2001;108:E21.
Kinney HC, Burger PC, Hurwitz BJ, et al. Degeneration of the central Nervous system associated with celiac disease. J Neurol Sci. 1982;53:9–22.
Kowal C, Degiorgio LA, Lee JY, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci U S A. 2006;103(52):19854–9.
Kristoferitsch W, Pointer H. Progressive cerebellar syndrome in adult coeliac disease. J Neurol. 1987;234:116–8.
Korponay-Szabo IR, Laurila K, Szondy Z, et al. Missing endomysial and reticulin binding of celiac antibodies in transglutaminase 2 knockout tissues. Gut. 2003;52:199–204.
Korponay-Szabó IR, Halttunen T, Szalai Z, et al. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53:641–8.
Li M, Pang SYY, Song Y, Kung MHW, Ho SL, Sham PC. Whole exome sequencing identifies a novel mutationin the transglutaminase 6 gene for spinocerebellar ataxia in a Chinese family. Clin Genet. 2013;83:269–73.
Lu CS, Thompson PD, Quin NP, et al. Ramsay Hunt syndrome and coeliac disease: a new association. Mov Disord. 1986;1:209–19.
Ludvigsson JF, Olsson T, Ekbom A, et al. A population based study of celiac disease, neurodegenerative and neuroinflammatory diseases. Alliment Pharmacol Ther. 2007;25:1317–27.
Ludvigsson JF, Zingone F, Tomson T, Ekbom A, Ciacci C. Increased risk of epilepsy in biopsy-verified celiac disease. Neurology. 2012;78:1401–7.
Lundin KE, Sollid LM. Advances in coeliac disease. Curr Opin Gastroenterol. 2014;30(2):154–62.
Luostarinen LK, Collin PO, Paraaho MJ, et al. Coeliac disease in patients with cerebellar ataxia of unknown origin. Ann Med. 2001;33:445–9.
Luostarinen L, Himanen SL, Luostarinen M, et al. Neuromuscular and sensory disturbances in patients with well treated celiac disease. J Neurol Neurosurg Psychiatry. 2003;74:490–4.
Manto MU, Laute MA, Aguera M, et al. Effects of anti-glutamic acid decarboxylase antibodies associated with neurological diseases. Ann Neurol. 2007;61:544–51.
Marsh M. Gluten, major histocompatibility complex and the small intestine. Gastroenterology. 1992;102:330–54.
Marks J, Shuster S, Watson AJ. Small bowel changes in dermatitis herpetiformis. Lancet. 1966;ii:1280–2.
Mata S, Renzi D, Pinto F, et al. Anti-tissue transglutaminase IgA antibodies in peripheral neuropathy and motor neuronopathy. Acta Neurol Scand. 2006;114:54–8.
Mavroudi A, Karatza E, Papastavrou T, et al. Successful treatment of Epilepsy and celiac disease with a gluten-free diet. Pediatr Neurol. 2005;33:292–5.
Morris JS, Ajdukiewicz AB, Read AE. Neurological disorders and adult celiac disease. Gut. 1970;11:549–54.
Mesin L, Sollid LM, Di Niro R. The intestinal B-cell response in celiac disease. Front Immunol. 2012;3:313.
Muller AF, Donnelly MT, Smith CML, et al. Neurological complications of Coeliac disease-a rare but continuing problem. Am J Gastroenterol. 1996;91:1430–5.
Nurminskaya MV, Belkin AM. Cellular functions of tissue transglutaminase. Int Rev Cell Mol Biol. 2012;294:1–97.
Paltola M, Kaukinen K, Dastidar P, et al. Hippocampal sclerosis in refractory temporal lobe epilepsy is associated with gluten sensitivity. J Neurol Neurosurg Psychiatry. 2009;80(6):626–30. doi:10.1136/jnnp.2008.148221.
Pan LL, Huang YM, Wang M, et al. Positional cloning and next-generation sequencing identified a TGM6 mutation in a large Chinese pedigree with acute myeloid leukaemia. Eur J Human Genet. 2015;23(2):218–23. doi:10.1038/ejhg.2014.67.
Paulley JW. Observations on the aetiology of idiopathic steatorrhoea, jejunal and lymph node biopsies. Br Med J. 1954;2:1318–21.
Reed AC, Ash JE. Atypical sprue. Arch Intern Med. 1927;40:786–99.
Pellecchia MT, Scala R, Filla A, et al. Idiopathic cerebellar ataxia associated with celiac disease: lack of distinctive neurological features. J Neurol Neurosurg Psychiatry. 1999;66:32–5.
Pellecchia MT, Scala R, Perretti A, et al. Cerebellar ataxia associated with subclinical celiac disease responding to gluten-free diet. Neurology. 1999;53:1606–7.
Pennisi G, Lanza G, Giuffrida S, et al. Excitability of the motor cortex in de novo patients with celiac disease. PLoS One. 2014. doi:10.1371/journal.pone.0102790.
Pereira AC, Edwards MJ, Buttery PC, et al. Choreic syndrome and coeliac disease: a hitherto unrecognised association. Mov Disord. 2004;19(4):478–82.
Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5(12), e327.
Ranua J, Luoma K, Auvinen A, et al. Celiac disease-related antibodies in an epilepsy cohort and matched reference population. Epilepsy Behav. 2005;6:388–92.
Romanos J, Rosén A, Kumar V, et al. PreventCD Group. Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut. 2014;63(3):415–22.
Salmi TT, Collin P, Korponay-Szabó IR, et al. Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut. 2006;55(12):1746–53.
Sander HW, Magda P, Chin RL, et al. Cerebellar ataxia and celiac disease. Lancet. 2003;362:1548.
Sanders DS, Patel D, Stephenson TJ, et al. A primary care cross-sectional study of undiagnosed adult celiac disease. Eur J Gastro Hep. 2003;15:407–13.
Sárdy M, Kárpáti S, Merkl B, Paulsson M. Epidermal transglutaminase (TGase3) is the autoantigen of Dermatitis Herpetiformis. J Exp Med. 2002;195:747–57.
Sarrigiannis PG, Hoggard N, Aeschlimann D, Sandres DS, Grunewlad RA, Unwin ZC, Hadjivassiliou M. Myoclonus ataxia and refractory coeliac disease. Cerebellum Ataxias. 2014. www.cerebellumandataxias.com/content/1/1/11.
Selva-O’Callaghan A, Casellas F, De Torres I, et al. Celiac disease and antibodies associated with celiac disease in patients with inflammatory myopathy. Muscle Nerve. 2007;35:49.
Serratrice J, Disdier P, De Roux C, et al. Migraine and celiac disease. Headache. 1998;38:627–8.
Simon-Vecsei Z, Király R, Bagossi P, et al. A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects. Proc Natl Acad Sci U S A. 2012;109(2):431–6.
Sollid LM, Molberg O, McAdam S, Lundin KE. Autoantibodies in coeliac disease: tissue transglutaminase – guilt by association? Gut. 1997;41:851–2.
Stamnaes J, Fleckenstein B, Sollid LM. The propensity for deamidation and transamidation of peptides by transglutaminase 2 is dependent on substrate affinity and reaction conditions. Biochim Biophys Acta. 2008;1784(11):1804–11.
Stamnaes J, Dorum S, Fleckenstein B, Aeschlimann D, Sollid LM. Gluten T cell epitope targeting by TG3 and TG6; implications for dermatitis herpetiformis and gluten ataxia. Amino Acids. 2010;39(5):1183–91.
Taylor TB, Schmidt LA, Meyer LJ, Zone JJ. Transglutaminase 3 present in the IgA aggregates in dermatitis herpetiformis skin is enzymatically active and binds soluble fibrinogen. J Invest Dermatol. 2015;135(2):623–5.
Thomas H, Beck K, Adamczyk M, et al. Transglutaminase 6: a protein associated with central nervous system development and motor function. Amino Acids. 2013;44(1):161–77.
Tison F, Arne P, Henry P. Myoclonus and adult celiac disease. J Neurol. 1989;236:307–8.
Toti P, Balestri P, Cano M, et al. Celiac disease with cerebral calcium and silica deposits: X-ray spectroscopic findings, an autopsy study. Neurology. 1996;46:1088–92.
Tye-Din JA, Stewart JA, Dromey JA, et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med. 2010;2(41):41ra51.
Ventura A, Magazu G, Gerarduzzi T, Greco L. Coeliac disease and the risk of autoimmune disorders. Gut. 2002;51:897–8.
Volta U, Bardella MT, Calabro A, Troncone R, Corazza GR. An Italian prospective multicentre survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014;12:85.
Wang JL, Yang X, Xia K, et al. TGM6 identified as a novel causative gene of spinocerebellar ataxias using exome sequencing. Brain. 2010;133:3510–8.
Ward ME, Murphy JT, Greenberg GR. Celiac disease and spinocerebellar degeneration with normal vitamin E status. Neurology. 1985;35:1199–201.
Woltman HW, Heck FJ. Funicular degeneration of the spinal cord without pernicious anemia. Arch Intern Med. 1937;60:272–300.
Zone JJ, Schmidt LA, Taylor TB, et al. Dermatitis herpetiformis sera or goat anti-transglutaminase-3 transferred to human skin-grafted mice mimics dermatitis herpetiformis immunopathology. J Immunol. 2011;186(7):4474–80.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hadjivassiliou, M., Sanders, D.S., Aeschlimann, D. (2016). The Neuroimmunology of Gluten Intolerance. In: Constantinescu, C., Arsenescu, R., Arsenescu, V. (eds) Neuro-Immuno-Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-319-28609-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-28609-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28607-5
Online ISBN: 978-3-319-28609-9
eBook Packages: MedicineMedicine (R0)