Abstract
Multiple sclerosis (MS) and neuromyelitis optica (NMO) are chronic, potentially disabling, inflammatory autoimmune demyelinating diseases of the central nervous system. Although they share clinical, pathological and immunological features, MS and NMO are now considered two separate entities, and there is evidence that their pathogenesis is different. The latter is now known to be mediated by antibodies against the water channel, aquaporin-4, associated with complement-mediated damage. Environmental factors have been implicated in the pathogenesis of both of these conditions. Among these, infectious factors seem to play a key role. One mechanism whereby infection triggers autoimmunity is molecular mimicry resulting in immune cross-reactivity between infectious antigens and autoantigens. Recently, a number of studies have pointed to an immunological cross-reactivity between intestinal bacteria and aquaporin-4, providing a potential pathophysiological mechanism for NMO. The bacteria involved were Clostridium and E. coli. The immune cross-reactivity is not restricted to antibodies but also involves T cells against aquaporin-4 that also recognises clostridium epitopes. Interestingly, Clostridium perfringens and its immunological or direct neurotoxic effects (e.g. disruption of the blood-brain barrier) have also been implicated in MS. This chapter reviews the relevant data regarding the role of these gut bacteria and the immune responses they trigger in MS and NMO with some insights into the pathogenesis of these inflammatory demyelinating diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neuromyelitis optica
- Intestinal bacteria
- Clostridium
- E. coli
- Multiple sclerosis
- Molecular mimicry
- Cross-reactivity
- Blood-brain barrier
- Toxin
Introduction
Multiple sclerosis (MS) is an immune-mediated inflammatory demyelinating disease of the central nervous system (CNS) affecting an estimated 2.5 million people worldwide and more than 120,000 in the United Kingdom [1, 2]. MS is a major cause of long-term neurological disability in young people. The pathology of MS is characterised by inflammation, demyelination, axonal loss and gliosis in the CNS in a multifocal distribution. Clinically, it manifests as relapsing and remitting neurological deficits (relapsing remitting MS, RRMS), which often evolve subsequently into a gradual progressive deterioration (secondary progressive MS, SPMS). A minority of patients (~15 %) experience a gradual deterioration from the start (primary progressive MS, PPMS). The cause of MS is unknown, but it believed to be the result of a combination of multiple genetic susceptibility factors and environmental triggers [1].
Comprehensive large genetic studies including genome-wide association studies have identified more than 100 genes linked to MS [3]. These genes are virtually all involved in the immune response, underscoring the immune-mediated mechanisms. Although the functional contributions of these immune response genes to the aetiology of MS are only beginning to be explored [4, 5], the further development of immunotherapies for the disease, as well as the use of relevant experimental models, seems justified.
All elements of the immune system contribute to the pathogenesis of MS. Accordingly, autoreactive T cells, elements of the innate immune system and B cells are all implicated in a complex, dysregulated network. Immunoregulatory factors including regulatory T cells seem to be defective in MS.
The first attack and the subsequent relapses of MS often follow infections or global immune activation by vaccines. A number of infectious agents have been implicated as environmental risk factors for MS, and currently the one that appears most consistently associated with MS, based on immunological, epidemiological and virological evidence, is the human gamma herpesvirus, the Epstein-Barr virus [6, 7].
Neuromyelitis optica (NMO) is also a chronic inflammatory demyelinating disease of the CNS, affecting preferentially the optic nerves and the spinal cord [8]. Like MS, it can cause significant disability. In fact this is often more severe than that seen in MS. It can also have a relapsing and remitting or a chronic progressive course. NMO is much less frequent than MS and was considered for a long time a rare, more aggressive variant of MS. It was not until a decade ago that the discovery of the NMO autoantibodies [9], directed against the water channel aquaporin-4 (Aqp4) [10], and the evidence implicating these antibodies directly in NMO pathogenesis [11] provided proof of distinct immunopathogenic mechanisms in MS and NMO.
Despite the immunological complexity of MS, the prevailing concept is that T lymphocyte plays a central and decisive role [12]. Although therapies targeting B cells have shown promise in MS, the most important role of B cells in MS appears to be their function as antigen-presenting cells. In contrast, NMO is mediated primarily by antibodies which can fix complement, and this is the main pathogenesis mechanism [8]. Indeed, NMO is frequently associated with other antibody-mediated autoimmune diseases, including systemic lupus erythematosus (SLE) or myasthenia gravis (MG). Aqp4-reactive T cells have also been demonstrated in NMO, but their exact pathogenic role is currently unclear. Therapies aiming at reducing circulating anti-Aqp4 antibodies such as plasma exchange, intravenous immunoglobulins and in particular the B cell-depleting anti-CD20 antibody rituximab have been shown to have a positive effect on NMO.
Infections as Triggers of MS and NMO
Despite the distinct pathophysiological mechanisms, infections have been implicated as triggers for both MS and NMO. In more general terms, autoimmune diseases are often thought to be triggered by infections. The mechanisms can be multiple, and they have all been implicated in MS and its experimental model, experimental autoimmune encephalomyelitis (EAE). Superantigens are infectious agent-derived substances that can activate a larger number of T (or sometimes B) cells bearing the same receptor. Superantigens have been linked to MS and EAE, in particular in the generation of relapses, which may be cytokine mediated [11, 13]. Bystander activation and exposure of otherwise sequestered autoantigens due to infection-induced tissue damage and subsequent epitope spreading is another possible mechanism [14]. Also, some infections act through disruption of immune regulatory mechanisms. Activation of innate immune receptors by pathogen-associated molecular patterns (PMP), for example, activation of TLR2 by its ligands that mimic bacterial infections, transforms regulatory T cells that are normally anti-inflammatory into cells that actually mediate inflammation by producing interleukin-17 (IL-17) [15]. The process may be mediated by infection-induced IL-6 and is more prominent in MS than in a healthy volunteer [16].
Molecular Mimicry: E. coli and Clostridia in NMO as Examples
Finally, the concept of immunological cross-reactivity, closely linked to that of molecular mimicry, explains how some infections trigger autoimmunity. Molecular similarity between components of infectious agents and self-antigens can lead to a strong cross-reactive immune response (humoral or cellular) and autoimmune disease. The clearest and best-established example is the molecular similarity between Campylobacter lipopolysaccharide and gangliosides present on nerve roots; antibodies against Campylobacter triggered by gastrointestinal infections with this agent cross-react with gangliosides and lead to the peripheral nerve-directed autoimmunity seen in some forms of acute inflammatory demyelinating polyradiculoneuropathy, or Guillain-Barre syndrome. This topic is discussed in detail in the chapter on Guillain-Barre syndrome and Campylobacter jejuni enteritis.
Molecular mimicry has been explored extensively in MS. Of note is that when eluting peptides from the MS-associated major histocompatibility complex (MHC) class II MBP-presenting groove (DRB1*1501), Wuchepfenning and colleagues found that some of these peptides were derived from EBV, the infectious agent most consistently associated with MS. Some of these peptides were able to stimulate myelin basic protein-specific T cells from MS patients [17].
Molecular Mimicry in NMO
Molecular mimicry and immunological cross-reactivity have been strongly implicated in NMO.
An early study exploring this phenomenon was performed by Ren and colleagues in 2012 [18]. Since water channels (aquaporins) are expressed in most species, they searched for sequence homologies between Aqp4 and E. coli aquaporin-Z (AqpZ). Some regions of relevance to the immune response to Aqp4 in NMO, possibly representing B cell or T cell epitopes (the latter from experimental models), had high homology. The authors raised sera against AqpZ in mice, which showed high reactivity against both AqpZ and Aqp4 in multiple assays. Sera from patients with NMO showed strong reactivity against AqpZ protein. Moreover, anti-AqpZ antibodies were demonstrated to have cytotoxic activity against astrocytes in culture. Also, intracerebral injection of antibodies against the peptide AqpZ 174–190 induced inflammation in the CNS of injected mice, associated with behavioural and clinical abnormalities (withdrawal, reduced movement). Finally, active immunisation with AqpZ in complete Freund’s adjuvant (CFA) induced CNS inflammation in mice. This inflammation was comparable in composition (mainly CD3+ T cells) and location with that induced by immunisation with Aqp4. The immunisation generated AqpZ-reactive T cells that presented a Th17 phenotype, consistent with predominant involvement of Th17 cells in NMO and the more severe forms of MS [18].
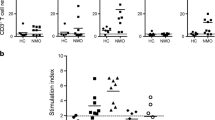
Another study analysed the T cell responses to Aqp4 in NMO [19]. The rationale was that antibodies against Aqp4 in NMO patients are of the IgG1 subclass, which requires T cell help, and there is evidence for IL-17 upregulation, implying Th17 mediation, in NMO [20]. The authors found consistent strong immunoreactivity against peptide 61–80 of Aqp4. This was inhibited by blocking antibodies against HLA-DR, suggesting MHC class II dependence of these T cell responses. They found that Aqp4 p61-80 reactive T cells of NMO patients exhibited a strong Th17 phenotype. Moreover, monocytes from NMO patients also showed pro-inflammatory polarisation. When searching for peptides with proteins with sequence homology with Aq4 p61-80, the researchers found a peptide of the Clostridium perfringens ABC transporter. C. perfringens is a ubiquitous spore-forming gram-positive bacterium found in the human gut, responsible for many cases of food poisoning [21]. The homology with Aqp4 extends to the ABC transporter of other Clostridium species [19]. Interestingly, in addition to its role in inducing potential autoimmunity via cross-reactivity, Clostridium species and other closely related have been associated with skewing the cytokine response towards a Th17 type and thus facilitating inflammation and autoimmunity [22].
It is thus plausible that Aqp4 molecular mimicry in NMO stimulates the Th17 responses known to be prominent in NMO.
Aqp4 molecular mimicry was explored in another study [23]. In this study, structural neighbour searches were performed for primary, secondary and tertiary structure similarities to Aqp4. Similarities were confirmed with AqpZ of E. coli, but very high similarity was observed with the corn protein ZmTIP4-1. The study went on to demonstrate that NMO patient sera contained antibodies to ZmTIP4-1, which were cross-reactive with antibodies against Aqp4 [23]. Not only does this study strengthen the evidence for an environmental trigger in NMO but also supports the concept of molecular mimicry and immunological cross-reactivity as a mechanism of pathogenesis in NMO.
Other Roles of Clostridium in Autoimmune Demyelination
An intriguing study by Rumah and colleagues points to another role of C. perfringens in MS, mediated through its associated epsilon toxin (ETX), a toxin produced by the type B and type D C. perfringens but not by the commensal type A [24].
The authors isolated C. perfringens type B from the stool of a young woman with recent onset of clinically isolated syndrome (CIS), the first manifestation of MS. At that time she had actively gadolinium-enhancing lesions on the magnetic resonance imaging (MRI) brain scan, indicating an acute disruption of the blood-brain barrier (BBB). Since, in experimental models, ETX disrupts the BBB and produces inflammatory lesions in myelinated brain areas such as the corpus callosum [25, 26], and given its affinity for endothelial cells and myelinated areas of the brain [27], the authors postulated that ETX or perhaps a similar gut microbiota-derived toxin may contribute to some of nascent lesions in MS. This is in line with the observation of Prineas and colleagues that BBB disruption, demyelination and oligodendrocyte apoptosis may precede inflammatory T cell infiltration in nascent lesions [28].
The researchers went on and analysed stool samples from 30 people with MS and 31 healthy controls involved in a prospective study to look at their gut microbiota. They found a lower prevalence of the human commensal C. perfringens type A in MS patients compared to controls (23 % versus 52 %) [24]. This is interesting, because the presence of type A is generally associated with the absence of type B and D, the types that secrete ETX, probably due to competition.
The authors found that 10 % of the patients with MS displayed immunoreactivity to ETX based on a Western blot analysis, whereas only one of the control subjects had positive reactivity in the cerebrospinal fluid (CSF) and none of the controls had reactivity in the serum. There was typically a good correlation between the positivity of the reaction between the CSF and blood [24]. The seropositivity is postulated to represent an underestimation of exposure to the bacterial toxin, given the high rate of seroreversion and of seronegativity following vaccinations of goats, suggesting that some samples may have been negative despite prior exposure to C. perfringens.
The study points to the possibility that C. perfringens contributes directly to the pathogenesis of MS through the action of ETX on the blood-brain barrier and myelin. The findings would support the epidemiological observations of Kurtzke and Hyllested (reviewed in [29]) who showed that the first MS epidemic in the Faroe Islands was associated with the arrival of the British troops during World War II. The pioneer MS epidemiologists also noted an increased incidence of gastrointestinal infections and argued for a faecal-oral transmission of the MS environmental (infectious) agent.
Murrell [30] then introduced the intriguing hypothesis of a connection with sheep, and one postulated sheep-associated pathogen was C. perfringens and its epsilon toxin ETX. This was further substantiated by the neurotoxic and endothelial cell-toxic effects demonstrated for ETX as discussed above.
Further studies will elucidate further the role of clostridia in MS. The increased interest in the gut microbiome in MS, EAE and other inflammatory diseases will facilitate further discoveries. Of interest is the observation from experimental studies in mice that the segmented filamentous bacteria or other similar bacteria that contribute to the pathogenicity and pro-inflammatory, predominantly Th17, profile in experimental autoimmune disease are thought to be spore forming and related to clostridia [22].
Clostridium difficile
C. difficile is a major cause of health concern due to its association with antibiotic-associated colitis, a major cause of morbidity and mortality worldwide. It is transmitted by faecal-oral route and is established in the human colon in 2–5 % of the population. It is unclear whether patients with MS and other inflammatory demyelinating diseases are colonised in a higher proportion compared to the general population, but since colonisation is associated with lengthy hospitalisation or nursing home residence, it is conceivable that MS patients, especially at more advanced stages, are more likely to be colonised. The implications of this infection are very important. This group of patients is more likely to receive antibiotics and to have bowel dysfunction (as discussed in the chapter on the impact of MS on the gastrointestinal function in this book).
Faecal transplantation is advocated and employed increasingly for severe, refractory C. difficile colitis, and its spectrum of application has expanded beyond the primary indication of colitis [31]. Anecdotal reports note the success of the intervention in people with MS.
Clostridium difficile infection can complicate autologous stem cell transplantation in MS, as discussed below.
The Gut Microbiome in MS and Its Relationship with MS Treatment
The gut microbiome and its role in MS have been receiving increasing attention in the recent years, since the observations that manipulations of the gut flora can modulate experimental MS-like inflammatory demyelination [32]. The gut microbiome and the effect of its modulation on clinical and experimental MS are discussed in separate dedicated chapters in this book.
Here, we only discuss the potential effects of MS therapies on the gut flora and the implications for MS.
The effect of the most commonly used MS drugs on the gut flora or MS patients is largely unknown and studies are in their infancy. Cantarel and colleagues have published pilot data comparing the gut microbiome of MS patients and healthy volunteers and also studied the effect of treatment with glatiramer acetate or vitamin D [33]. They noted considerable overlap between operational taxonomic units between MS patients and controls, but Faecalibacterium was less represented in MS patients. Specific changes were observed after treatment with glatiramer acetate and vitamin D [33]. Although this study was very exploratory and future studies in larger populations are needed to confirm the findings, the results indicate that the gut flora in MS is subject to modulation by drugs used in the treatment of MS.
Important recent experimental studies in cancer have pointed out the role of the gut flora in determining the response to cancer chemotherapeutic agents, notably cyclophosphamide [34]. The antitumour immune response and the efficacy of cyclophosphamide are influenced by the gut flora, and antibiotics compromise the antitumour effects of chemotherapy [34, 35]. Since cyclophosphamide is a drug that has been used in the treatment of MS [36] and also is used in bone marrow ablation prior to haematopoietic stem cell transplantation (HSCT), the evidence that changes in the gut microbiota induced, for example, by antibiotics can influence its effect needs to be considered further in MS. In addition, whether other immunosuppressive treatments used in MS are subject to the same influences remains to be determined.
In the recent months, autologous HSCT, particularly the non-myeloablative type [37], has been reported to be a promising treatment for aggressive forms of MS. Interestingly, a population of immune cells that is significantly depleted by the treatment are the IL-17-producing, pro-inflammatory mucosal-associated invariant T cells (MAIT) [38]. Targeting these cells, with potential beneficial effects in EAE, can also be achieved through manipulations of the gut flora.
It is important to note, however, that the rate of C. difficile infections seems to be higher in MS patients than in patients receiving the transplantation for haematological malignancies. This may reflect the longer hospitalisation in part due to more challenging mobilisation following the procedure.
Awareness of the possibility of C. difficile infections which may complicate HSCT is therefore crucially important [39].
Summary and Conclusions
This chapter has reviewed the evidence of the role of infections, with particular attention to the infections with gut pathogens, in NMO and MS. The evidence of molecular mimicry between the known autoantigen for NMO, Aqp4 and proteins from gut bacteria such as E. coli AqpZ and Clostridium ABC transporters, as well as some plant aquaporins, has been discussed. The functional consequence of this mimicry may be immunological cross-reactivity triggering autoimmune damage. The evidence for molecular mimicry is less robust in MS, at least in part due to the fact that the autoantigen is not as clearly established as for NMO, and the possibility that there are several autoantigens.
Direct effects of substances produced by gut bacteria such as clostridia may also be involved in the pathogenesis of MS. The epsilon toxin of C. perfringens is a candidate for a contribution to the pathology of some of the nascent lesions, supported by studies showing its neurotoxic effects and by epidemiology data implicating in triggering MS.
This chapter does not deal with the gut flora in MS or NMO, as this is addressed in other chapters. The manipulation of the gut microbiota can have therapeutic effects in MS. This chapter discusses briefly the opposite regulation: the potential effects of MS treatment on the gut microbiota. The role of gut flora in the effects of cyclophosphamide as shown in experimental cancer studies suggests that the issue needs to be considered when using this drug as MS treatment. A potential promising treatment, HSCT, which in part works through depletion of MAIT, may carry the risk of C. difficile infection, which needs to be taken into account when pondering such powerful treatment.
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17.
Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O'Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry. 2014;85(1):76–84. Pubmed Central PMCID: 3888639.
International Multiple Sclerosis Genetics C, Wellcome Trust Case Control C, Sawcer S, Hellenthal G, Pirinen M, Spencer CC, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–9. Pubmed Central PMCID: 3182531.
Hartmann FJ, Khademi M, Aram J, Ammann S, Kockum I, Constantinescu C, et al. Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells. Nat Commun. 2014;5:5056.
van Luijn MM, Kreft KL, Jongsma ML, Mes SW, Wierenga-Wolf AF, van Meurs M, et al. Multiple sclerosis-associated CLEC16A controls HLA class II expression via late endosome biogenesis. Brain. 2015;138(Pt 6):1531–47.
Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. 2007;61(6):504–13.
Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61(4):288–99.
Wingerchuk DM, Weinshenker BG. Neuromyelitis optica. Curr Treat Options Neurol. 2008;10(1):55–66.
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–12.
Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–7. Pubmed Central PMCID: 2212860.
Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69(24):2221–31.
Constantinescu CS, Gran B. The essential role of T cells in multiple sclerosis: a reappraisal. Biomed J. 2014;37(2):34–40.
Constantinescu CS, Wysocka M, Hilliard B, Ventura ES, Lavi E, Trinchieri G, et al. Antibodies against IL-12 prevent superantigen-induced and spontaneous relapses of experimental autoimmune encephalomyelitis. J Immunol. 1998;161(9):5097–104.
Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358(6382):155–7.
Nyirenda MH, Sanvito L, Darlington PJ, O'Brien K, Zhang GX, Constantinescu CS, et al. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187(5):2278–90.
Nyirenda M, Morandi E, Vinkemeier U, Constantin-Teodosiu D, Drinkwater S, King L, et al. Toll-like receptor 2 stimulation regulates the balance between Treg and Th17 function: a novel mechanism of reduced Treg function in multiple sclerosis. J Immunol. 2015;194(12):5761–74.
Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80(5):695–705.
Ren Z, Wang Y, Duan T, Patel J, Liggett T, Loda E, et al. Cross-immunoreactivity between bacterial aquaporin-Z and human aquaporin-4: potential relevance to neuromyelitis optica. J Immunol. 2012;189(9):4602–11. Pubmed Central PMCID: 3586280.
Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, Nelson PA, Stroud RM, Cree BA, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol. 2012;72(1):53–64. Pubmed Central PMCID: 3405197.
Uzawa A, Mori M, Arai K, Sato Y, Hayakawa S, Masuda S, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16(12):1443–52.
Lindstrom M, Heikinheimo A, Lahti P, Korkeala H. Novel insights into the epidemiology of Clostridium perfringens type A food poisoning. Food Microbiol. 2011;28(2):192–8.
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. Pubmed Central PMCID: 2796826.
Vaishnav RA, Liu R, Chapman J, Roberts AM, Ye H, Rebolledo-Mendez JD, et al. Aquaporin 4 molecular mimicry and implications for neuromyelitis optica. J Neuroimmunol. 2013;260(1–2):92–8. Pubmed Central PMCID: 3682654.
Rumah KR, Linden J, Fischetti VA, Vartanian T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS One. 2013;8(10):e76359. Pubmed Central PMCID: 3797790.
Finnie JW. Ultrastructural changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J Comp Pathol. 1984;94(3):445–52.
Finnie JW. Histopathological changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J Comp Pathol. 1984;94(3):363–70.
Zhu C, Ghabriel MN, Blumbergs PC, Reilly PL, Manavis J, Youssef J, et al. Clostridium perfringens prototoxin-induced alteration of endothelial barrier antigen (EBA) immunoreactivity at the blood–brain barrier (BBB). Exp Neurol. 2001;169(1):72–82.
Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55(4):458–68.
Kurtzke JF. Epidemiology in multiple sclerosis: a pilgrim's progress. Brain J Neurol. 2013;136(Pt 9):2904–17.
Murrell TG, O'Donoghue PJ, Ellis T. A review of the sheep-multiple sclerosis connection. Med Hypotheses. 1986;19(1):27–39.
Xu MQ, Cao HL, Wang WQ, Wang S, Cao XC, Yan F, et al. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J Gastroenterol WJG. 2015;21(1):102–11. Pubmed Central PMCID: 4284325.
Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173(6):1714–23. Pubmed Central PMCID: 2626383.
Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Invest Med 63(5):729–34.
Viaud S, Daillere R, Boneca IG, Lepage P, Langella P, Chamaillard M, et al. Gut microbiome and anticancer immune response: really hot Sh*t! Cell Death Differ. 2015;22(2):199–214. Pubmed Central PMCID: 4291500.
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70.
Awad A, Stuve O. Cyclophosphamide in multiple sclerosis: scientific rationale, history and novel treatment paradigms. Ther Adv Neurol Disord. 2009;2(6):50–61. Pubmed Central PMCID: 3002608.
Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. Jama. 2015;313(3):275–84.
Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain J Neurol. 2013;136(Pt 9):2888–903. Pubmed Central PMCID: 3754461.
Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8(3):244–53.
Acknowledgement
Work in Prof. Constantinescu’s laboratory relevant to this chapter has been supported by the MS Society of Great Britain and Northern Ireland, an unrestricted educational grant from Biogen and the Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Constantinescu, C.S., Chou, IJ. (2016). Intestinal Bacterial Antigens, Toxin-Induced Pathogenesis and Immune Cross-Reactivity in Neuromyelitis Optica and Multiple Sclerosis. In: Constantinescu, C., Arsenescu, R., Arsenescu, V. (eds) Neuro-Immuno-Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-319-28609-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-28609-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28607-5
Online ISBN: 978-3-319-28609-9
eBook Packages: MedicineMedicine (R0)