Abstract
Three different standard types of liver bipartition producing six different types of grafts can be created by following a plane directed on the right or the left line of the middle hepatic vein (MHV): a) splitting for adult and pediatric recipients with left lateral graft (LLG) and right extended graft (REG), b) splitting for two adults or for adult and pediatric recipients of large size with creation of left graft (LG) and right graft (RG), c) splitting for two adult recipients with creation of full left graft (FLG) and full right graft (FRG). The absence of an extrahepatic portal vein bifurcation is an absolute contraindication to liver splitting. Division of the portal branches to Segment I optimizes the freeing/lengthening of the left portal vein for the implantation. Identifying the portal tract entering the caudate process at its lower aspect is helpful in preparing for the division of the hilar plate. Early division of the Arantius remnant allows a safe encircling and control of the left hepatic vein. During in situ splitting technique for adult and pediatric recipients, a 1–2-min. selective clamping of the left hepatic vein (LHV) may provide assurance that the hepatic venous drainage of Segment IV is not jeopardized. Recognition of independent segment II and III suprahepatic venous outflow (<5 % of cases) is crucial in the adult and pediatric splitting procedure. Segment IV hypoperfusion is a potential pitfall during adult and pediatric liver splitting. During adult and pediatric split-liver procedure, parenchyma transection can be achieved according to the transhilar (TH) approach or transumbilical (TU) approach. In the liver-splitting technique for two adults, the “hanging manoeuvre” can be helpful to define the correct plane of transection from the bifurcation of the hepatic artery and portal vein to a point between the right and middle hepatic veins. In some case of MHV dominancy during split-liver procurement for two adults, the ex situ splitting of the vena cava and/or MHV can be considered possible options to avoid complex reimplantation of multiple tributaries of the MHV and the congestion of segments IV, V, and VIII.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Tips, Tricks, and Pitfalls-
Three different standard types of liver bipartition producing six different types of grafts can be created with different hepatic segments (S):
-
Splitting for adult and pediatric recipients with left lateral graft, S II-III (LLG) and right extended graft, S I, IV–VIII (REG)
-
Splitting for two adult recipients or for adult and pediatric recipient of large size recipients with creation of right graft, S I, V–VIII (RG) and left graft, S II–IV (LG)
-
Splitting for two adult recipients with creation of full left graft, S I–IV (FLG) and full right graft, S V–VIII (FRG)
-
-
The absence of an extrahepatic portal vein bifurcation is an absolute contraindication to liver splitting.
-
Division of the portal branches to S I optimizes the freeing/lengthening of the left portal vein for the implantation
-
Identifying the portal tract entering the caudate process at its lower aspect is helpful in preparing for the division of the hilar plate.
-
Early dissection of the Arantius remnant allows a safe encircling and control of the left hepatic vein (LHV)
-
In the adult and pediatric in situ splitting technique, a 1–2-min selective clamping of the LHV may provide assurance that the hepatic venous drainage of S IV is not jeopardized.
-
Recognition of independent S II and III suprahepatic venous outflow (<5 % of cases) is crucial in the adult and pediatric splitting procedure.
-
Segment IV hypoperfusion is a potential pitfall during liver splitting for adult and pediatric recipients.
-
During adult and pediatric split-liver procedure, parenchyma transection can be achieved according to the transhilar approach or the transumbilical approach.
-
In the liver-splitting technique for two adult recipients, the “hanging maneuver” can be helpful in defining the correct plane of transection.
-
In the rare cases of remarkable MHV dominancy during split-liver procurement, for two adult recipients, the ex situ splitting of the vena cava and/or MHV can be considered possible options to avoid the complex reimplantation of all tributaries of the MHV and the congestion of S IV, V, and VIII.
1 Introduction
The shortage of liver grafts available for transplantation from deceased donors (DD) prompted several transplant centers during the early 1990s to seek alternatives to conventional liver transplantation such as split-liver transplantation and partial grafts from living donors.
Waiting list mortality is approximately 15–20 % in Europe and 14 % in the United States [1, 2], and approximately 2,500 patients die every year in the United States for lack of a suitable liver donor. Split-liver transplantation (SLT), a procedure in which one donor liver is divided into two hemilivers, is an important method to overcome organ shortage. To date, the principal beneficiaries of this procedure have been adult/pediatric pairs, and excellent outcomes have been reported by the majority of pediatric transplant centers; where the waiting list mortality has been approximately 0 % in the last few years.
2 Historical Background
The transplantation of partial-liver allografts in children was initially advocated by Smith, who in 1969 proposed that the left lateral graft was suitable for children [2]. This technical option was revisited in the 1980s when the increasing demand for pediatric liver transplantation resulted in an insufficient pool of deceased donors (DD) and an increased waiting list mortality.
Initial attempts to reduce pediatric waiting list mortality targeted the surgical reduction of an adult DD liver to fit the abdominal cavity of a child, a procedure called “reduced-liver transplantation” (RLT). Split-liver transplantation (SLT) began in the 1980s as a response to the disparity between adult and pediatric recipients; the waiting list mortality in the pediatric population exceeded 25 % at major transplant referral centers.
The first successful RLT was simultaneously reported by Bismuth [3] and Broelsh [4] in 1984. Later studies demonstrated that the use of RLT grafts in children achieved success rates equal to or better than those of whole cadaver organs. However, the simple surgical reduction of a liver graft from an adult deceased donor (DD) failed to expand the donor pool and simultaneously increased competition problems between adult and pediatric transplant candidates. After substantial ethical debate [5–8], the surgical reduction techniques were also extended to adults of small size.
In 1989, Pichlmayr et al. and Bismuth et al. [9, 10] almost simultaneously reported the successful ex situ division of a deceased donor liver into a left lateral graft (LLG), segments (S) II and III, for transplantation into a pediatric recipient, and a right extended graft (REG), S I, IV–VIII for transplantation into an adult. As a prerequisite of this new SLT surgical procedure, a dedicated “benching” period was required for further dissection, which substantially increased the organ cold ischemia time. Goss and colleagues at University of California, Los Angeles (UCLA) proposed in situ separation as a procedure to reduce cold ischemia time and enhance the identification of biliary and vascular structures. During this procedure, the liver was divided within the adult DD during procurement without any special equipment. The in situ technique was initially performed at UCLA in 1992. Important clinical reports in 1997 by Goss [11], Azoulay [12], and Rogiers [13] showed the significant advantages of this technique in avoiding the period of bench surgery necessary to perform the ex situ splitting procedure with the related complications of the prolonged cold ischemia time of the graft. Performed on selected DDs, the in situ liver-splitting procedure offered the benefits of pediatric living donor liver transplantation (LDLT) without the donor risk, and this has now become the first choice for the transplantation of infants and small children in the majority of centers.
In 1999, Colledan et al. reported the first successful in situ split liver procedure from a deceased donor for two adults, obtaining a full right graft (FRG) and a full left graft (FLG).
3 Anatomic Principles
Any technical description of split, reduced, or living donor liver transplantation must begin with the acknowledgment of the anatomic classification of the liver described by Couinaud [13] and refined by Bismuth [14] (Fig. 13.1), which has been universally accepted by the transplant communities of Europe, Asia, and North America as the reference for describing different portions of partial-organ allografts. The liver is divided into eight functional units, termed “segments” which receive separate hilar pedicles. Each pedicle contains a portal venous branch, hepatic arterial branch, and a bile duct pedicle with unique drainage through individual venous branches. Hepatic parenchyma transection corresponds so called to “scissurae,” connective tissue planes that separate each individual liver “segment”.
The segmental anatomy of the liver as described by Couinaud and Bismuth. Each anatomic segment receives a unique portal pedicle consisting of a portal venous branch, hepatic arterial inflow, and bile duct. Each segment is drained by unique hepatic venous outflow branches and separated by connective tissue scissurae
Couinaud’s classification permits the creation of functionally distinct partial-organ allografts (Fig. 13.2). The division of the hepatic parenchyma at the falciform ligament yields an S II/III left lateral graft (LLG), which is 25 % of the total liver volume and approximately 250–350 cc, for pediatric recipients, and a remnant right extended graft (REG) (Couinaud S I and IV–VIII) of approximately 900–1100 cc, for transplantation into adults. The LLG can be further reduced to a “monosegment graft” (S III) for very small infants and neonates.
Surgical division of the liver along the middle hepatic vein (yellow line labeled “A”) yields a full left graft (FLG) S I–IV and full right graft (FRG) S V–VIII that can be utilized in SLT for two adults. Division along the falciform ligament (green line labeled “B”) yields the pediatric left lateral graft (LLG) S II–III and the remnant, adult right extended graft (REG) S I, IV–VIII
In order to transplant two recepients from one adult DD, the liver can be divided along a sagittal plane directed to the right or to the left side of the middle hepatic vein. The parenchyma transection along the chosen specific plane is able to create three types of liver bipartition with different percentages of parenchyma volume for the two hemi-grafts (Fig. 13.2). Depending on the recipient’s body weight, there are three standard techniques to split the liver and six different potential grafts:
-
(a)
Split liver for adult and pediatric recipients with a LLG (S II, III) of approximately 25 % of total volume and a REG (S I, IV–VIII) of approximately 75 % of total volume. (Fig. 13.3a).
Fig. 13.3 Schematic representation of split liver for adult and pediatric recipients (a) and split liver for two adults (b). For simplification and to expose details of the supra-hepatic veins the vena cava has not been drawn. In our experience in NITp area, the common bile duct (CBD) is usually retained with the right extended graft and with the full right graft. The celiac trunk (CT) is usually retained with the left lateral graft and with the full left graft whereas the main portal trunk is retained with the right extended graft (a) and with full left graft (b)
-
(b)
Split liver for two adult or for an adult and pediatric recipients of large size of about 25–35 kg) with the creation of a left graft (LG) including S II, III, and IV, with approximately 35 % of total volume, and a right graft (RG) (S I, V–VIII) with approximately 65 % of total volume.
-
(c)
Splitting for two adult recipients with the creation of a full left graft (FLG) including S I–IV with approximately 40 % of total volume and a full right graft (FRG) including S V–VIII with approximately 60 % of total volume (Fig. 13.3b).
Liver graft volume can be roughly estimated preoperatively. Its volume is about a 1.8 % and a 2.2 %, for female and male respectively (Table 13.1), of the total body weight of the donor. A more accurate formula taking into account the body surface and gender can give more accurate values of estimated liver volume [15]. Because a remarkably close correlation exists between the liver weight and the volume of water at 25 ° C, liver volume can be converted to liver weight on a one-to-one basis.
Partial-liver graft recipients with a graft weight to recipient weight ratio (GW/RW) less than 0.8–1 % are reported to have a higher incidence of postoperative complications [16], including small-for-size syndrome (SFSS) especially in patients with portal hypertension.
The decision whether to split a DD liver and whether to perform the procedure in situ or ex situ depend on a number of variables that have different degrees of impact on decision-making [5, 17–22]. These variables include formal donor-related data, the given anatomical situation, the macroscopic appearance of the liver, the weight and clinical status of the potential recipients; moreover we have to take into account the availability of an experienced surgeon and logistics. An ideal liver to be split (Table 13.2) would be from a young donor with no history of liver disease, normal liver values (particularly the γ-glutamyl-transpeptidase), and short intensive care stay. The optimal donor should be hemodynamically stable before and during the donor operation. The ideal liver should have a macroscopically soft consistency, with sharp edges, and preferably with a large left lateral lobe (unless the recipient of this part is a small child); a separate right or left replaced hepatic artery can be advantageous. Whenever possible and in consideration of optimal logistics, the donor should be submitted to multiorgan procurement in the transplant center itself, thus providing the best conditions for in situ splitting. However, this condition is rarely achievable
4 Split-Liver Transplantation: General Aspects
Split-liver transplantation for adult and pediatric recipients has become a standard procedure with results equivalent to those with whole liver transplantation (Figs. 13.3 and 13.4).
Schematic drawing of split liver for adult and pediatric recipients. The parenchyma transection line is immediately lateral to the falciform ligament, yielding a left lateral graft (LLG, S II/III) for pediatric recipients of approximately 250–300 cc (25 %) and a right extended graft (REG S I, IV–VIII) of approximately 900–950 cc (75 %). Usually in NITp area the celiac trunk (CT) is retained with LLG (S II–III); the main portal trunk (MPT) and common bile duct (CBD) are retained with REG (S I, IV–VIII)
Two different techniques can be distinguished: split liver ex situ, i.e., performed on the back-table in the ice bath after the perfusion and harvesting of the whole liver; in situ split liver, i.e., preformed inside the heart-beating donor prior to the perfusion and harvesting of the liver.
All types of splitting procedures can principally be performed ex situ as well as in situ. Because contrast-enhancement scans are rarely available for the majority of DDs, the anatomy of the biliary and hepatic vein system remains unknown until the liver is surgically divided. The liver’s extrahepatic vascular anatomy should be determined during the procedure by recognizing the different types of the vascular anatomical pattern. Identifying an extrahepatic left portal vein or a variant, the presence of multiple or a standard single hepatic arterial supply and their branching modality into the right and left arterial supply are the most important early surgical steps. Surgeons are sometimes required to modify the transection line according to their intraoperative observations and their personal knowledge of the liver’s anatomy and its variations [21, 23].
4.1 Ex Situ Split-Liver Technique for Adult and Pediatric Recipients
In the ex situ split-liver technique, the whole organ is retrieved according to the standard techniques of multiple organ procurement and whole liver procurement. The whole liver is preserved with the preferred perfusion solution. Grafts are usually prepared at the recipient transplant center and placed in an ice bath of perfusion solution. Predissection, cholangiography and arteriography can be performed at the back table during the ex situ technique in order to delineate the anatomy more precisely, but these procedures are time consuming. Otherwise, a thin metal cannula may be used to gently probe the hepatic artery and the bile duct to facilitate the detection of aberrant anatomy. As a general rule a successful liver division should share vascular and biliary structures between the two sides but without handicapping either and, when possible, provide either graft with single first order arterial and biliary elements. Dissection of the portal triad is performed to separate the branches of the hepatic artery, portal vein, and right and left hepatic ducts. It is matter of debate which half of the liver should retain the entire hepatic/celiac trunk and the main trunk of the portal vein. In the majority of cases, the common bile duct is retained with the right graft. The rationale for determining which graft should receive the major vascular pedicle is explained by the anatomy of the components of the porta hepatis [24]. In the majority of cases, the left portal vein, the right hepatic artery, and the left bile duct should be sectioned because they are anatomically longer than the contralateral pedicles, thus facilitating the anastomoses to the recipient vessels. The absence of extrahepatic portal vein bifurcation is a contraindication to liver splitting. Because microsurgical hepatic artery reconstruction is now commonly performed, retaining the sectioned left hepatic artery with the LLG is usually preferred and is more commonly performed in North Europe centers during in situ splitting (Fig. 13.5). Biliary anatomy can be carefully explored by probing the main bile ducts, because an extensive dissection of the bile ducts may hamper the peri-biliary vascular plexus. The left hepatic duct is preferably sectioned because it is usually single. When the left hepatic duct is absent, the left lobe drains S IV and S II–III, thus configuring with the right duct a bile duct trifurcation; this allows a favourable plane of transection between S IV and S II–III in cases of liver splitting for adult and pediatric recipient. With regard to the possible extension of the arterial graft, interposition grafts by allogeneic iliac, splenic, superior, or inferior mesenteric arteries are usually employed. For portal vein extension, donor iliac veins can be used to extend both the right and left sides. Dissection of the hepatic hilum should be performed only from the left side keeping the right side untouched. After completing the resection of the gallbladder, the portal vein, the hepatic artery, the bile duct, and the hepatic vein are identified as well as the segment IV artery. After transaction of the left hepatic artery distally to the origin of segment IV artery, the small portal branches from the left portal vein supplying S IV are ligated and transected. In case of an adult and pediatric liver transplant recipients, the line of parenchyma transection extends from the confluence of the middle and left hepatic veins 0.5 cm on the right side of the falciform ligament to approximately 1 cm until the right side of the umbilical fissure up to the hilar plate. Splitting the liver parenchyma step by step along with umbilical scissure is continued from downward to upward ligating all tiny vessels. Parenchyma dissection can usually be performed with the mosquito or Kelly fracture technique or with scalpel transection with ligation of the single elements of the intrahepatic portal triad structures. The left bile duct is finally transacted as well as the left portal vein and the left hepatic vein close to suprahepatic inferior vena cava, leaving a suitable stump. The left hepatic vein is retained with the left graft. The right and middle hepatic veins in continuity with the vena cava are retained with the REG. Infusion of cold preservation solution via portal vein and hepatic artery can help to check for leaks. In order to reduce bleeding from the surfaces of the grafts, the majority of surgeons use sealing products such as fibrin glue, collagen, or polyglactin mesh.
In early experience and until the late 1990s, the ex situ liver split procedure was the most widely used method to transplant two patients with one liver [25]. Recently, few centers are still using this approach [26–28]. The ex situ splitting of the liver allograft on the bench is considered a time-consuming procedure and usually results in a long ischemic time. During the splitting procedure into right and left grafts, some allograft rewarming occurs; even if slight, it can be associated with increased susceptibility to hepatic ischemic/reperfusion injury. When a second recipient operating room is not immediately available, or if one of the hemiliver grafts must be transported to another center, the prolonged ischemic time can hamper the recipient outcome. Prolonged ischemia and rewarming during the ex situ split procedure exposes the graft to injury, with a higher incidence of poor graft function unless the split-liver transplant is organized in a very favourable environment and conditions. Thus, the ex situ technique may be restricted to some elective and selective cases, particularly for adult and pediatric recipients who can be simultaneously transplanted in the same transplant centers [17–20] or when a donor becomes unstable during the in situ procedure. Some authors [29] have reported encouraging results with the ex situ split technique for two adult recipients by splitting the vena cava and the middle hepatic vein (see below Sect. 13.4.3).
4.2 In Situ Splitting Technique for Adult and Pediatric Recipients
In situ splitting in the heart-beating DD is a modification of the ex situ splitting technique; it is an extension of the techniques established for living related donor procurement, which is associated with a lower rate of biliary complications, intra-abdominal hemorrhage, and primary nonfunction of the graft compared with other series of ex situ splitting techniques [26, 27]. As for the ex situ technique, only hemodynamically stable DDs are considered suitable for in situ splitting. It is important that donor hospitals and other procurement teams are notified as soon as possible of the decision to split the liver in situ, and the decision to proceed should be unanimous among different organ teams. No special equipment is needed; standard surgical facilities for a multiorgan procurement are usually used. The procedure requires an extra 2–3 h compared to the standard multiorgan technique. Before starting the splitting procedure, the standard surgical steps of abdominal organ procurement, including supraceliac and infrarenal aortic dissection and cannulation of the inferior mesenteric vein, should be completed. With this strategy, if a donor becomes unstable, the splitting procedure could be aborted with quick aortic cannulation, aortic cross-clamping, and organ cold perfusion.
Isolation of the Left Hepatic Vein (LHV)
Segments II and III of the liver are mobilized. The dissection is always initiated with the division of the umbilical ligament, which is tied and gently held up to expose the umbilical fissure. Dissection of the falciform ligament is prolonged to the level of the diaphragm, with identification of the hepatic veins. By opening the gastrohepatic ligament and pulling up the left lobe (Fig. 13.6), it is possible to identify and section the fibrotic remnant of the ductus venosus Arantii which connects the left portal vein to the root of the left hepatic vein. Section and division of the fibrotic remnant near to the LHV enable isolation and encircling of the LHV with a vessel loop; this manoeuvre becomes easier and safer if directed both ventrocranially and dorsocaudally by an Overholt dissector. A 1–2 min selective clamping of the LHV can ensure that the middle hepatic venous drainage of S IV, V, and VIII is not jeopardized. Occasionally, S II and III have independent orifices to the vena cava. The recognition of independent S II and S III veins is critical, and inadvertent injury to these veins should be carefully avoided. However, this condition requires that both orifices are incorporated on a common caval patch. A common middle and left hepatic vein requires careful separation after 1–2 cm of parenchyma division.
Parenchyma Dissection and Division of the Umbilical Plate
There are two primary ways to dissect and divide the umbilical plate: (a) the transhilar division and (b) the transumbilical division. Both techniques have been well described by Broelsh [30] and more recently by J. De Ville De Goyet [31], and their use has largely depended on the surgeon’s education and personal preference.
(A) In the transhilar (TH) approach, the LLG should be prepared beginning with hilar dissection at the base of the round ligament, with isolation of the left hepatic artery, the left portal vein branch, and the left bile duct. During the last decade, a significant debate has developed around how and when to preserve the artery for S IV (Fig. 13.7). The segment IV artery originates very near the arterial bifurcation, rarely from the right and more commonly from the left hepatic artery with some anatomical variability as follows:
-
(a)
A unique branch from left hepatic artery (80 %)
-
(b)
As a middle trunk of trifurcation of the hepatic artery (middle hepatic artery)
-
(c)
From the right hepatic branch when a replaced left artery arises from the left gastric artery
-
(d)
From the right hepatic branch when a replaced right hepatic artery arises from SMA
-
(e)
Multiple small branches, the main from the left hepatic artery
However, one should be aware of the fact that the majority of S IV artery branches are functionally accessory branches rather than replaced arteries and that intrahepatic collateral circulation exists with a valuable supply. As a result, when the S IV artery arises from the left hepatic artery and do not appear to provide significant arterial inflow, it can be tied and sacrificed. In some particular cases, when the S IV artery shows a caliber ≥ 2 mm or if partial discoloration of segment IV is produced after 1–2 min occlusion of the same artery, it is better to preserve it, leaving the celiac trunk with REG or cutting the S IV branch at its origin of the left hepatic artery and re-anastomosing it with a microvascular technique with the gastroduodenal artery. Some concerns have also been raised for S I, the caudate lobe: when the right hepatic artery is divided at its origin and the celiac trunk is retained with the LLG, the vascular elements for the caudate lobe are removed with the left graft. In our experience during several hundred adult and pediatric split liver procurements in the North Italy Transplant program (NITp) area, the celiac trunk was usually retained with the LLG, and the S IV artery has been ligated and sacrificed if considered functionally an accessory branch. Only in some few cases when the S IV artery was considered functionally relevant (discoloration after clamping test or diameter ≥ 2 mm), the celiac trunk was retained with the REG. This technical option of keeping the celiac trunk usually with the LLG still remains a common agreement among surgeons of the NITp area and was never considered in our experience a possible cause of graft loss for ischemic necrosis of S I or S IV. Furthermore, one should be aware that the ultimate evaluation of S IV and S I viability can be better obtained after graft reperfusion during the transplant procedure. When REG reperfusion shows some areas of marked discoloration in S IV or S I, removing the segments or a portion of them it is an option that can be weighed against the high risk of ischemic necrosis and possible biliary fistula.
The TH phase continues with the dissection of small portal venous branches to S IV (usually 6–8), which are ligated with 5–6/0 Prolene suture and divided laterally to the umbilical fissure to isolate the entire left portal branch (Fig. 13.8). Some small portal branches to segment I should be preserved, because they originate from the main and not the left portal vein. When total vascular control of the left lateral segments is completed, parenchyma transection should began by marking with electrocautery the liver capsule 5 mm to the right side of the falciform ligament (Fig. 13.9). The parenchyma division proceeds until the hilar plate is divided at the right side of the umbilical fissure line (0.5–1 cm to the right) and ends precisely at the Arantius remnant line with the exclusion of the caudate segment (S I), which is not included in the graft. Dissection of the anterior liver parenchyma is obtained by a Harmonic scalpel, by CUSA, by bipolar electrocautery and water sealing, or by simply electrocautery and gentle Kelly fracture. The dissection is directed between the LLG and S IV and should be carried out to 1 cm above the ductal plate surrounding the left bile duct in the umbilical fissure. The MHV is retained with the right graft. Some small penetrating vessels draining S IV in the left venous system and some small biliary orifices should be divided and suture ligated as required. However, if any large vein from S IV is draining in the LHV, a short test clamping of 1–2 min of the LHV can be helpful to test the functional relevance of the same vessel.
Ligation and transection of the portal branches to segment IV (transumbilical approach) [32]
Transection line of the liver parenchyma during split liver for adult and pediatric recipient runs 5 mm on the right side of the falciform ligament [32]
The main advantage of the TH approach is that the surgeon can easily move the division line to the right providing a larger LLG including S II, III, and the small part of S IV). This flexibility can be helpful when the LLG is relatively small or when there is a need to provide a larger liver mass. The parenchyma division should proceed until the hilar plate is divided at the right of the umbilical fissure line (0.5–1 cm), and it should end precisely at the Arantius remnant line. In this way, S I is not included in the graft. It is necessary to divide the plate and the portal pedicle for S I to completely free the hilar plate and the LLG not only from the caudate lobe but also from its paracaval portion by ligating some very small ascending portal branches. Finally, a sharp division of the hilar plate is performed between the main bifurcation of the hepatic vascular structures and the umbilical fissure; this allows to obtain a single biliary orifice in the majority of cases.
(B) In the transumbilical (TU) approach, the Rex recessus (the distal portion of the left portal vein running after a sharp bend between S IV and the LLG) should be exposed soon after the preparation of the extrahepatic structures. The peritoneum of the umbilical fissure is opened from the umbilical ligament to the porta hepatis. All venous branches of the Rex recessus draining into S IV are divided. The left portal vein at the Rex recessus is shifted to the left until the umbilical plate where division for S II and S III will take place and then fully exposed (Fig. 13.10). The left hepatic artery is also progressively mobilized during the latter manoeuvre; eventually, the artery for S IV is divided, and the plate attached to S IV will go with the right split graft. The division of the S IV artery, arising more often from the left hepatic artery, follows the same general rules described for the TH approach. After a common agreement among different surgical teams of the NITp area, the celiac trunk is always retained with the LLG unless different anatomical evidence for possible ischemia of S IV is recognized (Fig. 13.4). The division of the liver parenchyma exactly follows the line of insertion of the falciform ligament at first and ends at the convergence between the LHV and MHV. In the same manner as for TH, the parenchyma division is guided to reach anteriorly the middle line of the umbilical plate, previously prepared, and along the Arantius remnant posteriorly. This approach results in dividing the plate more to the left compared with the TH technique, leaving in place the portal pedicle supplying the caudate lobe process. The remaining left hilar plate and bile duct are sharply transected with scissors or a scalpel close to the liver surface (Fig. 13.11), and biliary drainage to segment IV should be preserved. Vascularization and the perfusion of S IV can be more easily evaluated during the in situ procedure in the heart-beating DD. Hypoperfusion of S IV is a potential pitfall, and segmentectomy or subsegmentectomy of S IV may sometimes be considered. At the end of the parenchyma dissection, the LLG is separated from the remaining extended right liver graft parenchyma with its own vascular pedicle and venous drainage.
At the end of the dissection, two liver grafts are procured, each with a preserved vascular pedicle and venous drainage in a bloodless field. Some microfibrillar collagen sheets or a hemostatic sponge can be applied to the cut surfaces, and organ procurement continues with a subsequent perfusion phase and cooling of the donor organs. After organ perfusion and cooling, the right hepatic artery, the left portal vein, and the left hepatic veins are divided. At the end of the procedure, the main portal vein, the common bile duct, and the right hepatic artery stay with the right graft unless some particular anatomical conditions are evident as discussed before. The right graft is removed in the usual fashion, retaining the entire vena cava, while the LLG retains the left suprahepatic vein. The left bile duct and the common bile duct are gently flushed with 50 ml of perfusion solution prior to the storage of both grafts.
Main Steps for Bench Surgery
The transplantation of the LLG retaining the LHV requires the preservation of the native inferior vena cava in the recipient. After further flushing with the perfusion solution throughout the portal vein, the parenchyma surface is carefully inspected during back-table preparation for possible vascular and biliary leaks that are oversewn. Bench surgery depends on the particular technique of transplantation of the LLG. During recipient operation, the right hepatic vein orifice to the vena cava is suture ligated, as are all the smaller accessory hepatic veins along the inferior vena cava. The left and middle hepatic vein orifices are opened in order to form a large common trunk for hepatic venous anastomosis. Anastomosis of the portal vein will be performed end-to-end utilizing nonabsorbable monofilament suture. For infants and neonates, the anastomosis may be a running (continuous) suture on the posterior wall and interrupted suture anteriorly. If the celiac trunk has not been retained with the LLG, the donor left hepatic artery can be anastomosed to the recipient common hepatic artery provided a long branching of the left artery can be obtained in the recipient. Otherwise, the anastomosis can be performed with the infrarenal aorta by artery interposition of a graft harvested from the DD. Biliary anastomosis is occasionally performed duct-to-duct but is more frequently performed by an end-to-side Roux-en-Y hepaticojejunostomy.
Preparation of the REG during bench surgery for transplantation includes the removal of remnant diaphragm from the liver bare area, ligature of phrenic vein origins, and closure of the orifices of the left hepatic vein, left portal vein, and left hepatic artery origin in those cases where the celiac trunk is retained with the right graft. In some cases of an S IV relevant artery, its revascularization, using the recipient gastroduodenal artery, can be considered; the left bile duct remnant is oversewn. Gently flushing each structure may help to identify small vascular orifices. The orifice of the LHV is finally sutured by a transverse oversewn. The REG is ready for transplantation, utilizing standard whole-organ techniques.
4.3 Split-Liver Procedure for Two Adult Recipients
The initial steps in the donor operation are performed as in any other multiple organ harvesting procedure. One should remember that before pereforming any split procedure, the standard techniques of abdominal organ procurement, including supraceliac and infrarenal aortic dissection and cannulation of the inferior mesenteric vein, should be completed. In this way if a donor becomes unstable, the splitting procedure can be aborted with rapid progression to aortic cannulation, aortic cross-clamping, and organ cold perfusion.
The right hepatic pedicle is first dissected with the usual extrahepatic intra-Glissonean approach, and the right hepatic artery and right branch of the portal vein are isolated and encircled with different colored vessel loops (Fig. 13.12). The right liver lobe should be fully mobilized, and all the short hepatic veins to the retrohepatic vena cava are isolated and saved to preserve adequate venous outflow. The parenchyma bridge, when present, from S IV to S III around the IVC must be divided. The right hepatic vein is isolated and taped with a vessel loop. After the isolation of all short hepatic veins, a tape can be positioned from the groove between the RHV and MHV to the groove between the right and left Glissonean sheaths via the posterior hepatic surface (hanging maneuver). The lateral end of the tape is carried behind all the retrohepatic vein branches draining from the right liver lobe. To complete the hanging maneuver, the end of the tape is passed ventral to the right hepatic artery and right portal vein. In this way, the vessel loop defines a transection plane leading from the bifurcation of the hepatic artery and portal vein to a point between the right and middle hepatic veins. Before transection, ultrasound can be performed intraoperatively, whenever possible, to detect major S V and S VIII veins crossing the transection plane at the line of Cantlie. The “tape-assisted” parenchyma transection leads the surgeon more easily to the anterior wall of the inferior vena cava, potentially with better preservation of the caudal lobe venous outflow (Fig. 13.13). At the end of the parenchyma transection and division of the right bile duct, the organ-procurement procedure is continued with the standard technique, and organ perfusion and cooling can be initiated. The right hepatic vein, right hepatic artery, and right portal vein are divided at the end of organ perfusion, usually leaving the right hepatic branch, the right portal vein, and the common bile duct with the right graft. A FRG and a FLG are obtained, and the left and right bile ducts are gently flushed with the perfusion solution prior to the storage of both grafts. Almost all centers in the NITp area typically retain the common bile duct with the right graft and the common trunk of the portal vein and the celiac trunk with the left graft. However, in particular anatomical situations concerning both the donor and the recipient, some variations from the standard technique can be discussed. There are three modalities for liver-splitting techniques for two adults: (a) liver splitting into FLG S I–IV and FRG S V–VIII which is the most used in our experience, (b) liver splitting into FLG S II–IV and FRG S I, V–VIII, and (c) ex situ splitting with standard technique or splitting the vena cava and middle hepatic vein (FLG S I–IV and FRG S V–VIII).
The right hepatic pedicle is dissected with the extrahepatic intra-Glissonean approach; the right hepatic artery and right branch of the portal vein are isolated and encircled with different colored vessel loops [32]
The “tape-assisted” parenchyma transection leads more easily to the anterior wall of the inferior vena cava, with better preservation of the caudal lobe venous outflow [32]
-
(a)
Split Liver for Two Adult Recipients with Creation of FLG S I–IV and FRG S V–VIII
This is the technique most frequently used for adult recipients, and it has developed in parallel with the one of the right lobe living donor procurement [31–34] (Fig. 13.14). Usually, left lobe grafts of approximately 450–500 g with S I–IV are used for adults weighing from 45 to 50 kg and in select circumstances, depending on donor size, for heavier recipients. The FRG, S V–VIII, of approximately 750–800 g, generally allows donor graft-to-recipient body weight ratios of more than 1.0 %. The procedure is similar to the one described earlier (Sect. 13.4.3). The hepatic veins are identified, and the right hepatic vein is encircled with a vessel loop. All diaphragmatic attachments to the liver are released, and the dissection proceeds from the right lobe to the inferior vena cava. There is no need to dissect the left border of the inferior vena cava. Minor and major accessory hepatic veins are usually encountered in about one half of DDs; these are individually preserved with a small caval patch for implantation only if ≥ 5 mm in diameter.
The hepatoduodenal ligament is opened to expose the hilum after retrograde cholecystectomy. The right hepatic artery is identified and exposed lateral to the common hepatic duct. Lateral exposure can avoid skeletonization of the proper hepatic bifurcation, thereby preserving any possible arterial supply to S IV from the right hepatic artery. The right portal vein should be approached from the lateral right side of the hilum and dissected to the level of the bifurcation where it is encircled with a vessel loop. A short and selective Pringle maneuver of the left hilum is then performed to create a demarcation line for parenchyma division. Once the hilar plate has been identified, the left bile duct (unique or double duct orifices) is sharply divided, and the remnant orifice is closed with a 6/0 monofilament; bleeding from hilar plate points can be secured with 5–0 nonabsorbable monofilament suture. Parenchyma division will continue along the main portal fissure with the surgeon’s left fingertips positioned behind the right lobe anterior to the inferior vena cava. The hanging maneuver can be helpful and leads the surgeon more easily to the anterior wall of the inferior vena cava, with better preservation of the caudal lobe venous outflow. The MHV is retained with the FLG; for this reason, some S V and S VIII venous tributaries draining in the MHV are sharply divided and ligated when of small diameter (≤4 mm) (Fig. 13.15). Later revascularization of some venous tributaries to the MHV can be evaluated for vessels with a diameter larger than 5 mm or when a Makuuchi 5-min clamping test indicates its utility. In living donor liver transplantation (LDLT), Makuuchi [35] advocates aggressive reconstruction of all veins draining the right paramedian sector in the living donor right lobe when the MHV is not harvested with the right lobe. This author suggests the use of intraoperative ultrasound Doppler evaluation after a 5-min test by clamping both the hepatic artery and the branches of the MHV; the evidence of a portal hepatofugal flow in the paramedian portal branch will suggest the reconstruction of the occluded paramedian venous branch. After the completion of parenchyma division, the right hepatic vein, right portal vein, and right hepatic artery remain intact for organ cold perfusion. At this point, heparin is administered and aortic cannulation is achieved. At the end of cool perfusion, graft separation should be performed including sharp division of the right portal vein immediately distal to the bifurcation and transection of the right hepatic artery immediately distal to its takeoff from the proper hepatic artery (Fig. 13.16). The rationale for preserving the celiac axis with the left graft is to maximize arterial supply to S IV as its arterial supply is routinely derived more from branches of the left than from branches of the right hepatic artery. The right hepatic vein is divided from the suprahepatic vena cava as a patch, and the FRG S V–VIII is removed. Because more common biliary variants are described in the right lobe, the common bile duct is retained with FRG (Fig. 13.16) and is flushed prior to cold storage in the cold perfusion solution. The FLG S I–IV graft is also removed, utilizing standard organ recovery techniques followed by the irrigation of the left bile duct and storage in cold preservation solution.
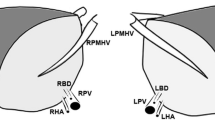
Split liver for two adults. FRG and FLG. Graft separation should be performed including sharp division of the right portal vein (RPV) immediately distal to the bifurcation and transection of the right hepatic artery (RHA) immediately distal to its takeoff from the proper hepatic artery. The common bile duct (CBD) is retained with FRG
-
(a1)
Main steps of bench surgery and recipient operation
The ex situ preparation of the FRG S V–VIII graft includes suture ligature of small biliary radicles and the potential restoration of all MHV branches draining S IV, S V, and S VIII to avoid congestion of the paramedian sector. Additionally, large accessory hepatic veins from S VI and S VII, when larger than 5 mm in diameter, should be anastomosed either directly to the vena cava or more optimally to some other venous conduit harvested from the donor (Fig. 13.17). This anastomosis can be performed with different techniques by employing donor iliac venous grafts and their secondary branches or the donor mesenteric vein. Ex situ preparation includes closure of the right portal vein orifice, the right hepatic vein orifice, and the right hepatic artery orifice from the common hepatic artery of the FLG S I–IV. All small parenchyma biliary orifices should be recognized and ligated. The FRG S V–VIII requires the recipient’s inferior vena cava. The FRG is positioned orthotopically with a graft hepatic vein anastomosis to the recipient right hepatic vein orifice or to a common trunk formed by the recipient’s remnant left, middle, and right hepatic vein orifices. End-to-end anastomosis of the portal vein is frequently possible, as the anastomosis of the right hepatic artery with the recipient common hepatic artery. Donor iliac arteries or veins may also be used for interposition grafting. Biliary drainage may be achieved in the recipient with an end-to-end anastomosis to the common bile duct. The FLG can be transplanted in the standard orthotopic manner with or without venovenous bypass or by a piggyback technique; biliary drainage is usually obtained with the left bile duct by Roux-en-Y bilio-jejunostomy or by an end-to-end anastomosis of the left duct with the donor common or left duct.
-
(b)
Split Liver for Two Adult Recipients or for Adult and Pediatric Recipient of Large Size with the Creation of a LG S II–IV and an RG S I, V–VIII
Grafting of the left lobe S II–IV weighing approximately 400–450 g is usually performed for smaller adults or for larger pediatric recipients weighing 35–45 kg. This procedure is technically more difficult than the previous ones and requires particular skill and experience in splitting the liver [28]. The middle and left hepatic veins should be retained together with the FLG; they are encircled together with a vessel loop to guide parenchyma dissection. In this procedure, the “hanging manoeuvre” by retrohepatic tape can also be helpful to guide parenchyma dissection. Unlike the previous technique, the tape should be passed not on the right but on the left side of the caval border, leaving the caudate lobe with the RG S I, V–VIII. For this purpose, the tape should be positioned from the groove between the right and middle veins to the groove between the right and left Glissonean sheaths along the posterior hepatic surface of the LLS and lying on the remnant of the ductus Arantii. The left bile duct, left hepatic artery, and left portal vein are identified and encircled by a vessel loop. The dissection should be performed distally along the entire extrahepatic length to the level of the round ligament. Left hepatic artery branch (or branches) servicing S IV must be preserved. The main difference in this technique is that the left portal vein should be freed along its entire length, and careful division of some small portal branches for caudate lobe (usually 1–5) is paramount to completely free the LG from the caudate lobe, which should be retained with RG S I, V–VIII. However, because some small portal branches are servicing the caudate lobe from the posterior wall of the portal vein, a complete dissection of these posterior small branches from the left portal vein can be better and safely performed only after cool perfusion and during bench surgery when their orifices can be suture ligated.
A temporary left pedicle occlusion, of both the left portal vein and the left hepatic artery, generates a clear demarcation plane for parenchyma transection. The plane is marked by electrocautery on the Glissonean capsule, and dissection proceeds to the hilar plate with the available surgical tools (CUSA, Harmonic Scalpel, monopolar electrocautery, and water cooling or simply by Kelly fracture and bipolar electrocautery). During this step, some parenchyma vessels are encountered and ligated. The left bile duct is sharply transected at the level of the hilar plate, whereas the left hepatic artery and left portal vein are preserved to ensure organ cold perfusion. After the administration of heparin, aortic cannulation, cross-clamp, and organ cold perfusion are started. Post-perfusion time requires the procedure to continue rapidly with sharp transection of the left portal vein immediately distal to the bifurcation and with transection of the right hepatic artery immediately distal to its takeoff from the proper hepatic artery. This technique requires that the common trunk of the portal vein and common hepatic duct are maintained with the right graft while preserving the celiac axis with the left graft as in the adult-to-pediatric technique. Because less collateral circulation is available in a small left lobe, the preservation of the celiac axis with the left graft can be paramount to maximize arterial supply to S IV, although some small branches can originate both from the left and from the right hepatic artery. The vena cava is retained with the RG S I, V–VIII. The left and middle hepatic veins are taken from the suprahepatic vena cava as a common venous cuff, and the left bile duct retained with the LG is flushed with perfusion solution prior to cold storage. This technique increases the risk of vascular and biliary complications because the perfusion of S IV may be sometime suboptimal. Complete dissection of the left portal vein can sacrifice some small portal branches to S IV; this manoeuvre associated with the arterial hypoperfusion of the same segment can lead to partial necrosis and bile leakage in that area.
-
(b1)
Main Steps for Bench Surgery and Recipient Operation
Ex situ graft preparation of LG S II–IV only requires the identification and repair of cut-surface biliary orifices. For both FLG S II–IV and FRG S I, V–VIII, after standard organ recovery, the irrigation of the common bile duct should be performed, and grafts should be stored in cold perfusion solution. Vascular reconstruction with donor-derived conduit vessels may be required for the FRG.
The implantation of RG S I, V–VIII into an adult is accomplished in the standard orthotopic manner with or without venovenous bypass with a piggyback technique. An oversewing of the common vein orifice of the left and middle hepatic vein can compromise the suprahepatic vena caval cuff in width; the orifice can be kept open for a running suture to the recipient caval cuff using the piggyback technique. The right hepatic artery and the common trunk of the portal vein are anastomosed end-to-end with the recipient hepatic artery and portal vein. Interposition vascular venous and arterial grafts must be used for anastomosis to a suitable source of arterial inflow. Biliary reconstruction can be performed by choledochocholedochostomy for RG with a T-tube, which reduce the biliary back pressure in order to prevent some bile leakage from the cut surface of the liver. The LG can be transplanted into a child or small adult with the preservation of the recipient vena cava. The middle and left hepatic vein cuff is anastomosed to the suprahepatic vena cava of the patient. However, because of size discrepancy, various venoplasty maneuvers must be often performed to avoid graft kinking. The majority of these techniques have been described by several authors [31–34]. Portal vein reconstruction must be individualized to the recipient’s anatomy. In some cases, a direct end-to-end anastomosis is contraindicated, and anastomosis to the confluence of the splenic and superior mesenteric veins is required. In some cases, an extension venous graft is necessary to provide a tension-free anastomosis, but the use of venous grafts should be limited while the longest recipient portal axis should be preserved during hepatectomy. Hepatic artery reconstruction can be performed either to the hepatic artery of the recipient or to the aorta with a transmesocolic infrarenal iliac graft arterial conduit. If the left hepatic artery is retained with the LG, a microsurgical reconstruction by end-to-end anastomosis to the proper hepatic artery of the recipient should be performed. The left graft biliary tract reconstruction is usually accomplished by a Roux-en-Y left hepaticojejunostomy, and in one fourth of LG S II–IV, there are two or more separate bile ducts.
-
(c)
Ex Situ Splitting for Two Adult Recipients: Standard Technique and Splitting of the Retrohepatic Vena Cava and Middle Hepatic Vein
The main surgical steps for ex situ splitting technique have been described above in paragraph Sect. 13.4.1. Only the lack of an extraepatic portal vein biforcation can be considered an absolute barrier to ex situ splitting. The liver can be divided through the middle of segment IV, retaining the MHV with the right graft. In some cases, the liver can be divided along the Cantlie line (the main portal scissure) separating the right and the left lobes and obtaining a FRG (S SV–VIII) and a FLG (SI, S II–IV) (Figs. 13.18, 13.19, 13.20); in this case all portion of S IV is allocated to the left graft to increase the graft-to-recipient body weight ratio. The middle hepatic vein can be kept on the left in continuity with the common trunk of the left and middle hepatic veins. The cutting lines are the same as for left hemihepatectomy in living donors. In this case, ex situ splitting may offer the advantage of full anatomical access to create the best optimal venous outflow in both grafts.
Sometimes the main problem associated with liver splitting for two adults is the possible congestion of the paramedian segments, S V, S VIII, and S IV, which can be evident only after revascularization; all these segments have some venous effluent to the MHV. Congestion of one or more than one segment with a higher probability of “small-for-size syndrome” and post transplant liver failure can be clearly evident during parenchyma division during in situ technique. In ex situ technique the lack of optimal blood flow in the paramedian segments can be recognized only after revascularization. At this regard some Authors have proposed the possibility to split longitudinally the inferior vena cava (IVC) into two parts [29]. Hilar dissection should start by the usual identification and preparation of the hepatic artery bifurcation and the S IV artery. The artery transection will depend on the origin of the S IV artery and on its functional relevance. The portal vein is dissected down to the main bifurcation, and the main portal vein is retained, as usual, with the left hemiliver to preserve the S I branches. The division of the bile duct retains the main bile duct with the right liver lobe due to the frequency of more biliary variants in the right hemiliver. Before starting the parenchyma transection, the dorsal and ventral wall of the IVC is cut along the midline, acquiring two hemicava patches (Fig. 13.21). Transection of the dorsal and ventral wall of the IVC in the midplane to conceive two hemicava patches is performed before starting the parenchyma transection, which is conducted later by the sharp knife technique along the line of Cantlie.
The MHV is then cut from inside the IVC, preserving the half of the MHV for each of the two hemilivers. The parenchyma can be cut outside the IVC from S VIII 1–2 cm on the right side of the MHV, thus leaving its main orifice for the left graft and two portions of the MHV for each graft. At the end we will have two graft each of them with a large hemicava patch including two halves of the MHV with orifices of all draining veins (Fig. 13.22). Then, the split portion of the MHV of the left hemiliver is reconstructed with half an iliac artery, and for the right portion, an entire iliac vein graft is used (Fig. 13.23). Implantation of the grafts is performed using the standard techniques. For the venous outflow, a large venous anastomosis is performed using a cavo-cavostomy technique. The hemicava patch of the right graft can also be anastomosed by longitudinal extension of the opening of the recipient’s right hepatic vein. Splitting of the MHV requires extra time for the ex situ venoplasty reconstruction with a longer ischemic time, which may increase the recipient morbidity, especially for biliary complications. For this reason, in our opinion this technique has a very limited application in clinical practice. It can be taken into consideration when a high number of accessory hepatic veins from S IV, S V and S VIII are draining into the MHV with a strong dominance which may hamper the vascularization the right paramedian sector and S IV.
5 Conclusions
The widespread utilization of split-liver transplantation is hampered by difficulties in sharing liver grafts between centers, especially when the liver is split for two adults. Most centers agree to partial-liver grafts from deceased donors only when shared between adult and pediatric recipients, as excellent outcomes have been described [26]. Considering the good results in a large series of split-liver transplantation for adult and pediatric recipients and the excellent results also reported in living donor liver transplants [26–28], many centers are questioning the value of split-liver procedures for two adults in light of the difference between the benefit of the transplant community and the cost to the individual transplant recipients. As a matter of fact, a higher risk of morbidity and mortality for patients after liver transplantation exists with marginal whole organs compared to optimal split-liver grafts, although no randomized studies exist or will most likely ever exist on this issue. Some concern remains about the significant learning curve for the splitting procedures for two adults, and some questions remain unanswered about the risk of low volume of the split grafts, which can put the recipient at risk of small-for-size syndrome with subsequent liver failure, in particular for those patients with portal hypertension. However, a multicenter study has recently reported encouraging results when donors and recipients are carefully selected and meticulous techniques are adopted [1, 26–28, 33, 34]. A cooperative split-liver transplant program among different centers may investigate better allocation policies and most likely will allow better results provided that close supervision is ensured by more experienced centers.
Abbreviations
- DD:
-
Deceased donor
- FLG:
-
Full left graft
- FRG:
-
Full right graft
- GW/RW:
-
Graft weight to recipient weight boby ratio
- LHV:
-
Left hepatic vein
- LLG:
-
Left lateral graft
- LDLT:
-
Living donor liver transplantation
- LG:
-
Left graft
- LHV:
-
Left hepatic vein
- MHV:
-
Middle hepatic vein
- NITp:
-
North Italy Transplant program
- RHV:
-
Right hepatic vein
- RLT:
-
Reduced liver transplantation
- REG:
-
Right extended graft
- RG:
-
Right graft
- S:
-
Segment
- SLT:
-
Split-liver transplantation
- SFSS:
-
Small-for-size syndrome
- TH:
-
Transhilar
- TU:
-
Transumbilical
References
Aseni P, De Feo TM, De Carlis L, Valente U, Colledan M, Cillo U, Rossi G, Mazzaferro V, Donataccio M, De Fazio N, Andorno E, Burra P, Split-Liver Study Group. A prospective policy development to increase split-liver transplantation for 2 adult recipients: results of a 12-year multicenter collaborative study. Ann Surg. 2014;259:157–65.
Smith B. Segmental liver transplantation from a living donor. J Pediatr Surg. 1969;4:126–32.
Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367–70.
Broelsch CE, Neuhaus P, Burdelski M. Orthotopic transplantation of hepatic segments in infants with biliary atresia. In: L K, Klinische Forschung, editors. Chirurgisches Forum ‘84f. Experim U. Berlin: Heidelberg-Springer; 1984.
Rogiers X, Malago M, Gawad K, Jauch KW, Olausson M, Knoefel WT, Gundlach M, Bassas A, Fischer L, Sterneck M, Burdelski M, Broelsch CE. In situ splitting of cadaveric livers. The ultimate expansion of a limited donor pool. Ann Surg. 1996;224:331–9.
Vulchev A, Roberts JP, Stock PG. Ethical issues in split versus whole liver transplantation. Am J Transplant. 2004;4:1737–40.
Busuttil RW, Goss J. Split liver transplantation. Ann Surg. 1999;229:313–21.
Giacomoni A, Lauterio A, Donadon M, De Gasperi A, Belli L, Slim A, Dorobantu B, Mangoni I, De Carlis L. Should we still offer split‐liver transplantation for two adult recipients? A retrospective study of our experience. Liver Transpl. 2008;14:999–1006.
Pichlmayr R, Ringe B, Gubernatis G. Transplantation of a donor liver to two recipients (splitting transplantation) – a new method in the further development of segmental liver transplantation. Langenbecks Arch Chir. 1989;373:127–30.
Bismuth H, Morino M, Castaing D, Gillon MC, Descorps Declere A, Saliba F, Samuel D. Emergency orthotopic liver transplantation in two patients using one donor liver. Br J Surg. 1989;76:722–4.
Goss JA, Yersiz H, Shackleton CR, Seu P, Smith CV, Markowitz JS, Farmer DG, Ghobrial RM, Markmann JF, Arnaout WS, et al. In situ splitting of the cadaveric liver for transplantation. Transplantation. 1997;64:871–7.
Azoulay D, Astarcioglu I, Bismuth H, Castaing D, Majno P, Adam R, Johann M. Split-liver transplantation. The Paul Brousse policy. Ann Surg. 1996;224:737–46.
Couinaud C. Le Foie. Paris: Masson; 1957.
Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9.
See Ching C, Chi Leung L, Chung Mau L, Lam BK, Lee EW, Yik W, Sheung Tat F. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12:2217–22.
Hill MJ, Hughes M, Jie T, Cohen M, Lake J, Payne WD, Humar A. Graft weight/recipient weight ratio: how well does it predict outcome after partial liver transplants? Liver Transpl. 2009;15:1056–62.
Rela M, Voregas V, Miniesan P, et al. Split liver transplantation: King’s College Hospital experience. Ann Surg. 1998;227:282–8.
Yersiz H, Renz JF, Hisatake GM, Farmer DG, Busuttil RW. The conventional technique of in-situ split-liver transplantation. J Hepatobiliary Pancreat Surg. 2003;10:11–5.
Renz JF, Yersiz H, Reichert PR, Hisatake GM, Farmer DG, Emond JC, Busuttil RW. Split-liver transplantation: a review. Am J Transplant. 2003;3:1323–35.
Humar A, Ramcharan T, Sielaff TD, et al. Split liver transplantation for two adult recipients: an initial experience. Am J Transplant. 2001;1:366–72.
Broering DC, Wilms C, Lenk C, Schulte am Esch 2nd J, Schönherr S, Mueller L, Kim JS, Helmke K, Burdelski M, Rogiers X. Technical refinements and results in full-right full-left splitting of the deceased donor liver. Ann Surg. 2005;242:802–12; discussion 812–813.
Xavier Rogiers, Henri Bismuth, Ronald W. Busuttil, Dieter C. Broering, Daniel Azoulay (Eds). Split liver transplantation. Springer Edition. Heidelberg 2002. ISBN: 978-3-7985-1256-6 (Print) 978-3-642-57523-5.
Chaib E, Bertevello P, Saad WA, Pinotti HW, Gama-Rodrigues J. The main hepatic anatomic variations for the purpose of split-liver transplantation. Hepatogastroenterology. 2007;54:688–92.
Broering DC, Schulte am Esch J, Fischer L, Rogiers X. Split liver transplantation. HPB. 2004;6:76–82.
Emond JC, Whitington PF, Thistlethwaite JR, Cherqui D, Alonso EA, Woodle IS, Vogelbach P, Busse-Henry SM, Zucker AR, Broelsch CE. Transplantation of two patients with one liver. Analysis of a preliminary experience with ‘split-liver’ grafting. Ann Surg. 1990;212:14–22.
Hong JC, Yersiz H, Farmer DG, Duffy JP, Ghobrial RM, Nonthasoot B, Collins TE, Hiatt JR, Busuttil RW. Long term outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. J Am Coll Surg. 2009;208:682–9; discusion 689–691.
Hashimoto K, Quintini C, Aucejo FN, Fujiki M, Diago T, Watson MJ, Kelly DM, Winans CG, Eghtesad B, Fung JJ, Miller CM. Split liver transplantation using hemiliver graft in the MELD Era: a single center experience in the United States. Am J Transplant. 2014;14:2072–80.
Lee WC, Chan KM, Chou HS, Wu TJ, Lee CF, Soong RS, Wu TH, Lee CS. Feasibility of split liver transplantation for 2 adults in the model of end-stage liver disease era. Ann Surg. 2013;258:306–11.
Broering DC, Pamela B, Lars M, Christian W, Xavier R. Splitting of the middle hepatic vein in full-right -full-left splitting of the liver. Liver Transpl. 2005;11:350–2.
Broelsch CE, Whitington PF, Emond JC, Heffron TG, Thistlethwaite JR, Stevens L, Piper J, Whitington SH, Lichtor JL. Liver transplantation in children from living related donors surgical techniques and results. Ann Surg. 1991;214:428–37.
de Ville de Goyet J, di Francesco F, Sottani V, Grimaldi C, Tozzi AE, Monti L, Muiesan P. Splitting livers: trans-hilar or trans-umbilical division? Technical aspects and comparative outcomes. Pediatr Transplant. 2015;19:517–26.
Forti D. Transplantul cu ficat redus (“reduced size”) si impartit (“split”). Capitolul 2004;48:1098–1110. In: Irinel Popescu.Chirurgia Ficatului, Vol II. Editura Universitara “Carol Davila”
Yersiz H, Renz JF, Hisatake G, Reichert PR, Feduska NJ Jr, Lerner S, Farmer DG, Ghobrial RM, Geevarghese S, Baquerizo A, Chen P, Busuttil RW. Technical and logistical considerations of in situ split-liver transplantation for two adults: part I. Creation of left segment II, III, IV and right segment I, V-VIII grafts. Liver Transpl. 2001;7:1077–80
Yersiz H, Renz JF, Hisatake G, Reichert PR, Feduska Jr NJ, Lerner S, Farmer DG, Ghobrial RM, Geevarghese S, Baquerizo A, Chen P, Busuttil RW. Technical and logistical considerations of in situ split-liver transplantation for two adults: part II. Creation of left segment I–IV and right segment V–VIII grafts. Liver Transpl. 2002;8:78–81
Makuuchi M, Sugawara Y. Technical progress in living donor transplantation for adults. HPB. 2004;2:95–8
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Aseni, P., Sguinzi, R., De Carlis, R., Giacomoni, A., Mangoni, I., De Carlis, L. (2016). Split Liver: Surgical Techniques for Adult and Pediatric Recipients and for Two Adult Recipients. In: Aseni, P., Grande, A., De Carlis, L. (eds) Multiorgan Procurement for Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-319-28416-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-28416-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28414-9
Online ISBN: 978-3-319-28416-3
eBook Packages: MedicineMedicine (R0)