Abstract
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas that can result in a moderately severe or severe disease with high morbidity and/or mortality. Gall stones and alcohol account for nearly 70 % of the cases. Local complications include acute peripancreatic fluid collections and acute necrotic collections that can evolve into pseudocyst and walled off necrosis respectively. Persistent organ failure and presence of local complications are associated with higher mortality and morbidity. Currently there is no effective tool to predict severity of the disease at the onset of illness. The Revised Atlanta Classification classifies AP into three groups—mild with no local complications or organ failure, moderately severe with local complications and/or transient organ failure, and severe with persistent organ failure. While there is no targeted treatment for AP, management is focused at providing support for organ failure and at treating complications. Aggressive intravenous hydration is essential in the initial 12–24 h that can significantly alter the outcome of AP. Enteral feeding to prevent infectious complications is necessary in AP. Infected necrosis is associated with high mortality and requires minimally invasive routes of intervention in the majority of patients. With aggressive management, 70–80 % patients with AP require brief hospitalization and improve quickly. However, 15–30 % patients go on to develop moderately severe or severe disease with high rate of mortality. Advances in understanding the pathogenesis of AP, development of effective prediction strategies, and targeted therapeutic options (drugs) can lead to further improvement in the outcomes in AP.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
What Causes Acute Pancreatitis?
Suggested response to patient: About 4 in 10 cases are caused by gall stones. In these cases, small (<5 mm) stones escape from the gall bladder and pass into the ducts that drain pancreatic juices causing blockade. This results in damage to the pancreas resulting in inflammation and pancreatitis. Another 3 in 10 cases are caused by heavy alcohol drinking. Exactly how alcohol causes pancreatitis in not well known. There are many other known causes that account for the rest although in many patients no cause can be found by current methods. It is thought that damage to the pancreas results from auto-digestion by action of powerful pancreatic enzymes that get activated within the pancreas. Inflammatory cells from the blood are recruited to the area of damage. These cells along with the cells of the pancreas lead to systemic inflammatory response. This can lead to fluid leakage into various spaces in the body including into the lungs causing problems in breathing, low blood pressure, and damage to multiple organs. With current aggressive treatment, this inflammatory response can be managed in as many as 70–80 % of the patients resulting in quick recovery. However, 15–30 % of patients will go on to develop moderately severe or severe disease.
Brief Review of the Literature
Introduction
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas which often affects many organ systems. Historically, AP was associated with a high mortality. While the overall mortality rate has dropped to 2–4 %, patients with severe disease continue to have a high mortality with estimates ranging from 20 % to 40 % for specific subgroups [1]. In the US, it affects about 40 individuals per 100,000 each year and its incidence is estimated to be increasing [2]. Based on US data from 2009 [3], AP was the most common gastroenterology discharge diagnosis with more than 275,000 hospitalizations, an estimated 2.6 billon US dollars in direct and indirect costs and 2600 deaths, making it the fifth leading cause of in-hospital deaths.
Diagnosis
AP is diagnosed clinically when any two of the following are present: (1) abdominal pain consistent with the disease, (2) serum amylase and/or lipase greater than three times the upper limit of normal, (3) consistent radiologic imaging (CT or MRI) findings [4].
The pain is typically described in the epigastric region or left upper quadrant and usually severe in intensity. Patients may describe pain radiating to the back, flank or chest although this is nonspecific. Serum lipase has better specificity than amylase and remains elevated longer making it preferable over amylase measurements. It is to be emphasized that neither the severity of the pain or the degree of enzyme elevations correlates with severity of the disease [4]. Imaging is not required for establishing diagnosis and should be reserved for patients in whom diagnosis is not clear or who fail to show improvement within 48–72 h or to evaluate for development of complications in the later stages of the disease [4]. Diffuse or localized swelling of the pancreas is consistent for AP on imaging with or without varying degree of peripancreatic fat stranding.
Etiology
Gall stones account for ~40 % and heavy alcohol abuse accounts for another ~30 % of cases of AP in the US [2, 5]. An abdominal ultrasound should be performed in all patients with AP to identify gall stones and biliary dilatation due to possible choledocholithiasis [4]. Heavy alcohol use for greater than 5 years is typically considered necessary for development of pancreatitis. Further, only 5 % of patients with gall stones or heavy alcohol use develop pancreatitis in their lifetime. Pancreatic tumors should be considered as a possible etiology in patients older than 40 or 50 years of age, without gall stone disease or history of alcohol abuse. Intra-ductal papillary mucinous neoplasms (IPMN), usually main duct type but sometimes even side-branch type may cause AP. The other infrequent causes include drugs, following procedures like ERCP (rarely after EUS and fine needle aspiration), hypercalcemia, hypertriglyceridemia (>1000 mg/dl), trauma, ischemia, autoimmune pancreatitis, and certain infections. These are quite uncommon, and caution should be exercised in ascribing one of these uncommon causes as the etiology. Often, these causes, especially drugs, are falsely implicated as the causative agent of AP. Post-ERCP AP is usually obvious and can occur in 5 % of diagnostic ERCPs, 7 % of therapeutic ERCPs and up to 25 % in patients with sphincter of Oddi dysfunction [6].
Idiopathic AP is defined as cases of AP where the etiology is not evident after imaging (trans-abdominal ultrasound and CT/MRI in the appropriate setting) and standard laboratory investigations including calcium and triglyceride levels [4]. Idiopathic AP may account for up to 30 % of all cases. Hereditary pancreatitis accounts for a small proportion of idiopathic AP. There is no clear data on risks and benefits of further endoscopic examination for evaluation of etiology of idiopathic AP. Further, no causative etiology may ever be identified in many of these patients. Current guidelines recommend referral of idiopathic AP to centers of expertise for further work-up of etiology [4].
Natural Course and Complications
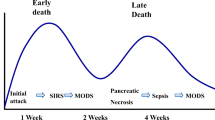
Regardless of the etiology, AP involves an early phase usually lasting a week or two which can progress in some patients into a late phase lasting weeks to months [4]. The early phase correlates to the time-course and effects of intense systemic inflammatory response elicited due to pancreatic injury. The late phase correlates to development of local complications from pancreatic injury. Persistent dysfunction of one or more organ systems can occur in both phases. Among the patients who succumb to AP, approximately half of them die during the first week or two (early phase) and another half in the late phase [7–10]. Deaths in the early phase occur due to organ failure and complications related to the severe inflammatory response. In the late phase, deaths occur due to local complications including infection of pancreatic necrosis or interventions for these complications [11].
Development of local complications and persistent organ failure are both associated with increased mortality, prolonged course of illness and increased rate of complications [1, 12]. This understanding has been the basis of the evolution of strategies for classifying patients with AP. The concepts of local complications and of organ failure are explained in detail below. The long-standing 1992 Atlanta Classification has been recently revised in 2013, resulting in a widely accepted classification system that has three groups—mild, moderately severe, and severe AP [12]. Another parallel classification system with an additional group of critical AP was developed around the same time, known as Determinant Based Classification (DBC) of AP [13]. Initial validation studies have shown both strategies to be effective in classifying patients appropriately with the aim of identifying patients at the highest risk of mortality and morbidity [14, 15]. In this chapter we discuss this Revised Atlanta Classification of AP in detail.
Organ failure conceptually refers to failure of cardiovascular, pulmonary, or renal systems commonly associated with conditions resulting in profound systemic inflammatory response. For objective assessment of organ failure, a modified Marshall scoring system (the following Web based resource may be used: http://www.pmidcalc.org/?sid=23100216&newtest=Y) is recommended in the Revised Atlanta Classification [12]. A score of 0–4 is assigned for each organ system depending on the severity of dysfunction assessed by worst observations over a 24 h period for PaO2/FiO2 for pulmonary, creatinine rise for renal, and hypotension for cardiovascular system. A score of 2 or more in any system (corresponds to PaO2/FiO2≤300, creatinine >1.9 mg/dl, and systolic blood pressure < 90 not fluid responsive) defines organ failure. While organ failure may be transient, its persistence for greater than 48 h is defined as persistent organ failure.
Local complications refer to acute necrotic collections and acute peripancreatic fluid collections and their morphologic counterparts: walled-off necrosis (WON) and pseudocyst, respectively, which evolve over 4–6 weeks [12]. These are defined in Table 2.1. In the early phase of AP, acute necrotic or peripancreatic fluid collections can be diagnosed by CT scan or MRI. However, it is unreliable to determine the extent of necrosis within the first few days, and the extent of morphological changes does not necessarily correlate with the severity of organ failure. Further, an acute fluid collection may occur in 30–50 % of the patients but are not predictive of organ failure as most of them resolve spontaneously. Because of the above reasons, assessment of local complications by multi-detector contrast enhanced CT is most reliable when performed at least after 5–7 days after admission [4]. In patients with renal failure, MRI without contrast can be used to evaluate pancreatic necrosis on T2 weighted imaging. Local complications should be suspected when there is lack of expected clinical improvement.
The presence of pancreatic and/or peripancreatic necrosis defines acute necrotizing pancreatitis [12]. Its absence defines acute interstitial pancreatitis [12]. Brief hospital stay and early recovery are typical in acute interstitial pancreatitis although some patients may have acute peripancreatic fluid collections and a small proportion may develop pseudocysts late in the disease. Although useful in understanding the natural course of AP, this morphologic classification is not evident in the early stage of AP and therefore not effective in predicting outcomes.
(Peri-)pancreatic necrosis can be sterile or infected. Infection of (peri-)pancreatic necrosis typically occurs 7–10 days after admission in the late phase of AP. Infected necrosis should be suspected in patients with persistent organ failure or signs of sepsis exceeding beyond 7 days [4]. Both sterile and infected necrosis can result in persistent organ failure. Infected necrosis is usually associated with higher mortality rates [1]. However, many patients with infected necrosis may lack persistent organ failure and infected necrosis without persistent organ failure has significantly lesser mortality than those with persistent organ failure.
Other local complications of AP include gastric outlet obstruction which can delay enteral nutrition, biliary obstruction, splenic and portal vein thrombosis, celiac and splenic artery pseudo-aneurysms that can lead to brisk bleeding, disruption of main pancreatic duct leading to refractory fluid collections, and rarely colon necrosis. Recurrent AP can be seen in about 15–20 % of patients with AP related to heavy alcohol abuse and also in a few patients with idiopathic AP [16].
Prognosis , Risk Stratification , and the Revised Atlanta Classification
Historically, AP has been associated with a high mortality and adverse outcomes. Advances in management including aggressive supportive care for patients with AP has led to significant improvement in the outcomes of AP overall. With institution of current management recommendations, about 70–80 % patients with AP have a mild course requiring only a brief hospitalization and showing good recovery without progressing to the late phase of the disease. However, a subset of patients (estimates ranging from 15 to 30 %) will go on to develop moderately severe or severe disease with high morbidity (prolonged hospital stay and/or need for interventions), and mortality rates of 25–40 % [1, 12, 17, 18]. Predicting which patient will show mild disease with good outcomes and who will develop moderately severe or severe disease has historically been a challenge and continues to be very challenging today despite decades of efforts aimed at developing prediction tools and strategies. There have been no studies about predicting the moderately severe disease and those that addressed severe disease lacked high positive predictive value.
Many prediction tools including Ranson’s criteria, BISAP score, APACHE-II score, Harmless AP score, and other lab values in various combinations including BUN, hematocrit, CRP have been tested extensively for their utility of predicting severity [4, 6]. Most of these require 48 h for predicting severe disease by which time the clinical condition of the patient makes the severity obvious. None of the presenting symptoms or signs on physical exam or initial CT/MRI is helpful in predicting severity of AP.
Local complications and persistent organ failure are the most important determinants of mortality and outcomes in AP and therefore form the backbone of strategy to classify AP patients into high risk and low risk groups [12]. It is to be emphasized that none of these features can be defined at admission, full characterization of local complications may not be possible in the early stages of AP and persistent organ failure can only be defined at 48 h or later. In the Revised Atlanta Classification [12] (Table 2.2), persistent organ failure (or death) defines severe AP while lack of any local complications or organ failure defines mild AP. Transient organ failure and/or local complications define moderately severe AP. The mortality for mild AP is <1 % while that for severe AP is estimated to be between 20 and 40 % [4, 12, 17]. The mortality rate of moderately severe AP is similar to mild AP but is associated with much higher morbidity with local complications needing interventions and prolonged hospital stays [18].
Management
-
(a)
Initial assessment
As discussed above, none of the clinical or imaging or scoring systems are predictive of severity of AP. For all practical purposes, for the initial 48 h, all patients with AP should be considered as severe AP. Frequently patients without local complications evident on initial imaging are mislabeled as mild AP leading to adverse outcomes. For initial assessment, a careful attention should be paid to general high risk features in the patient characteristics (age >55, obesity), presence of SIRS, signs of hypovolemia, features of focal infections, altered mental status, and presence of other comorbid conditions. While most patients can be aggressively managed in medical wards, patients with hemodynamic instability or respiratory decompensation or altered mental status needing intubation should be admitted to ICU. In general, providers should maintain a low threshold for ICU transfer, and patients with persistent SIRS or organ failure in the first 24 h in particular should be considered for ICU care when possible or for intermediary care setting at the minimum [4].
-
(b)
Hydration
Aggressive hydration is most beneficial within the first 12–24 h [4]. The importance of early aggressive hydration cannot be overemphasized. A rate of 250–500 ml/h or 5–10 ml/h/kg of isotonic crystalloid (lactated Ringer’s solution preferred) should be provided unless cardiac, renal, or other comorbidities are prohibitive in which case the rate should be tailored to the patient’s comorbid conditions [4]. In patients with severe volume depletion, rapid boluses may be needed initially. An objective measure of early fluid resuscitation should be to decrease hematocrit, BUN and maintain good hourly urine output [4]. Fluid requirement should be reassessed frequently within initial 6 h and for the next 24–48 h.
-
(c)
Need for ERCP
In patients presenting with AP who concurrently have acute cholangitis (fever, jaundice, elevated alkaline phosphatase), ERCP should be performed within 24 h [4]. In most AP patients with gall stones without evidence of ongoing biliary obstruction, ERCP is not beneficial. When choledocholithiasis is suspected but there is no cholangitis or jaundice, MRCP or EUS can be performed for evaluation rather than a diagnostic ERCP. In patients who require ERCP, rectal indomethacin or prophylactic pancreatic duct stents should be used to reduce the risk of post-ERCP pancreatitis [4].
-
(d)
Supportive care
Oxygen by nasal cannula (or additional respiratory support as needed) is recommended in all patients with AP. In patients with organ failure, supportive care targeted to each affected organ system should be administered. Antibiotics should only be given when an extra-pancreatic infection is suspected or established. Prophylactic use of antibiotics in severe AP or in patients with sterile necrosis is not recommended [4].
-
(e)
Nutrition
Traditionally, the concept of resting the pancreas in AP by avoiding enteral route has been floated. On the other hand, increased intestinal and colonic permeability due to disruption of gut mucosal barrier because of absence of food in the gut is thought to increase infectious complications in AP. In most patients with mild AP, oral feeding with soft low fat diet can be started as soon as nausea, vomiting, and pain have lessened and advanced as tolerated [4]. A recent trial showed that oral feeding initiated at 72 h with provision for nasoenteric feeding if oral route not tolerated was as effective as early nasoenteric feeding enteral feeding initiated within 24 h in preventing infectious complications in AP with high risk of complications [19]. Parenteral feeding should be avoided unless enteral route is not feasible or effective. Nasogastric and nasojejunal feeding appear to be comparable in tolerability and efficacy.
-
(f)
Steps for prevention of recurrent episodes
In AP patients with gall stones, cholecystectomy is recommended within the index admission for mild AP and should be performed after active inflammation subsides and fluid collections resolve or stabilize in moderate or severe AP [4]. In patients with AP related to alcohol abuse, counseling and support for alcohol cessation should be offered.
-
(g)
Management of local complications
Local complications can lead to lack of expected clinical improvement or even relapse of symptoms, especially pain, nausea, and failure of oral intake. No intervention is necessary for asymptomatic local complications including fluid collections, pseudocysts, or pancreatic necrosis regardless of size, location, or extension [4]. These patients can be safely followed.
Infected necrosis should be suspected after 7–10 days in patients with persistent organ failure or obvious sepsis or in patients who deteriorate after initial improvement. A contrast enhanced CT should be obtained if not already performed to evaluate for presence of gas in the necrotic collection which can establish the diagnosis of infected necrosis. In the absence of CT features, either empiric approach with necrosis penetrating antibiotics (Ciprofloxacin, metronidazole, imipenem or piperacillin/tazobactam) or occasionally CT guided FNA sampling for gram stain and culture are appropriate [4]. Role of FNA is diminishing in recent years and either empiric antibiotics for suspected infection or intervention if there is no improvement seems to be more commonly used. Traditionally, infected necrosis was managed with surgical necrosectomy. However, recent studies demonstrated higher mortality in stable patients with infected necrosis treated surgically (~50 %) compared to minimally invasive methods of intervention (15–20 %) [11, 20, 21]. Prompt surgical debridement should only be performed in unstable patients with infected necrosis. In otherwise stable patients with infected necrosis, conservative approach is to administer antibiotics as noted above and closely monitor clinical status with a plan to perform minimally invasive necrosectomy (endoscopic, percutaneous, or surgical) after 4 weeks once the collection is walled off (WON) and the necrotic contents have liquefied (which otherwise is cement like and not amenable to minimally invasive debridement) [4, 20]. A subgroup of patients may be managed by antibiotics alone without needed minimally invasive debridement if on close follow-up, the patients continue to be asymptomatic. However, it is to be emphasized that patients with infected necrosis have a high mortality and therefore should be clinically monitored very closely [4, 11, 21].
If further improvements in morbidity and mortality were to be seen, a drug that can be used safely at presentation to prevent organ failure and necrosis is highly necessary. Pentoxifylline has been recently reported to have some effect in a small pilot study [22] and a large NIH funded study is currently in progress.
Conclusion
Acute Pancreatitis is an inflammatory disorder of the pancreas which can cause a severe disease with high mortality and morbidity. With advances in management, the outcomes of acute pancreatitis have improved considerably. Local complications and organ failure are important determinants of mortality. Patients can be classified into mild acute pancreatitis in the absence of local complications or organ failure and severe acute pancreatitis in the presence of persistent organ failure. The remainder with local complications and/or transient organ failure is classified into moderately severe acute pancreatitis. Aggressive management including hydration, feeding, and treatment of complications are crucial in acute pancreatitis.
References
Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–20.
Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–61.
Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–87 e1–3.
Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–15. 16.
Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep. 2009;11(2):97–103.
Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96.
Mutinga M, Rosenbluth A, Tenner SM, Odze RR, Sica GT, Banks PA. Does mortality occur early or late in acute pancreatitis? Int J Pancreatol. 2000;28(2):91–5.
Renner IG, Savage 3rd WT, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30(10):1005–18.
McKay CJ, Buter A. Natural history of organ failure in acute pancreatitis. Pancreatology. 2003;3(2):111–4.
Gloor B, Muller CA, Worni M, Martignoni ME, Uhl W, Buchler MW. Late mortality in patients with severe acute pancreatitis. Br J Surg. 2001;88(7):975–9.
Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):333–40. e2.
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11.
Dellinger EP, Forsmark CE, Layer P, Levy P, Maravi-Poma E, Petrov MS, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256(6):875–80.
Choi JH, Kim MH, Oh D, Paik WH, Park do H, Lee SS, et al. Clinical relevance of the revised Atlanta classification focusing on severity stratification system. Pancreatology. 2014;14(5):324–9.
Nawaz H, Mounzer R, Yadav D, Yabes JG, Slivka A, Whitcomb DC, et al. Revised Atlanta and determinant-based classification: application in a prospective cohort of acute pancreatitis patients. Am J Gastroenterol. 2013;108(12):1911–7.
Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096–103.
Spanier BW, Dijkgraaf MG, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: an update. Best Pract Res Clin Gastroenterol. 2008;22(1):45–63.
Vege SS, Gardner TB, Chari ST, Munukuti P, Pearson RK, Clain JE, et al. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis”. Am J Gastroenterol. 2009;104(3):710–5.
Bakker OJ, van Brunschot S, van Santvoort HC, Besselink MG, Bollen TL, Boermeester MA, et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014;371(21):1983–93.
Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–94.
van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–502.
Vege SS, Atwal T, Bi Y, Chari ST, Clemens MA, Enders FT. Pentoxifylline treatment in severe acute pancreatitis: a pilot, double-blind, placebo-controlled, randomized trial. Gastroenterology. 2015;149(2):318–20. e3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sah, R.P., Vege, S.S. (2016). Acute Pancreatitis. In: Dua, K., Shaker, R. (eds) Pancreas and Biliary Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-28089-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-28089-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28087-5
Online ISBN: 978-3-319-28089-9
eBook Packages: MedicineMedicine (R0)