Abstract
A wide variety of diseases may involve the basal ganglia and thalami, and neuroimaging plays a major role in their diagnosis. The causes of abnormalities in deep gray structures in adults may be broadly classified as toxic, acquired metabolic disorders, inflammatory and infectious diseases, vascular and neoplasms, and many of them represent emergencies and must be promptly reported.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Toxic encephalopathies
- Basal ganglia
- Thalamus
- Central nervous system
- Diffusion-weighted imaging
- Magnetic resonance imaging
Background
The thalami and basal ganglia (comprised primarily by the caudate nuclei, putamina, and globus pallidi) are part of the most important and better demonstrated deep gray matter structures on neuroimaging, especially on magnetic resonance imaging (MRI).
The basal ganglia are part of the extrapyramidal motor system, participating in the production of movement, but they are also involved in memory, emotion, and other cognitive functions. Clinical signs and symptoms related to lesions in these structures may vary from movement disorders (dystonia, bradykinesia, chorea, tremors) to coma [1].
The thalami are crucial in regulating consciousness, sleep, and alertness, also being responsible for relaying sensory and motor signals to and from the cerebral cortex. Lesions affecting the thalami often result in disorders of consciousness and sensory disturbances [2].
A wide variety of diseases may involve the basal ganglia and thalami, and neuroimaging plays a major role in their diagnosis. The causes of abnormalities in deep gray structures in adults may be classified as toxic, acquired metabolic disorders, inflammatory and infectious diseases, vascular and neoplasms [3].
Key Points
Etiology
Toxic Encephalopathies
Carbon monoxide (CO) poisoning: Brain damage after CO exposure results from complex and partially unknown mechanisms, but is mostly related to hypoxia. Typically it affects the globus pallidi and the caudate nucleus; injuries to the thalami and putamina are unlikely [4].
Methanol intoxication: It is a rare accidental or suicidal condition, which has also been described as a result of fraudulent adulteration of alcoholic drinks. Symptoms as headache, dizziness, weakness, and visual disturbances (due to optic nerve necrosis and/or demyelination) are prominent in acute intoxication. Toxicity may result from metabolism of methanol to formic acid [5].
Acquired Metabolic Disorders
Wernicke encephalopathy: It is a life-threatening condition that results from vitamin B1 (thiamine) deficiency and is characterized by the classic clinical triad of changes in consciousness, ocular dysfunction, and ataxia. Wernicke encephalopathy is often associated with chronic alcohol abuse, but many other conditions can also cause it (so-called nonalcoholic Wernicke) such as celiac and Crohn’s disease, hyperemesis gravidarum, and parenteral nutrition [3].
Manganese accumulation: In patients with cirrhosis or portalsystemic shunts, serum manganese is elevated and transferred to the brain. Manganese neurotoxicity (“manganism”) is characterized by psychological and neurologic abnormalities, similar to Parkinsonism (hypokinesia, rigidity, and tremor). Manganese has a preferential deposition in the central nervous system at the level of the globus pallidi, subthalamic nuclei, and substantia nigra [6]. Moreover, manganese accumulation has been described in other conditions such as maintenance hemodialysis, total parenteral nutrition, occupation exposure to manganese from welding, non-cirrhotic portal vein thrombosis, and congenital portalsystemic bypass with no intrinsic hepatocellular disease [6, 7].
Hyperammonemia: Acute brain damage may occur when the serum ammonia concentration suddenly increases especially in patients with chronic cirrhosis and manifests as hepatic encephalopathy [6].
Nonketotic hyperglycemia: It is an uncommon cause of chorea-ballismus in diabetic patients. Pathophysiologic mechanisms remain controversial, but include petechial hemorrhages, myelin breakdown products, or hyperviscosity, leading to T1 shortening of the putamen and head of caudate nucleus on MRI [8].
Osmotic myelinolysis: It is associated with rapid overcorrection of hyponatremia and may be seen in chronic alcoholic patients, malnourished patients, or chronically debilitated organ transplant recipients. Symptoms are usually related to a brainstem lesion and include seizures, disturbed consciousness, gait disturbances, and decrease or cessation of respiratory function [9].
Hypoglycemia: Typically occurs in diabetic patients who accidentally overdose while receiving treatment with oral hypoglycemic agents. Involvement of the basal ganglia seems to portend a poor prognosis [10].
Inflammatory and Infectious Diseases
Behçet disease: It is a recurrent multisystem vasculitis of unknown origin, and its classical triad includes oral and genital ulcerations with uveitis. The CNS is affected in 4–49 % of patients, and the most commonly reported findings are a preference for brainstem–diencephalic involvement with a tendency to resolve over time [11].
Viral encephalitis: The family of flaviviruses typically affects the subcortical gray matter, including thalami, basal ganglia, substantia nigra, and also the cerebellum. The geographic distributions are characteristic, with Japanese encephalitis being common in Asia, West Nile fever in Middle East, and Murray Valley fever in Australia [12].
Creutzfeldt–Jakob disease (CJD): It is a spongiform encephalopathy caused by an infectious protein (prion) characterized by progressive dementia, myoclonus, and periodic discharges on the electroencephalogram. Usually CJD leads to death within 1 year of disease onset [13, 14].
Vascular Disorders
Bithalamic stroke: The artery of Percheron is an uncommon anatomic variant, in which a single dominant thalamoperforating artery supplies both medial thalami with variable contributions to the rostral midbrain. Occlusion results in bilateral paramedian thalamic infarcts with or without midbrain involvement. Typical clinical onset is the triad of altered mental status, vertical gaze palsy, and memory impairment [9].
Spectacular shrinking deficit: Refers to a sudden major hemispheric stroke syndrome followed by rapid improvement within a few hours period, leaving mild or no deficits. In it there is selective neuronal death and it has been called “incomplete infarction” [15].
Deep venous occlusion: Thrombosis of the internal cerebral veins, basal veins, vein of Galen, or straight sinus is less common than that affecting the superficial sinuses with most patients presenting symptoms of elevated intracranial pressure that may rapidly progress to coma [9].
Hypoxic-ischemic injury: Severe hypoxic-ischemic injury in adults primarily affects the gray matter structures, including the basal ganglia, thalami, cerebral cortex (perirolandic and visual cortex), cerebellum, and hippocampi [16].
Neoplasms
Bilateral thalamic glioma: This rare glioma, usually an astrocytoma, is characterized by bilateral thalamic involvement and may affect children and young adults presenting with behavioral impairment. Despite its low-grade classification (World Health Organization grade II), the prognosis is poor [9].
Best Imaging Modality
MRI is the modality of choice for evaluating these pathologic conditions affecting the deep gray matter structures. Computed tomography (CT) may be used as the first diagnostic tool especially in an emergency setting [3].
T2-weighted imaging (T2WI) is the best MRI sequence to study abnormalities in the deep gray matter since most diseases demonstrate increased signal intensity. In specific cases, T1-weighted imaging (T1WI) may be informative, showing high signal areas in basal ganglia or thalamus and helping to narrow the differential diagnosis. The role of diffusion-weighted images (DWI) in the detection of acute cytotoxic brain damage in acute infarction, hypoxia, hypoglycemia, CJD, and Wernicke encephalopathy is critical and has been well described [3].
Calcifications present in metabolic conditions, such as Fahr disease and parathyroid disorders and hemorrhage can be found in poisoning, venous infarctions, and encephalitis, are easily detected using non-contrasted CT or MRI susceptibility-weighted imaging (SWI) phase sequences [3].
The MRI protocol must include fluid-attenuated inversion recovery (FLAIR), T2WI and T1WI, gradient echo (GRE), or SWI and DWI, and intravenous gadolinium administration is required when lesions are identified. In some situations, advanced MRI sequences, such as perfusion-weighted imaging (PWI) and MR spectroscopy (MRS), may help to distinguish a neoplastic from a nonneoplastic condition [3].
With regard to vascular disorders such as Percheron artery infarct, basilar artery occlusion, or deep venous thrombosis, MR angiography/venography and CT angiography/venography are alternatives to detect the sites of occlusions and collaterals. Conventional catheter angiography is reserved for doubtful cases [3, 9].
Major Findings
Most of the disorders that affect the basal ganglia and thalami show hyperintensity on T2WI. However, a constellation of imaging findings may help narrow the differential diagnosis as follows.
Toxic Encephalopathies
CO poisoning: The typical findings on MRI are bilateral hyperintensities in the globus pallidi and cerebral white matter on T2WI resulting from necrosis and demyelination (Fig. 19.1). Damage to the caudate nucleus, thalamus, or putamen is unlikely in this condition. The globus pallidi often show findings related to hemorrhagic infarction. DWI frequently demonstrates areas of restricted diffusion in the acute stage. Delayed leukoencephalopathy and T1 shortening in the globus pallidi may be found in chronic stages [4].
Methanol intoxication: The classic MRI findings are bilateral putaminal necrosis with varying degrees of hemorrhage. White matter edema, especially in the frontal lobes, and edema and contrast enhancement of optic nerves are additional imaging features [5].
Acquired Metabolic Disorders
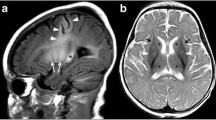
Wernicke encephalopathy: MRI demonstrates symmetric lesions in the medial thalami and the periventricular area of the third ventricle [3]. Symmetric alterations in the mammillary bodies have been observed in 57 % of patients (Fig. 19.2). There is a powerful correlation between contrast enhancement in the mammillary bodies and chronic alcohol consumption [17].
Different patterns of two common metabolic disorders. Wernicke’s encephalopathy in a patient with chronic alcohol abuse. (a, b) Axial FLAIR images show involvement of the mammillary bodies (white arrow on a) and of the periaqueductal gray matter (black arrow on a). There is also bilateral putaminal involvement and symmetric lesions involving the medial aspect of the thalami and the periventricular area around the third ventricle (white arrow on b). (c, d) A 46-year-old cirrhotic man presents with Parkinsonism due to CNS manganese accumulation. Axial T1WI demonstrate symmetric increased signal in the globus pallidi and substantia nigra
CNS manganese accumulation: Characteristi-cally the globus pallidi, subthalamic nuclei, and substantia nigra exhibit bilateral symmetric high signal intensity on T1WI (Fig. 19.2) [6]. The signal abnormality usually disappears after liver transplantation [6, 7]. Similar findings can be seen in patients with hereditary hemorrhagic telangiectasias, liver vascular shunts, and anemia and may be symptomatic.
Hyperammonemia: It presents as bilateral brain swelling and hyperintensity on FLAIR and T2WI as well as restricted diffusion in the basal ganglia, insular cortex, and cingulate gyrus. MRS characteristically demonstrates decreased levels of myoinositiol (3.5 ppm) and choline (3.2 ppm) and increased levels of glutamine (glutamate/glutamine peak at 2.2–2.4 ppm) (Fig. 19.3) [6].
A 50-year-old man with chronic liver disease and hyperammonemic encephalopathy. (a–c) FLAIR images (a, b) depict swelling and areas of increased signal in the insular cortex and cingulate gyrus. There are bilateral thalamic lesions (arrows on a). (c) Areas of cortical restricted diffusion are demonstrated on DWI (c). (d) MRS demonstrates decreased levels of myoinositiol (mI) (3.5 ppm) and choline (Co) (3.2 ppm) and increased levels of glutamine (Glx) (glutamate/glutamine peak at 2.2–2.4 ppm). NAA= n-acetyl aspartate which is also low
Nonketotic hyperglycemia: It is characterized by hyperdense changes in the putamen on CT that correspond to increased signal intensity on T1WI (Fig. 19.4). The process is either unilateral or bilateral, and, if unilateral, the imaging findings are typically contralateral to the affected hemibody. It has been reported that they usually resolve within a few months after glucose level normalization [8].
Acquired metabolic disorders. (a, b) A diabetic patient presenting with nonketotic hyperglycemia. There is abnormal hyperintensity on T1WI involving the left putamen and caudate nucleus (arrows). (c, d) Osmotic myelinolysis after a rapid overcorrection of hyponatremia. FLAIR images show abnormal hyperintensities areas in the cerebellar white matter (c) and central pons, typically sparing the peripheral fibers (d). (e, f) Hypoglycemia in a newborn. Axial DWI demonstrating multifocal lesions (restricted diffusion) in the parieto-occipital cortex and white matter. Notice the restricted diffusion in the splenium of corpus callosum as a consequence of excitatory mechanisms
Osmotic myelinolysis: The classic described symmetric trident-shaped or bat-shaped area of increased T2 signal in the pons may be associated with extra-pontine lesions involving symmetrically and bilaterally the deep gray matter (basal ganglia and thalami) as well as the white matter (Fig. 19.4). The affected areas may show restricted diffusion in the early stages [9].
Hypoglycemia: The MRI presentation is broad and includes bilateral and symmetric T2 prolongation and restricted diffusion in the cerebral cortex, basal ganglia, subcortical white matter, posterior limb of internal capsule, and splenium of the corpus callosum (Fig. 19.4) [10].
Inflammatory and Infectious Diseases
Behçet disease: Bilateral basal ganglia and midbrain involvement occurs in one-third of patients, and these lesions are hyperintense on T2WI, hypointense in T1WI, enhance after contrast administration, and are typically associated with vasogenic edema (Fig. 19.5) [12].
Neuro-Beçhet and flavivirus encephalitis. (a, b) Relapsing-remitting neuro-Behçet disease. Axial FLAIR images depict a left diencephalic–midbrain junction lesion and affecting the basal ganglia and the internal capsules. (c, d) Epstein-Barr virus encephalitis in 5-year old boy with selective bilateral abnormal hyperintensity in the basal ganglia on axial FLAIR (c) and coronal T2WI (d)
Viral encephalitis: Flaviviruses typically affect the subcortical gray matter, including thalami, basal ganglia, substantia nigra, and cerebellum manifesting as T2WI hyperintensities in involved areas (Fig. 19.5). Intralesional hemorrhages and restricted diffusion have also been reported. Common patterns may raise suspicions for specific etiologies as follows: Epstein–Barr virus typically presents as bilateral striatal encephalitis. Western Nile virus and Japanese encephalitis usually manifest as bithalamic hyperintensities on T2WI/FLAIR also affecting the substantia nigra [12].
CJD: Initially MRI demonstrates restricted diffusion usually limited to cerebral cortex and sometimes caudate nuclei, and over time this abnormality may spread to the anterior portion of putamina or involve them entirely (Fig. 19.6). In late stages, restricted diffusion may disappear and there may be abnormal T2 hyperintense areas in the cerebral cortex and basal ganglia and rapidly progressive brain atrophy. The classic described “hockey stick sign” (restricted diffusion in the bilateral pulvinar and medial aspects of the thalami) was described in the variant form of the disease, but can also be seen in the sporadic form [13, 14].
Spongiform encephalopathy. A 62-year-old man presenting with a rapidly progressive dementia and myoclonus diagnosed with Creutzfeldt–Jakob disease. (a–c) Axial DWI acquired respectively after 2 (a), 4 (b), and 6 (c) months from the symptoms onset show progressive hyperintensities in the cortex and basal ganglia
Vascular Disorders
Bithalamic stroke: Percheron artery occlusion results in bilateral paramedian thalamic infarcts with or without midbrain involvement [9]. This condition shows restricted diffusion in the affected areas and a distinct pattern of V-shaped hyperintensity on axial FLAIR and/or DWI might be seen along the anterior pial surface of the midbrain adjacent to the interpeduncular fossa (Fig. 19.7) [18].
Spectacular shrinking deficit: This condition is characterized on MRI by persistent hyperintense signal in the basal ganglia on T1WI and relative hypointensity on T2WI 7–10 days from the ictus (Fig. 19.8). The hyperintensity on T1WI gradually subsides with time, and the affected structures show atrophy over time [15].
Spectacular shrinking deficit. A 62-year-old man suddenly developed left hemiplegia and paresthesias that improved within 20 min suggestive of a transient ischemic attack. (a–c) The right putamen and caudate nucleus are mildly hyperintense on T1WI (a) and hypointense on T2WI (b). There are no significant changes on DWI (c)
Deep venous occlusion: Thalamic edema, characterized by increased signal on T2WI and FLAIR, is the imaging hallmark of this condition, and abnormalities may extend to the caudate nuclei and deep white matter. MR venography demonstrates absent flow in the deep venous structures (Fig. 19.9). Hemorrhage and areas of restricted diffusion are seen in some cases [9].
Deep venous occlusion. A 32-year-old woman using oral contraceptives presents with decreased level of consciousness. (a–c) Edema is depicted on FLAIR (a) especially in the left caudate nucleus and thalamus associated with hypointensity in the internal cerebral veins and straight sinuses on a T2*-weighted image (b). (c) MR venography shows absent flow in the deep venous system confirming the diagnosis of deep venous thrombosis
Hypoxic-ischemic injury: DWI demonstrates bilateral and symmetric restricted diffusion in gray matter structures including basal ganglia, thalami, perirolandic and visual cortex, as well as cerebellum and hippocampi usually within the first few hours after a hypoxic-ischemic event (Fig. 19.10). At the same time, T1WI and T2WI are often normal or demonstrate only subtle abnormalities. T2WI become positive in the early subacute stage (24 h to 2 weeks), showing hyperintensity and swelling. DWI abnormalities usually pseudonormalize by the end of the first week [16].
Neoplasms
Bilateral thalamic glioma: MRI and CT demonstrate a mass that enlarges and infiltrates bilaterally the thalami, usually with no contrast enhancement (Fig. 19.11) [9].
Imaging Follow-Up
There is no consensus for a standardized imaging follow-up for all deep gray matter lesions, and it should be clinically determined based on the initial diagnosis and evolution of the patients’ symptoms [3].
Main Differential Diagnosis
Although inherited metabolic disorders are not the focus of this chapter, they should be remembered as an important differential diagnosis for basal ganglia lesions in young adults, due to its classical presentation and good response to early treatment [19].
Wilson’s disease is a rare genetic condition in which copper accumulates in tissues, particularly in the liver and brain. Levels of copper might be at levels sufficient to destroy nerve cells. Kayser–Fleischer rings are a pathognomonic sign and are seen as deposits of copper in a ringlike fashion around the cornea. The most common brain finding among patients with neurologic symptoms is bilateral high T2 signal intensity in the striatum. In addition, the occurrence of high T1 signal intensity in the globus pallidus, putamen, and mesencephalon is associated with hepatic dysfunction [19].
Enlarged perivascular spaces (Virchow–Robin spaces) are very common along lenticulostriate arteries through the basal ganglia and become more prominent with age. On imaging, they characteristically display similar signal to cerebral spinal fluid (CSF) in all sequences and no surrounding gliosis unless giant (>10 mm) [20].
Globus pallidi calcifications are more frequently seen in older patients and are considered a normal incidental and idiopathic finding. It is important to remember that basal ganglia calcification may demonstrate high signal on T1WI mimicking manganese deposition due to calcium, reducing the relaxation of adjacent water molecules. SWI or GRE sequences can easily differentiate between them as calcium typically displays blooming effect not seen with manganese [21].
Progressive iron deposition in the brain also accompanies normal aging. In healthy adults, the maximum iron concentration is found in the globus pallidus, red nucleus, and pars reticulate of the substantia nigra. However, it is important to recognize that excessive brain iron deposits may be related to many neurodegenerative diseases [22].
Tips
-
Correlation of imaging findings with clinical and laboratory data is critical to make the correct diagnosis.
-
Putaminal necrosis typically occurs in methanol intoxication.
-
Bithalamic symmetric lesions hyperintense on T2WI are commonly seen in Wernicke’s encephalopathy. However, they may also be seen in gangliosidosis and ADEM.
-
Bilateral and symmetrical globus pallidi lesions in adults may be related to CO poisoning (hyperintense on T2WI) and manganism (hyperintense on T1WI).
-
Unilateral striatal T1 hyperintensity may be seen in the spectacular shrinking deficit and nonketotic hyperglycemia. Increased serum glucose level helps to distinguish these conditions.
-
Mammillary body involvement is typically seen in Wernicke’s encephalopathy and often in association with lesions in the medial thalami and periventricular areas of the third ventricle.
-
Symmetric abnormalities involving the thalami and substantia nigra may be seen in viral encephalitis (specially Western Nile virus and Japanese encephalitis).
-
Relapsing asymmetric lesions in the midbrain and diencephalon has been described in Behçet disease.
-
Bilateral basal ganglia and/or thalamic lesions may be associated with cortical lesions and restricted diffusion in hypoxic-ischemic encephalopathy, hypoglycemia, and CJD, which can be differentiated by their clinical presentations.
References
Milton WJ, Atlas SW, Lexa FJ, Mozley PD, Gur RE. Deep gray matter hypointensity patterns with aging in healthy adults: MR imaging at 1.5 T. Radiology. 1991;181(3):715–9. doi:10.1148/radiology.181.3.1947087.
Schmahmann JD. Vascular syndromes of the thalamus. Stroke J Cereb Circ. 2003;34(9):2264–78. doi:10.1161/01.STR.0000087786.38997.9E.
Hegde AN, Mohan S, Lath N, Lim CC. Differential diagnosis for bilateral abnormalities of the basal ganglia and thalamus. Radiograph: Rev Publ Radiol Soc N Am Inc. 2011;31(1):5–30. doi:10.1148/rg.311105041.
Beppu T. The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: a review of the literature. AJNR Am J Neuroradiol. 2014;35(4):625–31. doi:10.3174/ajnr.A3489.
Blanco M, Casado R, Vazquez F, Pumar JM. CT and MR imaging findings in methanol intoxication. AJNR Am J Neuroradiol. 2006;27(2):452–4.
Rovira A, Alonso J, Cordoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol. 2008;29(9):1612–21. doi:10.3174/ajnr.A1139.
da Silva CJ, da Rocha AJ, Jeronymo S, Mendes MF, Milani FT, Maia Jr AC, Braga FT, Sens YA, Miorin LA. A preliminary study revealing a new association in patients undergoing maintenance hemodialysis: manganism symptoms and T1 hyperintense changes in the basal ganglia. AJNR Am J Neuroradiol. 2007;28(8):1474–9. doi:10.3174/ajnr.A0600.
Wintermark M, Fischbein NJ, Mukherjee P, Yuh EL, Dillon WP. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. AJNR Am J Neuroradiol. 2004;25(6):975–6.
Smith AB, Smirniotopoulos JG, Rushing EJ, Goldstein SJ. Bilateral thalamic lesions. AJR Am J Roentgenol. 2009;192(2):W53–62. doi:10.2214/AJR.08.1585.
Johkura K, Nakae Y, Kudo Y, Yoshida TN, Kuroiwa Y. Early diffusion MR imaging findings and short-term outcome in comatose patients with hypoglycemia. AJNR Am J Neuroradiol. 2012;33(5):904–9. doi:10.3174/ajnr.A2903.
Kocer N, Islak C, Siva A, Saip S, Akman C, Kantarci O, Hamuryudan V. CNS involvement in neuro-Behcet syndrome: an MR study. AJNR Am J Neuroradiol. 1999;20(6):1015–24.
Gupta RK, Jain KK, Kumar S. Imaging of nonspecific (nonherpetic) acute viral infections. Neuroimaging Clin N Am. 2008;18(1):41–52; vii. doi:10.1016/j.nic.2007.12.004.
Ukisu R, Kushihashi T, Tanaka E, Baba M, Usui N, Fujisawa H, Takenaka H. Diffusion-weighted MR imaging of early-stage Creutzfeldt-Jakob disease: typical and atypical manifestations. Radiograph: Rev Publ Radiol Soc N Am Inc. 2006;26 Suppl 1:S191–204. doi:10.1148/rg.26si065503.
Ukisu R, Kushihashi T, Kitanosono T, Fujisawa H, Takenaka H, Ohgiya Y, Gokan T, Munechika H. Serial diffusion-weighted MRI of Creutzfeldt-Jakob disease. AJR Am J Roentgenol. 2005;184(2):560–6. doi:10.2214/ajr.184.2.01840560.
Fujioka M, Taoka T, Hiramatsu KI, Sakaguchi S, Sakaki T. Delayed ischemic hyperintensity on T1-weighted MRI in the caudoputamen and cerebral cortex of humans after spectacular shrinking deficit. Stroke J Cereb Circ. 1999;30(5):1038–42.
Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiograph: Rev Publ Radiol Soc N Am Inc. 2008;28(2):417–39. doi:10.1148/rg.282075066; quiz 617.
Zuccoli G, Gallucci M, Capellades J, Regnicolo L, Tumiati B, Giadas TC, Bottari W, Mandrioli J, Bertolini M. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol. 2007;28(7):1328–31. doi:10.3174/ajnr.A0544.
Lazzaro NA, Wright B, Castillo M, Fischbein NJ, Glastonbury CM, Hildenbrand PG, Wiggins RH, Quigley EP, Osborn AG. Artery of percheron infarction: imaging patterns and clinical spectrum. AJNR Am J Neuroradiol. 2010;31(7):1283–9. doi:10.3174/ajnr.A2044.
Kim TJ, Kim IO, Kim WS, Cheon JE, Moon SG, Kwon JW, Seo JK, Yeon KM. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. AJNR Am J Neuroradiol. 2006;27(6):1373–8.
Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiograph: Rev Publ Radiol Soc N Am Inc. 2007;27(4):1071–86. doi:10.1148/rg.274065722.
Adams AE. Basal ganglia calcification. Characteristics of CT scans and clinical findings. Neurosurg Rev. 1980;3(3):201–3.
Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini L. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252(1):165–72. doi:10.1148/radiol.2522081399.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Guedes, B.d.V.S., da Rocha, A.J., Hoffmann Nunes, R. (2016). Basal Ganglia and Thalamic Lesions. In: Hoffmann Nunes, R., Abello, A., Castillo, M. (eds) Critical Findings in Neuroradiology. Springer, Cham. https://doi.org/10.1007/978-3-319-27987-9_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-27987-9_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27985-5
Online ISBN: 978-3-319-27987-9
eBook Packages: MedicineMedicine (R0)