Abstract
The 21-year-old patient had breast augmentation with Silimed polyurethane foam-covered anatomical gel implants in the submuscular dual plane. At two and a half weeks postoperative, a double fold was noted. The discussion included transfer of implant to subglandular position, the cause of double fold, freeing of the inframammary fold, delaying surgery to see if double fold resolves, polyurethane foam-covered prostheses and lowering of the inframammary fold.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Submitted by Mayson: March 13, 2013
Age: 21 years
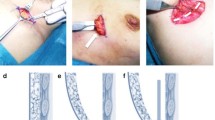
Preoperative: B cup, normal skin envelope, base width of each breast 12.3 cm (Fig. 66.1).
Primary breast augmentation: February 20, 2013.
Implants: Silimed polyurethane foam-covered anatomical gel implants (hereinafter called PUFs), style 30637, 315MD (315 mL).
Dimensions: 12.3 cm × 12.3 cm (round base) ×5.0 cm projection.
Placement: Although the operation was termed ‘submuscular’, the submuscular plane was dissected with enough detachment of the pectoralis infero-medially to make it more of a ‘dual plane’ rather than a ‘true’ submuscular. Therefore, the implants in the region of the double folds were in direct contact with the parenchyma in the lower poles.
Despite concerted efforts to prevent it, a ‘double fold’ still occurred (Fig. 66.2) and was first noticed by the patient 2 and a half weeks postoperatively once the swelling had subsided.
1.1 Relevant Operative Technique Details
-
Right incision: 19 mm below existing inframammary fold (IMF).
-
Left incision: 11 mm below existing IMF.
Inframammary incision, dissection down to the prepectoral fascia and then extended superiorly in the subglandular plane up to the level of the former IMF and beyond to detach the subcutaneous attachments of the former IMF to the pectoral fascia and to facilitate three vertical ‘releases’ of the former IMF area and lower pole parenchyma using cutting cautery as well as blunt ‘stretching’ of the lower pole in a concerted attempt to reduce the chance of ‘double fold’. The submuscular plane was then dissected with enough detachment of the pectoralis insertions from the infero-medial quadrant to make it more of a ‘dual plane’ rather than a true submuscular placement. Therefore, the lower part of the implant was in direct contact with the lower pole parenchyma.
Admittedly, it is only early days, and the implant could press out the former IMF, but the patient is justifiably concerned.
1.2 Previous Experiences
Over the last 3 years, I have implanted many more PUFs than smooth or textured implants, the great majority being anatomical as opposed to round PUFs. However, this case does highlight a disconcerting possibility that PUFs might be more likely to cause ‘double folds’ than non-PUF implants. I say this because since using PUFs, I have seen far more ‘double folds’ than I ever did with non-PUFs in patients where the IMF needed to be lowered. After much thought as to why it is happening more with PUFs (with no major changes in operative techniques), I have a theory which I invite members of our group to comment on, especially those who regularly use PUFs.
1.3 Theory
As we all know, a double fold is the original inframammary fold that has not been pressed out. It is a fold in the dermis primarily, but also in the subcutaneous tissues attached, that has not been stretched out to a round shape to fit the contour of the new breast. The dermis ends up retaining its shape and does not stretch out to the rounded contour we want. Most importantly, to free the original IMF, one needs to perform a subcutaneous dissection, not a submuscular one. The proper plane is to dissect the subcutaneous fat free from the underlying muscle and fascia, leaving the fat attached to the skin.
This is exactly what I did with this patient as well as all others that needed lowering of the IMF. Yet, despite a subglandular dissection up to and beyond the former IMF (prior to any submuscular dissection) plus vertical ‘releases’ by blunt stretching of the lower pole in the region of the former IMF, I am still plagued by the occasional ‘double fold’ which I cannot seem to totally avoid.
Admittedly, in many instances, pressure from the implant over time on the former IMF will cause a ‘double fold’ to improve or even totally disappear, but it can take time, and with PUFs I have seen it take up to 2 years to fully disappear. However, there is never a guarantee that it will, and in the meantime you have a disappointed and unhappy patient. Why could it be more likely with PUFs?
My theory is that the ‘Velcro’ effect of the PUF on the pocket wall prevents or limits the implant from acting as an obturator or dilator to stretch out the former IMF. In those instances where the ‘double fold’ improves or totally disappears over time, I suspect that the implant, acting like a tissue expander, on the skin and subcutaneous tissue eventually ‘wins out’ over the ‘Velcro’ effect!
Over to the Group for their comments, experiences and any suggestions.
P.S. Apologies for the quality of the photos. These were sent to me by the patient who lives interstate.
Mangubat: March 14, 2013
The key here is the date of the procedure, February 20, 2013. It has been less than 3 weeks and even a mild pseudoptosis can retain a double bubble effect for a while. I would like to see the preoperative pictures. But I think this will resolve over the next 3–6 months without further intervention.
Ah, the curse and the challenge of the ptotic breast. Here, patience is a virtue.
Beldholm
I would tell the patient that they need to wait at least 6 months and I would not contemplate doing anything for 12 months. I have had a few double folds especially in the ptotic patients and with bigger size implants. All except for one patient had near resolution of the issue after 12 months, and the one patient that still had a small double fold was happy to leave this.
I find it very helpful to take photos every time they see me and show them that the double fold is settling. Most are happy when they see that the fold is getting better over time.
Szalay
I agree that Brazilians are more likely to cause a double fold, and I agree with your proposed theory as to the cause. I have mainly experienced a double fold previously with the round Brazilians below muscle, and as a consequence, I feel it is unwise to lower the IMF when you are placing a round Brazilian below the muscle (in contrast to non-Brazilians where you can safely lower the IMF 1 cm when below muscle). When going above muscle, I have not had any problem when the IMF has been lowered up to a maximum of 1 cm when a round Brazilian is used. I find it interesting that you have experienced a double fold with a teardrop on the left side which you only lowered just over 1 cm. I have not yet had that happen; however, most of the teardrops I use are extra high, which fall away more sharply and are therefore less likely to double fold.
This double fold might go away but it might persist and require revision. Your hands are pretty much tied now though because it is a little over 3 weeks and these implants might have become stuck, so you need to wait until 6 months and do your revision then. You would then have some choices. Raise the IMF and switch to 30646 is one option (these are less likely to double fold because of their shape, but even with these it is unwise to lower the IMF more than 1 cm when below muscle). Another choice would be to go above the muscle or stay below the muscle and switch to non-Brazilian (but even then I think you need to raise your fold so the amount it was lowered does not exceed 1 cm.
I am now making a mental note not to lower the IMF when a medium-profile 30637 is used below the muscle.
Thanks for sharing this interesting case.
Mayson: March 16, 2013
Thank you for your input and suggestions.
However, you will note from the operative technique I describe that although the placement was termed ‘submuscular’, the implant was in direct contact with the breast parenchyma at the lower pole, so I cannot see that switching the rest of the implant to a subglandular plane is going to solve the problem.
As regards the 30646 range (for those members not familiar with 30646 shape, they are a PUF where the width is noticeably greater than the height), their advantage is that their reduced height may enable one to locate the incision in the existing IMF. However, many patients to whom I offer this option reject them on the basis of their shape.
Michael (Szalay), I have had double folds with all shapes and sizes of PUFs. I do not think that makes any difference, but I will keep on monitoring nevertheless.
Mangubat
After seeing preoperative photos, Tony (Mangubat) commented further. Very minor pseudoptosis. Be patient and I think it will resolve without further intervention. Thanks. This was a very good case to discuss.
Higgs: March 16, 2013
Thank you for your interesting case.
I would like to comment further after the following information. How far, if at all, did you dissect the native IMF ligament medially and laterally beyond the ends of your skin incisions?
Mayson: March 17, 2013
Three important points:
-
1.
In reply to your specific question, the previously described subglandular dissection in the area of the original IMF was not confined to the area immediately above the incision but instead across the entire width of the breast from medical to lateral borders at that level. I would be interested to hear what you had in mind when asking this.
-
2.
We must also keep in mind another cause of ‘double folds’ that is unrelated to polyurethane foam-covered implants (PUFs) and which I intentionally omitted from my previous discussion so as not to cloud or confuse the issue. I am now referring to those ‘double folds’ that develop because of different thicknesses of subcutaneous tissues above and below the original IMF when there is a need to create a new, lower IMF in order to correctly position the nipple over the implant. If there is a significant disparity in the thickness of breast tissue immediately above the original IMF compared to the thickness of the subcutaneous tissues below the original IMF, a ‘double fold’ will develop irrespective of what type of implant one uses. It will occur whether a smooth or textured PUF of whatever shape is used, and it will be permanent. Preoperative consideration and evaluation of this are essential in the planning stages because if a significant disparity is found, one must select an implant that will allow the inframammary incision to be placed in the original IMF.
-
3.
Mike (Higgs), on an unrelated matter, when we spoke recently on the telephone you mentioned that there have been reports of increased risks of infection with PUFs. I was not aware of this. For the benefit of the Group, could you please expand on this for us? Personally, I have only ever had one pocket infection in 5 years (case submitted) and that was not a PUF.
Higgs: March 18, 2013
George (Mayson), what I had in mind was how adequately you had destroyed the inframammary fold ligament. While I rarely use PU implants, I do not have the double fold problems you describe. I frequently use the subpectoral plane, lower the fold and deliberately divide the inframammary fold ligament under vision, especially laterally.
Regarding increased infection rates with PU foam-coated implants, I have had two surgeons communicate with me by email.
December 7, 2011: ‘5–6 patients with pocket infections’.
November 29, 2012: ‘4 explantations for suspected infection’.
It stands to reason, on infection control principles, that a prosthesis covered with foam would have a higher infection risk than a smooth prosthesis due to the interstices in which contaminating organisms could rest away from host defences and antibiotics. The risk of infection may be so small with both devices that in practice no meaningful difference is apparent. However, this is contrary to the anecdotal evidence I have received and the references I have attached [1, 2].
Walker: March 19, 2013
Why aren’t these available in the USA?
Hodgkinson
Darryl Hodgkinson sent the group a link to his website [3] that contains an article that is repeated below.
The fuzzy logic behind furry Brazilian breast implants: Many patients want to know about ‘furry’ Brazilian implants which are being promoted heavily and marketed in Australia by both cosmetic and plastic surgeons. Having used these implants under their correct name as polyurethane implants and having been involved with this implant for over 30 years, I feel qualified to comment on some of the myths leading information that is turning out in the media, the Internet, printed media and magazines.
The implants have been recently introduced: Not true. They were first introduced in the 1970s and were called the Ashley implant. Dr Ashley was a plastic surgeon in Los Angeles.
The foam is incorporated into the body: That is the problem. It degenerates with time and its by-products and the process of degeneration is chronic inflammation. There have been serious worries about the by-products of the implant in the past and whether it could have been carcinogenic or not. After the implant foam has degenerated, the body is exposed then to a plain underlying gel implant.
Foam implants do not move: That is true and that is a problem. They are fixed, unlike real breasts which move. They are glued in place and give a ‘headlamp’-like appearance.
Capsular contraction is reduced: Not really. Because of the chronic inflammation, the scar tissue is delayed from forming. Eventually, when the foreign body reaction dissolves the foam, the old silicone implant is exposed, and capsular contraction then occurs. There is a honeymoon period. Dr Ben Cohney reviewed his cases of this type of implant with the 1980s version of the polyurethane implant, and they ended up with a high rate of capsular contraction. Removing the capsule and putting in a new implant is really difficult because the foam has broken off and is nearly impossible to remove and it is a real chore to try and clean it up.
Other disadvantages:
-
1.
There is a bigger scar and a big incision is necessary to insert the implant. If you are pushing or shoving the implant in, it is probably breaking and fracturing the polyurethane which is unadvisable. You cannot put this implant through the armpit or the bellybutton.
-
2.
It is generally placed under the breast tissue alone which with time the breast tissue degenerating the implant will show, and this gives an obvious ‘boob job’ especially in the upper pole.
-
3.
Texture products are more palpable than smooth and the furry Brazilian is a textured implant.
Other concerns:
-
1.
This implant is not FDA approved in the USA, the biggest market where still saline implants have a very high percentage of utilisation because of the long-term satisfaction that can be achieved with surgeons who know how to use the implant.
-
2.
KY jelly is used by some surgeons to insert the implant, but there are serious questions as to whether this off-label use of KY jelly and KY jelly may have products in it that we do not know whether that is a good thing for them to be introduced around an implant or into a body cavity.
-
3.
The most fuzzy of logic is the one about the reduction of breast capsular contraction. In single series by surgeons over the last 20 years, smooth-walled saline implants put under the muscle or in a partial subpectoral pocket have a rate of capsular contraction as low as less than 2 %. So why would one want to change from using the safest of all of implant fillers, intravenous saline? Our body is 50 % saline.
The polyurethane implant has a place in the very established most difficult cases of capsular contraction which these days is fortunately not so common, due to the fact that people are not getting injections of silicone into their breasts and the new cohesive gel implants are not giving quite as much trouble in the long run of causing bad capsular contractions. I have used foam implants but only in these very selected cases which is the only indication, not in a primary operation. I see no place for it in the primary breast augmentation.
Surveillance of saline implants is virtually nil as if they fail or they break, then one knows instantly. Saline breaks at the same rate as any gel implant, but the surveillance necessary in a gel implant is much greater as one is not sure if it is broken, leaking or rotated, and in these cases, special tests which are expensive need to be performed to evaluate the patient and are often inconclusive.
In conclusion:
Finally, beware of any product and beware of any surgeon who promotes any new product and be aware that many of these surgeons are paid by the manufacturer or get special deals by the manufacturer. Ascertain the surgeon’s disclosures of why he is promoting this implant so strongly. I have no disclosures myself regarding breast implants. All I have is an experience of over 30 years and operating on over 6,000 women for breast implants, and for this reason, I feel obliged to alert women considering breast implants to have an open mind and not necessarily choose the newest and most sexy-sounding implant, especially if it is being highly marketed by surgeons enthusiastic to get you on the operating table.
Fleming
Firstly, a disclosure that I have acted as a paid consultant from time to time for Silimed, one of the manufacturers of polyurethane foam-covered implants. I agree with George Mayson’s analysis of the genesis of double folds. The only way to 100 % avoid a double fold is never to lower the IMF or to only operate on patients who do not have a native IMF. Once the IMF is lowered, a double fold can occur and it may or may not persist. This is true even if a premuscular plane is used and all of the releasing and lower pole expansion methods that George (Mayson) correctly performed are utilised. In some patients, there will be a demarcation line between where the implant is covered with breast tissues and where it is covered by the tissues that preoperatively were part of the distal chest wall. The best way to avoid double folds, and to reduce the severity of those that do occur, is to develop an operative plan that minimises the risk.
-
1.
Identify the relative risk for the patient. This includes, but is not limited to, the nipple-IMF distance, the density of the breast tissue, the mobility of the IMF (does it disappear when the patient raises her hands above her head), the degree of constriction of the lower pole, the nature of the chest wall distal to the IMF and the patient’s desired size.
-
2.
In anybody other than a low-risk patient, avoid lowering the IMF by more than 1 cm and use the subglandular, subfascial or a muscle-splitting dual plane. Limiting the lowering of the IMF often means either using a small round base implant or a shaped implant where the vertical height is less than the horizontal width. For most patients, the small round option will be too small and the shaped implant will be the best choice. Since shaped implants have a high risk of rotation if they do not adhere to the capsule, it is best to use a polyurethane foam-covered shaped implant.
This is the style 30646 in the Silimed range that Mike Szalay and George (Mayson) mentioned earlier. This is the commonest shape I use not only to avoid double folds but also to preferentially create cleavage without too much vertical height. The upper pole fullness is determined by which profile and size is chosen. I have never knowingly had a patient who has declined this type of implant because of its shape. I show the patient how its use will reduce the risk of a double fold and then show examples of double folds and pre- and postoperative results with the 30646 implant. George’s (Mayson) patient was at a high risk of double fold, and this could have been reduced or avoided by using an appropriately sized style 30646 implant and not the round-based anatomical implant she now has. I suspect this double fold will persist and a replacement with 30646 will be indicated after about 6–12 months. When I first started using PU foam implants in 2004, only the round style was available, and I found that double folds were not more likely to occur compared with smooth and textured implants but were more likely to be persistent. I am sure George (Mayson) is right, and this is because the Velcro effect means the lower pole tissues are not supporting the weight of the implant. However, this lack of pressure on the lower pole is desirable in every other respect as it reduces the chance of downward displacement. Also, it reduces the likelihood of the subtle bottoming out of non-adherent smooth and textured implants that commonly detract from the result after several years, all the more commonly in larger implants.
I am saddened by Darryl Hodgkinson’s contribution. I have great respect for him, but in this instance his triumphant posting of his advertising material in this forum is, with respect, embarrassing. Darryl is entitled to his opinions and preference for saline implants, but he is not entitled to his own facts regarding polyurethane foam implants. I do not propose to go through his material and expose its errors and anachronisms one by one. For those of you who would like to read the evidence-based, peer-reviewed and up-to-date facts about polyurethane foam, Silimed can provide you with a copy of the chapter I wrote on the subject in ‘Biomaterials in Plastic Surgery: Breast Implants’ [3]. I have copied the abstract and conclusions below (Appendix A).
Regarding infection rates, there is no evidence of an increase in infection rates with PU foam implants compared to smooth and textured implants. The data Mike Higgs has submitted is from the mid-1980s, and I note the surgeons were using a previous iteration of the foam implant technology explained in full in the chapter. Although this is not relevant for infection rates, it has implications for some of the other comments in the second of Mike’s references. The first reference reported in 290 patients is a 0.7 % infection rate per patient (0.35 % per implant), the other a 9 % infection rate per patient with predominantly Staphylococcus epidermidis in a series of 88 cases. This group suggested methods by which they might reduce their contamination of the implants. It is not logical that these data sets are evidence that the type of implant, as opposed to the surgical technique, is the cause of the high infection rate in one group. Mike’s (Higgs) anonymous anecdotal reports are just that. Quite what ‘4 explantations for suspected infection’ means is uncertain. Presumably, specimens were sent for culture at explant. The suggestion that bacteria might secrete in the non-smooth surface is reasonable but not supported by any comparative evidence showing this theoretical risk results in an increased infection rate with polyurethane foam when proper techniques are used. Also, if this was a significant issue, then textured implants should have a higher infection rate compared with smooth, and there is again no evidence of this that I am aware of.
Shiffman: March 20, 2013
Polyurethane implants were shown to slough the covering (at times) and break down into carcinogenic substances.
Fleming
The sloughing Mel (Shiffman) refers to was in fact delamination of the polyurethane foam layer that could sometimes occur when the foam layer was glued to the shell. This problem was solved in the late 1980s when the use of glue was discontinued and instead the foam layer vulcanised to the shell. Silimed has manufactured PU foam implants continuously since 1988 and has always used the vulcanisation process, and delamination does not occur with their implants.
Regarding the alleged breakdown of the foam into carcinogenic substances, this is covered in considerable detail in the book chapter in the section on safety. In summary, concerns were raised in a biochemistry journal paper in 1991 that identified the presence of raised levels of 2,4 TDA in the urine, but none in the blood, of two patients who had PU foam implants. 2,4 TDA is a known carcinogen in high doses in rodents but has not been shown to be a human carcinogen in any dose. Since in excess of 100,000 US women had had PU foam implants at that time, research was commissioned by the FDA to investigate these reports. The conclusions were that the original report methodology was flawed and that 2,4 TDA had been created in vitro by the authors when they prepared the urine samples by boiling them in 6× normal hydrochloric acid for 1 hour prior to analysis. This was why, despite its presence in the urine samples, no 2,4 TDA was detected in the same patients’ blood. There was none because, in vivo, insignificant amounts of 2,4 TDA are produced as a result of the slow degradation of PU foam by inflammatory cell esterases. The FDA announced there was no significant risk (‘negligible’ was the word they used), to patients with PU foam implants in a published statement in 1995. Details of the research, and the same conclusions, were published in PRS by Hester in 1997 [4].
The reason that PU foam-covered implants are not available in the USA today is because neither of the two manufacturers has applied for them to be approved by the FDA. US supply ceased in 1991 when Surgitek, a division of Bristol Myers, stopped selling their US-made PU foam implants. This was a commercial decision taken at the time of the subsequently discredited report of raised levels of urinary 2,4 TD and the imminent moratorium on all silicone gel implants in the USA that ran from 1992 until 2006. Silimed product rights are owned in the USA by Sientra, who has FDA approval for Silimed’s smooth and textured implants. Sientra also made a commercial decision not to apply for PU foam approval at the same time as the non-foam implants.
Fortunately, for patients and surgeons here in Australia, the TGA approved the unrestricted use of PU foam implants from January 2008.
Shiffman
I find Daniel’s (Fleming) comments very interesting.
-
1.
The cause of the double fold or double bubble has nothing to do with the implant. It is a result of placing the implant under the muscle where the original fold attaches from the pectoralis fascia to the dermis. Simply replacing the implant over the muscle with excision of any residual fibrosis in the dermis of the skin will solve the problem of double bubble.
-
2.
Polyurethane implants are illegal in the USA.
-
3.
The attributes of polyurethane implants over textured implants are interesting; however, capsule contracture is reduced if the depths of the impressions are over 100 μ (not present in Mentor implants) in textured implants. There is no adequate comparison study between Mentor and McGhan implants regarding capsule contracture.
-
4.
Lowering the inframammary fold is a standard procedure in breast augmentation. It is required for almost all patients. The pectoralis attachments to the ribs would have to be released but adequate postoperative compression at the new inframammary fold is essential.
Any postoperative inferior displacement of the implant can be treated with Roy Morgan’s transcutaneous suturing [5] or simply suturing the fibrous capsule (capsulorrhaphy) at the appropriate level with an open surgical procedure or use the hammock capsulorrhaphy by Moufarrège [6].
-
5.
The patient in question has a raised right implant that is possibly due to inadequate compression of the top of the implant postoperatively, the tendency for the pectoralis muscle to push the implant upward, and possible capsule contracture. The treatment would be to replace the implant over the muscle or do capsulectomy or capsulotomy with transection of the fibrous band in the subdermis at the original inframammary fold (possibly using an expander to stretch the fold). I would avoid the expense of new implants at this time.
References
Umansky C (1985) Infection with polyurethane-coated implants. Plast Reconstr Surg 75(6):925
Herman S (1985) Infection surrounding Meme implants. Plast Reconstr Surg 75(6):926
Fleming D (2012) Polyurethane foam covered breast implants. In: Peters W, Brandon H, Jerina KL, Wolf C, Young VL (eds) Biomaterials in plastic surgery: breast implants. Woodhead, Cambridge, UK, pp 96–120
Hester RT Jr, Ford NF, Gale JP, Hammett JL, B.S, Raymond R, Turnbull D, Phil D, Frankos VH, Cohen MB (1997) Measurement of 2,4-toluenediamine in urine and serum samples from women with Même or Replicon breast implants. Plast Reconstr Surg 100(5):1291–1298
Yoho R (2009) Inframammary fold elevation and external synmastia repair: Morgan-Metcalf method. In: Shiffman MA (ed) Breast augmentation: principles and practice. Springer, Berlin, pp 601–604
Moufarrège R (2009) Hammock capsulorrhaphy. In: Shiffman MA (ed) Breast augmentation: principles and practice. Springer, Berlin, pp 569–572
Handel N (2006) Long-term safety and efficacy of polyurethane foam-covered breast implants. Aesthet Surg J 26(3):265–274
Vázquez G, Pellón A (2007) Polyurethane-coated silicone gel breast implants used for 18 years. Aesthet Plast Surg 31(4):330–336
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix A
Appendix A
Abstract
Polyurethane foam-covered silicone gel breast implants have been proven to dramatically reduce the incidence of capsular contracture in primary and secondary patients in every study published over more than four decades. Analysis of capsular contracture at the molecular level and of the difference between textured and polyurethane foam surfaces shows why polyurethane foam is effective in preventing capsular contracture and texturing is not. Concerns about the safety of polyurethane foam have been exhaustively investigated and disproved.
Alleged difficulties in the use and removal of these implants are discussed and revealed to be unfounded. There is no logical reason not to use polyurethane foam-covered implants as first choice in all patients.
Conclusions
Polyurethane foam-covered breast implants have the lowest rates of capsular contracture, the commonest complication of breast augmentation and the commonest reason for reoperation. They also reduce the second commonest reason for reoperation, implant displacement. The low rates of these complications are known to be sustained for at least 15 years post-implantation. There is no evidence that the advantages of polyurethane foam-covered implants over smooth and mechanically textured implants diminish in the long term. Polyurethane foam-covered implants have been proven to be safe over more than four decades of use in humans. Theoretical concerns about possible carcinogenicity have been exhaustively investigated and disproved.
Textured implants were developed as an attempt to mimic the proven efficacy of polyurethane foam-covered implants before the safety of foam had been established beyond doubt. Textured implants have failed to deliver the benefits of foam.
Foam-covered implants are not difficult to use but they are different to use.
The learning curve for surgeons wishing to give their patients the reduced risks of polyurethane foam is simple once understood. Surgeons often start using foam implants in secondary cases where the patient already has contracture, rotation or displacement. Other than a fear of making a mistake in a primary patient, there is no logical reason for this, and patients are better served with primary use of foam-covered implants to reduce their risk of becoming a secondary patient. Other authors agree.
There is nothing . . . to suggest that polyurethane foam, or its in vivo breakdown products, pose a threat to the health or safety of patients. Polyurethane implants have measurable advantages over smooth and mechanically textured gel-filled prostheses and do not appear to be associated with an increased risk of complications or morbidity.’ [7]
“Currently, given our wide experience with the use of polyurethane-coated silicone gel implants, we may state they are the best option for augmentation mammoplasty, and have the lowest incidence of fibrous contracture” [8]. ‘During the span of this author’s practice, he has never been able to match the number and quality of superior results exemplified by these patients when using other devices.’ [4]
‘I think the evidence in favour of preferable use of PU covered implants is overwhelming compared to smooth or textured implants and it is clinically negligent to not put these facts to the patient.’ (J. Frame, Professor of Aesthetic Plastic Surgery, Anglia Ruskin University, UK, 2011, personal communication). Frame’s view is based on more than 40 years of accumulated evidence of the safety and efficacy of polyurethane foam as a covering for breast implants and the undeniable results of the core studies, showing the much higher rate of complications with both smooth and textured implants. Unless the patient is in one of the few countries where polyurethane foam implants are not available, it is unethical and is increasingly likely to be found to be negligent, not to inform patients seeking breast augmentation of the fact that using polyurethane foam-covered implants reduces the risk of the surgery. In the USA, which gave the world polyurethane foam-covered implants in 1968, they remain unavailable. Because of the FDA’s policy of requiring US-based trials prior to approval of a specific breast implant, no matter what the duration and weight of evidence from other countries, patients will be denied access to the safest type of breast implants for many years yet. Ironically, senior surgeons in the USA already know this. Dr Leroy Young commented following his presentation updating delegates about polyurethane foam-covered implants at the 2009 American Association of Aesthetic Plastic Surgeons’ annual breast meeting in Santa Fe, ‘…there were a number of surgeons who had used the devices when they were available in the US who uniformly said they were the best implants they ever used.’ (L. Young, Plastic Surgeon, St Louis, 2009, personal communication).
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mayson, G. et al. (2016). Case 160: Double Folds and Polyurethane Foam-Covered Implants. In: Higgs, M., Shiffman, M. (eds) Cosmetic Breast Cases. Springer, Cham. https://doi.org/10.1007/978-3-319-27714-1_66
Download citation
DOI: https://doi.org/10.1007/978-3-319-27714-1_66
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27712-7
Online ISBN: 978-3-319-27714-1
eBook Packages: MedicineMedicine (R0)