Abstract
Subxiphoid and suprapubic hernias pose a difficult challenge as the fascial defects abut bony structures. Additionally, care must be taken during fixation of the mesh for repair in order to avoid viscera and minimize potential long-term pain. Laparoscopy is an effective approach to repair these defects. Thorough knowledge of the anatomy and careful dissection for mesh will minimize complications and allow for a durable hernia repair.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Background
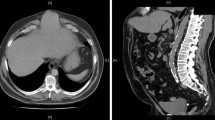
Subxiphoid defects can be congenital or incisional , usually following coronary bypass procedures or subcostal incisions for liver or foregut procedures (Fig. 24.1). Congenital epigastric defects can approach the xiphoid as well. Frequently, epigastric defects can be multiple and well suited for laparoscopy to avoid missed defects. Solitary defects can be addressed in an open fashion either with suture alone or mesh reinforcement. The incidence of subxiphoid hernias is unknown, as most authors do not routinely separate these types of defects in their reports.
Suprapubic hernias are almost always incisional in nature. Fascial defects that are within 5 cm of the symphysis pubis are considered suprapubic (Fig. 24.2). These types of hernias are more common in females due to gynecologic procedures via a lower midline or Pfannenstiel approach. Additionally, colorectal procedures and urologic procedures through a lower midline incision can result in suprapubic-type defects. The true incidence of suprapubic hernias is not well reported, as the definition varies by author. In our database of 860 laparoscopic ventral repairs, 15% required bladder mobilization and were classified as suprapubic [2].
Many times the subxiphoid or suprapubic areas are approached during a routine incisional defect that involves the midline. For incisions that course from “stem to stern,” incisional hernias may result that are both subxiphoid and suprapubic. These are especially challenging when it comes to placing sutures for mesh fixation. In this chapter, I will discuss the nuances of the laparoscopic approach to subxiphoid and suprapubic hernias.
Preoperative Consideration s
By definition, a subxiphoid or suprapubic hernia is one in which the extent of the fascial defect is within 5 cm of the bony prominence. Preoperative imaging of the abdomen and pelvis using computed tomography (CT) is critical to plan one’s approach. On CT imaging, it is important to measure the number of “cuts” from the xiphoid down to the superior aspect, or from the symphysis up to the inferior-most point of the fascial defect. This determination is more important for suprapubic defects because preoperative knowledge will prompt the surgeon to plan for potential saline infusion of the bladder prior to draping. This technique will be described in further detail in the “Technical Considerations” section.
Apart from imaging, all other preoperative concerns mimic those of any incisional hernia patient that is being considered for a laparoscopic approach. The patient must be able to tolerate general anesthesia. Preoperative optimization should include tobacco cessation, management of blood glucose, and reasonable weight control.
Following laparoscopic repair of subxiphoid and suprapubic hernias, pain management is definitely an issue. The subxiphoid repair is especially uncomfortable due to placement of sutures and fixation constructs along the sensitive costal margin. Proper preoperative consent should address this concern with patients. Non-narcotic measures for pre-emptive pain control should be considered and addressed preoperatively, not after the fact. The use of preoperative “pain cocktails” to include intravenous non-steroidal analgesics, epidural catheters to assist with postoperative analgesia, and low-dose ketamine infusions can be utilized to improve patient satisfaction and pain control postoperatively. A collaborative approach with anesthesiology can help to establish enhanced recovery pathways for a better patient experience.
Technical Considerations
Subxiphoid
For epigastric and subxiphoid hernias, it is not critical to tuck the patient’s arms. The surgeon will be positioned typically at the patient’s lower quadrant and working cephalad. We recommend always tucking the arms, however, to avoid any potential unexpected surprises like adhesions extending down to the inferior aspect of the midline, or an unanticipated umbilical defect.
For adhesiolysis during a subxiphoid hernia, the transverse colon should always be identified. Once its location is established and shown to be well away from the defect, takedown of adhesions can proceed rather quickly. The liver and stomach can be involved in subxiphoid defects; however, they typically reduce easily and are much easier to deal with if injuries occur to them. Once the upper abdomen is cleared of adhesions, the falciform should be taken down. This maneuver requires energy for hemostasis, which is why confirmation of the location of the transverse colon is critical. Monopolar or ultrasonic energy can be used to mobilize the falciform ligament at its juncture with the abdominal wall. This dissection should extend to at least 5 cm superior to the edge of the defect to allow for flush mesh placement. Not infrequently, the falciform ligament may be involved in the hernia defect. It should be grasped and brought into the abdominal cavity to visualize its insertion point into the underside of the fascia.
Mesh Orientation and Fixatio n
For atypical location hernias, placement of the mesh can be the most difficult step of the operation. Due to the bony structures and the vicinity of important structures like diaphragm, pericardium, iliac vessels, etc., placement of sutures and orientation of the mesh can be tricky. Some additional time should be given to these steps to avoid improper overlap and potential recurrences long term.
Following safe adhesiolysis , the defect is prepared for mesh placement. Spinal needles can be used to mark the edges of the defect in a lateral and cephalad-to-caudad orientation. Many techniques to measure the size of the defect can be employed. We use an internal metric ruler to determine the distance between the edges of the defect. Umbilical tape or suture can be stretched between the two marks as well. Some measure while the abdomen is desufflated. The midpoints of the defect should be determined and marked externally on the patient. These marks will be important to position the mesh precisely.
Particular note should be made of the distance from the superior aspect of the defect and the tip of the xiphoid process . The determination of mesh size and location of the superior suture (if used) will be based on this measurement. If the superior aspect of the hernia defect is at the xiphoid, in order to achieve a 5-cm mesh overlap, the superior suture should be placed 5 cm off the mesh edge. For example, if the defect is 10 cm long, a mesh that is 20 cm in length will be selected. However, if the superior aspect of the defect is 3 cm from the xiphoid process, the overlap will be calculated to allow for 5 cm of overlap onto the ribs in addition to the distance from the xiphoid. So, for the same 10 cm long defect, a mesh that is 23 cm in length would be chosen. It is also important to note the distance from the lateral edges of the defect and the costal margin. For patients with steeply sloped ribs, sutures at the lateral edge may have to be placed away from the mesh edge to avoid passing them through the chest wall.
Once the mesh is introduced into the abdominal cavity through a trocar, the mesh is unfurled. The first suture to be retrieved is the superior suture at the level of the xiphoid. One of the lateral sutures is then placed along the grid that was created earlier. The assistant pulls up on these two sutures and the mesh is stretched inferiorly to gauge the location of the inferior suture. The same technique is used to place the final lateral suture. Once the sutures are secured, tacks are placed. The decision to use permanent versus absorbable tacks is surgeon dependent . However, the use of absorbable tacks does not change the fact that no fixation constructs should be placed above the costal margin! A double-crown approach may be utilized as long as all tacks are caudal to the costal margin. The superior aspect of the mesh is left to be held in place by the liver, by holding the mesh in place during desufflation of the abdominal cavity. Frequently, suturing the edge of the mesh or utilization of the glue is needed to ensure that no bowel is trapped between the mesh and the diaphragm. No tacks should be used cephalad to the costal margin and xiphoid process in order to avoid devastating cardio-pulmonary injuries (Fig. 24.3).
Suprapubic
Positioning of the patient is more critical in suprapubic hernia repai rs. The arms must be carefully padded and tucked at the side of the patient. Given that the fascial defect is inferior and the surgeon will be standing at the patient’s head, both arms should be tucked to prevent harm to the patient’s arm while leaning against the arm board. Tucking the arms will also prevent undue stress on the surgeon’s back that results from twisting and other gyrations used to avoid the outstretched arm. The patient should be secured to the bed with the waist strap and additional tape around the thighs if necessary. During dissection, steep Trendelenburg positioning helps to assist with retraction of the intestinal contents. Pads that minimize sliding of the patient can be considered as well.
Intra-operative bladder infusion is critical to facilitate its safe dissection. A three-way urinary catheter should be placed and the bladder infusion should be set up prior to draping. Standard intravenous infusion tubing is attached to the infusion port using the luer-lock tip. When it is time for bladder infusion, the nurse should place a clamp on the tubing that drains the urinary catheter close to the catheter. This clamp should not be placed on the urinary catheter itself or the tubing. Experience has taught us to instruct the nurse prior to prepping and draping where to place the clamp to avoid any confusion during the case. We infuse 250–500 cm3 of Normal saline into the bladder to identify its superior extent so that the peritoneal flap can be safely developed superior to this margin. Signs of injury to the bladder include visualization of the urinary catheter balloon, excessive bleeding, or a rush of fluid. Once the peritoneal flap is raised, the remaining portion of the dissection to develop the space of Retzius is largely blunt. There may be small venous tributaries to the bladder, but these are easily controlled with light touches of the cautery. Even in multiply operated patients, keeping the dissection close to the abdominal wall when lowering the bladder flap will help to avoid injury. The bladder is much thicker than the peritoneum. If the dissection does not progress bluntly, or if the tissue that is being dissected is very thick or bleeds a lot, the surgeon should reassess the plane. Once the pubic symphysis is visualized, dissection should continue for 1–2 cm inferior to symphysis to allow for subsequent mesh overlap. Cooper’s ligaments should be identified bilaterally (Fig. 24.4). At the lateral edge of Cooper’s ligament, the entrance to the femoral canal and iliac vessels has to be identified. Careful dissection of the medial aspect of the myopectineal orifice is essential for sufficient mesh overlap, but extreme caution in that area is necessary to avoid devastating injuries to major vascular structures.
Bladder injuries can occur. Usually, cystotomy results from impatience and not instilling the bladder with saline. Injuries should be repaired based on the comfort level of the surgeon. A two-layer repair with absorbable suture is ideal. Since the injury occurs at the dome of the bladder, large bites can be taken without concern for compromising the bladder lumen or injuring the ureters. The decision to proceed with the hernia repair is again the choice of the surgeon. Urine is technically sterile, and multiple reports describe repair of the bladder laparoscopically and completion of the hernia repair without any infectious complications. This approach is our preference, but it is also acceptable to abandon the repair and bring the patient back to the operating room in 3–5 days to complete the repair and place the mesh. This time frame is chosen because adhesions will not have formed and there will be enough time to clear any bacterial contamination.
Once bladder mobilization is complete, the defect size is assessed as described earlier. The distance from the symphysis to the inferior aspect of the fascial defect should be determined. In contrast to the subxiphoid hernia, the inferior suture in the suprapubic defect will need to be positioned at a distance from the mesh edge to allow for appropriate overlap onto the pubis. By leaving 5 cm of overlap beyond the symphysis, the mesh can be secured to Cooper’s ligaments bilaterally. The potential weak point of the repair of suprapubic defects is inferior. Recurrences are more likely inferior due to improper mesh overlap and/or fixation. In our experience, those recurrences are due to failure to take down the bladder flap. The surgeon is then unable to provide adequate mesh overlap or fixation due to fear of injury to the bladder. By identifying the bladder upfront, injuries from sutures and fixation constructs can be avoided.
Mesh Orientation and Fixatio n
After introducing the mesh, the preplaced inferior suture is retrieved first just off the pubic symphysis (Fig. 24.5). The superior suture should be the next one to be pulled up. The site for suture placement is determined by stretching the mesh taut. An alternative method of mesh fixation is to utilize a mesh-positioning system. For suprapubic defects, placement of additional inferior sutures is critical. Once the sutures are secured, circumferential tacks are placed. Permanent, metallic tacks are preferred here as they more reliably penetrate the ligaments along the superior ramus. Absorbable tacks may be also used if placed just superior to the ramus and not into the bone directly. Tacks may be placed in a double crown configuration with the inner row around the hernia orifice. One additional suture is then placed on either side of the inferior, cardinal suture for more secure fixation inferiorly. It is important not to put any tacks below Cooper’s ligaments (Fig. 24.6). Also, the inferior-lateral aspect of the mesh could be in the “Triangles of Doom and Pain”. No tack fixation should be done in that area. This is accomplished by identifying the iliopubic tract and providing external palpation for EVERY tack in that area. Fibrin glue is a very helpful adjunct for fixation of the inferior aspect of the mesh. Following mesh fixation, the bladder is desufflated and the flap left in situ. There is no need to attempt to re-approximate the flap, since a barrier-coated mesh was used. Incomplete closure of the flap may actually create potential openings that may result in internal hernias involving the small bowel.
For large defects or recurrent defects in the suprapubic position, more secure fixation can be provided by bone anchors [3]. A small, stab incision is made over the pubic symphysis. The bone guide is placed through the skin incision and rested against the symphysis. A pilot hole is created with the drill, and the bone anchor is inserted into the symphysis. The bone anchors contain a double-armed braided suture (Fig. 24.7). The needles are cut off and the tails of the suture are passed through the stab incision and into the mesh. Additional anchors may be placed along the superior ramus as well.
Postoperative Concerns
For the most part, the postoperative management of the patient undergoing laparoscopic subxiphoid or suprapubic hernia repair is similar to standard laparoscopic ventral hernia repair. All patients are admitted predominantly for pain control. The concerns for ileus versus small bowel obstruction, seroma, and infection are the same as with all ventral herni a repairs. Early ambulation and generous use of analgesics is encouraged. Urinary catheters are removed on the morning of postoperative day one unless there was a bladder injury that required repair.
Seromas frequently complicate suprapubic repairs. Attempts to close the defect laparoscopically at the time of repair may help mitigate some of this concern. The defect may be closed with a series of stab incisions and figure-of-eight sutures in a “shoelace” fashion (Chapter 22). Intracorporeal suturing of the defect has also been described, including recent modification with the use of the robot. Fascial closure of the subxiphoid defects can be attempted as well, however, the benefit is not as great and the trade-off is increased pain.
Conclusion
Laparoscopic approach to repair of subxiphoid and suprapubic defects represents an additional challenge. Familiarity and proficiency with laparoscopic repairs of routine to midline ventral defects is mandatory prior to embarking on the repair of the atypical defects. Understanding of anatomic nuances of both the upper and lower abdomen is paramount to avoid visceral and vascular injuries as well as providing durable and lasting repairs. Understanding and implementation of strategies for safe urinary bladder identification and mobilization is critical for suprapubic repairs. Mesh placement in both locations should be aimed to extend beyond the bony margins with fixation performed off the edge of the mesh. Importantly, maintaining the xiphoid process and costal margin as cranial safety margins for safe tacker/suture placement is absolutely necessary to avoid pulmonary/cardiac injuries. Defect closure may be of particular use for suprapubic defects to minimize postoperative seromas and bulging. Overall, suprapubic and subxiphoid defects can be effectively repaired laparoscopically, provided the important principles of safe dissection and mesh positioning described in this chapter are always maintained.
References
Cobb WS, Kercher KW, Heniford BT. Laparoscopic repair of incisional hernias. Surg Clin North Am. 2005;85(1):91–103.
Carbonell AM, Kercher KW, Matthews BD, Sing RF, Cobb WS, Heniford BT. The laparoscopic repair of suprapubic ventral hernias. Surg Endosc. 2005;19(2):174–7.
Yee JA, Harold KL, Cobb WS, Carbonell AM. Bone anchor fixation for complex laparoscopic ventral hernia repair. Surg Innov. 2008;15(4):292–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
Laparoscopic Subxiphoid hernia repair, by Belyansky (MP4 889,359 kb)
Laparoscopic Suprapubic Hernia repair, by Novitsky (MP4 50,457 kb)
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cobb, W.S. (2016). Laparoscopic Subxiphoid and Suprapubic Hernia Repair. In: Novitsky, Y. (eds) Hernia Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-27470-6_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-27470-6_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27468-3
Online ISBN: 978-3-319-27470-6
eBook Packages: MedicineMedicine (R0)