Abstract
Termites cause economic losses by directly injuring and destroying both living and dead vegetation. They can damage right from sowing the crops till harvest. Billions of dollars are spent annually throughout the world to control and prevent termite infestation. Many bacteria, fungi, and nematodes occurring naturally in soils are known to suppress termite activity. Entomopathogenic nematodes (EPNs) and their associated bacterial symbionts are highly specific in their host range and compatible with many pesticides. EPNs, also called beneficial nematodes, are commercially used to control insect pests. These nematodes offer an environmentally safe alternative to chemical insecticides, and a wide range of EPNs are effective against various termite species. Only a limited number of field studies have been conducted using EPNs as control agents for termites. New isolates of EPNs may prove potential against termite pests in the field. This chapter outlines the potentials of entomopathogenic nematodes in termite management.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
For many decades organochlorines formed the backbone of termite management worldwide. Synthetic insecticidal compounds became popular due to the long residual action and the wide toxicity spectrum. However, these pesticides were banned or withdrawn from the market for human health and environmental reasons from an increasing number of countries in the late 1980s and the 1990s. Indiscriminate, inadequate, and improper use of these synthetic organic pesticides had caused tremendous damage to the environment. Consequently, negative impacts on soil, groundwater quality, human health, wildlife, and ecological balance within agroecosystems are increasingly becoming a concern. To overcome the hazards associated with chemical pesticides, the use of biopesticides is increasingly being adopted. As a consequence of these developments, the focus in termite management has shifted increasingly to alternative methods in dealing with termite problems.
Biological control constitutes a more environmentally acceptable alternative to traditional chemical control measures. When successfully implemented, it can yield permanent, cost-effective management of pest populations with minimal environmental disturbance. It refers to the application or manipulation of predators, parasitoids, or pathogens in order to suppress and manage insect pest population. The literature contains numerous reports of microorganisms that may have potential to cause the death of termites. Lenz (2005) highlighted the potential of nematodes and fungal pathogens in termite management. A partial review by Myles (2002) lists two viruses, five bacteria, 17 fungi, and five nematodes. The full list of such organisms is no doubt larger. Entomopathogenic nematodes have been found parasitizing species in the orders Hemiptera, Diptera, Hymenoptera, Lepidoptera, Orthoptera, Coleoptera, Thysanoptera, Siphonaptera, as well as Isoptera (Nickle and Welch 1984). These nematodes offer an environmentally safe alternative to chemical insecticides in the management of termites. This chapter outlines the potentials of entomopathogenic nematodes in termite management.
2 Termites
Termites are a group of social insects which are widely distributed throughout the tropical and subtropical regions, with the highest diversity found in tropical forests (Eggleton 2000) while few occur in different temperate areas of the world. They are of paramount importance as pests and belong to the insect order Isoptera, an ancient insect group that dates back more than 100 million years. The Latin name Isoptera means “equal wing” and refers to the fact that the front set of wings on a reproductive termite is similar in size and shape to the hind set. Since the first truly scientific work on termites in 1779, which was carried out in India by J. G. Konig, much work has been done all over the world. The presence of termites is often not readily noticed because of their hidden activities. They act as herbivores as well as decomposers, feeding on a wide range of living, dead, or decaying plant material (Bignell and Eggleton 2000; Traniello and Leuthold 2000). Their feeding habits make them ecosystem engineers, which over long periods of time can modify the physical properties of soil, at various spatial scales (Dangerfield et al. 1998). These termites comprise some 2900 species (Krishna and Grimaldi 2003), of which 300 species are of economic importance as pests in agriculture, forestry, and urban situations worldwide.
Termites present in a locality are pests and do not depend on their species and a number of colonies, more precisely their food habits, whether they are competitors for food resources or not. Based on their habitat, termites can be grouped into three general categories: subterranean, damp-wood, and dry-wood termites (Paul and Rueben 2005). Subterranean termites live in the soil and wood that is in contact with soil. Dry-wood and damp-wood termites live inside wood of varying levels of decay and moisture content. Subterranean termites derived their name because of their association with the soil. These are serious pests of wooden structures causing tremendous amounts of damage (Su and Scheffrahn 1998) and are reported responsible alone for at least 80 % of losses caused by termites (Su and Scheffrahn 1990).
Dry-wood or powder-post termites are primitive termites whose damage often goes unnoticed by homeowners. Dry and powdery pellets, occasionally kicked out by dry-wood termites from infested wood, are very characteristic of their presence. Unlike the subterranean termites, dry-wood termites form colonies within sound dead wood rather than in the soil below. Their ecology and behavior are distinctly different from the subterranean termites, a fact which alters their monitoring and controls procedures from those methods used for standard subterranean termites. They have been reported to cause less than 20 % of damage (Su and Scheffrahn 1990). The pest status of damp-wood termites, however, is minor compared to the other termite groups. The wide distribution, large colonies, cryptobiotic lifestyle, and aggressiveness of subterranean termites make it difficult to manage and control them (Su and Scheffrahn 1988; Culliney and Grace 2000). The annual economic cost of termite damage and prevention worldwide is estimated in billions. Yu et al. (2008) reported damage to wooden structures attributed to termites in the United States can exceed $3 billion annually. Rust and Su (2012) reported global economic impact of termite to be at least $40 billion. Estimates, however, vary considerably by region. High frequency of damage caused by termites and the high losses sustained have resulted in multimillion-dollar pest control industry.
2.1 Biology and Habitat
Termites undergo hemimetabolous (incomplete) metamorphosis. Important biological roles are divided among physically distinct termites, called as castes. Termite colonies contain three principal castes: workers (pseudergates), soldiers, and reproductives (king, queen, alates or swarmers). The worker caste is responsible for the damage that makes termites an economically important pest. They are wingless and sterile and comprise the majority of the population within a colony. Their mouthparts are adapted for chewing through wood or other cellulose materials. Workers do all the physical labor like building nests, foraging, cleaning, feeding all other dependent castes, grooming the queen, etc. Soldiers, however, have only one mission, to defend the colony against invaders with their specialized strong mandibles. They cannot forage for food or feed themselves and depend on the workers to care for them. Soldiers, when disturbed, readily attack approaching object and may secrete a white gluey defensive secretion. Termite queen lays about 3000 eggs a day through its enlarged abdomen (Thompson 2000). The eggs are yellowish-white and hatch after 50–60 days of incubation. The queen lives around 25 years.
Winged reproductives are also called swarmers or alates. Alates are flying form of termites, which fly out in great numbers from mature colonies at certain times of the year. This process is known as swarming. Environmental factors trigger the emergence of swarmers, and they leave the colony in large numbers during spring or early summer in search of new habitats. Following swarming, alates shed their wings, and males and females pair off and seek out a suitable place to establish a new colony. Swarming alates are usually the first sign of infestation noticed. Swarming time, season, and conditions differ among species and locations. Only a small percentage of swarmers survive to develop a new colony. The colony reaches its maximum size in approximately 4–5 years.
The size of a termite colony depends on location, food availability, and environmental conditions. Some colonies remain small; others contain up to several thousand individuals. A mature colony of Coptotermes formosanus Shiraki may have 1–4 million termites (Su et al. 1984). While other termites like Reticulitermes flavipes (Kollar) may contain ca. 200,000 termites in a colony (Howard et al. 1982) with a foraging range up to 100 m in any direction (King and Spink 1969) (Fig. 1). These data indicated the presence of a large subterranean termite colony beneath an infested structure. A termite mound is the most familiar form of termite nest. Typically, each species builds a characteristic mound, although there may be geographical variation in the size and shape of the mound within species. Apart from grass-eating species, which forage in the open, all termites build tunnels between their nest and source of food through covered runways. These shelter tubes connecting soil with structures are common signs of subterranean termite infestation. Termites construct a variety of tube types as summarized by Ebeling (1978). These covered tunnels provide humidity conditions, thus preventing desiccation, and darkness necessary for their movement and protection against predators. Symbiotic flagellate protozoa are found in the hindgut of lower termites, namely, Kalotermitidae, Rhinotermitidae, Hodotermitidae, Mastotermitidae, Serritermitidae, and Termopsidae. Higher termites, the Termitidae, host no or very few protozoa.
2.2 Distribution and Identification
Many ecological factors influence termite distribution but vegetation and soil type remain the most important. Termites are widely distributed by people who unknowingly transport infested furniture. As a result, many pest termites have very wide distributions. The Formosan subterranean termite C. formosanus is the most widely distributed and most economically important. This species of termite is probably endemic to southern China and was apparently transported to Japan prior to the 1600s and to Hawaii in the late 1800s (Su and Tamashiro 1987). Another species of termite, C. havilandi, was supposed to have been introduced from Southeast Asia to parts of South and North America and to a number of Caribbean islands, where it became as a serious pest of woodwork in buildings (Scheffrahn et al. 1994; Su et al. 1997a, b). However, in the countries from where it was supposed to have originated, it was never considered a serious pest. Environmental changes can drastically change the species composition, and some rare species in the natural landscape can be more easily detectable in these areas (Dambros et al. 2012). Formosan termites attack a variety of wood products and cause significant damage within a short time period. C. formosanus, R. flavipes, and R. hesperus Banks are the three most important subterranean termite in the United States (Su and Scheffrahn 1990). C. formosanus coexists with Reticulitermes spp. in some areas of Florida (Scheffrahn et al. 1988a). Termite fauna of Saudi Arabia is predominantly subterranean; however, surveys revealed the presence of dry-wood termites in the Western region, most probably introduced with wood and timber imports from abroad (Faragalla 2002). The majority of the pest species in India are soil inhabiting, either as mound builders or as subterranean nest builders. The major mound-building species are Odontotermes obesus, O. redemanni, and O. wallonensis. The major subterranean species are Coptotermes ceylonicus, C. heimi, Heterotermes indicola, Odontotermes homi, Microtermes obesi, Microcerotermes beesoni, and Trinervitermes biformis (Rajagopal 2002) (Table 1).
Soldier termites and alates are the only types of termites that can be accurately identified (Scheffrahn and Su 1994). Soldiers are generally large than the workers, with brownish heads and, in most species, large, toothed mandibles. Unfortunately, dry-wood termites do not maintain large number of soldiers in their colonies, and majority of the nest is composed of worker but there is no identification key available for worker individuals. Identification must be done with care, as the alate castes of some dry-wood species found in Alabama were similar in size and color with those of the Formosan subterranean termite. Kirton and Brown (2003) mentioned that C. gestroi and C. havilandi are, in fact, the same species. C. gestroi had been described from the soldier form of the termite by Erich Wasmann in 1896, while C. havilandi had been described later by Nils Holmgren in 1911 from the alates of the same species. The difficulty of matching alates to soldiers had led to this situation. If there is taxonomic confusion or the wrong name is applied to the species, then the pest management decisions we make could be based on misleading information. The importance of accurate termite taxonomy in the broader perspective of termite management was highlighted by Kirton (2005). However, Haverty et al. (2005) studied identification of termite species by the hydrocarbons in their feces. They mentioned that hydrocarbons extracted from fecal pellets were qualitatively and quantitatively similar to cuticular extracts and can be used to determine the termite species responsible without the termites present.
2.3 Economic Importance
The economic importance of termites is twofold, extremely beneficial and extremely injurious to man. These small creatures are a part of the natural ecosystem and contribute significantly to most of the world’s ecosystems. Their greatest contribution is the role they play in recycling wood and plant material. The breakdown and release of organic matter as termites eat and digest plant material plays an important role in maintaining soil health. Aeration of the soil due to termite burrowing activities helps in maintenance of soil microflora (Wood 1988). Soil fertility improves when termite mounds, rich in minerals, are crushed down and incorporated into the soil. Review by Freymann et al. (2008) on importance of termites for the recycling of herbivore dung highlights the economic importance of termites in tropical ecosystems. Moreover, termites provide a source of protein-rich food for many organisms including ants, guinea fowl, and other mammals including humans. In African countries, use of termites as a protein source for poultry production has been investigated.
Termite species, however, gain pest status when they damage building materials or agronomic and forestry commodities. As the principal food of some of the termite castes is cellulose, they cause economic losses by directly injuring and destroying both living and dead vegetation, buildings, bridges, dams, etc. Termites are among the few forest insects that are able to live in and on both decayed and living plant tissue. They injure living plants originally by attacking from outside but may continue the destruction of living tissues from outside or from within (Fig. 2). Termites also damage man-made fabrics (textile materials), plastics (polytene, polyvinyl chloride), and some metal foils (Howse 1970). Termites ate through notes worth Rs. 10 million (about $222,000) stacked in a steel chest inside the State Bank of India, in a northern Indian town (Sacks 2011). The bank was said to be housed in an old building infested with termites. A similar incident happened in Bihar state of India when a trader lost his life savings after termites infested his bank’s safe deposit boxes (Tewary 2008). Damage to human habitations by termites varies in its impact. It is estimated that 20 % of Australian homes are infested by termites (Scholz et al. 2010), whereas in China up to 90 % of Chinese homes south of the Yangtze river are affected by termite damage. In what may be the most extreme example of damage by termites, an entire township in India was gradually destroyed by the termite Heterotermes indicola and eventually resembled a bombed-out ghost town (Roonwal 1955). Economic losses associated with termite damage in the United States and Japan are around 1000 and 800 million US$ a year, respectively (www.chem.unep.ch/pops/termites), and Japan may be the third largest user of pesticides for structural pest control in the world. Economic losses due to termite in India have been estimated around 35.12 million US$ (Joshi et al. 2005); however, in Malaysia 8–10 million US$ are spent toward termite treatment every year (Lee 2002). Heavy infestation of termite on agricultural crops (field and vegetables) including maize, sorghum, sweet peppers, tomatoes, okra, and millets have also been reported from the western region of Saudi Arabia (Faragalla et al. 1998). Annual economic losses associated with termite activities have also been mentioned by Ghaly and Edwards (2011) (Table 2). Characterization of termite pest problem starts with identification of termite species, knowledge of basic biology and ecology of the species, and evaluation of the magnitude of the economic damage.
2.4 Losses in Agriculture
Termites can damage right from sowing the crops till harvest. It is difficult to locate termites and usually it is too late when the typical termite damage symptoms are noticed in field, leading to severe losses. Credible information on the economic losses caused by termites is difficult to obtain. Ground-based monitoring devices, however, have been developed and used experimentally for identification of subterranean termite colony ramifications (Potter 1997). Termites damage was greater in rain-fed than irrigated crops (Sharma et al. 2009). By increasing the number of irrigations, termite damage was reduced (Sharma et al. 2004). Hence, in dry areas where proper facilities for irrigation are not usually available, termite infestation is inimical. In the dryer parts of India, infestation by termite has also been reported by Roonwal (1979) in maize, pearl millet, pulses, sugarcane, cotton, paddy, groundnut, potato, citrus, vegetables, spices, and fruit crops. Annual crops are attacked toward harvest time while perennial crops are attacked most destructively during dry seasons. Severe losses in different regions of India have been recorded on highly susceptible crops such as wheat and sugarcane in North India; maize, groundnuts, and sunflower in South India; cotton in Western India; and tea in Northeast India (Rajagopal 2002). Subterranean termites attack sugarcane crop from its germination through shoot emergence, and finally it affects the quality of canes. At germination stage, the losses up to 90–100 % have been recorded (Salihah et al. 1988). As many as 13 species of termites are reported to cause damage to sugarcane in India. Alam and Miah (1997) reported five species of termites destructive to sugarcane in Bangladesh. Sharma et al. (2004) conducted a survey to determine termite damage on wheat crop covering five mega wheat-growing zones in India. They reported that termites were a real problem in Rajasthan and some parts of Madhya Pradesh. The range of infestation indicated that ~50 % of the fields had low termite damage, ~30 % had medium damage, and ~20 % had severe termite damage. The damage was low in clay and black soils, high in sandy loam soils, and severe in red soils. About 16 species of termite were found to damage the wheat crop in India, of these two species, viz., Odontotermes obesus (Rambur) and Microtermes obesi Holmgren, were found dominant (Chhillar et al. 2006). Soft wooded tea plants are known to be easily attacked by termites. Live wood-eating termite Microcerotermes and dead wood-eating termite Odontotermes are tea pest in India (TBI 2014). Ohiagu (1979) reported that termites damage three major cereal crops, maize, millet, and sorghum, in various parts of Northern Nigeria (Table 3).
3 Entomopathogenic Nematodes
Due to environmental concerns and human safety, efforts have been focused on biological control using entomopathogenic nematodes (EPNs). EPNs are soft-bodied, non-segmented roundworms. They are mobile, highly virulent insect parasites with high reproductive potential. Naturally they occur in soil environments and are capable of parasitizing many economically important insect pests. They locate their host in response to carbon dioxide, vibration, and other chemical cues and in some cases provide a level of insect control equivalent to that of chemical insecticides (Poinar 1986; Georgis 1990). Despite their broad host range and high virulence, these nematodes have shown no mammalian pathogenicity (Gaugler and Boush 1979) and are safe to vertebrates, plants, earthworms, honeybees, and other nontarget organisms (Kaya and Gaugler 1993). In cryptic habitats, EPNs have proven superior to chemicals in controlling the target insect (Gaugler 1981).
The first entomopathogenic nematode was described by Steiner as Aplectana kraussei (now Steinernema kraussei) in 1923. In 1929, Glaser and Fox (1930) found a nematode, S. glaseri (Steiner), infecting grubs of the Japanese beetle Popillia japonica, at New Jersey. Jaroslav Weiser described S. carpocapsae in 1955. The genus Heterorhabditis was described in 1976. The genus Steinernema and Neosteinernema, family Steinernematidae, and genus Heterorhabditis, family Heterorhabditidae (Rhabditida: Nematoda), contain the most important species of entomopathogenic nematodes. Now Steinernema contains more than 50 species and Neosteinernema only one species, N. longicurvicauda. Heterorhabditis, however, contains more than a dozen species. Steinernematids and heterorhabditids received great attention as a group of biological control agents in the 1990s. The history of entomopathogenic nematology is briefly reviewed by Poinar and Grewal (2012).
Steinernematids and heterorhabditids are obligate insect parasites (Poinar 1979) and are associated with symbiotic bacteria of the genera Xenorhabdus spp. and Photorhabdus spp., respectively (Akhurst and Boemare 1990). These Xenorhabdus and Photorhabdus are motile, gram-negative, facultative, non-spore-forming anaerobic rods in the family Enterobacteriaceae. Together, nematodes and their symbiotic partners form an insecticidal complex that is effective against a wide range of insect hosts (Gaugler and Kaya 1990; Kaya and Gaugler 1993). Symbiotic bacterium of H. bacteriophora was earlier characterized as Xenorhabdus luminescens in 1979 which was later transferred to the genus Photorhabdus (Boemare et al. 1993). Most Photorhabdus spp. are luminescent and catalase positive, whereas Xenorhabdus spp. have no luminescence and are catalase negative. Studies suggest each Steinernema species has an apparent specific natural association with only one Xenorhabdus species, though a single Xenorhabdus bacterial species may be associated with more than one nematode species (Fischer-Le Saux et al. 1999; Boemare 2002). Poinar (1966) and Poinar and Leutenegger (1968) demonstrated the location of bacteria in the infective-stage juveniles, using light microscopy and later electron microscopy. In Steinernema bacterial symbionts are harbored in a specialized structure known as “bacterial receptacle.” In Heterorhabditis bacterial symbionts are distributed along a broad stretch of the anterior portion of the nematode intestine. Entomopathogenic nematode species and isolates show substantial variation in behavior, host range, infectivity, reproduction, and environmental tolerances. EPNs find their hosts either actively or passively (Smart 1995). Directional response to electrical fields varies among EPN species which may be used by nematodes for host finding or other aspects of navigation in the soil (Shapiro-Ilan et al. 2012a).
Entomopathogenic nematodes, also called beneficial nematodes, are commercially used to control insect pests. S. carpocapsae was the first nematode product marketed while S. scapterisci became available commercially only in 1993 and S. riobravis in 1994 (Smart 1995). These EPNs offer excellent potential for control of insects in soil habitats but are not well suited for foliar application, as they are sensitive to desiccation and ultraviolet radiation. In laboratory tests, S. carpocapsae alone infected more than 250 species of insects from over 75 families in 11 orders (Poinar 1975). The broad host range and high virulence of entomopathogenic nematodes make them suitable for use as augmentative-release biocontrol agents (Hui and Webster 2000). They can be mass produced easily in liquid culture at economically reasonable costs (Ehlers et al. 1998) and are sufficiently small to pass through standard spraying equipment. These nematodes are compatible with many pesticides, can be mass produced and formulated, and have been exempt from registration in many countries. Insects controlled with entomopathogenic nematodes have been reviewed by various researchers (Klein 1990; Georgis and Manweiler 1994; Smart 1995; Hazir et al. 2003; Lacey and Georgis 2012). Several books on the potential use and field techniques of ENPs in various cropping systems has been published (Gaugler and Kaya 1990; Bedding et al. 1993; Lacey and Kaya 2000; Gaugler 2002). Similarly, Lacey and Georgis (2012) highlight EPN development for control of insect pests, above and below ground, including those from foliar, soil surface, cryptic, and subterranean habitats.
Subterranean termites live and forage in habitats that are moist, cool, and without direct sunlight such as soil or wood materials. These environmental conditions are ideal for the survival and movement of steinernematid and heterorhabditid nematodes and, therefore, provide the basis for the interest in their role in control of termites. Research shows that the entomopathogenic nematodes have a potential to use as environmentally safe alternative control tactic for termites (Wang et al. 2002; Yu et al. 2006). The rapid development in the study and commercial application of EPNs in pest control during the last 30 years instigated further interest in finding useful nematodes and better application methods to control subterranean termites. EPNs market continues to grow rapidly with US$14.5 million in 2007 and >US$20 million in 2013 (CPL 2006, 2013a, b).
3.1 Biology
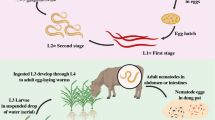
Nematode infective juveniles (IJs) live in soil where they search for susceptible insect hosts. Nonfeeding juveniles invade the body of the susceptible insect by direct penetration either through natural openings (mouth, anus, and spiracles) or, in some cases, through the cuticle. Heterorhabditis use an anterior tooth to penetrate directly into the hemocoel. The parasitic cycle of nematodes is initiated by the third-stage infective juveniles (IJs), the only free-living stage. These infective juveniles act as vectors to transport the bacteria (Xenorhabdus and Photorhabdus spp.) into an insect host within which they can proliferate, as bacteria alone are incapable of penetrating the alimentary tract and cannot independently gain entrance to the host’s hemocoel. IJs penetrate the alimentary tract of the host insect and release symbiotic bacteria, which are held in the intestine. At this point in their life cycles, bacteria and nematodes exist separately although in close proximity to one another. The released bacteria cause a lethal septicemia, killing the host within 24–48 h. The bacteria produce extracellular enzymes that break down proteins and lipids in the insect carcass, thereby providing nutrients for bacterial and nematode growth. The bacteria also suppress secondary infection of the host by producing antibiotic substances. Inside the hemocoel, IJs of Heterorhabditis release the symbionts by regurgitation, while bacteria are defecated by Steinernema spp. (Ciche et al. 2006). Nematode and its symbiotic bacteria act together in overcoming the insect’s immune response (Dowds and Peters 2002). Heterorhabditis spp., if deprived from their symbionts, are nonpathogenic to insects (Han and Ehlers 1999), whereas symbiont-free Steinernema spp. will kill their insect hosts, although it takes them about ten times longer to do so (Ehlers et al. 1997). A review by Stock and Blair (2008) on entomopathogenic nematodes and their bacterial symbionts provides detailed information. Even though the bacterium is primarily responsible for the mortality of most insect hosts, the nematode also produces a toxin that is lethal to the insect (Burman 1982).
Symbiont bacteria create conditions necessary for nematode survival and reproduction within the insect cadaver. The nematode initiates its development, feeding on rapidly multiplying bacterial cells and host tissues that have been metabolized by the bacterium. Nematodes develop through four juvenile stages to the adult and then reproduce. They complete 1–3 generations within the host cadaver, depending on host size. When nematode numbers become high and nutrients become limiting in the insect cadaver, nematode progeny reassociate with bacteria and differentiate into the colonized, nonfeeding IJ form that emerges into the soil to forage for a new host (Kaya and Gaugler 1993). Reproduction differs in heterorhabditid and steinernematid. IJs of heterorhabditid nematodes become hermaphroditic adults, but individuals of the next generation produce both males and females, whereas in steinernematid (with the exception of Steinernema hermaphroditum (Griffin et al. 2001)), all generations are produced by males and females (gonochorism) (Grewal et al. 2005).
Infective juveniles do not feed but can live for weeks on stored reserves as active juveniles and for months by entering a near-anhydrobiotic state. The length of time that juveniles survive in the soil in the absence of a host depends upon temperature, humidity, natural enemies, and soil type. Generally, survival is measured in weeks to months and is better in a sandy soil or sandy loam soil at low moisture than in clay soils. The heterorhabditids do not survive as well as do steinernematids (Molyneux 1985). Their survival is better in sterilized soil than in non-sterilized soil. EPN population in non-sterilized soil is reduced by bacteria, fungi, mites, predatory nematodes, tardigrades, and other soil organisms (Smart 1995). Mites appear to be especially voracious nematode feeders.
3.2 Rearing
EPNs in the genera Steinernema and Heterorhabditis are commercially available for the control of soil-inhabiting insects. Several companies in Asia, Europe, and North America mass-produce EPNs either on a small scale or in large scale using bioreactors (Shapiro-Ilan and Gaugler 2002). EPNs can be reared by different methods either in vivo or in vitro (Bedding 1984; Georgis 1990; Shapiro-Ilan and Gaugler 2002; El-Sadaway 2011). For solid medium culture, a substrate such as beef kidney or liver or chicken offal may be used. The substrate usually is made into a paste that is coated onto a porous substrate such as sponge. The medium is sterilized and inoculated with the bacterium and then nematodes are added 24 h later. Infective juveniles are harvested after about 15 days. This method is labor intensive and is particularly well suited for laboratory use and small-scale field experiments. Production in liquid medium can be done in small containers or in fermentation tanks. Greater numbers of juveniles can be produced per unit area in fermentation tanks, which makes this method especially suited for large-scale commercial production at reasonable quality and cost (Shapiro-Ilan et al. 2012b). Ru (2001) and Sharma et al. (2011) highlight mass production, commercialization, and utilization of EPNs as microbial biopesticides for plant protection.
3.3 Distribution and Dispersal
Steinernematids and heterorhabditids are ubiquitous in distribution and have been recovered from soils throughout the world (Hominick et al. 1996). S. carpocapsae and S. feltiae are widely distributed in temperate regions, H. bacteriophora is common in regions with continental and Mediterranean climates, and H. indica is found throughout much of the tropics and subtropics. Other species such as S. rarum, S. kushidai, S. ritteri, and H. argentinensis appear to have a much more restricted distribution (Hazir et al. 2003). Juveniles of nematodes can be dispersed at great distances, vertically and horizontally, both actively and passively (Kaya 1990; Parkman et al. 1993). Passively, they may be dispersed by rain, wind, soil, phoresis, infected hosts, or human activity which may, in part, account for their widespread global distribution. Zadji et al. (2014b) observed nematode dispersal occurred by infected termites or phoresis. In addition to jumping for some nematode species, the infective juveniles can disperse in soil up to 90 cm in both the horizontal and vertical directions within 30 days (Kaya 1990). Soil porosity can also affect nematode dispersal with less dispersal occurring as soil pores become smaller. Although some entomopathogenic nematodes have been isolated from insects naturally infected in the field, they are most commonly recovered from soil by baiting with susceptible insects (Bedding and Akhurst 1974). The wax moth larva Galleria mellonella (L.) is most commonly used as bait. Molecular techniques have also been applied to measure genetic diversity of the nematodes and provide an initial screen to identify useful strains.
3.4 Genetic Manipulation
A limitation of nematodes for insect control is their susceptibility to environmental stress like extreme temperature, solar radiation, and desiccation, which prevents them from being used to maximum advantage as bio-insecticides under field conditions. The ability of different species to tolerate adverse conditions varies enormously. The potential of genetic engineering to enhance nematode environmental tolerance is being explored. To enhance tolerance of high temperatures, hsp70A (heat shock protein genes) from the free-living nematode Caenorhabditis elegans was introduced to H. bacteriophora juvenile (Hashmi et al. 1995; Wilson et al. 1999). Resulting transgenic strain, however, was not successful at field level. Isolation of natural populations that can survive harsh environments, such as deserts, indicated that some populations have enhanced abilities to survive desiccation. Fodor et al. (2010) isolated a tps-1 gene from the yeast Saccharomyces cerevisiae and transformed it to H. bacteriophora which showed increased osmotolerance. In another study, indigenous gut bacteria of the subterranean termite C. formosanus were genetically modified with entomopathogenic bacterium Photorhabdus luminescens subsp. laumondii TT01, a symbiont of the entomopathogenic nematode H. bacteriophora. When termites were fed on filter paper inoculated with these recombinant bacteria, the chromosomal expression of the introduced genes showed that there were insecticidal activities against termite workers and soldiers. Termite mortality was reported 3.3 % at day 5, and it increased from 8.7 % at day 9 to 93.3 % at day 29 (Zhao et al. 2008).
4 Control Measures of Termite
Successful termite management requires many special skills and varies depending on the species causing infestation. An understanding of termite biology and its identification can help to detect problem and methods of control. Subterranean and, less frequently, damp-wood termites can have nests at or near ground level, so control methods for these can be similar. However, dry-wood termites nest above ground; therefore the approach for eliminating them is different. The predominant control strategies for subterranean termite consist of chemical treatment and baiting systems using a matrix containing a slow-acting insecticide or insect growth regulator. Dry-wood termites, however, must be treated by removing infested wood, using spot treatments or by fumigation. An integrated program to manage termites must be practiced.
4.1 Cultural Practices
Cultural practices regulate termite numbers rather than eliminate them so that the benefits provided by termites are not lost. Removal of the queen or destruction of the nest has frequently been used by farmers as a traditional method for control of mound-building termites. Mounds are physically destroyed, flooded, or burnt with straw to suffocate and kill the colony. Deep plowing exposes termites to desiccation and to predators; therefore repeated plowing and digging of the soil may reduce termite damage. The removal of residues and other debris from the field may reduce potential termite food supplies and hence lead to a reduction in termite numbers and subsequent attack. Wood ash around the base of the trunk of coffee bushes and date palms has been recorded as preventing termite infestations. Intercropping maize and beans is the most effective cultural practice used by small-scale farmers in sub-Saharan Africa. Although termites may damage healthy plants, water-stressed and diseased plants are generally more susceptible to termite attack. Inorganic fertilizers enhance plant vigor and hence the ability to withstand pest damage. Application of nitrogen, phosphorus, and potassium in wheat, barley, and yam has been observed to reduce termite incidence. Sowing of indigenous varieties is recommended. In general, crops susceptible to termites are exotic while resistant crops are indigenous.
4.2 Barrier Control
Barriers can be physical, chemical, or hybrid. Physical barriers are good for prevention of termite damage in buildings and storage structures. As chemical barriers, soil applications of liquid termiticides to form a termite-impermeable barrier are in vogue these days especially for protecting buildings and other structures. The standard measure of acceptable performance is that the chemical barrier must keep termites from penetrating 90 % of the barriers for at least 5 years (Gold et al. 1996).
4.3 Chemical Control
For the last four decades, pest control industry has depended heavily on soil termiticides, and still chemical control is playing a vital role in controlling subterranean termites (Su and Scheffrahn 1998). With cryptic insects such as termites, effective delivery of insecticides to kill the population is particularly challenging. Reproductive and nymphs of subterranean termites are concentrated in nests near or below ground level, out of reach of other control methods. Therefore, common methods for controlling these termites are the application of termiticides or baiting campaigns. Active ingredients in currently available termiticides can be broadly classified as repellent or nonrepellent.
Repellent means that the termites are able to detect the insecticide, which basically serves as a barrier, and termites are repelled by it without receiving a dose that will kill them. Therefore, when using these materials, it is important to make sure there are no gaps remaining in the barrier. Repellent termiticides provide immediate protection for the structure. Currently available repellent termiticides include pyrethroids such as permethrin (Dragnet® FT, Prelude®), cypermethrin (Prevail® FT, Demon® TC), deltamethrin (Suspend® SC), and bifenthrin (Talstar®). Apart from repellent, some termiticides prevent termite invasion by nonrepellent or lethal contact. They include imidacloprid (Premise®), fipronil (Termidor®), chlorfenapyr (Phantom®), etc. Chlorpyrifos, iodofenphos, isofenphos, carbosulfan, and carbofuran have been used as alternatives; however their low persistence often necessitates repeated applications. Environmental factors, viz., soil type, weather, and application techniques, influence the mobility of insecticides in the soil. Use of pesticides containing conventional active ingredients in both agriculture and urban areas had led to increased exposures and unacceptable risks (NRC 1993; Wright et al. 1994). They were also a threat to water quality in many areas (Johnson 2005). Due to increasing concerns about these side effects, there has been great interest in finding other methods of controlling termites and reducing the use of chemicals. Moreover, treatment of soil under the structure may kill only a small portion of the colony. It is very likely that the majority of the colony population will survive to re-infest the structure, either by flying alates or foragers entering through an untreated or an inadequately treated portion of soil.
4.4 Baits
The last decade has seen the rapid development of baiting technology. Termite baits are a promising alternative to soil termiticide treatment. Where liquid termiticides are not normally suitable to apply, termite baits can be used. Bait matrix may contain a slow-acting insecticide or chitin synthesis inhibitor. Foraging termites feed on termite baits and carry the digested cellulose and termiticide back to the colony where they regurgitate their stomach contents to feed other termites, a process known as trophallaxis, leading to the demise of the entire colony population. Effectiveness of these treatment methods varies depending on the skills of the applicator, type and dosage of chemical used, and target species (Rust and Su 2012).
4.5 Trap-Treat-Release
Trap-treat-release (TTR) was developed by Dr. T. G. Myles at the University of Toronto. TTR is a technique for suppressing or killing social insect colonies, particularly subterranean termite colonies. In this technique the toxicant is applied externally to termite bodies as a groomable coating. Coated termites carry effectively larger loads of toxicant than do bait-fed termites. Under laboratory conditions, it is possible to achieve extraordinarily high kill ratios among members of the colony (one treated termite can kill over 1000 untreated termites). Under field conditions, an estimated 50–100 termites are killed for each termite treated.
4.6 Host Plant Resistance
Natural resistance of wood to termite attack is due in part to chemicals deposited during heartwood formation (Kumar 1971; Scheffrahn et al. 1988b). Chemicals in termite-resistant wood may be contact toxic to termites or act as antifeedants, repellents, or protozoacides (Carter et al. 1983). A specific chemical that causes resistance to insects may occur only in one plant species and not in others. Cornelius and Osbrink (2015) evaluated the wood consumption of the subterranean termite C. formosanus on ten different species of wood, used as commercial lumber. They reported that teak was the most resistant wood tested, and toxic chemical components of teak hold the most promise as wood preservatives. Indigenous crops are more resistant to termites than exotic crops. In Africa, sorghum and millet are more resistant to termites than maize and cowpea. The indigenous crop bambara nuts are not attacked while groundnuts suffer serious damage.
4.7 Biopesticides
Some plant biomass contains insecticidal activity which can be exploited for termite control. A rich source of new pesticides is plant essential oils. Singh and Kumar (2008) evaluated the anti-termite activity of Jatropha curcas Linn. oil and its toxic fraction at 1, 5, 10, and 20 % dilutions against Microcerotermes beesoni Snyder. They reported maximum wood protection against termites of both the treatments at 20 % concentration. Orange oil is currently available as an insecticide and has been registered in California under the brand name XT-2000™ for control of dry-wood termites (Mashek and Quarles 2008). Hu et al. (2011) reported anti-termitic properties of Lantana camara cultivars against subterranean termite Reticulitermes flavipes. Elango et al. (2012) evaluated the anti-termitic activity of crude leaf hexane, ethyl acetate, acetone, and methanol extracts of medicinal plants against C. formosanus. All the crude extracts were reported to show anti-termitic activity in a dose-dependent manner and exhibited a significant activity after 24 and 48 h of exposure.
4.8 Biological Control Agents
Natural enemies of insect pests, also known as biological control agents, include predators, parasitoids, and pathogens. Biological control is generally perceived as both providing more permanent insect control and as having less potential damage to the environment or nontarget organisms. Therefore, biocontrol should be considered as a long-range research goal rather than an immediate solution. Brazil has a history of success with biological control projects involving the use of parasitoids, insect pathogenic fungi, and viruses (Campanhola et al. 1995). The shortcomings associated with conventional chemical control methods have prompted policymakers and scientists to evaluate the potential for natural enemies to suppress termite populations. The use of biological control agents to hunt or to infect termites within their hidden galleries is appealing. Reviews by Grace (1997)on biological control strategies for the suppression of termite, by Culliney and Grace (2000) on prospects for the biological control of subterranean termites, and by Verma et al. (2009) on biological alternatives for termite control provide complete knowledge for nonchemical control of termite.
Specialized natural enemies of termites are rather limited in numbers, possibly because of cryptic and protected habitats in which termites live. As termites provide a good source of protein, their predators include spiders, beetles, flies, wasps, and especially ants. Other predators include frogs, reptiles, birds, and mammals such as pangolins, bats, and monkeys. Bushes and trees around farms are a home for many of these useful creatures. Termite predators are both opportunist and specialist, but ants are the greatest predators of termites and may have a considerable local impact on termite populations in some areas of the world. Although ants limit termite numbers under natural conditions, their suitability for use as biological control agents for target termite management has yet to be ascertained. Larvae of Lomamyia latipennis (Neuroptera: Berothidae) are carnivorous, live within termite nests, and prey upon damp-wood termites, Zootermopsis angusticollis (Hagen) (Tauber and Tauber 1968; Johnson and Hagen 1981). A few parasitoids of termites are known, but their potential for regulating termite populations seems negligible. A larval parasitoid Verticia fasciventris Malloch (Diptera: Calliphoridae) was reported to develop in the head of soldier termite in some species of Macrotermes (Sze et al. 2008). Parasitized soldiers possess a short and square-shaped head capsule, and these soldiers were statistically less aggressive than healthy soldiers (Neoh and Lee 2011). The use of naturally occurring pathogens of termites offers unique advantages over chemically based termiticides. Characteristics of the colony, such as a protected, underground location, are likely to limit the impact of predators and parasitoids on subterranean termites. The study of pathogens for termite control started as early as 1965 (Yendol and Paschke 1965). Since then there has been renewed interest in using pathogenic organisms, such as bacteria, viruses, nematodes, and most fungi for controlling termites in recent years.
4.8.1 Entomopathogenic Fungi
Fungal diseases are known to cause insect mortality naturally (Vimaladevi and Prasad 2001). Entomopathogenic or disease-causing fungi have received considerable attentions as they are exceptionally virulent and function as lethal parasites of insect pests. These fungi are among the first microorganisms to be used for the biological control of insect pests. They are cosmopolitan organisms, invade their host directly through the cuticle, and have been isolated from soils and infected insects from around the world. More than 700 species of fungi from around 90 genera are pathogenic to insects (Wraight et al. 2007; Hemasree 2013); however, only a few have been thoroughly investigated for their use against insect pests in agriculture. Fungal pathogens for the management of spodopteran pests were reviewed by Khan and Ahmad (2015).
These fungi also offer the best prospect as termite control agents. Conidia of entomopathogenic fungi could be spread through the colony by contact and grooming between contaminated and uncontaminated termites. Investigations with termites have largely focused on two fungal species, Beauveria bassiana and Metarhizium anisopliae, and recently also Paecilomyces fumosoroseus. Green muscardine fungus M. anisopliae is especially recommended for practical control of termites as a bio-insecticide and is virulent to all species of termites tested. Conidia from M. anisopliae can survive >18 months in termite nests (Milner and Staples 1996). Myles (2002) isolated a virulent strain (pathotype) of M. anisopliae from the eastern subterranean termite Reticulitermes flavipes in Toronto. Dong et al. (2007) evaluated the efficacy of a new virulent of M. anisopliae var. dcjhyium (obtained from Odontotermes formosanus in China) against the subterranean termite O. formosanus in the laboratory. The new isolate was reported to be highly infectious and virulent against termites and could cause approximately 100 % mortality of termites 3 days post-inoculation at a concentration of 3 × 108 conidia/ml. In the market, there is a bio-control product BioBlast™ containing M. anisopliae as active ingredient to be used against subterranean termites. Strains of M. anisopliae and B. bassiana, when employed in baiting schemes, can be transferred among nest mates and may offer the potential for subterranean termite control (Jones et al. 1996; Wright et al. 2002, 2004). Isolates of B. bassiana from soil were as effective as those isolated directly from insect hosts for Heterotermes tenuis Snyder (Almeida et al. 1997). B. bassiana ATCC 90519 was reported sufficiently pathogenic against subterranean termites (Wright and Lax 2013). Germ tubes of B. bassiana can penetrate the integument of Reticulitermes flavipes as early as 16 h after application. On the other end antifungal volatile compounds have been identified in C. formosanus nests that may contribute to the inability of entomopathogenic fungi to propagate within the colony (Wiltz et al. 1998). Review by Hussain et al. (2012) on the current status of entomopathogenic fungi as mycoinsecticides is worthy to mention.
4.8.2 Bacteria and Viruses
Among the bacteria, Bacillus thuringiensis stands out, representing approximately 95 % of microorganisms used in biological control of insect pests in different cultures (Lambert et al. 1992). Castilhos-Fortes et al. (2002) evaluated the effects of B. thuringiensis subspecies against termite Nasutitermes ehrhardti under laboratory conditions. They reported that Bt. kurstaki registered <72 % mortality at the seventh day after the bacterial application. Singha et al. (2010) evaluated B. thuringiensis and B. thuringiensis subsp. israelensis for their pathogenicity against two species of tea termites, viz., Microtermes obesi and Microcerotermes beesoni. They reported that B. thuringiensis strains caused >80 % mortality in both the termite species. B. thuringiensis subsp. israelensis, however, was noticed to be more virulent compared to B. thuringiensis. Osbrink et al. (2001) isolated Serratia from dead termites and reported that three of the Serratia isolates were recorded to induce >85 % mortality within 19 days in Petri dish tests. In another study Pseudomonas fluorescens CHA0 was reported to kill subterranean termite Odontotermes obesus by inhibiting cytochrome c oxidase of the termite respiratory chain (Devi and Kothamasi 2009). Insect viruses can provide safe, effective, and sustainable control of a variety of insect pests. They are highly specific in their host range, usually limited to a single type of insect. Al-Fazairy and Hassan (1988, 1993) reported that nuclear polyhedrosis virus isolated from Spodoptera littoralis infects termite castes of Kalotermes flavicollis. They mentioned that all tested castes of termites were quite equal in their response to the virus infection and produced over 90 % mortality at 6.4 × 107 polyhedra/ml concentration.
5 Entomopathogenic Nematodes
Majority of the termite control practices are ineffective and ecologically unsustainable and, above all, do not address the root cause of termite infestation and thus merely provide temporary relief to the problem (Mugerwa 2015). Most termite management practices are focused on total elimination of termite population rather than sustaining their population. EPNs are being produced commercially and used as biological control agents in many parts of the world. Because these nematodes are adapted to the soil environment, the principal hosts are soil insects and insects in cryptic habitats. These nematodes have been utilized in classical, conservative, and augmentative biological control programs. S. scapterisci originally isolated from Uruguay was successfully introduced into Florida for the classical biological control of mole cricket pests (Parkman and Smart 1996). In the field, however, entomopathogenic nematodes attack a significantly narrower host range than in the laboratory. Indigenous nematodes are exempted from registration in many European countries, Australia, and the United States, while in other countries, they are subject to similar registration procedures as for a chemical pesticide. Use of these nematodes offers a number of advantages and will be useful in countries where alternative control approaches are needed to replace the more toxic insecticides that are currently in use. With the general public becoming increasingly concerned about pesticide usage, the use of nematodes for termite control is a potentially promising market. Current use of Steinernema and Heterorhabditis nematodes as biological control organisms has been summarized by Shapiro-Ilan and Gaugler (2010) (Table 4).
5.1 Under Laboratory Conditions
Hundreds of different species from most orders of insects were susceptible to various entomopathogenic nematodes under laboratory conditions. Entomopathogenic nematodes Steinernema carpocapsae, Steinernema riobrave, Heterorhabditis bacteriophora, and Heterorhabditis indica were evaluated by Wang et al. (2002) for their activity against Reticulitermes flavipes and Coptotermes formosanus in the laboratory. All were noticed effective against C. formosanus at ≥400 nematodes per termite. S. riobrave, however, had no detectable effect against R. flavipes even at a rate of 2000 nematodes per termite. They also demonstrated that H. indica was more efficacious (H. indica > H. bacteriophora > S. carpocapsae > S. riobrave) at α = 0.10 level, and nematodes were able to reproduce from R. flavipes and C. formosanus. In contrast, Manzoor (2012) reported that S. carpocapsae and H. bacteriophora when applied individually against the eastern subterranean termite R. flavipes showed no detrimental effects on workers and nymphs of the termites. These efficacy differences of the same nematode species between two studies may be due to the differences in the strains of nematode species used in each study.
In another study, Yu et al. (2006) evaluated parasitism of subterranean termites by S. riobrave, S. carpocapsae, S. feltiae, and H. bacteriophora in laboratory sand assays. They reported that all tested EPNs were capable of infecting and killing termite species Heterotermes aureus, Reticulitermes flavipes, and Gnathamitermes perplexus (Banks). S. riobrave, however, was found to be particularly effective in sand assays and caused significant mortality (≥80 %) of worker H. aureus and G. perplexus at 22 °C. Nematode concentration and incubation time had significant effects on the mortality. Yu et al. (2008) reported that S. carpocapsae, S. riobrave, and H. bacteriophora successfully reproduced in termite H. aureus and infective juveniles (IJs) exited the termite cadavers successfully. However, no progeny were produced by S. feltiae. However, Yu et al. (2010) compared virulence of three novel strains of S. riobrave (3-8b, 7-12, and TP) against subterranean termites H. aureus, R. flavipes, and C. formosanus workers. H. aureus was very susceptible to all the S. riobrave strains, and termites in all nematode treatments were dead after 4 days. The TP strain of S. riobrave, however, caused 75 % and 91 % mortality in R. flavipes and C. formosanus, respectively, which was more than 300 % and 70 % higher than the mortality caused by other strains. Screening indicated superior virulence in the 355 (=TX) strain of S. riobrave to H. aureus compared with the virulence of S. carpocapsae, S. feltiae, and H. bacteriophora (Yu et al. 2006). Thus, S. riobrave 355 may have substantial potential to be developed as a biocontrol agent for H. aureus.
Higher mortalities in subterranean termite Macrotermes caused by S. pakistanense were reported by Shahina and Tabassum (2010) in a filter paper and sand assay. Mankowski et al. (2005) examined the attachment and infectivity of two entomopathogenic nematode species, S. carpocapsae and H. indica, on soldiers and workers in two subterranean termite species, C. formosanus and C. vastator. In attachment tests with S. carpocapsae, they noticed that more nematodes attached to soldiers of C. formosanus and C. vastator. When soldiers alone or workers alone are exposed to the nematodes, there is a differential susceptibility of soldiers and workers to nematode infection with soldiers being more susceptible than workers. The reason for this differential response to nematode infection is that soldiers do not exhibit grooming behavior. El-Bassiouny and El-Rahman (2011) reported the possibility of using entomopathogenic nematodes H. baujardi and H. indica against the Egyptian subterranean termite Psammotermes hypostoma (Desn.) and Anacanthotermes ochraceus (Burm.). The bioagent H. baujardi was noticed more effective for control of the two tested termites, but P. hypostoma was highly susceptible than A. ochraceus. Overall, the mortality increased as the nematode concentrations increased and vice versa.
Differential susceptibility of two termite species, Macrotermes bellicosus and Trinervitermes occidentalis, against EPNs isolates H. indica Ayogbe1, H. sonorensis Azohoue2, H. sonorensis Ze3, and Steinernema sp. Bembereke, from Benin (West Africa), was studied by Zadji et al. (2014a). The results at forty-eight hours post-exposure of workers of M. bellicosus to 50 IJ of H. indica Ayogbe1, H. sonorensis Azohoue2, H. sonorensis Ze3, and Steinernema sp. Bembereke for each termite were 96.3 %, 87.9 %, 94.5 %, and 75.0 % mortality, respectively. In the case of workers of T. occidentalis, under the same conditions, these EPN isolates caused 91.7, 98.5, 75.0, and 95.0 % mortality. Based on concentration-mortality data, they reported that isolates H. indica Ayogbe1 and H. sonorensis Ze3 were more virulent against M. bellicosus with LC50 values of 11 IJ, whereas Steinernema sp. Bembereke was the most virulent against T. occidentalis with LC50 values of 12 IJ. All tested EPN isolates can be recycled in both M. bellicosus and T. occidentalis and the soldiers of both termites studied were noticed more susceptible than workers. Twenty-nine Beninese isolates of H. sonorensis and one local isolate of H. indica were screened by Zadji et al. (2014b) for their pathogenicity against the termite pest Macrotermes bellicosus. Most of the isolates (73 %) killed more than 80 % of the insects. Bioassays, however, showed significant differences among isolates of all tested traits. The greatest survival of infective juveniles to heat (8 h), desiccation (8 h), and hypoxia (72 h) was observed with the H. sonorensis isolates Kassehlo (72.8 %), Setto1 (72.5 %), and Kissamey (81.5 %, respectively). Sri Lankan isolates of ENPs were compared with two commercial formulations of entomopathogenic nematodes, namely, Biosafe (S. carpocapsae) and Nemasys (S. feltiae), against the live-wood termite Postelectrotermes militaris. Sri Lankan isolate Heterorhabditis spp. (HSL 6) and S. carpocapsae were reported better controlling agents against P. militaris than other isolates tested (Amarasinghe and Hominick 1993a). Wilson-Rich et al. (2007) reported that damp-wood termite Zootermopsis angusticollis exhibits a dose-dependent susceptibility to the soil nematode S. carpocapsae (Weiser) (Mexican strain).
5.2 Under Field Conditions
Only a limited number of field studies have been conducted using EPNs as control agents for termites. Certain species of nematodes although effective in laboratory control is often quite variable under field conditions. Soil moisture and soil type appear to limit the nematode’s ability to move in the soil and locate termites. In the 1930s Glaser and colleagues applied S. glaseri to 73 different field plots in New Jersey to control the Japanese beetle, Popillia japonica (Glaser 1932; Glaser et al. 1940). They recovered parasitized grubs from 72 plots 2 weeks after application. Parasitism of the grub population by the nematode in the various plots ranged from 0.3 to 81.0 %. A study testing the efficacy of S. carpocapsae (Weiser) against foraging workers of Reticulitermes tibialis in pastureland was published by Epsky and Capinera (1988). Study showed potential for control of R. tibialis in laboratory and field trials. They also established LD50 values for specific nematode/termite combinations. Amarasinghe and Hominick (1993b) reported that higher doses of S. carpocapsae and S. feltiae showed promising control of live-wood termite Postelectrotermes militaris in tea plantations. Nguyen and Smart (1994) isolated EPN Neosteinernema longicurvicauda Nguyen and Smart from naturally infected subterranean termites, Reticulitermes flavipes.
Heterorhabditis sp. have given encouraging results in Australia. Studies indicate that colonies of Neotermes attacking in the South Pacific Islands can be eliminated using Heterorhabditis sp. from palms and other trees (Dolinski and Lacey 2007). Populations of the damp-wood termite Glyptotermes dilatatus that form colonies of only several thousand members have also been successfully managed with Heterorhabditis sp. with a dose of 4000 ml and 8000 ml nematode suspension in doses of 40 ml and 30 ml per tea bush, respectively (Danthanarayana and Vitarana 1987).
In contrast, workers reported that EPNs showed effectiveness against subterranean termites in the laboratory but did not cause colony elimination in the field with C. formosanus (Fujii 1975; Tamashiro 1976), R. flavipes (Mauldin and Beal 1989), or R. tibialis (Epsky and Capinera 1988) which have shown to be minimally controlled by nematodes. Nematodes sold commercially in the United States failed to eliminate colonies of R. flavipes, in controlled field experiments (Mauldin and Beal 1989). The reasons suggested were termite social behavior like walling-off dead termites and avoiding foraging in nematode infested areas (Fujii 1975; NPCA 1985). Epsky and Capinera (1988) also reported an ability of the subterranean termite R. tibialis to detect and avoid S. feltiae. Yanagawa et al. (2012) hypothesized that termites can perceive pathogens and this ability plays an important role in effective hygiene behavior. For the successful application of biopesticides to termites in nature, it would be beneficial to identify substances that could disrupt the termite’s ability to perceive pathogens. Different isolates or species of entomogenous nematode species that are tolerant to higher temperatures are required for control of subterranean termite in the fields. Insect susceptibility to entomopathogenic nematodes in the families Steinernematidae and Heterorhabditidae varies among insect species and is influenced by nematode species, strain, and an assembly of abiotic and biotic factors. Entomopathogenic nematodes also differ in their abilities to survive different environmental conditions.
5.3 Synergism of EPNs
Several previous studies have demonstrated additive or synergistic relationships between the combined use of low-impact insecticides and biological control agents (Kaakeh et al. 1997; Quintela and McCoy 1998; Koppenhofer and Kaya 1998; Nishimatsu and Jackson 1998). Infective juveniles are tolerant of short exposures (2–6 h) to most agrochemicals including insecticides, herbicides, fungicides, and acaricides (Rovesti and Deseo 1990; Ishibashi 1993) and, therefore, can often be tank mixed. However, some pesticides can reduce nematode infectivity and survival (Grewal et al. 1998). Heterorhabditids tend to be more sensitive to physical challenges, including pesticides, than steinernematids (Grewal et al. 2001). Murugan and Vasugi (2011) evaluated bioactivities of S. glaseri in combination with neem seed kernel extract (NSKE) against Reticulitermes flavipes. On the fourth day 40 % and 70 % mortality were reported at lower (1.0 %) and higher (4.0 %) concentrations of NSKE, respectively. Neem at various concentrations did not affect the survival of nematodes but had considerable impact on the survival of termites which may be due to the presence of bioactive compounds azadirachtins, salanin, etc. Entomopathogenic nematodes and the chloronicotinyl insecticide imidacloprid interact synergistically on termite mortality. The degree of interaction, however, varies with nematode species. Manzoor (2012) reported synergism between imidacloprid and nematodes species S. carpocapsae and H. bacteriophora that caused more than 50 % mortality of workers and nymphs of R. flavipes within all three colonies tested.
6 Survey of New Species/Strains
Screening of entomopathogenic nematode species or strains for the control of specific insect pest, which are adapted to local environmental conditions, could achieve a higher level of efficacy. Therefore, it is important to survey and preserve indigenous entomopathogenic nematode population. Kary et al. (2009) conducted survey in Iran throughout the three provinces in the northwest of the country and concluded that out of the 833 soil samples, 27 (3.2 %) were positive for entomopathogenic nematodes with 17 (2.0 %) containing Heterorhabditis and 10 (1.2 %) Steinernema isolates. H. bacteriophora and S. feltiae were the common species. A survey of entomopathogenic nematodes was conducted for the first time in Nepal by Khatri-Chhetri et al. (2010). Of the 276 soil samples studied, 29 (10.50 %) were positive of EPNs containing seven samples of heterorhabditids (24.14 %) and 22 samples (75.86 %) of steinernematid. To determine the occurrence of EPNs, Zepeda-Jazo et al. (2014) conducted a survey on the Pacific coast of the State of Colima, Mexico. Of the 19 soil samples collected, 14 (73.7 %) were positive for EPNs, containing 12 steinernematid isolates (85.7 %) and two heterorhabditid isolates (14.3 %). Further they reported that most of the isolates were recovered from cultivated habitats, suggesting that there is a higher prevalence of EPNs in cultivated soils. Jawish et al. (2015) conducted a survey of EPNs of the families Steinernematidae and Heterorhabditidae from January 2011 to December 2013 in Damascus countryside of Syria. Of 189 soil samples studied, 17 (9 %) were positive of entomopathogenic nematode with 11 (65 %) samples containing Heterorhabditis and six (35 %) Steinernema isolates. Therefore survey and evaluation of new species or strains of entomopathogenic nematodes against termite pests may achieve a higher level of management.
7 Conclusion and Future Prospects
EPNs and their associated bacterial symbionts have been proven safe to vertebrates and other nontarget organisms. They are highly specific in their host range and compatible with many pesticides, can be easily produced in vivo and in vitro, can be applied with standard spray equipment, and have been exempt from registration in many countries. A wide range of entomopathogenic nematodes are effective against various termite species. However, limited number of field studies have been conducted against termites using EPNs. New isolates of EPNs from various parts of the world are being studied, which may prove effective against termite pests in the field. Therefore use of EPNs would bring several benefits to sustainable agriculture and develop a better understanding of biodiversity.
References
Akhurst RJ, Boemare N (1990) Biology and taxonomy of Xenorhabdus. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, FL, pp 75–87
Alam MA, Miah MAH (1997) Akher Anistakari pokamaker (in Bangla). In: Ahmed TU, Jalil AFMA (eds) Bangladesher Krisher Anistakari Pokamakor, vol 2. Bangla Academy, Dhaka, Bangladesh, pp 1–49
Al-Fazairy AA, Hassan FA (1988) Infection of termites by Spodoptera littoralis nuclear polyhedrosis virus. Int J Trop Insect Sci 9:37–39
Al-Fazairy AA, Hassan FA (1993) Histopathology of termite Kalotermes Flavicollis Fabr. infected with a nuclear polyhedrosis virus. Int J Trop Insect Sci 14:127–134
Almeida JEM, Alves SB, Pereira RM (1997) Selection of Beauveria spp isolates for control of the termite Heterotermes tenuis (Hagen, 1858). Z Angew Entomol 121:539–543
Amarasinghe LD, Hominick WM (1993a) Efficacy of entomopathogenic nematodes to control up-country live-wood termite. Sri Lanka J Tea Sci 62:16–24
Amarasinghe LD, Hominick WM (1993b) Potential of using entomopathogenic nematodes to control up-country live-wood termite (Postelectrotermes militaris). Sri Lanka J Tea Sci 62:66–78
Bedding RA (1984) Large-scale production, storage, and transport of the insect-parasitic nematodes Neoaplectana spp. and Heterorhabditis spp. Ann Appl Biol 104:118–120
Bedding RA, Akhurst RJ (1974) A simple technique for the detection of insect parasitic nematodes in soil. Nematologica 21:109–110
Bedding R, Akhurst RJ, Kaya H (1993) Nematodes and the biological control of insect pests. CSIRO, East Melbourne, VIC
Bignell DE, Eggleton P (2000) Termites in ecosystems. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publications, Dordrecht, pp 363–387
Boemare N (2002) Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford
Boemare NE, Akhurst RJ, Mourant RG (1993) DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacteriol 43:249–255
Burman M (1982) Neoaplectana carpocapsae: toxin production by axenic insect parasitic nematodes. Nematologica 28:62–70
Campanhola C, de Moraes GJ, de Sa LAN (1995) Review of IPM in South America. In: Mengech AN, Saxena KN, Gopalan HNB (eds) Integrated pest management in the tropics. John Wiley and Sons, Chichester, pp 121–152
Carter FL, Jones SC, Mauldin JK, de Camargo CRR (1983) Responses of Coptotermes formosanus Shiraki to extracts from five Brazilian hardwoods. Z Angew Entomol 95:5–14
Castilhos-Fortes R, Matsumura ATS, Diehl E, Fiuza LM (2002) Susceptibility of Nasutitermes ehrhardti (Isoptera: termitidae) to Bacillus thuringiensis subspecies. Braz J Microbiol 33:219–222
Chhillar BS, Saini RK, Roshanlal K (2006) Emerging trends in economic entomology. Chaudhary Charan Singh Haryana Agricultural University Press, Hissar, pp 191–192
Ciche TA, Darby C, Ehlers RU, Forst S, Goodrich-Blair H (2006) Dangerous liaisons: the symbiosis of entomopathogenic nematodes and bacteria. Biol Control 38:22–46
Cornelius ML, Osbrink WLA (2015) Natural resistance of exotic wood species to the Formosan subterranean termite (Isoptera: Rhinotermitidae). Int Biodeterior Biodegradation 101:8–11
CPL (2006) Biopesticides 2007. CPL Business consultants, Wallingford
CPL (2013a) Europe: biopesticides market 2013. CPL Business consultants, Wallingford
CPL (2013b) North America: biopesticides market 2013. CPL Business consultants, Wallingford
Culliney TW, Grace JK (2000) Prospects for biological control of subterranean termite (Isoptera: Rhinotermitidae), with special reference to Coptotermes formosanus. Bull Entomol Res 90:9–21
Dambros CS, Mendonca DRM, Morais JW, Rebelo TG (2012) Termite species list in a terra firme and ghost forest associated with a hydroelectric plant in Presidente Figueiredo, Amazonas, Brazil. Check List 8:718–721
Dangerfield JM, Mccarthy TS, Ellery WN (1998) The mound-building termite Macrotermes michaelseni as an ecosystem engineer. J Trop Ecol 14:507–520
Danthanarayana W, Vitarana SI (1987) Control of the live-wood tea termite Glyptotermes dilatatus using Heterorhabditis sp. (Nemat.). Agric Ecosyst Environ 19:333–342
Devi KK, Kothamasi D (2009) Pseudomonas fluorescens CHA0 can kill subterranean termite Odontotermes obesus by inhibiting cytochrome c oxidase of the termite respiratory chain. FEMS Microbiol Lett 300:195–200
Dolinski C, Lacey LA (2007) Microbial control of arthropod pests of tropical tree fruits. Neotrop Entomol 36:161–179
Dong C, Zhang J, Chen W, Huang H, Hu Y (2007) Characterization of a newly discovered China variety of Metarhizium anisopliae (M. anisopliae var. dcjhyium) for virulence to termites, isoenzyme, and phylogenic analysis. Microbiol Res 162:53–61
Dowds BCA, Peters A (2002) Virulence mechanisms. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, pp 79–98
Ebeling W (1978) Urban entomology. Div. Agric. Sci., Univ. Calif, Berkeley, CA, p 695
Eggleton P (2000) Global patterns of termite diversity. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer, Dordrecht, pp 25–51
Ehlers RU, Wulff A, Peters A (1997) Pathogenicity of axenic Steinernema feltiae, Xenorhabdus bovienii, and the bactohelminthic complex to larvae of Tipula oleracea (Diptera) and Galleria mellonella (Lepidoptera). J Invertebr Pathol 69:212–217
Ehlers RU, Lunau S, Krasomil-Osterfeld K, Osterfeld KH (1998) Liquid culture of the entomopathogenic nematode-bacterium-complex Heterorhabditis megidis/Photorhabdus luminescens. Biocontrol 43:1386–6141
Elango G, Abdul Rahuman A, Kamaraj C, Bagavan A, Abduz Zahir A, Santhoshkumar T, Marimuthu S, Velayutham K, Jayaseelan C, Vishnu KA, Rajakumar G (2012) Efficacy of medicinal plant extracts against Formosan subterranean termite, Coptotermes formosanus. Ind Crops Prod 36:524–530
El-Bassiouny AR, El-Rahman RMA (2011) Susceptibility of Egyptian subterranean termite to some entomopathogenic nematodes. Egypt J Agric Res 89:121–135
El-Sadaway HA (2011) Mass production of Steinernema spp. on in-vitro developed solid medium. World Appl Sci J 14:803–813
Epsky ND, Capinera JL (1988) Efficacy of the entomogenous nematode Steinernema feltiae against a subterranean termite, Reticulitermes tibialis (Isoptera: Rhinotermitidae). J Econ Entomol 81:1313–1317
Faragalla AA (2002) Ecozoogeography of termites (Isoptera) in Saudi Arabia. Sociobiology 39:195–212
Faragalla AA, Al-Ghamdi KMS, Taher MO (1998) Performance of some insecticides against subterranean termites infesting field crops in Southern Saudi Arabia. Ann Agric Sci Moshtohor 36:1115–1124
Fischer-Le Saux M, Arteaga-Hernandez E, Mracek Z, Boemare NE (1999) The bacterial symbiont Xenorhabdus poinarii (Enterbacteriaceae) is harbored by two phylogenetic related host nematodes: the entomopathogenic species Steinernema cubanum and Steinernema glaseri (Nematoda: Steinernematidae). FEMS Microbiol Ecol 29:149–157
Fodor A, Banfalvi Z, Jenes B, Lehoczky E, Alexa A (2010) Desiccation tolerant transgenic nematodes. In: Proceeding of International Symposium of European Society of Nematologists, Vienna, 19–23rd September, p 194
Freymann BP, Buitenwerf R, Desouza O, Olff H (2008) The importance of termites (Isoptera) for the recycling of herbivore dung in tropical ecosystems: a review. Eur J Entomol 105:165–173
Fujii JK (1975) Effects of an entomogenous nematode Neoaplectana carpocapsae Weiser, on the Formosan subterranean termite, Coptotermes formosanus. PhD thesis, University of Hawaii, Honolulu, HI
Gaugler R (1981) Biological control potential of neoaplectanid nematodes. J Nematol 13:241–249
Gaugler R (2002) Entomopathogenic nematology. CABI Publishing, Wallingford
Gaugler R, Boush GM (1979) Nonsusceptibility of rats to the entomogenous nematode, Neoaplectana carpocapsae. Environ Entomol 8:658–660
Gaugler R, Kaya HK (1990) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, FL
Georgis R (1990) Formulation and application technology. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, FL, pp 173–191
Georgis R, Manweiler SA (1994) Entomopathogenic nematodes: a developing biological control technology. Agric Zool Rev 6:63–94
Ghaly A, Edwards S (2011) Termite damage to buildings: nature of attacks and preventive construction methods. Am J Eng Appl Sci 4:187–200
Glaser RW (1932) Studies on Neoaplectana glaseri, a nematode parasite of the Japanese beetle (Popillia japonica). Circular No. 211, New Jersey Department of Agriculture, Trenton, New Jersey
Glaser RW, Fox H (1930) A nematode parasite of the Japanese beetle (Popillia japonica Newm.). Science 71:16–17
Glaser RW, McCoy EE, Girth HB (1940) The biology and economic importance of a nematode parasitic in insects. J Parasitol 26:479–495
Gold RE, Howell RN, Pawson JBM, Wright MS, Luts JC (1996) Persistence and termiticides to Subterranean termites (Isoptera: Rhinotermitidae) from five soil types and locations in Texas. Sociobiology 28:337–363
Grace JK (1997) Biological control strategies for suppression of termites. J Agric Entomol 14:281–289
Grewal PS, Webber T, Batterley DA (1998) Compatibility of Steinernema feltiae with chemicals used in mushroom production. Mushroom News 46:6–10
Grewal PS, De Nardo EAB, Aguillera MM (2001) Entomopathogenic nematodes: potential for exploration and use in South America. Neotrop Entomol 30:191–205
Grewal PS, Ehlers RU, Shapiro-Ilan DI (2005) Nematodes as biological control agents. CABI Publishing, New York, NY
Griffin CT, Callaghan KM, Dix I (2001) A self-fertile species of Steinernema from Indonesia: further evidence of convergent evolution amongst entomopathogenic nematodes. Parasitology 122:181–186
Han R, Ehlers RU (1999) Trans-specific nematicidal activity of Photorhabdus luminescens. Nematology 1:687–693
Hashmi S, Hashmi G, Gaugler R (1995) Genetic transformation of an entomopathogenic nematode by microinjection. J Invertebr Pathol 66:293–296
Haverty MI, Woodrow RJ, Nelson LJ, Grace JK (2005) Identification of termite species by the hydrocarbons in their feces. J Chem Ecol 31:2119–2151
Hazir S, Kaya HK, Stock SP, Keskin N (2003) Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turk J Biol 27:181–202
Hemasree E (2013) A critical review on the natural occurrence of entomopathogenic fungi in agricultural ecosystem. Int J Appl Biol Pharma Technol 4:372–375
Hominick WM, Reid AP, Bohan DA, Briscoe BR (1996) Entomopathogenic nematodes: biodiversity, geographical distribution and the convention on biological diversity. Biocontrol Sci Technol 6:317–331
Howard RW, Jones SC, Mauldin JK, Beal RH (1982) Abundance, distribution and colony size estimates for Reticulitermes spp. (Isoptera: Rhinotermitidae) in southern Mississippi. Environ Entomol 11:1290–1293
Howse PE (1970) Termite: a study in social behaviour. Rentokil Ltd, West Sussex, p 150
Hu XP, Yuan Z, Ding W (2011) Anti-termitic properties of Lantana camara (Lamiales: Verbenaceae). In: Proceeding of seventh International conference on urban pests, 7–10th August, 2011, Ouro Preto, Brazil, pp 217–221
Hui E, Webster JM (2000) Influence of insect larvae and seedling roots on the host-finding ability of Steinernema feltiae (Nematoda: Steinernematidae). J Invertebr Pathol 75:152–162
Hussain A, Tian MY, Ahmed S, Shahid M (2012) Current status of entomopathogenic fungi as mycoinsecticides and their inexpensive development in liquid cultures. In: Garcia MD (ed) Zoology. InTech, Rijeka, Croatia, pp 103–122, Available from http://www.intechopen.com/books/zoology/current-status-of-entomopathogenic-fungi-as-mycoinecticides-andtheir-inexpensive-development-in-liq. Assessed on 24th June 2015
Ishibashi N (1993) Integrated control of insect pests by Steinernema carpocapsae. In: Bedding RA, Akhurst RJ, Kaya HK (eds) Nematodes and biological control of insects. CSIRO, East Melbourne, VIC, pp 105–113
Jawish A, Al-assas K, Basheer A (2015) A survey of entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in Damascus countryside of Syria. EPPO Bull 45:81–89
Johnson B (2005) Diazinon and pesticide related toxicity in bay area urban creeks: water quality attainment strategy and total maximum daily load (TMDL). Final Project Report, California Regional Water Quality Board, San Francisco Bay Region, 1515 Clay St., Oakland, CA, pp 132
Johnson JB, Hagen KS (1981) A neuropterous larva uses an allomone to attack termites. Nature 238:506–507
Jones WE, Grace JK, Tamashiro M (1996) Virulence of seven isolates of Beauveria bassiana and Metarhizium anisopliae to Coptotermes formosanus (Isoptera: Rhinotermitidae). Environ Entomol 25:481–487
Joshi PK, Singh NP, Singh NN, Gerpacio RV, Pingali PL (2005) Maize in India: production systems, constraints, and research priorities. DF CIMMYT, Mexico, p 22
Kaakeh W, Reid BL, Bohnert TJ, Bennet GW (1997) Toxicity of imidacloprid in the German cockroach (Dictyoptera: Blattellidae), and the synergism between imidacloprid and Metarhizium anisopliae (Imperfect fungi: Hyphomycetes). J Econ Entomol 90:473–482
Kary NE, Niknam G, Griffin CT, Mohammadi SA, Moghaddam M (2009) A survey of entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in the north-west of Iran. Nematology 11:107–116
Kaya HK (1985) Entomogenous nematodes for insect control in IPM systems. In: Hoy MA, Herzog DC (eds) Biological control in agricultural IPM systems. Academic, Orlando, FL, pp 283–302
Kaya HK (1990) Soil ecology. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, FL, pp 93–115
Kaya HK, Gaugler R (1993) Entomopathogenic nematodes. Annu Rev Entomol 38:181–206
Khan MA, Ahmad W (2015) The management of Spodopteran pests using fungal pathogens. In: Sree KS, Varma A (eds) Biocontrol of lepidopteran pests, vol 43, Soil biology. Springer International Publishing, Cham, pp 123–160
Khatri-Chhetri HB, Waeyenberge L, Manandhar HK, Moens M (2010) Natural occurrence and distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in Nepal. J Invertebr Pathol 103:74–78
King EG, Spink WT (1969) Foraging galleries of the Formosan termite, Coptotermes formosanus, in Louisiana. Ann Entomol Soc Am 62:537–542
Kirton LG (2005) The importance of accurate termite taxonomy in the broader perspective of termite management. In: Proceedings of 5th International Conference on Urban Pests, 10–13th July, 2005, Singapore, pp 1–7
Kirton LG, Brown VK (2003) The taxonomic status of pest species of Coptotermes in Southeast Asia: Resolving the paradox in the pest status of the termites, Coptotermes gestroi, C. havilandi and C. travians (Isoptera: Rhinotermitidae). Sociobiology 42:43–64
Klein MG (1990) Efficacy against soil-inhabiting insect pests. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, FL, pp 195–214
Koppenhofer AM, Kaya HK (1998) Synergism of imidacloprid and an entomopathogenic nematode: a novel approach to white grub (Coleoptera: Scarabaeidae) control in turfgrass. J Econ Entomol 91:618–623
Krishna K, Grimaldi DA (2003) The first cretaceous Rhinotermitidae (Isoptera): a new species, genus, and subfamily in Burmese Amber. Nat Hist 3390:10
Kumar S (1971) Causes of natural durability in timber. J Timber Dev Assoc India 18:1015
Lacey LA, Georgis R (2012) Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J Nematol 44:218–225
Lacey LA, Kaya HK (2000) Field manual of techniques in invertebrate pathology. Kluwer Academic Publishers, Dordrecht
Lambert B, Hofte H, Annys K, Jansens S, Soetaert P, Peferoen M (1992) Novel Bacillus thuringiensis insecticidal crystal protein with a silent activity against coleopteran larvae. Appl Environ Microbiol 58:2536–2542
Lee CY (2002) Subterranean termite pests and their control in the urban environment in Malaysia. Sociobiology 40:3–9
Lenz M (2005) Biological control in termite management: the potential of nematodes and fungal pathogens. In: Proc 5th Int conf on urban pests, 10–13th July, 2005, Singapore, pp 47–52
Mankowski ME, Kaya HK, Grace JK, Sipes B (2005) Differential susceptibility of subterranean termite castes to entomopathogenic nematodes. Biocontrol Sci Technol 15:367–377
Manzoor F (2012) Synergism of imidacloprid and entomopathogenic nematodes for the control of eastern subterranean termite, Reticulitermes flavipes (Isoptera: Rhinotermitidae). Pak J Zool 44:1397–1403
Mashek B, Quarles W (2008) Orange oil for drywood termites: magic or marketing madness. The IPM Practitioner 30(1/2):1–9
Mauldin JK, Beal RH (1989) Entomogenous nematodes for control of subterranean termites, Reticulitermes spp. (Isoptera, Rhinotermitidae). J Econ Entomol 82:1638–1642
Milner RJ, Staples JA (1996) Biological control of termites: results and experiences within a CSIRO project in Australia. Biocontrol Sci Technol 6:3–9
Molyneux AS (1985) Survival of infective juveniles of Heterorhabditis spp., and Steinernema spp. (Nematoda: Rhabditida) at various temperatures and their subsequent infectivity for insects. Rev Nematol 8:165–170
Mugerwa S (2015) Infestation of African savanna ecosystems by subterranean termites. Ecol Compl 21:70–77
Murugan K, Vasugi C (2011) Combined effect of Azadirachta indica and the entomopathogenic nematode Steinernema glaseri against subterranean termite, Reticulitermes flavipes. J Entomol Acarolog Res 43:253–259
Myles TG (2002) Isolation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) from Reticulitermes flavipes (Isoptera: Rhinotermitidae) with convenient methods for its culture and collection of conidia. Sociobiology 40:257–262
National Pest Control Association (1985) Termite control: use of nematodes. Tech Release ESPC 055047. National Pest Control Association, New York
Neoh KB, Lee CY (2011) The parasitoid, Verticia fasciventris causes morphological and behavioral changes in infected soldiers of the fungus-growing termite, Macrotermes carbonarius. J Insect Sci 11:47
Nguyen KB, Smart GC Jr (1994) Neosteinernema longicurvicauda n. gen., n. sp. (Rhabditida: Steinernematidae), a parasite of the termite Reticulitermes flavipes (Koller). J Nematol 26:162–174
Nickle WR, Welch HE (1984) History, development, and importance of insect nematology. In: Nickle WR (ed) Plant and insect nematodes. Marcel Dekker, New York, NY, pp 627–653
Nishimatsu T, Jackson JJ (1998) Interaction of insecticides, entomopathogenic nematodes, and larvae of the western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol 91:410–418
NRC (National Research Council) (1993) Pesticides in the diets of infants and children. National Academy Press, Washington, DC, p 386
Ohiagu CE (1979) Nest and soil population of Trinervitermes Spp. with particular references to Trinervitermes geminatus (Wasmann) Isoptera in Southern Guinea Savanna, near Mokwa, Nigeria. Ecología 40:167–178
Osbrink WLA, Williams KS, Connick WJ Jr, Wright MS, Lax AR (2001) Virulence of bacteria associated with the Formosan subterranean termite (Isoptera: Rhinotermitidae) in New Orleans, LA. Environ Entomol 30:443–448
Parkman JP, Smart GC Jr (1996) Entomopathogenic nematodes, a case study: introduction of Steinernema scapterisci in Florida. Biocontrol Sci Technol 6:413–419
Parkman JP, Frank JH, Nguyen KB, Smart GC Jr (1993) Dispersal of Steinernema scapterisci (Rhabditida: Steinernematidae) after inoculative applications for mole cricket (Orthoptera: Gryllotalpidae) control in pastures. Biol Control 3:226–232
Paul BB, Rueben JM (2005) Arizona termites of economic importance. University of Arizona Press, Tucson, AZ, pp 9–17
Poinar GO Jr (1966) The presence of Achromobacter nematophilus Poinar and Thomas in the infective stage of a Neoaplectana sp. (Steinernematidae: Nematoda). Nematologica 12:105–108
Poinar GO Jr (1975) Entomogenous nematodes. A manual and host list of insect-nematode associations. EJ Brill, Leiden, p 317
Poinar GO Jr (1979) Nematodes for biological control of insects. CRC Press, Boca Raton, FL, p 277
Poinar GO Jr (1986) Entomogenous nematodes. In: Franz JM (ed) Biological plant and health protection. G Fischer Verlag, Stuttgart, pp 95–121
Poinar GO Jr, Grewal PS (2012) History of entomopathogenic nematology. J Nematol 44:153–161
Poinar GO Jr, Leutenegger R (1968) Anatomy of the infective and normal third stage juveniles of Neoaplectana carpocapsae Weiser. J Parasitol 54:340–350
Potter MF (1997) Termite baits: a status report. Pest Control Technol 25:24–26
Quintela ED, McCoy CW (1998) Synergistic effect of two entomopathogenic fungi and imidacloprid on the behavior and survival of larvae of Diaprepes abbreviates (Coleoptera: Curculionidae) in soil. J Econ Entomol 91:110–122
Rajagopal D (2002) Economically important termite species in India. Sociobiology 40:33–46
Roonwal ML (1955) Termite ruining a township. Z Angew Entomol 38:103–104
Roonwal ML (1979) Termite life and termite control in tropical south Asia. Scientific Publishers, Jodhpur, p 25
Rovesti L, Deseo KV (1990) Compatibility of chemical pesticides with the entomopathogenic nematodes, Steinernema carpocapsae Weiser and S. feltiae Filipzev (Nematoda: Steinernematidae). Nematologica 36:237–245
Ru E (2001) Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol 56:623–633
Rust MK, Su NY (2012) Managing insects of social importance. Ann Rev Entomol 57:355–375
Sacks E (2011) Termites eat through $222,000 worth of rupee notes in Indian bank. Daily News. Available from http://www.nydailynews.com/news/world/termites-eat-222-000-worth-rupee-notes-indian-bank-article-1.111054. Accessed on April 25th, 2011
Salihah Z, Shah M, Sattar A (1988) Survey of sugarcane termites of Nowshera and Charsadda Tehsils. In: Proc 8th Pak Congr Zool 8:289–97
Scheffrahn RH, Su NY (1994) Keys to soldier and winged adult termites (Isoptera) of Florida. J FL Entomol 77:460–474
Scheffrahn RH, Mangold JR, Su NY (1988a) A survey of structure-infesting termites of peninsular Florida. J FL Entomol 71:615–630
Scheffrahn RH, Hsu RC, Su NY, Huffman JB, Midland SL, Sims JJ (1988b) Allelochemical resistance of bald cypress, Taxodium distichum, heartwood to the subterranean termite, Coptotermes formosanus. J Chem Ecol 14:765–766
Scheffrahn RH, Darlington JPEC, Collins MS, Krecek J, Su NY (1994) Termites (Isoptera: Kalotermitidae, Rhinotermitidae, Termitidae) of the West Indies. Sociobiology 24:213–238
Scholz G, Militz H, Gascon-Garrido P, Ibiza-Palacios MS, OliverVillanueva JV, Peters BC, Fitzgerald CJ (2010) Improved termite resistance of wood by wax impregnation. Int Biodeterior Biodegradation 64:688–693
Shahina F, Tabassum KA (2010) Virulence of Steinernema pakistanense against different insect species in laboratory condition. Pak J Nematol 28:279–284
Shapiro-Ilan DI, Gaugler R (2002) Production technology for entomopathogenic nematodes and their bacterial symbionts. J Ind Microbiol Biotechnol 28:137–146
Shapiro-Ilan DI, Gaugler R (2010) Nematodes: Rhabditida: Steinernematidae & Heterorhabditidae. In: Shelton A (ed) Biological control: a guide to natural enemies in North America. Cornell University. Available from http://www.biocontrol.entomology.cornell.edu/pathogens/nematodes.php. Accessed on June 24th, 2015
Shapiro-Ilan DI, Han R, Dolinksi C (2012a) Entomopathogenic nematode production and application technology. J Nematol 44:206–217
Shapiro-Ilan DI, Lewis EE, Campbell JF, Kim-Shapiro DB (2012b) Directional movement of entomopathogenic nematodes in response to electrical field: effects of species, magnitude of voltage, and infective juvenile age. J Invertebr Pathol 109:34–40
Sharma AK, Babu KS, Nagarajan S, Singh SP, Kumar M (2004) Distribution and status of termite damage to wheat crop in India. Ind J Entomol 66:235–237
Sharma AK, Sahaan MS, Babu KS (2009) Wheat crop. Health Newsl 14:23–27
Sharma MP, Sharma AN, Hussaini SS (2011) Entomopathogenic nematodes, a potential microbial biopesticide: mass production and commercialisation status – a mini review. Arch Phytopathol Plant Protect 44:855–870
Singh N, Kumar S (2008) Anti termite activity of Jatropha curcas linn. Biochemicals. J Appl Sci Environ Manag 12:67–69
Singha D, Singha B, Dutta BK (2010) In vitro pathogenicity of Bacillus thuringiensis against tea termites. J Biol Control 24:279–281
Smart GC Jr (1995) Entomopathogenic nematodes for the biological control of insects. J Nematol 27(Suppl):529–534
Stock SP, Blair HG (2008) Entomopathogenic nematodes and their bacterial symbionts: the inside out of a mutualistic association. Symbiosis 46:65–75
Su NY, Scheffrahn RH (1988) Foraging population and territory of the Formosan subterranean termite (Isoptera: Rhinotermitidae) in an urban environment. Sociobiology 14:353–359
Su NY, Scheffrahn RH (1990) Economically important termites in the United States and their control. Sociobiology 17:77–94
Su NY, Scheffrahn H (1998) A review of subterranean termite control practices and prospects for integrated pest management programmes. Integr Pest Manag Rev 3:1–13
Su NY, Tamashiro M (1987) An overview of the Formosan subterranean termite in the world. In: Tamashiro M, Su NY (eds) Biology and control of the Formosan subterranean termite. University of Hawaii, Honolulu, HI, pp 3–15
Su NY, Tamashiro M, Yates JR, Haverty MI (1984) Foraging behavior of the Formosan subterranean termite (Isoptera: Rhinotermitidae). Environ Entomol 13:1466–1470
Su NY, Scheffrahn R, Weissling T (1997a) Termite species migrates to Florida: a non-native subterranean species, Coptotermes havilandi, has been discovered in Miami. Pest Control 65:27–28
Su NY, Scheffrahn RH, Weissling T (1997b) A new introduction of a subterranean termite, Coptotermes havilandi Holmgren (Isoptera: Rhinotermitidae) in Miami, Florida. J FL Entomol 80:408–411
Sze TW, Pape T, Toole DK (2008) The first blow fly parasitoid takes a head start in its termite host (Diptera: Calliphoridae, Bengaliinae; Isoptera: Macrotermitidae). Syst Biodivers 6:25–30
Tamashiro M (1976) Susceptibility of termites to microbes. Final technical report. Office of Naval Research Department of Entomology, University of Hawaii, Honolulu, HI
Tauber CA, Tauber MJ (1968) Lomamyia latipennis (Neuroptera: Berothidae) life history and larval descriptions. Can Entomol 100:623–629
TBI (2014) Plant Protection code. Policy on usage of plant protection formulations in tea plantations of India. Tea Board of India, Ministry of Commerce and Industry, Govt. of India, pp 1–59
Tewary A (2008) Termites feast on trader’s money. BBC News. Available from http://news.bbc.co.uk/2/hi/south_asia/7334033.stm. Accessed on June 24th, 2015
Thompson G (2000) Termites. Tropical Topics News Letter No. 64, Tropical Savanna, Australia
Traniello JFA, Leuthold RH (2000) Behavior and ecology of foraging in termites. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer, Dordrecht, pp 141–168
Verma M, Sharma S, Prasad R (2009) Biological alternatives for termite control: a review. Int Biodeterior Biodegradation 63:959–972
Wang C, Powell JE, Nguyen K (2002) Laboratory evaluations of four entomopathogenic nematodes for control of subterranean termites (Isoptera: Rhinotermitidae). Environ Entomol 31:381–387
Wilson M, Xin W, Hashmi S, Gaugler R (1999) Risk assessment and fitness of a transgenic entomopathogenic nematode. Biol Control 15:81–87
Wilson-Rich N, Stuart JR, Rosengaus RB (2007) Susceptibility and behavioral responses of the dampwood termite Zootermopsis angusticollis to the entomopathogenic nematodes S. carpocapsae. J Invertebr Pathol 95:17–25
Wiltz BA, Henderson G, Chen J (1998) Effect of naphthalene, butylated hydroxytoluene, dioctyl phthalate, and adipic dioctyl ester, chemicals found in the nests of the Formosan subterranean termite (Isoptera: Rhinotermitidae) on a saprophytic Mucor sp (Zygomycetes: Mucorales). Environ Entomol 27:936–940
Wood TG (1988) Termites and the soil environment. Biol Fertil Soils 6:228–236
Wraight SP, Inglis GD, Goettel MS (2007) Fungi. In: Lacey LA, Kaya HK (eds) Field manual of techniques in invertebrate pathology, 2nd edn. Springer, Dordrecht, pp 223–248
Wright MS, Lax AR (2013) Combined effect of microbial and chemical control agents on subterranean termites. J Microbiol 51:578–583
Wright CG, Leidy RB, Dupree HE Jr (1994) Chlorpyrifos in the air and soil of houses eight years after its application for termite control. Bull Environ Contam Toxicol 52:131–134
Wright MS, Lax AR, Henderson G, Chen JA (2000) Growth response of Metarhizium anisopliae to two Formosan subterranean termite nest volatiles, naphthalene and fenchone. Mycologia 92:42–45
Wright MS, Osbrink WLA, Lax AR (2002) Transfer of entomopathogenic fungi among formosan subterranean termites and subsequent mortality. J Appl Entomol 126:20–23
Wright MS, Osbrink WLA, Lax AR (2004) Potential of entomopathogenic fungi as biological control agents against the Formosan subterranean termite. In: Nelson WM (ed) Agricultural applications in green chemistry. American Chemical Society, Washington, DC, pp 173–188
Yanagawa A, Fujiwara-Tsujii N, Akino T, Yoshimura T, Yanagawa T, Shimizu S (2012) Odor aversion and pathogen-removal efficiency in grooming behavior of the termite Coptotermes formosanus. PLoS One 7:e47412
Yendol WG, Paschke JD (1965) Pathology of an entomophthora infection in the eastern subterranean termite, Reticulitermes flavipes (Kollar). J Invertebr Pathol 7:414–422
Yu H, Gouge DH, Baker P (2006) Parasitism of subterranean termites (Isoptera: Rhinotermitidae:Termitidae) by entomopathogenic nematodes (Rhabditida: Steinernematidae; Heterorhabditidae). J Econ Entomol 99:1112–1119
Yu H, Dawn HG, Patricia SS, Paul BB (2008) Development of entomopathogenic nematodes (Rhabditida: Steinernematidae; Heterorhabditidae) in the desert subterranean termite Heterotermes aureus (Isoptera: Rhinotermitidae). J Nematol 40:311–317
Yu H, Gouge DH, Shapiro-Ilan DI (2010) A novel strain of Steinernema riobrave (Rhabditida: Steinernematidae) possesses superior virulence to subterranean termites (Isoptera: Rhinotermitidae). J Nematol 42:91–95
Zadji L, Baimey H, Afouda L, Moens M, Decraemer W (2014a) Characterization of biocontrol traits of heterorhabditid entomopathogenic nematode isolates from South Benin targeting the termite pest Macrotermes bellicosus. Biocontrol 59:333–344
Zadji L, Baimey H, Afouda L, Moens M, Decraemer W (2014b) Comparative susceptibility of Macrotermes bellicosus and Trinervitermes occidentalis (Isoptera: Termitidae) to entomopathogenic nematodes from Benin. Nematology 16:719–727
Zepeda-Jazo I, Molina-Ochoa J, Lezama-Gutierrez R, Skoda SR, Foster JE (2014) Survey of entomopathogenic nematodes from the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in Colima, Mexico. Int J Trop Insect Sci 34:53–57
Zhao R, Han R, Qiu X, Yan X, Cao L, Liu X (2008) Cloning and heterologous expression of insecticidal-protein-encoding genes from Photorhabdus luminescens TT01 in Enterobacter cloacae for termite control. Appl Environ Microbiol 74:7219–7226
Acknowledgments
Senior author is thankful to the Head of Biology Department, Faculty of Science, Jazan University, for his encouragement to study bio-management of termites. Financial assistance (No: CERS 7/2013) granted by the Research Center for Environmental Studies, Jazan University, is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Khan, M.A., Ahmad, W., Paul, B., Paul, S., Khan, Z., Aggarwal, C. (2016). Entomopathogenic Nematodes for the Management of Subterranean Termites. In: Hakeem, K., Akhtar, M., Abdullah, S. (eds) Plant, Soil and Microbes. Springer, Cham. https://doi.org/10.1007/978-3-319-27455-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-27455-3_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27453-9
Online ISBN: 978-3-319-27455-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)