Abstract
Cerebral venous sinus thrombosis (CVST) is a relatively rare but potentially devastating entity that mainly affects young adults and children and represents between 0.5 and 1 % of all strokes (Saposnik et al., Stroke 42(4):1158–1192, 2011). Despite significant improvements in outcomes (Coutinho et al., Stroke 45(5):1338–1341, 2014), CVST is a heterogeneous stroke subtype that can be challenging to diagnose and effectively treat and, as a result, is still a significant cause of death and disability (Coutinho et al., Stroke 45(5):1338–1341, 2014; Ferro et al., Stroke 35(3):664–670, 2004). Anticoagulation is currently the mainstay of treatment (Coutinho et al., Cochrane Database Syst Rev (8):CD002005, 2011; Einhaupl et al., Eur J Neurol 17(10):1229–1235, 2010; Weimar, Curr Neurol Neurosci Rep 14(1):417, 2014), but a multidisciplinary approach is usually required to achieve optimal outcomes. Expertise in neurologic diagnosis, medical/surgical management of intracranial hypertension and cerebral edema, neuro-endovascular therapy and critical care often places the neurosurgeon at the center of this multidisciplinary team. And as an integral part of this team, modern neurosurgeons must have an excellent understanding of this complex and interesting stroke subtype. The purpose of this chapter is to provide a basic review of CVST and its treatment with a focus on the role of systemic anticoagulation as a part of the overall treatment methodology. The specific objectives are to review the epidemiology and clinical significance of CVST, discuss the pathophysiology of CVST and its relation to clinical subtypes and potential treatments, provide evidence based review of modern treatment options for CVST with a focus on anticoagulation, offer a clinical algorithm for care of the CVST patient and highlight future directions for management of CVST. As awareness improves and more is known about how and why CVST affects certain groups, more can be done to prevent or eliminate CVST. The neurosurgeon is an integral part of the modern multidisciplinary approach to CVST and thus must maintain a high clinical awareness for CVST and be familiar with the use of anticoagulation and other treatment options for CVST.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cerebral venous sinus thrombosis (CVST) is a relatively rare but potentially devastating entity that mainly affects young adults and children and represents between 0.5 and 1 % of all strokes [1]. Despite significant improvements in outcomes [2], CVST is a heterogeneous stroke subtype that can be challenging to diagnose and effectively treat and, as a result, is still a significant cause of death and disability [2, 3]. Anticoagulation is currently the mainstay of treatment [4–6] but a multidisciplinary) approach is usually required to achieve optimal outcomes. Expertise in neurologic diagnosis, medical/surgical management of intracranial hypertension and cerebral edema, neuro-endovascular therapy and critical care, often places the neurosurgeon at the center of this multidisciplinary team. And as an integral part of this team, modern neurosurgeons must have an excellent understanding of this complex and interesting stroke subtype. The purpose of this chapter is to provide a basic review of CVST and its treatment with a focus on the role of systemic anticoagulation as a part of the overall treatment methodology. The specific objectives are to
-
1.
Review the epidemiology and clinical significance of CVST.
-
2.
Discuss the pathophysiology of CVST and its relation to clinical subtypes and potential treatments.
-
3.
Provide evidence based review of modern treatment options for CVST with a focus on anticoagulation.
-
4.
Offer a clinical algorithm for care of the CVST patient.
-
5.
Highlight future directions.
Epidemiology
CVST is reported to have been initially described as early as 1825 by French physician Ribes [7, 8]. However, due to a combination of poor diagnostic/imaging techniques and a highly variable clinical presentation, CVST was historically only diagnosed at autopsy [5]. Therefore, it is likely that its true incidence was underestimated. Widespread access to advanced, modern imaging techniques has lead to the ability to diagnose CVST more accurately and at earlier, more benign stages of presentation. As expected the overall incidence of CVST has increased accordingly. Currently, CVST is estimated to occur in three to four people per million in adults and between six and seven million in children and neonates [4, 5, 9]. Using data from Portugal, Hong Kong, Mexico, and Iran, the incidence has been estimated at 0.2–1.23 per 100,000. Most recently a Dutch population study looking at 9270 patients over a 2-year period found an incidence of approximately 1.32 per 100,000 in the general population [10, 11].
As mentioned, CVST is a disease of young adults and children. Indeed, based on the International Study on Cerebral Venous and Dural Sinuses Thrombosis (ISCVT), the largest cohort study to date, 78 % of those affected were less than 50 years of age [1, 3]. Similarly, in a retrospective analysis of the National Inpatient Sample (NIS) data for 11,400 patients older 15 years of age admitted to US hospitals (2001–2008), the mean age was 38 years old and 78 % of the patients were between the ages of 15 and 49 years old [1, 3, 12].
CVST also preferentially affects women. Nearly 75 % of adult CVST patients are women. This predilection for women is based on gender specific issues including pregnancy, puerperium, oral contraceptive pill (OCP) use (especially third generation), and hormone supplementation [9]. The incidence of CVST in female population is reported to be as high as 2.78 per 100,000 in certain groups [10]. Pregnancy/puerperium related cases alone account for between 5 and 20 % of all CVST in developed countries [6]. When controlling for gender specific factors, the incidence in women is similar to that of their male counterparts. This has been demonstrated in studies that have shown that rates of CVST in female children and elderly women are the same as in men. Moreover, a significant rise in the incidence of CVST in women occurred in the 1970s coincident with the introduction and widespread adoption of the OCP [9]. Interestingly, the presentation and outcomes associated with CVST can vary with gender as well [13, 14]. Women with gender-specific causes tend to have better outcomes as compared to their male counterparts [14].
Outcomes
Historically, CVST was associated with mortality rates as high as 40–50 % and was usually diagnosed upon the patient’s death. Recent studies have demonstrated that the prognosis has improved and current mortality is 2–6 % [3, 9, 11, 12]. A recent review of the literature highlights a significant reduction in mortality over the last 2 decades that can be linked to four main factors. Hospital care, including ICU and critical care, has significantly improved over the last several decades leading to improved outcomes for a variety of disease processes. The second major factor is improved imaging. Major improvements in the ability to diagnose CVST occurred with the advent of invasive catheter cerebral angiography. The utility of catheter angiography was limited by the fact that the technique was slow, required technical expertise and had significant risks. It was, and continues to be, somewhat cumbersome to use invasive angiography as an initial diagnostic or screening tool. Thus even further improvement occurred with the widespread adoption of noninvasive techniques such as magnetic resonance imaging (MRI), magnetic resonance venography (MRV), and CT venography (CTV) that, with some limitations, allowed safe and efficient diagnosis of CVST. In the future molecular based imaging techniques may yield further advances [15]. Shifts in risk factors have also contributed to overall decline in mortality from CVST. Indeed over the last decades the incidence of traumatic and infectious CVST has been on the decline. As will be discussed, these subtypes are associated with worse outcomes. Conversely, the relative incidence of CVST associated with gender specific causes, such as OCP use, has been increasing. In general, these subtypes have relatively lower risk profiles [3, 13]. Finally, the ability to treat CVST has improved. And although still controversial, the use of intravenous heparin or subcutaneous low molecular weight heparin (LMWH) is the recommended first line therapy and seems to be associated with lower mortality and improved outcomes. Other measures such as hemicraniectomy or endovascular techniques have also been shown to improve outcomes in certain cases. The combination of these factors and improved awareness of CVST has lead to marked improvements in mortality.
Currently, CVST is associated with good outcomes in over 85 % of patients and has an overall mortality of about 2–6 % and a rare recurrence rate of about 3 % [2, 3, 9, 12]. Despite improvement there are several factors that have been associated with poor outcomes. Some examples are: malignancy as a cause of the CVST, CNS infection, seizures, associated brain hemorrhage, GCS <9 on admission and/or altered mental status, hemiparesis, male gender, age over 37 years, and CVST affecting the straight sinus or deep venous system [3].
The best outcomes can be achieved when CVST is recognized and treated in a timely and appropriate manner. Indeed, delay in diagnosis and treatment is a significant problem with CVST. Data from the ISCVT study showed in the ISCVT, the median delay from onset of symptoms to hospital admission was 4 days. Further it took an additional 3 days in the hospital for the diagnosis of CVST to be made. Thus, the median delay from initial symptom to diagnosis was about 1 week [16]. Patients presenting with visible parenchymal or hemorrhagic lesions, seizures, or mental status changes, are usually diagnosed most quickly. While patients presenting without visible lesions on imaging and only symptoms of elevated intracranial pressure (usually headache) alone are a diagnostic challenge and face greater delays in diagnosis and initial of treatment. Interestingly men are also subject to relative diagnostic delay [16].
Pathophysiology
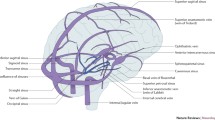
CVST is a stroke subtype that is characterized by thrombo-occlusion of major cerebral veins and/or dural venous sinuses. In general, these two broad interrelated pathophysiologic mechanisms contribute to the findings that characterize CVST. Thrombosis of major cerebral veins leads to local blockage of normal venous outflow pathways. In turn this contributes to the development of venous hypertension and cerebral edema. Gross and micropathologic examination reveals engorged thrombotic veins, ischemic neurons, edema, and microhemorrhages. Intracellular cytotoxic edema develops as a result of ischemia which damages the cellular Na/K pumps [9, 17, 18]. This is followed by development of reversible interstitial vasogenic edema that results from secondary disruption of the blood brain barrier. Venous infarction and/or hemorrhage are common sequelae [9, 18] (Fig. 20.1). Elevated intracranial pressure (ICP) and, sometimes, herniation can develop as a result of malignant edema or mass effect from large hemorrhagic lesions. The nature and viability of collateral venous drainage is an important consideration. Those with robust alternative venous drainage may be able to tolerate thrombosis for longer periods of time.
Case I: A young woman with hemorrhagic CVST in setting of OCP use. A 49-year-old right-handed woman with unremarkable medical history presented with acute confusional state. While at work, she began having difficulties with naming and became disoriented. Her initial head CT (A&B) showed an approximately 3 × 3 cm intraparenchymal hematoma (a—arrowheads) in the right frontal lobe with adjacent subarachnoid hemorrhage in the right sylvian fissure and overlying the right frontotemporal lobes (b—asterisk). There was significant surrounding edema but no significant mass effect or midline shift. There was hyperdensity at the right transverse sigmoid sinus (b—arrowhead). Further history from family revealed a 2-month history of intermittent right occipital headaches (worse over the last 2 weeks) and retroauricular pain. She had been given a diagnosis of mastoiditis and treated with augmentin. She had been taking oral contraceptive pills for many years. Her history and imaging (hyperdensity at the right transverse sinus) were concerning for CVST. She could not tolerate MRI due to her confusion and agitation. So a CTA/CTV (C&D) was obtained. It showed no evidence cerebral aneurysm on CTA. CTV showed dural venous sinus thrombus extending from the proximal right transverse sinus through the sigmoid sinus and into the proximal jugular vein (c and d—arrows). She was initially admitted and followed on the neurology service with serial CT scans showing stable intraparenchymal hemorrhage, and started on a therapeutic heparin drip for 3 days and subsequently started on coumadin (received one dose). On the fourth day she was noted to be progressively more confused and developed worsened LUE weakness. Repeat CT (e) head showed progressive midline shift and increased mass effect from IPH with early uncal herniation. Neurosurgery was consulted and she was taken for decompressive hemicraniectomy (f). She stabilized and made some improvement while in the hospital. She was discharged to rehab with mild language apraxia. She was restarted on LMWH and coumadin 10 days after surgery. She was continued on anticoagulation for 6 months total. Repeat MRI/MRV was obtained at 6 month follow-up and showed that normal contrast and flow related enhancement are demonstrated throughout the major dural sinuses and proximal internal jugular veins without occlusive filling defect with no evidence of sinus occlusion (g and h—arrows)
The next mechanism is intracranial hypertension that results from reduction of CSF reabsorption. As venous outflow is impaired, the gradient for CSF reabsorption via the arachnoid granulations is interrupted. As a result, patients develop signs and symptoms of intracranial hypertension such as headache or papilledema. Despite the presence of elevated intracranial pressure, CVST is not usually associated with ventricular dilatation or hydrocephalus. These two mechanisms usually occur in concert with each other leading to the more common clinical presentations associated with CVST. Isolated intracranial hypertension without cortical venous thrombosis occurs in about 20 % of patients presenting with CVST [3, 9].
In accordance with the Virchow triad of blood stasis, changes in the vessel wall and changes in the composition of the blood, CVST usually occurs in the setting of an inherited thrombophilia or an acquired prothrombotic state or condition. In the ISCVT study a thrombophilia was documented in 34 % of patients [3, 19]. Multiple specific predisposing conditions have been identified and are presented in Table 20.1 [3]. In many cases there is a double hit type of phenomenon and CVST occurs as a result of an antecedent prothrombotic cause in the setting the setting of a preexisting underlying thrombophilia (Fig. 20.2). As will be discussed, an important part of therapy for CVST is the identification and treatment of modifiable factors. Unfortunately, a likely risk factor for CVST can only be identified in only 85 % of cases [9].
Case II: A man with recent dehydration and underlying history of malignancy with CVST and dAVF. A 66-year-old male with a history of CML s/p bone marrow transplant on immunosuppression who presents after obtaining MRI brain this evening for workup of pulsatile tinnitus which incidentally showed hemorrhagic conversion of a area of stroke. He reported being on a hiking trip 3 weeks ago and dehydrated after significant exertion. At that time he had a severe 10/10 headache with dizziness that lasted for the day, and resolved by the following day. He did not seek medical care at that time. He did, however, start to take daily ASA since he thought his symptoms may be related to an MI. He reports only a couple of mild headaches since that time. He did have one episode of vomiting after dinner 1 week ago, but none since then. He has noted a “wooshing” sound in his R ear which was worked up by his primary care physician and ENT who recommended an MRI/MRA. He denied any diplopia, blurry vision, visual field disturbance, seizures, weakness/numbness/tingling. His initial non-contrast CT head (a—arrowhead) showed an approximately 7 cm area of non-confluent intraparenchymal hemorrhage and edema in the right temporal-occipital region with associated mass effect and midline shift, the MRI/MRA showed diffuse abnormal signal intensity involving the right temporal lobe with heterogeneous T2 signal intensity centrally consistent with blood products and surrounding edema (b—arrowhead). There is some thickening of the overlying cortex with mass effect causing effacement of the right lateral ventricle, midline shift from left to right, and minimal uncal herniation into the suprasellar cistern. It appeared to be consistent with hemorrhagic transformation of an ischemic event. There was concern for CVST and/or dAVF based on his history of cancer, dehydration, and tinnitus/swooshing in the ear. A dedicated MRI/MRV (c and d) was then obtained. Post-contrast images (c) demonstrated branched vascular enhancement (c—arrowhead) throughout most of the right temporal, occipital and inferior parietal lobes. This enhancement appears to converge on the anterior superior margin of the medial right tentorium cerebelli. These findings were concerning for dAVF. There was a large tubular filling defect within the transverse sinus, sigmoid sinus and visualized proximal internal jugular vein is consistent with a near occlusive venous thrombosis. This was well seen on the 3D reconstruction (d—bracket). Digital subtraction angiography (e–g) confirmed a high grade dAVF filling mainly from arterial feeders arising from the occipital artery as well as to a lesser extent the superficial temporal artery. There was egress into a venous pouch in the region of the transverse sigmoid sinus (f and g—arrows). There was marked retrograde reflux into the cortical veins. There was no obvious filling from the right internal carotid and the right-sided venous system in the region of the transverse sigmoid appears to be occluded (e—bracket). It is not clear whether the sinus thrombosis or the dAVF was the initial event. The dAVF was treated for cure using staged endovascular embolization. The patient made an excellent neurologic recovery and no longer has tinnitus
Historically, post-infectious CVST was common. However, recently this has been on the decline as a risk factor for most patients with CVST. However, it is more common as a risk in male patients, pediatric patients and in patients from developing countries [11, 13, 20–23]. Other less common but notable risk factors include lumbar puncture with intracranial hypotension [24], intracranial trauma, and neurosurgical procedures. These can present significant treatment challenges due to the inherent risk of bleeding when considering using anticoagulation for treatment following trauma or surgery. In a recent study of over 900 patients (ages 1–77, mean 36.1) admitted to a US University Hospital with skull fractures, over 10 % had a skull fracture associated with a cerebral venous sinus and, of those, over 34 % had an associated thrombus involving at least one venous sinus [25].
Presentation and Clinical Subtypes
The presentation of CVST varies widely and largely depends on the location of thrombosis, chronicity of presentation, presence of intraparenchymal lesions, and the underlying pathophysiology (Fig. 20.3, Table 20.2). Those with involvement of dural sinuses with cortical venous thrombosis may present with only features of elevated intracranial pressure such as headache or papilledema. CVST can present in an acute, subacute, and chronic time course. 90 % of patients present with headache [6, 9]. It is the initial symptom in nearly 80 % and the only symptom in 15–25 % of patients [1, 6, 26, 27]. Thus a high index of suspicion must be maintained in patients with isolated headache and risk factors for CVST. The type of headache is also quite variable but is often gradual, diffuse and subacute in nature. Rarely CVST can present with sudden, severe headache pattern similar to that usually associated with aneurysmal subarachnoid hemorrhage. Conversely, 5–30 % of patients have been reported to present without headache [28]. These patients tend to be older, male and show a trend towards worse clinical outcomes [28].
Focal neurologic deficits can be seen and usually correspond to the locale of intraparenchymal lesions [3, 9], while marked alterations in consciousness or coma are associated with deep venous thrombosis with associated thalamic involvement. In general focal deficits or coma as a presenting feature portend poor outcomes [1, 3, 9].
Seizure is also a common presenting feature and has been reported as a presenting feature in nearly 40 % of patients with CVST [29]. Logistic-regression analysis showed that CVST presenting with seizure was associated with supratentorial lesions, cortical vein thrombosis, sagittal sinus thrombosis and puerperal CVST. In patients at higher risk for seizure, prophylactic antiepileptic medical therapy may be considered [1, 29]. Seizures are much more common in CVST as compared to arterial stroke subtypes. Indeed, seizures with suspicious imaging findings should raise suspicion for CVST [1, 30].
Finally, there are variations based on demographic subgroups. As mentioned, women with gender specific causes usually present acutely with headache and seem to have better outcomes as compared to men. Subgroup analysis from ISCVT study showed that 81 % of women presenting with CVST had a complete recovery versus only 71 % of men [14]. The elderly often present with mental status changes and depressed consciousness. They have poor outcomes relative to younger patients. Only 49 % of the elderly (age >65 years old) make a complete recovery versus 82 % of younger patients and the elderly have a mortality of more than 25 % [31]. Children have unique characteristics. In a recent study, 55 % of children ages 0–12 years old were less than 6 months of age at presentation. Common presenting features included seizures (59 %), coma (30 %), motor weakness (21 %), and headache (18 %). Over half were found to have decreased level of consciousness. The most common risk factor was infection which was notable as a cause in over 40 %. In this study 13 % of children died and 46 % had a neurologic deficit at discharge [20].
Diagnosis
Clinical
As discussed above the presentation of CVST is highly variable and, as a result, clinical diagnosis can be quite challenging. The main stay of diagnosis is a thorough history and physical examination. Practitioners must maintain a high level of suspicion for CVST in high risk groups. With that in mind the clinical evaluation must be focused on uncovering and treating risk factors for CVST including significant past medical history, recent trauma, recent use of OCPs or pregnancy, infection, dehydration, or other clues as discussed above.
Laboratory
Focused laboratory investigations can be of benefit in patients in whom CVST is suspected. According to the AHA/ASA guidelines, a complete blood count, chemistry panel, sedimentation rate, prothrombin time, and activated partial thromboplastin time may be useful in uncovering occult infection, or underlying coagulopathy [1].
Measurement of the fibrin breakdown product, D-dimer, can also be useful in laboratory diagnosis of CVST but should be considered in combination with an estimation of clinical suspicion. Studies have reported that positive D-dimer levels (>500 μg/ml) have been shown to be highly sensitive (94–97 %) for detection of CVST but less specific (90 %) [1]. Indeed, D-dimer is highly associated with CVST in patients with positive D-dimer levels and neurological deficit. False negatives were most common in patients presenting with isolated headache. The AHA/ASA guidelines point out some controversy in the use of D-dimer and further recommend that normal D-dimer level according to a sensitive immunoassay or rapid enzyme-linked immunosorbent assay (ELISA) may also be useful to help identify patients with low probability of CVST [1, 6, 32]. Thus it seems that D-dimer is best used to establish a positive diagnosis of CVST in patients with high clinical suspicion, a negative diagnosis in patients with low clinical suspicion, but is not as useful to rule out CVST in patients with high clinical suspicion. In general lab evaluation is not used as the sole or primary means of diagnosis. Imaging is the mainstay of modern diagnosis.
Imaging
Computed Tomography (Figs. 20.1, 20.2, and 20.4)
Although not the test of choice for diagnosis of CSVT, standard non-contrast head CT (Figs. 20.1a, b, e, f, 20.2a, and 20.4a) is often the first imaging study obtained in a patient presenting acutely with headache. It is helpful to rule out other more common intracranial pathologies but is only positive in about 30 % of known CVST cases [1]. There are findings on CT that can raise suspicion of CVST. These include hyperdensity of a cortical vein or dural sinus, a dense delta sign involving the posterior sagittal sinus, and ischemic lesions in a non-arterial pattern at or near a dural sinus that are sometimes associated with a characteristic fluffy appearing hemorrhage. Although rare, subarachnoid blood can be noted and is usually along the convexity and not in basilar cisterns or within the sylvian fissure [33]. Contrast enhanced CT may show a filling defect within the sinus. The addition of CT venography significantly improves the sensitivity of CT (Figs. 20.1c, d, 20.2a, and 20.4b, c, f) With an overall sensitivity of about 95 % and specificity of 91 %, CTV with multiplanar reconstruction is about as reliable as MRI with MR venography [34]. It can be obtained quickly, is noninvasive and is tolerated well by patients and is most useful for subacute and chronic thrombosis. It has an overall accuracy of 90–100 % and is best for detection of major dural sinus thrombosis . Evaluation of cortical venous thrombosis and thrombosis of the deep venous system is relatively poor using CTV. Other drawbacks include relatively poor accuracy for visualization of smaller parenchymal lesions, radiation exposure and need for contrast in those with allergies, significant kidney dysfunction, or other contraindications [1, 19, 34].
Case III: A man with a history of seizures, CVST and a recent trauma. A 54-year-old left-handed male presented after a fall from standing 1 day prior to admission. He was amnestic to the event but thinks he may have slipped on some gravel. He hit the back of his head on the ground. He was neurologically intact on presentation but had right posterior parieto-occipital scalp bruising and bilateral racoon eyes (R > L) and right Battle’s sign. He reports headache but denied nausea, vomiting, or confusion. He did have a generalized tonic-clonic seizure 2 weeks prior. An initial non-contrast head CT head (a and b) obtained for rapid evaluation in the setting of trauma. It showed trace right temporal-parietal subarachnoid hemorrhage (a—arrowheads), a transverse fracture of the right temporal bone with extensive pneumocephalus (a—asterisk) intracranially as well along the right transverse and sigmoid sinuses (b—arrowheads and asterisk) as well as the right jugular fossa. These findings were concerning for sinus involvement and a CTA/CTV confirmed associated thrombosis of the right transverse sinus and sigmoid sinus (c—arrowheads) as well as non-occlusive thrombosis of the right jugular vein. One day later an MRI (d) with MRV (e) was performed which confirmed the findings on CTV. 3D reconstruction demonstrates thrombosis in the transverse/sigmoid sinus (e—bracket). He was admitted to the ICU with trauma evaluation as well as neurology, ENT and ophthalmology consults. He was started on seizure prophylaxis. He remained stable with stable CT head. He was started on an IV heparin drip on post-trauma day 2. In the setting of trauma, no bolus was given and he had a goal PTT of 50–70. Serial CT head evaluation was stable and he was transitioned to coumadin for INR of 2–3 on post trauma day 5. Follow-up CT venogram 8 weeks later showed improved flow through a partially recanalized right transverse sinus (f—arrowheads). He remained clinically stable without neurologic deficit until the completion of coumadin therapy
Magnetic Resonance
The AHA/ASA 2011 guidelines recommend MR with T2-weighted imaging and MR venography as the imaging test of choice for evaluation of suspected CVST (Figs. 20.1g, h, 20.2b–d, and 20.4d, e) MRI/MRV provides excellent visualization of cortical veins and deep and superficial venous systems, and shows good tissue detail including micro-hemorrhagic lesions and developing cerebral edema. MRI does not expose patients to ionizing radiation but takes longer, and is difficult for patients unable to tolerate closed spaces or those with defibrillators or ferrous metal in the body. Moreover, chronic venous thrombosis, anatomic variation and slow-flow states can confound the diagnosis of CVST using MR. Using a combination of static and dynamic MR imaging techniques has been shown to improve accuracy in these cases [1, 35–37].
Digital Subtraction Angiography
Intra-arterial digital subtraction angiography (DSA) is the gold standard for visualization of CVST. However, due to the invasive, time consuming and relatively risky nature of DSA and because reliable noninvasive alternatives are readily available, DSA is no longer the recommended first choice for initial diagnosis. Its use is reserved for cases in which CTV or MR/MRV is contraindicated or cannot provide a certain diagnosis. It can provide particularly useful visualization of cortical and deep venous thrombosis. DSA is also used if endovascular therapy is under consideration (Figs. 20.2 and 20.3e, g) [1, 9].
Other
Direct venous angiography has also been used but is rarely necessary. Advanced imaging techniques such as PET or perfusion studies are rarely used in the initial diagnosis of CVST. However, there is recent interest in the use of CT perfusion as a way to follow the progression of CVST after diagnosis and initiation of treatment using systemic anticoagulation [38] or endovascular [39] techniques. There are no large well designed studies evaluating CTP, so more work needs to be done establish its clinical value and justify its widespread use in evaluation of CVST after treatment .
Treatment
Approach
As discussed above CVST is complex and presents significant diagnostic challenges. Similarly, effective treatment of CVST is also complex and often requires a multidisciplinary team within a specialized environment. Neurosurgeons are uniquely qualified because of their experience with making neurological diagnosis, dealing with critical care issues and because of their expertise in surgical and endovascular techniques. However, involvement of other specialists including neurologists, neuroradiologists, hematologists, emergency medicine practitioners, pediatricians, obstetrician/gynecologists, and others is paramount to achieve optimal outcomes.
The goal of treatment is to stabilize the patient, eliminate any treatable thrombophilic conditions, reduce clot burden/revascularize to improve venous outflow and to prevent death and disability. Thus after stabilization of the comatose or critically ill patients, the initial phase of treatment entails discovery of any conditions, such as dehydration, that can be treated. In addition, after acute CVST treatment, long-term treatment of underlying thrombophilic conditions must be considered.
Anticoagulation
Treatment of CVST with anticoagulation is somewhat controversial but there is no true equipoise [1, 40]. The consensus in the literature supports the use of anticoagulation therapy for treatment of acute CVST in adults and children [9, 20, 26, 30]. Moreover, the AHA/ASA guideline [1], and the EFNS guidelines [5] and the EPNS/SFNP guidelines for children and neonates [41], recommend therapeutic dosing of IV heparin or low molecular weight heparin (LMWH) as the primary therapy for acute CVST in most adult and pediatric patients. The controversy stems from a logical concern about hemorrhagic complications after use of anticoagulation to treat CVST—especially those presenting with hemorrhage and the fact that there are only two well-designed randomized, controlled trials comparing anticoagulation to placebo in the general adult population. These two trials evaluate the use of IV unfractionated heparin (UFH) or subcutaneous LMWH (nandroparin) in 20 and 59 patients respectively. Meta analysis of these small trials revealed a nonsignificant trend toward reduction (−13 %) in absolute risk of death and severe disability with heparin treatment [4]. Importantly, there was no symptomatic ICH in either group after use of anticoagulation. A third randomized controlled trial of anticoagulation for CVST was performed in pregnancy-related CVST and demonstrated a benefit of UFH over no anticoagulation therapy in these women [26]. Much of the data regarding CVST comes from observational studies and in particular from the ISCVT which is the largest study of its kind. In this study 624 patients from 89 centers were enrolled. Most (>80 %) were treated with anticoagulation and the mortality was about 8 % at 16 months and nearly 80 % had complete recovery. These and other studies indicate that use of PTT adjusted IV heparin or body weight adjusted LMWH is safe and effective for treatment of CVST even in the setting of hemorrhage. Data from ISCVT indicates a range of risks for ICH after anticoagulation for CVST from 0 to 5.4 % [3]. In general treatment of CVST using IV heparin or LMWH has similar outcomes. However, a small number of small studies suggest that there may be a slight advantage of LMWH in terms of effectiveness and risk of hemorrhage [1, 6, 42]. In patients that may need surgical treatment; IV heparin may be advantageous due to the relatively short ½ life of IV heparin and ability to effectively reverse IV heparin if needed. In cases where a direct cause can be identified, stable patients should be converted to oral therapy using oral vitamin K antagonists such as warfarin and treatment continued for 3–6 months. In patients without an identifiable cause, long term oral therapy should be continued for 6–12 months. In cases of recurrence, CVST at additional sites or CVST in setting of severe underlying prothrombotic states (i.e., antithrombin deficiency), lifetime oral anticoagulation may be considered [5, 26, 43]. Newer generation oral anticoagulation agents, such as dabigatran or direct factor Xa inhibitors, have been tried with some success in case series but no high quality data is available to support the widespread use of these medications [44, 45]. In addition to prevention of progressive or recurrent CVST, long-term oral anticoagulation therapy is also aimed at preventing venous thromboembolism in the lung or other extracranial sites. Consultation with a hematologist for management of complex cases may be of benefit [1].
Endovascular Therapy
Anticoagulation therapy acts mainly to prevent extension of thrombosis and has significantly improved the prognosis associated with CVST. , However, 9–13 % of CVST patients have poor outcomes despite being adequately anticoagulated. High risk patients, especially those with large clot burdens and persistent underlying prothrombotic conditions, are more likely to fail anticoagulation treatment. This is often due to incomplete or partial recanalization that can occur in greater than 50 % of patients treated with anticoagulation alone. In patients that deteriorate or progress despite anticoagulation, endovascular measures including direct local thrombolysis or mechanical thrombectomy and others may be considered [1]. These therapies have the benefit of dissolving the thrombus leading to more rapid recanalization. There have been multiple small reports composed of case reports and series that have shown benefit of endovascular therapies in selected patients but no large randomized controlled studies have been performed. A recent review of the literature on mechanical thrombectomy for treatment of CVST reports on a total of 64 patients from case reports and case series. IV heparin use was reported in 60 patients, not used in one patient and not reported in three patients. 48 % of patients had a hemorrhage at presentation. There were nine reported complications and after a mean follow-up period of about 28 weeks nine patients had died. After the same follow-up period, 62 % had good outcome with documented MRS of 0–2 [46]. In the ISCVT study 38 % of patients treated using thrombolysis (local or systemic) died. There is a great deal of potential bias in the reports about thrombolysis for CVST. The patients usually have a more severe clinical condition but there is also publication bias for positive findings.
Several possible endovascular techniques have been reported. These include direct catheter thrombolysis, balloon assisted thrombectomy/thrombolysis, catheter based thrombectomy/thrombolysis, stenting or stent based thrombus retrieval. Further evaluation must be done to determine which among these techniques provides a meaningful benefit [47–50]. Endovascular therapy seems to be a safe and effective option and some have even suggested its use as a first line treatment [51]. However, at this time, endovascular therapy can only be recommended in cases of failed anticoagulation therapy [1, 52]. In a prospective case series of 20 patients with CVST, patients with large hemorrhagic infarcts did not benefit from endovascular thrombolysis. This may be due in part to the potential for increase in size of the lesion and the associated increased risk of herniation [6, 53].
The Thrombolysis or anticoagulation for cerebral venous thrombosis (TO-ACT) trial opened in 2011 and is currently ongoing with 50 patients enrolled (http://www.to-act-trial.org/). The TO-ACT trial is a multicenter, prospective, randomized, open-label, blinded endpoint trial designed to determine if ET improves the functional outcome of patients with a severe form of CVST Patients are included if they have a radiologically proven CVST, a high probability of poor outcome (defined by presence of one or more of the following risk factors: mental status disorder, coma, intracranial hemorrhagic lesion, or thrombosis of the deep cerebral venous system), and if the responsible physician believes that there is clinical equipoise between endovascular thrombolysis or standard anticoagulant treatment. One hundred sixty four patients (82 in each treatment arm) will be included to detect a 50 % relative reduction (from 40 to 20 %) of poor outcomes [54].
Systemic thrombolysis has also been used in limited cases. There are no randomized clinical trials, but a recent meta-analysis reports on a total of 26 patients from 16 reports. In this group of patients, most regained independence with 88 % achieving MRS 0–2 [55]. However, there were three serious bleeding events and two deaths. The results of systemic thrombolysis were similar to local thrombolysis noted in other studies. As with other therapies, there is a serious risk of hemorrhagic complication and more work needs to be done to establish the role and safety of systemic thrombolysis. But it may represent an additional tool for treatment of patients who fail anticoagulation therapy. It has the advantage of being noninvasive and potentially more widely available than local thrombolysis using endovascular techniques.
Surgery
As endovascular techniques have improved, the need for direct surgical treatment of CVST has nearly disappeared. On the rare occasion that indirect transvenous access cannot be obtained, surgical exposure of a dural sinus may be used to provide direct access for endovascular therapy.
Hemicraniectomy
The main cause of death in patients with CVST is transtentorial herniation due to a unilateral lesion or diffuse edema from multiple intraparenchymal lesions [56]. In recent studies as many as 4.3 % of patients die in the acute phases of CVST. In patients that fail medical and/or endovascular treatments, hemicraniectomy has been demonstrated to be an effective and life saving measure in patients with malignant edema, large space occupying lesions and/or clinical evidence of herniation (Fig. 20.1) [1, 57–60]. There are no well designed studies to evaluate the clinical effectiveness of hemicraniectomy for CVST, but in small patient series, despite more severe clinical presentations, most patients demonstrate good or excellent outcomes at follow-up [58, 59, 61, 62].
Treatment of Associated Problems
Seizures
Seizures are common in CVST occurring in 37 % of adults, 48 % of children, and 71 % of neonates [1, 29]. Seizures can be dangerous due to the increased risk of anoxic damage. Therefore treatment of documented seizure with appropriate antiepileptic medications is recommended to reduce the risk of further seizures [1, 5]. Prophylactic use of AEDs is controversial because there is a paucity of data to support its use. Despite this the EFNS suggests that prophylactic antiepileptic therapy may be a therapeutic option in patients deemed high risk for seizure such as those with focal neurological deficits and supratentorial lesions on admission CT/MRI [5], whereas the AHA/ASA guidelines states that in the absence of seizure, routine prophylactic use of AEDs is not recommended [1].
Intracranial Hypertension
Intracranial hypertension is common in CVST but frank hydrocephalus is not. However, in patients with persistently elevated intracranial pressures and associated problems such as severe headache, papilledema or vision loss, altered level of consciousness or cranial nerve palsy, specific measures to manage elevated ICP can be instituted. Reasonable measures include lumbar puncture, use of acetazolamide, ventriculostomy, or even shunting if indicated [1, 63, 64]. However, recent subgroup analysis of 15 patients from the ISCVT study suggests that CSF diversion may not be effective in preventing death from herniation [63]. Ophthalmologic consultation should be considered to evaluate for possible optic nerve fenestration in the setting of intractable intracranial hypertension.
Steroids
Steroids are not recommended [1]
Sequelae
The current prognosis of CVST is good. Over 85 % are reported to have good outcomes (MRS < 2) at 16 month follow-up and only about 10 % were dead or severely disabled [3]. Despite this some patients experience chronic headaches (50 %), late seizures (5 %), visual problems recurrence (4 %), or VTE in extracranial sites (5.5 %) [1]. Other more rare problems such as dural arteriovenous fistulae are also noted (Fig. 20.2). dAVF may also be causative in some cases of CVST.
Special Groups
Special consideration must be taken when treating pregnant women or children. Specialty consultation with obstetrics/gynecology and pediatrics or neonatology would be recommended. The clinical characteristics and presentations are similar but do have some important variations as mentioned above. The incidence ranges from 1 in 2500 to 1 in 10,000 during pregnancy. As much as 73 % of all CVST in women occur during pregnancy. It is most likely during the last trimester and first postpartum month. Treatment principles are similar in pregnant women and anticoagulation is the mainstay of therapy. However unfractionated heparin is teratogenic and warfarin is associated with fetal embryopathy and bleeding. Thus, LMWH is the treatment of choice and should be continued throughout the entire pregnancy followed by LMWH or warfarin in the post-partum period. In women with a history of CVST, future pregnancy is not contraindicated but prophylaxis with LMWH during the pregnancy may be advisable [1].
In children, neonatal CVST has the highest incidence. Outcomes are worse in neonates as compared to older children and adults. Forces on the child’s head during birth and a lack of circulating anticoagulant factors put neonates at increased risk. Careful monitoring must be undertaken because this population cannot easily communicate problems. Surveillance for visual disturbances and papilledema is recommended. Continuous EEG is advisable for comatose patients. Investigation for underlying thrombophilia should be undertaken [1, 41]. Anticoagulation for 3–6 months with UFH or LMWH followed by warfarin is the recommended treatment for children greater than 28 weeks old even in the presence of hemorrhage. The data regarding use of anticoagulation in neonates is not clear. The EPNS/SFNP guidelines suggest that anticoagulation for neonates with acute CVST may be safe and effective but that it must be evaluated on a case by case basis. If chosen, anticoagulation should be continued for a shorter period of 6–12 weeks [1, 41].
Clinical Care Algorithm (Fig. 20.5)
A potential clinical care algorithm is presented in Fig. 20.5.
Proposed clinical algorithm for CVST. It is not comprehensive and each patient must be considered on a case-by-case basis. This takes into account current data but may change as more information becomes available especially regarding newer treatments including novel anticoagulants and endovascular therapies. In addition to above patients must be evaluated and stabilized if necessary. Patients should also receive necessary treatment for associated conditions including intracranial hypertension, visual changes, seizures, and coma. Oral anticoagulation therapy should be continued for at least 3 months in adults depending on the underlying cause
Conclusion and Future Directions
CVST is a relative rare stroke subtype that preferentially affects the young. There are wide variations in clinical presentation that make timely diagnosis and effective treatment challenging. Fortunately, as a result of modern imaging and treatment paradigms, the prognosis for CVST has been improving. As study of this interesting problem continues, it is likely that the prognosis will continue to improve. Additional study needs to be undertaken to evaluate endovascular therapy for use in high risk patients and/or in patients after failing standard anticoagulation treatment. It is also being considered for use as a first line approach. The ongoing TO-ACT trial will hopefully address some of these issues. Laboratory investigation of molecular signaling and associated molecular imaging may lead to the ability to diagnose CVST even earlier. Screening in high risk groups could be considered in certain circumstances. Indeed, prevention along with screening, counseling or other methodologies will be an essential element in the overall “treatment” of CVST. As awareness improves and more is known about how and why CVST affects certain groups, more can be done to prevent or eliminate CVST. The neurosurgeon is an integral part of the modern multidisciplinary approach to CVST and thus must maintain a high clinical awareness for CVST and be familiar with the use of anticoagulation and other treatment options for CVST.
References
Saposnik G, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4): 1158–92.
Coutinho JM, Zuurbier SM, Stam J. Declining mortality in cerebral venous thrombosis: a systematic review. Stroke. 2014;45(5):1338–41.
Ferro JM, et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–70.
Coutinho J et al. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev. 2011(8):CD002005.
Einhaupl K, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17(10):1229–35.
Weimar C. Diagnosis and treatment of cerebral venous and sinus thrombosis. Curr Neurol Neurosci Rep. 2014;14(1):417.
Ribes M. Des recherches faites sur la phlebite. Rev Med Franc Etrang. 1825;3:5–41.
Yakovlev SB, et al. Endovascular treatment of acute thrombosis of cerebral veins and sinuses. Neuroradiol J. 2014;27(4):471–8.
Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352(17):1791–8.
Coutinho JM, et al. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43(12):3375–7.
Ruiz-Sandoval JL, et al. Cerebral venous thrombosis in a Mexican multicenter registry of acute cerebrovascular disease: the RENAMEVASC study. J Stroke Cerebrovasc Dis. 2012;21(5):395–400.
Borhani Haghighi A, et al. Mortality of cerebral venous-sinus thrombosis in a large national sample. Stroke. 2012;43(1):262–4.
Hinnell C, et al. Sex differences in adult cerebral venous sinus thrombosis: a 10-year experience. Can J Neurol Sci. 2012;39(1):74–7.
Coutinho JM, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356–61.
Stracke CP, et al. Molecular MRI of cerebral venous sinus thrombosis using a new fibrin-specific MR contrast agent. Stroke. 2007;38(5):1476–81.
Ferro JM, et al. Delay in the diagnosis of cerebral vein and dural sinus thrombosis: influence on outcome. Stroke. 2009;40(9):3133–8.
Pfefferkorn T, et al. Clinical features, course and outcome in deep cerebral venous system thrombosis: an analysis of 32 cases. J Neurol. 2009;256(11):1839–45.
Filippidis A, et al. Cerebral venous sinus thrombosis: review of the demographics, pathophysiology, current diagnosis, and treatment. Neurosurg Focus. 2009; 27(5):E3.
Piazza G. Cerebral venous thrombosis. Circulation. 2012;125(13):1704–9.
Wasay M, et al. Cerebral venous sinus thrombosis in children: a multicenter cohort from the United States. J Child Neurol. 2008;23(1):26–31.
Narayan D, et al. Risk factors, clinical profile, and long-term outcome of 428 patients of cerebral sinus venous thrombosis: insights from Nizam’s Institute Venous Stroke Registry, Hyderabad (India). Neurol India. 2012;60(2):154–9.
Sidhom Y, et al. Cerebral venous thrombosis: clinical features, risk factors, and long-term outcome in a Tunisian cohort. J Stroke Cerebrovasc Dis. 2014;23(6):1291–5.
Hedlund GL. Cerebral sinovenous thrombosis in pediatric practice. Pediatr Radiol. 2013;43(2):173–88.
Kate MP, Thomas B, Sylaja PN. Cerebral venous thrombosis in post-lumbar puncture intracranial hypotension: case report and review of literature. F1000Res. 2014;3:41.
Rivkin MA, Saraiya PV, Woodrow SI. Sinovenous thrombosis associated with skull fracture in the setting of blunt head trauma. Acta Neurochir (Wien). 2014;156(5):999–1007. discussion 1007.
Thorell SE, et al. Cerebral venous thrombosis-A primer for the haematologist. Blood Rev. 2015;29(1): 45–50.
Gameiro J, et al. Prognosis of cerebral vein thrombosis presenting as isolated headache: early vs. late diagnosis. Cephalalgia. 2012;32(5):407–12.
Coutinho JM, et al. Cerebral venous thrombosis in the absence of headache. Stroke. 2015;46(1):245–7.
Ferro JM, et al. Early seizures in cerebral vein and dural sinus thrombosis: risk factors and role of antiepileptics. Stroke. 2008;39(4):1152–8.
Kernan WN, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236.
Ferro JM, et al. Cerebral vein and dural sinus thrombosis in elderly patients. Stroke. 2005;36(9):1927–32.
Dentali F, et al. D-dimer testing in the diagnosis of cerebral vein thrombosis: a systematic review and a meta-analysis of the literature. J Thromb Haemost. 2012;10(4):582–9.
Panda S, et al. Localized convexity subarachnoid haemorrhage--a sign of early cerebral venous sinus thrombosis. Eur J Neurol. 2010;17(10):1249–58.
Wetzel SG, et al. Cerebral veins: comparative study of CT venography with intraarterial digital subtraction angiography. AJNR Am J Neuroradiol. 1999;20(2):249–55.
Meckel S, et al. Cerebral venous thrombosis: diagnostic accuracy of combined, dynamic and static, contrast-enhanced 4D MR venography. AJNR Am J Neuroradiol. 2010;31(3):527–35.
Idbaih A, et al. MRI of clot in cerebral venous thrombosis: high diagnostic value of susceptibility-weighted images. Stroke. 2006;37(4):991–5.
Ihn YK, Jung WS, Hwang SS. The value of T2*-weighted gradient-echo MRI for the diagnosis of cerebral venous sinus thrombosis. Clin Imaging. 2013;37(3):446–50.
Gupta RK, et al. Prognostic indices for cerebral venous thrombosis on CT perfusion: a prospective study. Eur J Radiol. 2014;83(1):185–90.
Lin N, et al. Reversible changes in diffusion- and perfusion-based imaging in cerebral venous sinus thrombosis. BMJ Case Rep. 2015. doi:10.1136/bcr-2014-011447.
Cundiff DK. Evidence-basis for anticoagulants for cerebral sinus venous thrombosis? Stroke. 2013;44(6):e67.
Lebas A, et al. EPNS/SFNP guideline on the anticoagulant treatment of cerebral sinovenous thrombosis in children and neonates. Eur J Paediatr Neurol. 2012;16(3):219–28.
Coutinho JM, et al. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575–80.
Caprio F, Bernstein RA. Duration of anticoagulation after cerebral venous sinus thrombosis. Neurocrit Care. 2012;16(2):335–42.
Geisbusch C, et al. Novel factor xa inhibitor for the treatment of cerebral venous and sinus thrombosis: first experience in 7 patients. Stroke. 2014;45(8): 2469–71.
Mendonca MD, et al. Oral direct thrombin inhibitor as an alternative in the management of cerebral venous thrombosis: a series of 15 patients. Int J Stroke. 2015;10(7):1115–8.
Haghighi AB, et al. Mechanical thrombectomy for cerebral venous sinus thrombosis: a comprehensive literature review. Clin Appl Thromb Hemost. 2013;20(5):507–15.
Khan IS, et al. Endovascular thrombolysis for pediatric cerebral sinus venous thrombosis with tissue plasminogen activator and abciximab. J Neurosurg Pediatr. 2014;13(1):68–71.
Choudhri O, et al. Endovascular management of cerebral venous sinus thrombosis. Neurosurg Focus. 2014;37(1 Suppl):1.
Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
Shaikh H, et al. Combined use of Solitaire FR and Penumbra devices for endovascular treatment of cerebral venous sinus thrombosis in a child. BMJ Case Rep. 2014. doi:10.1136/bcr-2013-011088.
Dashti SR, et al. Mechanical thrombectomy as first-line treatment for venous sinus thrombosis: technical considerations and preliminary results using the AngioJet device. J Neurointerv Surg. 2013;5(1): 49–53.
Coutinho JM, et al. Mechanical thrombectomy cannot be considered as first-line treatment for cerebral venous thrombosis. J Neurointerv Surg. 2013;5(6):621–2.
Stam J, et al. Endovascular thrombectomy and thrombolysis for severe cerebral sinus thrombosis: a prospective study. Stroke. 2008;39(5):1487–90.
Coutinho JM, et al. Thrombolysis or anticoagulation for cerebral venous thrombosis: rationale and design of the TO-ACT trial. Int J Stroke. 2013;8(2):135–40.
Viegas LD, et al. Systemic thrombolysis for cerebral venous and dural sinus thrombosis: a systematic review. Cerebrovasc Dis. 2014;37(1):43–50.
Canhao P, et al. Causes and predictors of death in cerebral venous thrombosis. Stroke. 2005;36(8): 1720–5.
Ferro JM, et al. Decompressive surgery in cerebrovenous thrombosis: a multicenter registry and a systematic review of individual patient data. Stroke. 2011;42(10):2825–31.
Aaron S, et al. Decompressive craniectomy in cerebral venous thrombosis: a single centre experience. J Neurol Neurosurg Psychiatry. 2013;84(9): 995–1000.
Raza E, et al. Decompressive surgery for malignant cerebral venous sinus thrombosis: a retrospective case series from Pakistan and comparative literature review. J Stroke Cerebrovasc Dis. 2014;23(1):e13–22.
Theaudin M, et al. Should decompressive surgery be performed in malignant cerebral venous thrombosis? A series of 12 patients. Stroke. 2010;41(4):727–31.
Zuurbier SM, et al. Decompressive hemicraniectomy in severe cerebral venous thrombosis: a prospective case series. J Neurol. 2012;259(6):1099–105.
Mohindra S, et al. Decompressive craniectomy for malignant cerebral oedema of cortical venous thrombosis: an analysis of 13 patients. Br J Neurosurg. 2011;25(3):422–9.
Lobo S, et al. Shunting in acute cerebral venous thrombosis: a systematic review. Cerebrovasc Dis. 2014;37(1):38–42.
Canhao P, et al. Safety of lumbar puncture in patients with cerebral venous thrombosis. Eur J Neurol. 2013;20(7):1075–80.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ashley, W.W. (2016). Cerebral Venous Sinus Thrombosis. In: Loftus, C. (eds) Anticoagulation and Hemostasis in Neurosurgery. Springer, Cham. https://doi.org/10.1007/978-3-319-27327-3_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-27327-3_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27325-9
Online ISBN: 978-3-319-27327-3
eBook Packages: MedicineMedicine (R0)