Abstract
Obstructed hemivagina in association with a double uterus and renal anomaly has been described as early as 1922. Since then, this anomaly has been described in the literature associated with several different names: double uterus with obstructed hemivagina and ipsilateral renal agenesis, obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome, and Herlyn-Werner-Wunderlich syndrome. Diagnosis should be suspected in an adolescent female with escalating dysmenorrhea as menstruation occurs from the patent outflow tract, but hematocolpos, hematometra, and retrograde menstruation occur in the obstructed side leading to pain. Treatment is typically primary resection of the vaginal septum with marsupialization to unite the vaginal cavities and relieve the obstruction. Alternative therapies include hemihysterectomy with hemocolpotomy, and drainage and decompression of the hematocolpos with hormonal suppression of menses. Fertility may be affected by extent of endometriosis and adhesive disease, but infertility is not common. Pregnancies occur more commonly in the non-obstructed horn, but may be present in the horn ipsilateral to obstruction in a significant percentage of cases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Double uterus with obstructed hemivagina and ipsilateral renal agenesis
- Obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome
- Herlyn-Werner-Wunderlich syndrome
- Vaginal septum resection with marsupialization
- Uterus didelphys
- Complete septate uterus
- Renal agenesis
Congenital obstructive malformations of the female reproductive tract constitute of a group structural malformations arising from the abnormal development of the Müllerian ducts. Obstructed hemivagina in association with a double uterus and renal anomaly has been described as early as 1922 [1]. Since then, this anomaly has been described in the literature associated with several different names: double uterus with obstructed hemivagina and ipsilateral renal agenesis [2], obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome [3], and Herlyn-Werner-Wunderlich syndrome [4].
Incidence
It is difficult to accurately estimate the true incidence of obstructive Müllerian duct malformations in the general population, as most data regarding the condition arise from studies involving patients with reproductive problems [5]. Furthermore, accurate assessment of uterine anatomy and morphology are lacking in such studies [5]. Despite these limitations, the overall incidence of obstructive Müllerian duct malformations is thought to range from 0.1 to 3.8 % [6].
Obstructed hemivagina is typically associated with a uterus didelphys or complete septate uterus as these conditions are often associated with a longitudinal vaginal septum [8] (Fig. 12.1). Purslow first described the combination of obstructed hemivagina and uterus didelphys in 1922 [1]. Since then, over 250 cases have been reported in the medical literature [7]. The combination of an obstructed hemivagina, uterus didelphys, and ipsilateral renal agenesis was first reported as a syndrome in 1971 [9] with an incidence ranging from 1 in 2000 to 1 in 28,000 [10]. Variability of the anatomic structures involved in this syndrome is well known [2]. While most reports have described the uterine anomaly as uterus didelphys, septate, and bicornuate uterus has also been reported [2, 11, 12]. Unilateral obstruction associated with this syndrome is mainly vaginal, but cervical obstruction has been occasionally described [2, 11, 12]. Reports of dysplastic or duplicated kidneys may also be found associated with the syndrome [2, 12].
The anomaly uterus didelphys or septate uterus with obstructed hemivagina is not captured or described by the AFS Classification system as this classification does not describe vaginal anomalies [13]. The newer classification systems do consider vaginal, cervical, and uterine anomalies and are able to adequately categorize and describe these anomalies [14–16].
Etiology
Müllerian anomalies generally occur around the eighth week of gestation [17]. An isolated vaginal septum is thought to arise due to failed resorption of the uterovaginal septum [18]. However, the pathogenesis of the triad of uterine didelphys, obstructed hemivagina, and ipsilateral renal agenesis is more complex. The putative embryonic mechanism is likely due to a disruption in the development of the caudal portion of one mesonephric duct with secondary involvement of the ipsilateral Müllerian duct [2]. On the affected side, the mesonephric duct anomaly accounts for failure of regular ureteric budding and kidney differentiation, with consequent renal agenesis, as well as an abnormal location of ipsilateral Müllerian duct. This results in the failure of the abnormal Müllerian duct fuse with both its opposite counterpart and with the urogenital sinus, thereby creating a double uterus and cervico-vaginal obstruction [2, 11].
The double uterus described with this anomaly may have many configurations, which have been described in a series of 87 patients [2] (Fig. 12.2). The most common presentation is that of a uterus didelphys with two separate uterine horns occurring in 77 % of patients [2, 19]. The second most common variant in this series is a complete septate uterus with duplicated cervices, or septate bicollis occurring in 14 % of cases [2, 19] In another study, the incidence of obstructed hemivagina with uterus didelphys or a complete septate uterus was 57 % and 29 %, respectively [19]. Less common variants described include a bicornuate bicollis configuration, didelphic uterus with unilateral cervical atresia, and bicornuate uterus with septate cervix and obstructed hemivagina. With the uterus didelphys and complete septate variants, the right and left sides are separate and noncommunicating. The obstruction may be complete or partial. With complete obstruction presenting symptoms occur earlier. Partial obstruction involves a communication or microperforation between the obstructed hemivagina and normal vagina. In addition, there may be a communication occurring higher up in the reproductive tract such as within the cervix or uterine cavities [2, 10, 11] (Fig. 12.3).
Double uterus with obstructed hemivagina is seen in association with renal anomalies in 90–95 % of cases, with 5–10 % of patients having two normal kidneys [3, 11]. The most commonly reported renal anomaly seen is ipsilateral renal agenesis occurring in approximately 95 % of patients. Other real anomalies reported include dysplastic kidney, duplicated ureter, and polycystic kidney. Right-sided obstruction with ipsilateral renal agenesis is seen more commonly than left-sided obstruction [20] with an incidence 62–77 % noted in the larger case series [4, 11, 19, 21]. In the largest systematic review of 138 patients, right-sided obstruction was noted in 65 % of the study population [11]. Similarly, in a series of 70 patients, right-sided predominance was noted in 62 % of patients [4]. Several mechanisms have been proposed to account for this asymmetry. Differences in gene expression on either side of the embryo at various times during development can result in unequal sensitivity of paired structures to teratogens and adverse genetic influences during organogenesis. This may lead to differences in the lateral distribution of some birth defects [22]. It is also thought that the left side of the embryo has greater mitochondrial maturity compared to the right, resulting in higher energy reserves and lesser tissue damage [23]. The right side, is therefore, more susceptible to hypoxic damage than the left [24]. Despite these mechanisms, the actual cause for this asymmetry still remains elusive [11].
Differential Diagnosis
When evaluating a patient with suspected double uterus with obstructed hemivagina menses occur normally from the non-obstructed side. As menstruation appears to be occurring normally, dysmenorrhea and pain symptoms are often not addressed and may be attributed to “bad menstrual cramps,” possible endometriosis or non-gynecologic causes. The challenge is to recognize the pain symptoms, which typically become increasingly severe over a short period of time, and consider a Müllerian anomaly in the differential diagnosis, especially if the adolescent has a known congenital renal agenesis. One must differentiate this condition from an obstructed uterine horn, which may have a similar presentation. Imaging studies such ultrasound and magnetic resonance imaging (MRI) will distinguish the location of the obstruction and will reveal the presence of cervix and hematocolpos in the setting of obstructed hemivagina. With uterus didelphys and obstructed hemivagina with microperforation, these patients typically present with regular menstruation, but complain of persistent vaginal spotting throughout the menstrual cycle or purulent vaginal discharge. This condition is often confused with abnormal uterine bleeding, vaginitis, or a sexually transmitted disease. Again imaging, preferably MRI, can define the anatomy and identify the uterine anomaly.
Diagnosis
The diagnosis of obstructed hemivagina is difficult due to its rarity, as well as its nonspecific signs and symptoms, and commonly a lack of consideration of a Müllerian anomaly in the differential diagnosis [25]. Patients usually remain asymptomatic until menarche [12], though rare presentations in the neonatal period have also been reported [26]. Symptoms usually become apparent within several months postmenarche and are described as progressively worsening abdominal pain or dysmenorrhea [12, 25]. Presenting symptoms occur as a result of a build up of menstrual fluid within the obstructed hemivagina and subsequently the uterus, fallopian tube, and abdominal cavity due to retrograde menstruation. These symptoms are often not recognized because menstruation occurs normally through the patent and non-affected side [3, 12]. As a result, the diagnosis is often delayed for months to years after menarche [4]. The most common presenting symptom at diagnosis is dysmenorrhea [4, 27] typically progressive, escalating, and severe eventually limiting the patient’s ability to participate in normal activities. Other symptoms include abdominal pain, pelvic or abdominal pressure, urinary frequency, urinary retention, constipation, and paravaginal mass [27].
In girls who have a communication between the obstructed and patent sides, symptoms typically include cyclic menstruation and dysmenorrhea, but with irregular and continuous spotting between periods reflecting a slow egress of the menstrual blood in the obstructed side through the microperforation and into the patent side [4, 27]. In addition, infection may develop in the obstructed side from ascending bacterial infection. If there is infection in the obstructed vaginal canal, there can be continuous, profuse, and malodorous purulent vaginal discharge. Frequently, this discharge is assumed to be vaginitis or a sexually transmitted disease, and the patient has undergone numerous courses of antibiotics without success, often over several months to years. The age and presenting symptoms of patients with obstructed hemivagina varies according to the degree of obstruction. In a retrospective study of 70 patients, mean age (±standard deviation) at diagnosis in patients with complete obstruction was 13.0 (±2.1) years while in patients with obstructed hemivagina with microperforation mean age at presentation was later 24.7 (±7.7) [4].
Delay in diagnosis can lead to the development of upper tract disease due to retrograde menstruation and development of endometriosis and adhesions [28, 29]. In one case series, common findings at laparoscopy in patients with double uterus with obstructed hemivagina included: endometriosis 37 %, hematosalpinx 22 %, pelvic adhesions 10 %, and 1 case of pysosalpinx [27]. These findings have been noted in other studies [3, 4]. These conditions are also correlated with development of abdominal pain and infertility. Therefore, the longer the delay in diagnosis, the greater the risk for development of these upper tract findings, and subsequent infertility.
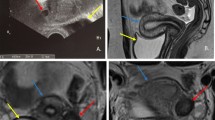
Abdominal examination may reveal a tender suprapubic or abdominal mass [12]. Pelvic or rectal examination can be significant for a paravaginal cystic mass [12]. Often, pelvic examinations in adolescents or virginal females may be significantly limited and in some cases traumatic for the individual and not helpful in clarifying the diagnosis [6]. Radiologic imaging, therefore, plays an important role in the diagnosis. When double uterus with obstructed hemivagina is suspected, ultrasonography (US) can be performed initially to delineate abnormalities of the genitourinary tract [7]. US serves as an important tool in the detection of hematocolpos, which usually appears as a rounded, smooth-walled, hypoechoic mass with absent flow on Doppler imaging [6, 10]. However, to facilitate accurate diagnosis, MRI should be performed. Compared to US, MRI provides more detailed information regarding uterine morphology and continuity with each vaginal canal, thereby facilitating the most appropriate treatment strategy [10] (Figs. 12.4 and 12.5). Three-dimensional ultrasound is helpful in distinguishing septate from bicornuate uterus, but may not be helpful in determining the specific anatomic defect or the level of the obstruction. Laparoscopic evaluation of pelvic anatomy should be considered when MRI is non-diagnostic or when an experienced radiologist is unavailable [2, 4, 6]. In general, computerized tomography is not helpful in delineating anatomy of Müllerian anomalies.
The presence of uterine didelphys and obstructed hemivagina is highly correlated with unilateral renal agenesis ipsilateral to the obstruction. Given the prevalence of fetal ultrasonography, unilateral renal agenesis is often diagnosed prior to birth. Therefore, increasing dysmenorrhea in a young adolescent with a history of renal agenesis should trigger an evaluation for uterine didelphys and obstructed hemivagina, which would facilitate early diagnosis.
It is important to clarify the specific uterine anomaly seen in association with the obstructed hemivagina so as to better counsel the patient regarding treatment options as well as future fertility and reproductive outcomes.
Treatment
There are several options to treat double uterus with obstructed hemivagina including primary resection, removal of the vagina and uterus on the obstructed side, and drainage techniques to acutely relieve symptoms of obstruction with subsequent hormonal suppression of menses to defer septum resection. When considering surgical management, it is also important to consider the maturity level of the patient and family dynamics. Social work and adolescent medicine specialists may be helpful in the discussion of treatment options.
Resection of Vaginal Septum and Marsupialization
Primary resection of the vaginal septum with marsupialization is considered the optimal approach as it involves a one-step definitive procedure [3, 4] (Fig. 12.6). Several observational studies have demonstrated that complete septum resection with marsupialization is superior to simple incision and drainage as this procedure is associated with a higher rate of re-occlusion and infection [6, 12, 18]. The approach to primary resection of the vaginal septum and marsupialization depends on the size of the hematocolpos and the location of the obstruction. If the vaginal obstruction is at the level of the lower vaginal segment, then the hematocolpos is relatively large and runs parallel to the normal vaginal canal [6, 25]. In this situation, it is relatively easy to identify and resect a large segment of the septum thereby creating a large window between the patent and obstructed hemivaginas. The larger the window the less likely it will close and re-occlude. If, however, the obstruction is high in the vaginal canal, then the hematocolpos is smaller and the area of septum that can be resected is also small and therefore more likely to re-occlude. It can also be more difficult to identify the smaller hematocolpos at the time of surgery. Techniques that facilitate identifying the hematocolpos include palpation, insertion of 19G needle with syringe to aspirate and confirm the presence of menstrual blood and transabdominal ultrasound guidance [6, 18], placement of probe through the uterine fundus ipsilateral to the obstruction and passing it through the cervix to tent the lower edge of the obstructed vagina (similar to what is done with transverse vaginal septum [30]), and hydrodissection of the plane in between the hemivaginal septum and the cervix [25]. Once the hematocolpos is identified, then the septum can be entered at the needle insertion site using a bovie or knife. A long clamp with narrow tip can then be used to further enlarge the opening and allow drainage of the hematocolpos. Following drainage, the septum can be palpated and resected using scissors/knife or cautery (bovie, ligasure). The septum can be very thick and vascular and suture ligature, cautery or vessel sealing techniques are helpful to control bleeding during marsupialization. In addition, the rectum and bladder are extremely close to the area of septum resection and after decompression of a hematocolpos the anatomy may not be distinct. Frequent rectal exams and cystoscopy can decrease the risk of, or identify, bowel and bladder injury.
Histologic evaluation of the resected vaginal septum in the obstructed side may reveal different histology then the typical expected squamous epithelium. Changes including columnar epithelium with glandular crypts of cervical type and adenosis have been reported [3, 21]. Long-term follow-up shows reversion to normal squamous epithelium over time [21].
Transvaginal septum resection using traditional specula or retractors may not be feasible in pediatric patients or patients who wish to preserve hymenal integrity. In such cases, resection of a vaginal septum can be undertaken using vaginoscopy [25, 31–34].
The risk of re-occlusion following resection has been reported to be approximately 5–24 % [3, 15, 35]. To reduce the risk of re-occlusion the resected portion of the vaginal septum should be as large as possible and constriction of the vaginal opening should be avoided if sutures are used for hemostasis. Placement of a vaginal dilator postoperatively to prevent re-occlusion is usually not indicated with this procedure.
The use of simultaneous laparoscopy at the time of vaginal septum resection for double uterus with obstructed hemivagina has been advocated [27]. However, others have recommended that routine laparoscopy at the time of vaginal septum resection is not indicated [3]. The concern with any obstructed Müllerian anomaly is retrograde menstruation and associated development of endometriosis that may lead subsequent issues of pain, adhesions, and infertility. However, the natural history of endometriosis that develops due to an obstructed Müllerian anomaly is not well understood. Spontaneous resolution of endometriosis in the setting of treatment of an obstructed hemivagina has been described [21, 36]; however, there are no large long-term studies evaluating this issue. When there is evidence of a hematosalpinx or ovarian endometrioma caused by an obstructed Müllerian anomaly, laparoscopy is recommended to surgically treat as these conditions do not resolve. The decision to perform simultaneous laparoscopy should therefore be based on the presence of hematosalpinx, ovarian endometrioma, severity of symptoms suggesting severe adhesive disease or endometriosis, interval between menarche and diagnosis, or need to confirm the diagnosis or anatomy.
When caring for a patient with obstructed hemivagina associated with a complete septate uterus, it is not advisable to resect the uterine septum at the same time. With distention of the endometrial cavity on the obstructed side, there is distortion of the anatomy and resecting the uterine septum may be more challenging increasing the risk of damage to the uterus with subsequent impact on reproductive function. Furthermore, although resection of a uterine septum may reduce miscarriage risk in some individuals, there are no data to recommend routine resection of a complete uterine septum in an asymptomatic individual. Individuals with complete septate uterus may have normal reproductive function.
Hemihysterectomy with Ipsilateral Hemicolpectomy
Hemihysterectomy with ipsilateral hemicolpectomy has been performed to treat double uterus with obstructed hemivagina [27] (Fig. 12.7). The vaginal tissue on the obstructed side must be removed with the uterus. If the vaginal mucosa is not removed, there is risk of development of a closed cavity lined with vaginal mucosa that may subsequently enlarge with vaginal secretions and cause pain. This procedure is more invasive than vaginal septum resection, involving either laparoscopy or laparotomy and extensive dissection. This procedure should probably be reserved for those cases where a safe vaginoplasty is difficult such as in the cases where the obstructed hemivagina is narrow, small, high, distant from the patent vagina, recurrent vaginal stenosis occurs, or there are other anatomic issues [3]. It is important to recognize that the obstructed hemiuterus can have normal reproductive function following vaginal septum resection as evidenced by the observation that 23–37 % of pregnancies occur in the side ipsilateral to the vaginal obstruction [4, 19]. Given that the hemiuterus on the obstructed side is reproductively competent, there is little reason to remove it unnecessarily.
Drainage of Hematocolpos
Another option to treat double uterus with obstructed hemivagina is to drain and decompress the hematocolpos. This procedure will immediately relieve the pain due to a dilated hemivagina and uterus. However, drainage must be considered a temporary solution as the obstruction is still there, so with subsequent menstruation the hematocolpos will reaccumulate and the patient will need to undergo another procedure in the future to relieve the obstruction. Drainage and decompression is accomplished by transvaginal or laparoscopic approach, although transvaginal drainage may be associated development of infection and pyocolpos given the bacterial flora in the vagina and perineum [6, 12, 18] (Fig. 12.8). If decompression is performed, then menstruation must be suppressed with combined hormonal contraceptives (oral contraceptives pills), progestins (depo-medroxyprogesterone acetate or norethindrone), or GnRH analogs with add back therapy. If menses are not suppressed, then the hematocolpos will reaccumulate relatively quickly (Fig. 12.9). Another concern with this approach is once the hematocolpos is decompressed, the hematocolpos is smaller and more difficult to locate for subsequent vaginal septum excision and marsupialization. Usually, the patient has to be taken off hormonal suppression and allow one or more menstrual cycles to distend the obstructed vaginal cavity and facilitate septum excision. In these cases, intraoperative ultrasound or placement of a probe through the hemiuterus on the obstructed side and through the cervix to tent the vaginal septum may facilitate localization and resection. Primary resection of vaginal septum is the preferred approach to treat double uterus with obstructed hemivagina. However, drainage and decompression may be utilized when there is uncertainty regarding the exact anatomic configuration, lack of surgical expertise to treat the condition, or other health or social conditions that would make primary resection problematic.
(a) Laparoscopic picture showing uterus didelphys with obstructed right hemivagina. The large mass is the hematocolpos. The left uterine horn is visible and not dilated. (b) Laparoscopic image showing laparoscopic drainage of the hematocolpos. (c) Laparoscopic image showing the decompressed right hematocolpos. The uterus didelphys is easily identified
Hormonal suppression of menstruation without first decompressing the hematocolpos is only appropriate if the patient is not in severe pain. It may take several weeks for the hematocolpos to decrease in size in response to the medications. As a result, the patient will continue to have symptoms she presented with for a long time requiring continued pain medication and in some cases hospital admission for pain control.
Treatment of Double Uterus and Obstructed Hemivagina with Microperforation
Managing patients who have double uterus and obstructed hemivagina with a microperforation can be challenging as it can be difficult to identify the microperforation. The microperforation may be located in the vaginal septum or there may be a communication between the obstructed and non-obstructed side anywhere along the cervical or uterine septum in the case of complete septate configuration, or between the two cervices or uterine cavities with other anomalies [2, 10, 11] (Fig. 12.10). If the microperforation is located in the vaginal septum, then identifying the perforation may be easier at the time of menstruation when menstrual blood may be seen at the perforation site. Alternatively, under anesthesia, pressure on the obstructed side will reveal egress of fluid through the microperforation either directly or with the use of vaginoscopy. Once the microperforation is identified, it can be dilated using lacrimal duct probes or a thin tipped clamp such as right angle to gently dilate the opening and identify the vaginal septal tissue. Resection of the vaginal septum then can proceed as usual. If the communication between the two sides is located between the cervices or uterine cavities, then hysterosalpingogram (under anesthesia for an adolescent) can be utilized to identify the location [37] (Fig. 12.11). In these cases, once the vaginal septum is resected on the obstructed side, there is no need to further resect or clarify the communication between the two sides as both sides will drain vaginally.
HSG used to identify a communication between the two cervices of a uterus didelphys with obstructed left hemivagina. Catheter placed into the right cervix. (a) Radiopaque dye entering the right cervix. (b) Radiopaque dye fills the right uterine cavity. Careful evaluation reveals a small extravasation of dye coming from the left side of the cervix representing the communication between the two cervices. (c) Radiopaque dye filling left cervix from the right side, confirming communication
Impact on Fertility
A few studies have evaluated reproductive function in women who have a history of double uterus with obstructed hemivagina. Due to the inherent delay in diagnosis seen with this disorder and subsequent retrograde menstruation, these individuals are at risk of developing adhesions, hematosalpinx, ovarian endometrioma, and undergoing salpingectomy and ovarian cystectomy or oophorectomy, all of which can adversely affect reproductive function. Interestingly, there does not seem to be an increased rate of primary infertility in these patients with studies reporting conception rates of 62–87 % [4, 19, 21]. Some studies have estimated the rate of spontaneous abortion to be as high as 40 %, most likely related to the uterine anomaly [38]. In the largest series to evaluate reproductive outcome following treatment of double uterus obstructed hemivagina, 33 women attempted conception with 28 women (84.8 %) reporting 52 pregnancies [4]. In another large series of 36 patients, 13 out of 15 patients who wanted children succeeded in conceiving (87 %) with a live birth rate of 77 % [21]. In another retrospective study, 13 out of 21 women (62 %) who attempted pregnancy conceived 22 pregnancies with a live birth rate of 91 % [19]. A smaller retrospective study reported 9 women with 20 pregnancies after septum resection with a live birth rate of 65 % [27]. Pregnancy occurred more commonly in the non-obstructive side, which may reflect prior damage to the ipsilateral tube, ovary, or adhesive disease due to retrograde menstruation with obstruction [4, 19]. However, a significant number of pregnancies have been reported to occur in the uterus ipsilateral to the obstruction with 23 % [19] and 36.5 % [4] reported in the two studies, respectively.
Conclusions
Obstructive Müllerian duct anomalies should be suspected in any adolescent with abdominal pain or cyclic dysmenorrhea, and obstructed hemivagina, HWW syndrome, and OHVIRA syndrome should be included in the differential diagnosis. If left undiagnosed or untreated, an obstructed hemivagina can lead to endometriosis, pelvic adhesions, pyometra, pyosalpinx, or intraabdominal abscesses. Thus, early diagnosis and septum resection should be undertaken to relieve hemivaginal obstruction and circumvent subsequent complications.
References
Purslow CE. A case of unilateral haematocolps, haematometria, and haematosalpinx. J Obstet Gynaecol Br Emp. 1922;29:643.
Fedele L, Motta F, Frontino G, Restelli E, Bianchi S. Double uterus with obstructed hemivagina and ipsilateral renal agenesis: pelvic anatomic variants in 87 cases. Hum Reprod. 2013;28(6):1580–3.
Smith NA, Laufer MR. Obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome: management and follow-up. Fertil Steril. 2007;87(4):918–22.
Tong J, Zhu L, Lang J. Clinical characteristics of 70 patients with Herlyn-Werner-Wunderlich syndrome. Int J Gynaecol Obstet. 2013;121(2):173–5.
Attar R, Yıldırım G, Inan Y, Küzılkale O, Karateke A. Uterus didelphys with an obstructed unilateral vagina and ipsilateral renal agenesis: a rare cause of dysmenorrhoea. J Turk Ger Gynecol Assoc. 2013;14(4):242–5.
Schutt AK, Barrett MR, Trotta BM, Stovall DW. Perioperative evaluation in Herlyn-Werner-Wunderlich syndrome. Obstet Gynecol. 2012;120(4):948–51.
Heinonen PK. Clinical implications of the didelphic uterus: long-term follow-up of 49 cases. Eur J Obstet Gynecol Reprod Biol. 2000;91:183–90.
Cetinkaya SE, Kahraman K, Sonmezer M, Atabekoglu C. Hysteroscopic management of vaginal septum in a virginal patient with uterus didelphys and obstructed hemivagina. Fertil Steril. 2011;96(1):e16–8.
Herlyn U, Werner H. Simultaneous occurrence of an open Gartner-duct cyst, a homolateral aplasia of the kidney and a double uterus as a typical syndrome of abnormalities. Geburtshilfe Frauenheilkd. 1971;31:340–7.
Del Vescovo R, Battisti S, Di Paola V, Piccolo CL, Cazzato RL, Sansoni I, Grasso RF, Zobel BB. Herlyn-Werner-Wunderlich syndrome: MRI findings, radiological guide (two cases and literature review), and differential diagnosis. BMC Med Imaging. 2012;12:4.
Vercellini P, Daguati R, Somigliana E, Viganò P, Lanzani A, Fedele L. Asymmetric lateral distribution of obstructed hemivagina and renal agenesis in women with uterus didelphys: institutional case series and a systematic literature review. Fertil Steril. 2007;87(4):719–24.
Gholoum S, Puligandla PS, Hui T, Su W, Quiros E, Laberge JM. Management and outcome of patients with combined vaginal septum, bifid uterus, and ipsilateral renal agenesis (Herlyn-Werner-Wunderlich syndrome). J Pediatr Surg. 2006;41(5):987–92.
Grimbizis GF, Campo R. Congenital malformations of the female genital tract: the need for a new classification system. Fertil Steril. 2010;94(2):401–7.
Acien P, Acien MI. The history of female genital tract malformation classifications and proposal of an updated system. Hum Reprod Update. 2011;17(5):693–705.
Oppelt P, Renner SP, Brucker S, Strissel PL, Strick R, Oppelt PG, et al. The VCUAM (Vagina Cervix Uterus Adnex-associated Malformation) classification: a new classification for genital malformations. Fertil Steril. 2005;85(5):1493–7.
American Fertility Society. The AFS classification of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Mullerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49:944–55.
Berger-Chen S, Ritch JM, Kim JH, Evanko J, Hensle TW. An unusual presentation of uterine didelphys and obstructed hemivagina. J Pediatr Adolesc Gynecol. 2012;25(6):e129–31.
Khong TL, Siddiqui J, Mallinson P, Horton D, Gandhi J, Daniel R. Herlyn-Werner-Wunderlich syndrome: uterus didelphys, obstructed hemivagina, and ipsilateral renal agenesis-role of sonographically guided minimally invasive vaginal surgery. Eur J Pediatr Surg. 2012;22(2):171–3.
Heinonen PK. Pregnancies in women with uterine malformation, treated obstruction of hemivagina and ipsilateral renal agenesis. Arch Gynecol Obstet. 2013;287:975–8.
Rock JA, Jones Jr HW. The double uterus associated with an obstructed hemivagina and ipsilateral renal agenesis. Am J Obstet Gynecol. 1980;138:339–42.
Candiani GB, Fedele L, Candiani M. Double uterus, blind hemivagina, and ipsilateral renal agenesis: 36 cases and long-term follow-up. Obstet Gynecol. 1997;90:26–32.
Paulozzi LJ, Lary JM. Laterality patterns in infants with external birth defects. Teratology. 1999;60:265–71.
Fantel AG, Person RE, Burroughs-Gleim C, Shepard TH, Juchau R, Backler B. Asymmetric development of mitochondrial activity in rat embryos as a determinant of the defect patterns induced by exposure to hypoxia, hyperoxia, and redox cyclers in vitro. Teratology. 1991;44:355–62.
Fantel AG, Juchau R, Tracy JW, Burroughs CJ, Person RE. Studies of mechanisms of niridazole-elicited embryotoxicity: evidence against a major role for covalent binding. Teratology. 1989;39:63–74.
Pereira N, Anderson SH, Verrecchio ES, Brown MA, Glassner MJ. Hemivaginal septum resection in a patient with a rare variant of Herlyn-Werner-Wunderlich syndrome. J Minim Invasive Gynecol. 2014;21(6):1113–7.
Wu TH, Wu TT, Ng YY, Ng SC, Su PH, Chen JY, et al. Herlyn-Werner-Wunderlich syndrome consisting of uterine didelphys, obstructed hemivagina and ipsilateral renal agenesis in a newborn. Pediatr Neonatol. 2012;53(1):68–71.
Haddad B, Barranger E, Paniel BJ. Blind hemivagina: long-term follow-up and reproductive performance in 42 cases. Hum Reprod. 1999;14(8):1962–4.
Olive DL, Henderson DY. Endometriosis and mullerian anomalies. Obstet Gynecol. 1987;69:412–5.
Fedele L, Bianchi S, Di Nola G, Franchi D, Candiani GB. Endometriosis and nonobstructive müllerian anomalies. Obstet Gynecol. 1992;79(4):515–7.
Rock JA, Zacur HA, Dlugi AM, Jones HW. Pregnancy success following surgical correction of imperforate hymen and complete transverse septum. Obstet Gynecol. 1982;59:448.
Kim TE, Lee GH, Choi YM, Jee BC, Ku SY, Suh CS, et al. Hysteroscopic resection of the vaginal septum in uterus didelphys with obstructed hemivagina: a case report. J Korean Med Sci. 2007;22:766–9.
Shih CL, Hung YC, Chen CP, Chien SC, Lin WC. Resectoscopic excision of the vaginal septum in a virgin with uterus didelphys and obstructed unilateral vagina. Taiwan J Obstet Gynecol. 2010;49(1):109–11.
Nassif J, Al Chami A, Abu Musa A, Nassar AH, Kurdi A, Ghulmiyyah L. Vaginoscopic resection of vaginal septum. Surg Technol Int. 2012;22:173–6.
Dorais J, Milroy C, Hammoud A, Chaudhari A, Gurtcheff S, Peterson CM. Conservative treatment of a Herlyn-Werner-Wunderlich müllerian anomaly variant, noncommunicating hemiuterus with Gartner duct pseudocyst. J Minim Invasive Gynecol. 2011;18(2):262–6.
Wang S, Lang JH, Zhu L, Zhou HM. Duplicated uterus and hemivaginal or hemicervical atresia with ipsilateral renal agenesis: an institutional clinical series of 52 cases. Eur J Obstet Gynecol Reprod Biol. 2013;170:507–11.
Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39–43.
Acien P, Acien M, Sanchez-Ferrer M. Complex malformations of the female genital tract. New types and revision of classification. Hum Reprod. 2004;19:2377–84.
Propst AM, Hill III JA. Anatomic factors associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18:341–50.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pereira, N., Pfeifer, S.M. (2016). Obstructed Hemivagina. In: Pfeifer, S. (eds) Congenital Müllerian Anomalies. Springer, Cham. https://doi.org/10.1007/978-3-319-27231-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-27231-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27229-0
Online ISBN: 978-3-319-27231-3
eBook Packages: MedicineMedicine (R0)