Abstract
DNA/RNA photo-cross-linking reactions have great potential for regulating the functions, structures, and characters of nucleic acids. The photo-responsive manner of the reactions are expected to enable spatiotemporal control of the behavior of nucleic acids, and the thermal irreversibility of the photo-cross-linked product is expected to enable construction of thermally stable nanostructured DNA.
Therefore, various artificial nucleic acids that can photo-cross-link to complementary DNA or RNA have been developed. This chapter focuses on the chemistry of these artificial nucleic acids and their application for molecular, cellular, and chemical biology, and also DNA nanotechnology, which is an interesting field for the construction of nanomaterials in a bottom-up manner, such as DNA origami.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
The photoreaction in DNA is one of the most important phenomena in the basic study of photodamage in genomic DNA. Since the first report about photo-induced pyrimidine dimer formation in double-stranded DNA by Setlow [1], many researchers have made huge efforts to understand the mechanism of the phenomenon [2–6] and the mechanism of DNA damage repair [7–11]. In this phenomenon, the cyclobutane ring formation through [2 + 2] photocycloaddition between C5 and C6 carbons on adjacent pyrimidine bases in a DNA strand is induced by UVB irradiation, and the photoproduct causes genomic damage and cell death.
Psoralen derivatives, which can photo-cross-link with C5–C6 carbons on a pyrimidine base in a DNA strand in the same manner of pyrimidine dimer formation by UVA irradiation; contrary to the case of pyrimidine dimer formation caused by UVB irradiation [12], are key compounds in the history of the development of photo-functionalized nucleic acids. Based on the findings of psoralen derivatives, until now, various artificial nucleic acids that can photo-cross-link with DNA or RNA with a sequence specific manner have been developed.
In this chapter, the mechanism of the photo-cross-linking reaction in nucleic acids including psoralen and other artificial DNA photo-cross-linkers is described. The application of the photo-cross-linking reaction on gene regulation, genome analysis, and DNA-based nanotechnology is also described.
7.2 Psoralen: A Natural DNA Photo-Cross-Linker
Naturally occurring plant furocoumarins , e.g., psoralen, methoxsalen, and trioxsalen (Fig. 7.1a), that can photoreact with a DNA double strand, have been used for the treatment of various skin disorders such as Atopic dermatitis, vitiligo, eczema, and cutaneous T-cell lymphoma. Psoralen derivatives effectively intercalate to the AT region of genomic DNA, and the fran ring and pyrone ring of psoralen derivative form cyclobutane ring with C5–C6 carbon on the thymine bases possessed at different two DNA strands with UVA irradiation (Fig. 7.1b). Thus, the two DNA strands can be bound covalently via a photo-cross-linked product consisting of a psoralen derivative and two thymine bases [12]. This induces cytotoxicity only at the photoirradiated area. Since the psoralen derivatives can be activated with UVA irradiation, treatment of skin disorders can be performed without significant photodamage of genomic DNA caused by UVB-induced pyrimidine dimer formation.
The photoreaction including psoralen derivative also occurs in AU regions in double-stranded RNA. Using this reaction, the secondary structures of RNAs were successfully explored [13–16].
7.3 Psoralen-Modified Artificial Nucleic Acids
With the development of the methodology for organic synthesis and modification of nucleic acids, psoralen derivatives have been conjugated with various synthetic oligonucleotides to give sequence specificity for the photo-cross-linking reaction of psoralen derivatives. Miller and co-workers conducted one of the most pioneering studies in this field. They introduced trioxsalen at the 5′ end of synthetic oligodeoxyribonucleotide (ODN(s)) (Fig. 7.2, Compound 1) and clearly demonstrated that the trioxsalen-modified ODN photo-cross-linked to complementary single-stranded DNA [17–19] and double-stranded DNA [20] with UVA (365 nm) irradiation. Furthermore, they also demonstrated that the trioxsalen-modified ODN having an antisense sequence for rabbit globin mRNA effectively inhibits the translation of rabbit globin mRNA in a photo-responsive manner [21]. Their findings opened the door for the development of photo-functionalized synthetic oligonucleotides. Indeed, in the early 1990s, various groups reported antisense ODN [22, 23] and triplex-forming ODN (TFO) modified with psolaren derivative [24–30]. In particular, oligonucleotide having 2′-trioxsalen-modified adenosine (Fig. 7.2, Compound 2) has the highest photoreactivity toward thymine or uracil base in a complementary DNA or RNA strand [31, 32]. Recently, coumarin-modified nucleic acid having photo-cross-linking ability to complementary DNA was reported (Fig.7.2, Compound 4 [33]). They successfully modified thymidine with coumarin using a Cu(I)-catalyzed click reaction. This is the first example of interstrand photo-cross-linking reaction by a modified pyrimidine nucleoside .
7.4 3-Cyanovinylcarbazole-Modified Artificial Nucleic Acids
Owing to its photoreactivity and the ease with which it is obtained from natural plants, psoralen derivative is widely used for photo-functionalization of synthetic ODNs; however, the UVA irradiation required for the photoreaction itself sometimes causes unexpected cytotoxicity to cells. Therefore, a more highly reactive photo-cross-linker that can photo-cross-link to nucleic acids with shorter irradiation time than psoralen derivatives was required. In 2008, as a DNA photo-cross-linker having higher photoreactivity compared to psoralen derivatives, 3-cyanovinylcarbazole-modified nucleoside (CNVK) was reported by Fujimoto and co-workers ([34, 35]; Fig. 7.3, Compound 1). Similar to the case of psoralen derivatives, the photo-cross-linking reaction of CNVK occurs through a [2 + 2] photocycloaddition reaction between the vinyl moiety of CNVK and C5–C6 double bond of the pyrimidine base with 365 nm irradiation. As the photoreactivity of ODN having CNVK is at least tenfold greater than that of psoralen-modified ODNs, CNVK is the most reactive DNA photo-cross-linker at that time. Since ODN having CNVK photo-cross-link with complementary DNA or RNA [36–38], and also double-stranded DNA [39], the same as the case of psoralen derivatives, they are expected to be a powerful tool for regulating the functions of nucleic acids, the same as psoralen-modified ODNs. Most recently, a novel DNA photo-cross-linker consisting of 3-cyanovinylcarbazole and d-threoninol (CNVD) has been reported ([40]; Fig. 7.3, Compound 2). As the photoreactivity of CNVD is 1.8–8-fold higher than that of CNVK, this is the most highly reactive DNA photo-cross-linker reported. Furthermore, recent research by Fujimoto’s group of JAIST revealed that the complementary base of the pyrimidine base that will be cross-linked with CNVK greatly affects the photoreactivity of CNVK in double-stranded DNA [41]. Particularly, in the case of cytosine as the target of CNVK, the decrease of the hydrogen bonds between the cytosine and its complementary base by the substitution of canonical guanine with a noncanonical complementary base, such as inosine and 2-aminopurine, drastically accelerates the photoreactivity 3.6–7.7-fold. These findings suggest that the local stability and/or flexibility of the photo-cross-linking site is an important factor for governing the photoreaction. In general, the reaction rate of the photo-cross-linking toward the cytosine base through [2 + 2] photocycloaddition is lower than that toward thymine or uracil [12, 34]. There is huge potential for improving photoreactivity toward cytosine by regulating the local stability and/or flexibility of the photo-cross-linking site with the substitution of a complementary base of cytosine.
7.5 Other Artificial Nucleic Acids
He and co-workers reported another class of DNA photo-cross-linker having a different mechanism from that of psoralen and 3-cyanovinylcarbazole derivatives: diazirine-modified nucleic acid analogue ([42]; Fig. 7.4, Compound 1). The diazirine group forms a carbene intermediate upon UVA-induced N2 elimination and cross-links to multiple nearby bases in the complementary strand. Contrary to the case of photo-cross-linking via [2 + 2] photocycloaddition, this type of photo-cross-linker can react with four kinds of nucleobases in the complementary DNA and RNA strand ([43]; Fig. 7.4, Compound 2 and 3).
As another class of the DNA photo-cross-linking reaction, recently, Asanuma’s group of Nagoya University reported stilbene-modified artificial nucleic acids (Fig. 7.5 [44]). They successfully demonstrated that two complementary synthetic ODNs having stilbene moiety can photo-cross-link each other with the 340 nm irradiation through [2 + 2] photodimerization of two stilbene moieties in the double-stranded DNA. Contrary to the case of psoralen or 3-cyanovinylcarbazole-modified nucleic acids, it is unclear whether the reaction occurs toward native nucleic acid bases; however, the combination of the photodimerization pair can be selected freely, in their case. Therefore, the strategy has far-reaching potential for improving the photoreactivity and for regulating the irradiation wavelength required for activating the photoreaction.
7.6 Applications of Photo-Cross-Linking Reaction in Nucleic Acids
The sequence specific photo-cross-linking reaction using various photo-cross-linkers mentioned above is applicable for regulating biological events including nucleic acid, such as replication, transcription, translation, and DNA damage repair, and also DNA nanostructures (Fig. 7.6). As the timing and area of photoirradiation can be regulated completely, the spatiotemporal regulation of the biological events or nanostructures mentioned above is expected to be regulated freely with photoirradiation.
7.6.1 Photoregulation of Gene Expression
The photodynamic antisense strategy (Fig. 7.7a) is a successful example of regulating gene expression in cells. In this strategy, photo-responsive ODNs having complementary sequence of target mRNA specifically cross-link and form irreversible photoadduct with target mRNA . Therefore, the translation of target mRNA is selectively inhibited by steric hindrance. The main concept of the photodynamic antisense strategy was advocated by Millar and co-workers as mentioned above [21]. They successfully demonstrated that trioxsalen-modified antisense ODN effectively downregulates rabbit globin gene expression in an in vitro translation system in a photo-responsive manner. In an early report of the cellular application of trioxsalen-modified antisense ODN, Chang et al. and Lin et al. successfully photo-regulated the translation of point-mutated ras protein in 453 cells [22] and collagenase I in dermal fibroblast [23], respectively. Murakami’s group of KIT energetically worked in this area [45, 46], and they successfully demonstrated that trioxsalen-modified antisense ODN effectively regulates the gene expression of HPV E6 and E7 mRNA and suppresses the proliferation of HPV positive SiHa cells with nanomolar treatment of trioxsalen-modified antisense ODN and UVA irradiation. Recently, the temporal regulation of constitutive GFP gene expression has been demonstrated by the use of CNVK-modified antisense ODNs [47]. The high photoreactivity of CNVK enables quick regulation of gene expression in cells with 10 s of UVA irradiation.
As another strategy for regulating gene expression in a photo-responsive manner, the photodynamic antigene strategy (Fig. 7.7b) has been reported by several researchers. Psoralen-modified triplex forming ODNs is one of the successful examples of this strategy. The ODNs are effective for regulating gene expression with sequence specific photo-cross-linking reaction between the psoralen moiety tethered with ODN and double-stranded genomic DNA. Based on this strategy, the downregulation of interleukin 2 receptor [27], human rhodopsin [48], and β-globin [49] genes in cells has been reported.
7.6.2 Photo-Cross-Linking Reaction for Nucleic Acids Analysis
Because of the highly thermal stability of the photo-cross-linked duplexes, photoreactive synthetic ODNs are applicable for highly sensitive detection of nucleic acids or as a nucleic acid capture probe.
The reactivity of the photo-cross-linking reaction through [2 + 2] photocycloaddition is quite different among pyrimidine bases . Using this character, 5-methyl modification of cytosine in the DNA strand was clearly discriminated with unmodified cytosine by the use of psoralen- or CNVK-modified ODN probes (Fig. 7.8 [50, 51]). Based on this selective photo-cross-linking reaction, the methodology for analyzing epigenetic modification of DNA can be further developed.
7.6.3 Photo-Cross-Linking Reaction for Nanotechnology
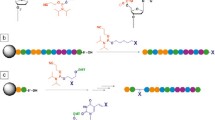
DNA-based nanotechnologies, such as DNA nanocrystal and DNA origami, are cutting-edge areas in nanotechnology and supramolecular science. The bottom-up manner of this technology, which relies on the simple hybridization property of DNA strands, is expected to lead to the construction of various nanostructures and functions induced by finely designed nanostructures. DNA photo-cross-linking is applicable also in this area. The thermally stable double-stranded DNA caused by the interstrand photo-cross-linking reaction of psoralen gives thermally stable nanostructured DNA (Fig. 7.9a), such as DNA origami tiles [52], DNA origami branches [53], and branched oligonucleotide networks [54]. The ability to use these nanostructures at higher temperature enables various applications such as the creation of nanoscale electronic devices and higher-temperature assembly of functional molecules on nanostructured DNA. CNVK-modified ODNs are also applicable for constructing thermally stable DNA nanostructures. Tagawa et al. and Nakamura et al. reported that the DNA double-crossover AB-staggered tiles having CNVK and DNA 2D array including CNVK, respectively, could be stabilized by UVA irradiation (Fig. 7.9b [55, 56]). Furthermore, Gerrard et al. successfully developed a method of integrating nanostructured DNA using thermally stable nanostructured DNA modules and orthogonal copper-free click chemistry (Fig. 7.9c [57]). Since the thermal stability of nanostructured DNA is an important issue for constructing higher-ordered DNA nanostructures, the photo-cross-linking strategies mentioned above are expected to contribute to the further development of DNA-based nanotechnology.
Most recently, Kanaras’s group of the University of Southampton successfully demonstrated that the assembly of nanoparticles is finely and reversibly regulated by the irradiation of UV light [58]. They used 15 nm two gold particles modified with only one DNA strand, one has CNVK and one has a thymine base as the photo-cross-linking site, and clearly demonstrated that the dimer assembly and dis-assembly were completely regulated by 365 and 312 nm irradiation, respectively. Since the triangle and tetrahedron structure, which has gold nanoparticles at its vertexes and photo-cross-linked duplexes at its sides, is also assembled by using a similar strategy, this new technique will be of particular applicability in several research fields using nanoparticle assemblies such as catalysis, photonics, and biosensors.
7.7 Conclusion and Prospects
Functionalized ODNs having photo-cross-linking ability possess great potential for regulating functions and structures of nucleic acids because of their sequence selectivity, thermally irreversibleness, and photo-responsive manner.
However, problems still remain with the clinical application of photo-cross-linking ODNs, e.g., the cytotoxicity of photoreactive moieties, unexpected photodamage caused by UVA [59], and low transparency of UVA in bio-organs. Further development of photoreactive groups having photo-cross-linking ability with longer wavelengths and low cytotoxicity is required and also a combination with advanced light sources such as femtosecond pulse lasers that can activate molecules by two or three photons with longer wavelengths.
References
Setlow RB (1966) Cyclobutane-type pyrimidine dimers in polynucleotides. Science 153(3734):379–386
Kao JL, Nadji S, Taylor JS (1993) Identification and structure determination of a third cyclobutane photodimer of thymidylyl-(3′-->5′)-thymidine: the trans-syn-II product. Chem Res Toxicol 6(4):561–567
Koning MG, van Soest JJ, Kaptein R (1991) NMR studies of bipyrimidine cyclobutane photodimers. Eur J Biochem 195(1):29–40
Rao SN, Keepers JW, Kollman P (1984) The structure of d(CGCGAAT[]TCGCG). d(CGCGAATTCGCG); the incorporation of a thymine photodimer into a B-DNA helix. Nucleic Acids Res 12(11):4789–4807
Schreier WJ, Schrader TE, Koller FO, Gilch P, Crespo-Hernández CE, Swaminathan VN, Carell T, Zinth W, Kohler B (2007) Thymine dimerization in DNA is an ultrafast photoreaction. Science 315(5812):625–629
Su DG, Kao JL, Gross ML, Taylor JS (2008) Structure determination of an interstrand-type cis-anti cyclobutane thymine dimer produced in high yield by UVB light in an oligodeoxynucleotide at acidic pH. J Am Chem Soc 130(34):11328–11337
Jiang N, Taylor JS (1993) In vivo evidence that UV-induced C-->T mutations at dipyrimidine sites could result from the replicative bypass of cis-syn cyclobutane dimers or their deamination products. Biochemistry 32(2):472–481
Kim ST, Sancar A (1991) Effect of base, pentose, and phosphodiester backbone structures on binding and repair of pyrimidine dimers by Escherichia coli DNA photolyase. Biochemistry 30(35):8623–8630
Niggli HJ, Cerutti PA (1983) Cyclobutane-type pyrimidine photodimer formation and excision in human skin fibroblasts after irradiation with 313-nm ultraviolet light. Biochemistry 22(6):1390–1395
Niggli HJ (1993) Aphidicolin inhibits excision repair of UV-induced pyrimidine photodimers in low serum cultures of mitotic and mitomycin C-induced postmitotic human skin fibroblasts. Mutat Res 295(3):125–133
Shwartz H, Shavitt O, Livneh Z (1988) The role of exonucleolytic processing and polymerase-DNA association in bypass of lesions during replication in vitro. Significance for SOS-targeted mutagenesis. J Biol Chem 263(34):18277–18285
Song P-S, Tapley KJ Jr (1979) Photochemistry and photobiology of psoralen. Photochem Photobiol 29:1177–1197
Calvet JP, Pederson T (1979) Photochemical cross-linking of secondary structure in HeLa cell heterogeneous nuclear RNA in situ. Nucleic Acids Res 6(5):1993–2001
Cantor CR, Wollenzien PL, Hearst JE (1980) Structure and topology of 16S ribosomal RNA. An analysis of the pattern of psoralen crosslinking. Nucleic Acids Res 8(8):1855–1872
Rabin D, Crothers DM (1979) Analysis of RNA secondary structure by photochemical reversal of psoralen crosslinks. Nucleic Acids Res 7(3):689–703
Wollenzien PL, Youvan DC, Hearst JE (1978) Structure of psoralen-crosslinked ribosomal RNA from Drosophila melanogaster. Proc Natl Acad Sci USA 75(4):1642–1646
Bhan P, Miller PS (1990) Photo-cross-linking of psoralen-derivatized oligonucleoside methylphosphonates to single-stranded DNA. Bioconjug Chem 1(1):82–88
Lee BL, Murakami A, Blake KR, Lin SB, Miller PS (1988) Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with single-stranded DNA. Biochemistry 27(9):3197–3203
Pieles U, Englisch U (1989) Psoralen covalently linked to oligodeoxyribonucleotides: synthesis, sequence specific recognition of DNA and photo-cross-linking to pyrimidine residues of DNA. Nucleic Acids Res 17(1):285–299
Lee BL, Blake KR, Miller PS (1988) Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with synthetic DNA containing a promoter for T7 RNA polymerase. Nucleic Acids Res 16(22):10681–10697
Kean JM, Murakami A, Blake KR, Cushman CD, Miller PS (1988) Photochemical cross-linking of psoralen-derivatized oligonucleoside methylphosphonates to rabbit globin messenger RNA. Biochemistry 27(26):9113–9121
Chang EH, Miller PS, Cushman C, Devadas K, Pirollo KF, Ts’o PO, Yu ZP (1991) Antisense inhibition of ras p21 expression that is sensitive to a point mutation. Biochemistry 30(34):8283–8286
Lin M, Hultquist KL, Oh DH, Bauer EA, Hoeffler WK (1995) Inhibition of collagenase type I expression by psoralen antisense oligonucleotides in dermal fibroblasts. FASEB J 9(13):1371–1377
Giovannangéli C, Thuong NT, Hélène C (1992) Oligodeoxynucleotide-directed photo-induced cross-linking of HIV proviral DNA via triple-helix formation. Nucleic Acids Res 20(16):4275–4281
Takasugi M, Guendouz A, Chassignol M, Decout JL, Lhomme J, Thuong NT, Hélène C (1991) Sequence-specific photo-induced cross-linking of the two strands of double-helical DNA by a psoralen covalently linked to a triple helix-forming oligonucleotide. Proc Natl Acad Sci USA 88(13):5602–5606
Cassidy RA, Kondo NS, Miller PS (2000) Triplex formation by psoralen-conjugated chimeric oligonucleoside methylphosphonates. Biochemistry 39(29):8683–8691
Grigoriev M, Praseuth D, Guieysse AL, Robin P, Thuong NT, Hélène C, Harel-Bellan A (1993) Inhibition of gene expression by triple helix-directed DNA cross-linking at specific sites. Proc Natl Acad Sci USA 90(8):3501–3505
Miller PS, Bi G, Kipp SA, Fok V, DeLong RK (1996) Triplex formation by a psoralen-conjugated oligodeoxyribonucleotide containing the base analog 8-oxo-adenine. Nucleic Acids Res 24(4):730–736
Miller PS, Kipp SA, McGill C (1999) A psoralen-conjugated triplex-forming oligodeoxyribonucleotide containing alternating methylphosphonate-phosphodiester linkages: synthesis and interactions with DNA. Bioconjug Chem 10(4):572–577
Wang G, Glazer PM (1995) Altered repair of targeted psoralen photoadducts in the context of an oligonucleotide-mediated triple helix. J Biol Chem 270(38):22595–22601
Higuchi M, Yamayoshi A, Yamaguchi T, Iwase R, Yamaoka T, Kobori A, Murakami A (2007) Selective photo-cross-linking of 2′-O-psoralen-conjugated oligonucleotide with RNAs having point mutations. Nucleosides Nucleotides Nucleic Acids 26(3):277–290
Higuchi M, Kobori A, Yamayoshi A, Murakami A (2009) Synthesis of antisense oligonucleotides containing 2′-O-psoralenylmethoxyalkyl adenosine for photodynamic regulation of point mutations in RNA. Bioorg Med Chem 17(2):475–483
Haque MM, Sun H, Liu S, Wang Y, Peng X (2014) Photo switchable formation of a DNA interstrand cross-link by a coumarin-modified nucleotide. Angew Chem Int Ed 53(27):7001–7005
Yoshimura Y, Fujimoto K (2008) Ultrafast reversible photo-cross-linking reaction: toward in situ DNA manipulation. Org Lett 10(15):3227–3230
Fujimoto K, Yamada A, Yoshimura Y, Tsukaguchi T, Sakamoto T (2013) Details of the ultrafast DNA photo-cross-linking reaction of 3-cyanovinylcarbazole nucleoside: cis-trans isomeric effect and the application for SNP-based genotyping. J Am Chem Soc 135(43):16161–16167
Fujimoto K, Konishi-Hiratsuka K, Sakamoto T, Yoshimura Y (2010) Site-specific photochemical RNA editing. Chem Commun (Camb) 46(40):7545–7547
Yoshimura Y, Ohtake T, Okada H, Fujimoto K (2009) A new approach for reversible RNA photocrosslinking reaction: application to sequence-specific RNA selection. ChemBioChem 10(9):1473–1476
Fujimoto K, Kishi S, Sakamoto T (2013) Geometric effect on the photocrosslinking reaction between 3-cyanovinylcarbazole nucleoside and pyrimidine base in DNA/RNA heteroduplex. Photochem Photobiol 89(5):1095–1099
Fujimoto K, Yoshinaga H, Yoshio Y, Sakamoto T (2013) Quick and reversible photocrosslinking reaction of 3-cyanovinylcarbazole nucleoside in a DNA triplex. Org Biomol Chem 11(31):5065–5068
Sakamoto T, Tanaka Y, Fujimoto K (2015) DNA photo-cross-linking using 3-cyanovinylcarbazole modified oligonucleotide with threoninol linker. Org Lett 17(4):936–939
Sakamoto T, Ooe M, Fujimoto K (2015) Critical effect of base pairing of target pyrimidine on the interstrand photo-cross-linking of DNA via 3-cyanovinylcarbazole nucleoside. Bioconjugate Chem 26(8):1475–1478. doi:10.1021/acs.bioconjchem.5b00352
Qiu Z, Lu L, Jian X, He C (2008) A diazirine-based nucleoside analogue for efficient DNA interstrand photocross-linking. J Am Chem Soc 130(44):14398–14399
Nakamoto K, Ueno Y (2014) Diazirine-containing RNA photo-cross-linking probes for capturing microRNA targets. J Org Chem 79:2463–2472
Doi T, Kashida H, Asanuma H (2015) Efficiency of [2+2] photodimerization of various stilbene derivatives within the DNA duplex scaffold. Org Biomol Chem 13:4430–4437
Murakami A, Yamayoshi A, Iwase R, Nishida J, Yamaoka T, Wake N (2001) Photodynamic antisense regulation of human cervical carcinoma cell growth using psoralen-conjugated oligo(nucleoside phosphorothioate). Eur J Pharm Sci 13(1):25–34
Yamayoshi A, Iwase R, Yamaoka T, Murakami A (2003) Psoralen-conjugated oligonucleotide with hairpin structure as a novel photo-sensitive antisense molecule. Chem Commun 12:1370–1371
Sakamoto T, Shigeno A, Ohtaki Y, Fujimoto K (2014) Photo-regulation of constitutive gene expression in living cells by using ultrafast photo-cross-linking oligonucleotides. Biomater Sci 2:1154–1157
Intody Z, Perkins BD, Wilson JH, Wensel TG (2000) Blocking transcription of the human rhodopsin gene by triplex-mediated DNA photocrosslinking. Nucleic Acids Res 28(21):4283–4290
Shahid KA, Majumdar A, Alam R, Liu ST, Kuan JY, Sui X, Cuenoud B, Glazer PM, Miller PS, Seidman MM (2006) Targeted cross-linking of the human beta-globin gene in living cells mediated by a triple helix forming oligonucleotide. Biochemistry 45(6):1970–1978
Yamayoshi A, Matsuyama Y, Kushida M, Kobori A, Murakami A (2014) Novel photodynamic effect of a psoralen-conjugated oligonucleotide for the discrimination of the methylation of cytosine in DNA. Photochem Photobiol 90(3):716–722
Fujimoto K, Konishi-Hiratsuka K, Sakamoto T (2013) Quick, selective and reversible photocrosslinking reaction between 5-methylcytosine and 3-cyanovinylcarbazole in DNA double strand. Int J Mol Sci 14(3):5765–5774
Rajendran A, Endo M, Katsuda Y, Hidaka K, Sugiyama H (2011) Photo-cross-linking-assisted thermal stability of DNA origami structures and its application for higher-temperature self-assembly. J Am Chem Soc 133(37):14488–14491
Liu J, Geng Y, Pound E, Gyawali S, Ashton JR, Hickey J, Woolley AT, Harb JN (2011) Metallization of branched DNA origami for nanoelectronic circuit fabrication. ACS Nano 5(3):2240–2247
Hartman MR, Yang D, Tran TN, Lee K, Kahn JS, Kiatwuthinon P, Yancey KG, Trotsenko O, Minko S, Luo D (2013) Thermostable branched DNA nanostructures as modular primers for polymerase chain reaction. Angew Chem Int Ed 52(33):8699–8702
Nakamura S, Fujimoto K (2013) Creation of DNA array structure equipped with heat resistance by ultrafast photocrosslinking. J Chem Technol Biotechnol 89:1086–1090
Tagawa M, Shohda K, Fujimoto K, Suyama A (2011) Stabilization of DNA nanostructures by photo-cross-linking. Soft Matter 7:10931–10934
Gerrard SR, Hardiman C, Shelbourne M, Nandhakumar I, Nordén B, Brown T (2012) A new modular approach to nanoassembly: stable and addressable DNA nanoconstructs via orthogonal click chemistries. ACS Nano 6(10):9221–9228
Harimech PK, Gerrard SR, El-Sagheer AH, Brown T, Kanaras AG (2015) Reversible ligation of programmed DNA-gold nanoparticle assemblies. J Am Chem Soc 137(29):9242–9245
Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ (2003) UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J 17(9):1177–1179
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sakamoto, T., Fujimoto, K. (2016). Photo-Cross-Linking Reaction in Nucleic Acids: Chemistry and Applications. In: Nakatani, K., Tor, Y. (eds) Modified Nucleic Acids. Nucleic Acids and Molecular Biology, vol 31. Springer, Cham. https://doi.org/10.1007/978-3-319-27111-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-27111-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27109-5
Online ISBN: 978-3-319-27111-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)