Abstract
Undifferentiated embryonal sarcoma (UES) is defined as a highly malignant mesenchymal neoplasm of the liver. UES mainly occurs in the pediatric age group and is characterized by the presence of an immature and myxoid mesenchyme that contains numerous pleomorphic and giant cells with distinct eosinophilic globular cytoplasmic inclusions. UES is the third most common malignant hepatic tumor in children, whereby about 50 % of patients are 6–10 years old at diagnosis. In contrast to hepatoblastoma, only a minority of UES is diagnosed in children younger than 5 years. UES is a highly aggressive neoplasm that forms large liver tumors mainly in the right liver lobe, typically undergoing cystic change, sometimes mimicking hydatid disease. Spontaneous hemorrhage and tumor rupture may occur. Apart from a basic histologic pattern, several variants are known, including small cell, anaplastic and rhabdoid phenotypes, and tumors with various heterologous components. The abnormal mesenchyme of UES may surround abnormal and dilated intratumoral bile duct-like structures. There is a pathogenetic relationship between UES and mesenchymal hamartoma of the liver.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Muscle Cell Differentiation
- 1Metastasis Associate Lung Adenocarcinoma Transcript
- Mesenchymal Hamartoma
- Malignant Hepatic Tumor
- Immunohistochemical Pattern
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Undifferentiated (embryonal) sarcoma (UES) is defined as a highly malignant mesenchymal neoplasm of the liver, predominantly in children, characterized by the presence of an immature and myxoid mesenchyme containing pleomorphic and giant cells with globular eosinophilic cytoplasmic inclusions.
UES was first described in 1978 as a separate pathologic entity, based on 31 observations (Stocker and Ishak 1978). This tumor type may have first been reported in 1946, under the term, mesenchymoma, based on an observation in a 6-year-old boy, but the fact that this patient survived well for 13 months in a pre-chemotherapy era and based on the two histologic figures in the article leaves some doubt whether this tumor was not rather mesenchymal hamartoma (Donovan and Santulli 1946). Several terms had been employed to denote this neoplasm, including primary sarcoma of the liver (Willeford and Stembridge 1950), malignant mesenchymoma, embryonal sarcoma (Lagacé et al. 1974; Blattner et al. 1977; Forster and Berman 1977; Cornut-Sipido 1979; Cozzutto et al. 1981), primary mesenchymoma (Lorimer 1955), fibromyxosarcoma, primary malignant mesenchymal tumors, or simply sarcoma (Anderson 1951; Keeling 1971; Stanley et al. 1973). Prior to the 31 cases described by Stocker and Ishak (1978), the largest series of this lesion was that by Foster and Berman (1977) with 12 cases. The term, undifferentiated sarcomas of the liver, had however previously been employed to denote a primary hepatic sarcoma in an adult patient (Esposito et al. 1977). Within few years after the first description, several further cases have been reported (Abramowsky et al. 1980; Kinjo et al. 1980; Gallivan et al. 1983; Gonzalez-Crussi 1983; Weinberg and Finegold 1983; Parichatikanond and Parichatikanond 1985).
Epidemiology
UES is the third most common malignant tumor of the liver in the pediatric age group, accounting for about 9–15 % of childhood hepatic malignant neoplasms (Stocker and Ishak 1978; Weinberg and Finegold 1983). Among the 31 patients reported by Stocker and Ishak (1978), 51.6 % of the children were 6–10 years of age, followed by the groups 11–15 years (19.4 %), 0–5 years (16.1 %), 16–20 years (6.5 %), and >20 years (6.5 %). Four patients of this series were not in the pediatric age group and were 19, 20, 22, and 28 years old at diagnosis. In some reports, there was a male preponderance, but in a larger study of 20 patients, the male to female ratio was 1:1 (Nicol et al. 2007). As already specified in a report of 31 cases (Stocker and Ishak 1978), the majority of patients present between 6 and 10 years of age, i.e., clearly later than patients with hepatoblastoma. The neoplasm is not related to preexisting liver disease (literature reviews: Aghajanzadeh et al. 2003; Sakellaridis et al. 2006; Iqbal et al. 2008; Pachera et al. 2008; Mohapatra and Krisnanand 2007; Boybeyi et al. 2009; Pathirana et al. 2010).
Albeit typically a tumor of older children, there are numerous reports documenting UES in adult patients. Adult primary UES is a rare disease that, similar to cases in the pediatric age group, develops in livers without preexisting disease, in particular without liver cirrhosis.
Selected References
Stocker and Ishak 1978; Chang et al. 1983; Miettinen and Kahlos 1989; Kanamaru et al. 1991; Kchir et al. 1991; Martins et al. 1992; McFadden et al. 1992; Reichel et al. 1994; Zaheer et al. 1994; Johnson et al. 1995; Mistry et al. 1995; Grazi et al. 1996; Houry et al. 1998; Tokunaga et al. 2000; Yedibela et al. 2000; Diedhiou et al. 2002; Shufaro et al. 2002; Nishio et al. 2003; Psatha et al. 2004; Alagiozian-Angelova et al. 2005; Dai et al. 2005; Agaram et al. 2006; Scudiere and Jakate 2006; McCarthy et al. 2007; Gourgiotis et al. 2008; Lenze et al. 2008; Faraj et al. 2010; Jiménez-Fuertes et al. 2008; Ma et al. 2008; Pachera et al. 2008; Kullar et al. 2009; Yang et al. 2009; Kaur et al. 2010; Lee et al. 2010; Li et al. 2010; Massani et al. 2010; Yoon et al. 2010; Xu et al. 2010; Gasljevic et al. 2011; Kalra et al. 2011; Lightfoot and Nikfarjam 2012; Varol et al. 2012; Chen et al. 2013; Lin et al. 2013
Clinical Features
The patients commonly present with an abdominal mass and upper abdominal pain. Among 31 patients, 12 had an abdominal mass only, 10 abdominal pain only, and 6 abdominal mass and pain (Stocker and Ishak 1978; Wei et al. 2008). Abdominal discomfort and pain were experienced for periods of 3 days–1 month. Asymptomatic abdominal masses had been noted for periods of 2 weeks–6 months. Jaundice and systemic signs such as fever, malaise, lethargy, and weight loss may occur, and sometimes the patient is referred to the hospital in a severely ill state, e.g., owing to tumor rupture with abdominal crisis (Weinberg and Finegold 1983; Lack et al. 1991). In UES, serum AFP is in the normal range (Stocker and Ishak 1978; Kanamaru et al. 1991; Lack et al. 1991; Wei et al. 2008), but lectin-reactive AFP (AFP-L3) may be elevated in a minority of patients (Okuda et al. 2005). UES tends to invasion and tissue destruction, including extension of the neoplasm along the inferior vena cava to the heart (Gallivan et al. 1983; Bassly et al. 2008). Bronchobiliary fistula has been observed in a child suffering from UES (Corapçioglu et al. 2004). In adult patients, the frequently cystic tumor may mimic hepatic hydatid disease (Faraj et al. 2010; Yoon et al. 2010; Kalra et al. 2011). In one 46-year-old female patient, UES was associated with systemic lupus erythematosus (Jia et al. 2013). In one adult case, UES radiologically mimicked Klatskin tumor (Lee et al. 2010). Complications of UES comprise hemorrhage (Suarez et al. 2000; Küpeli et al. 2008),spontaneous rupture followed by hematoperitoneum and eventually hemorrhagic shock (Stocker and Ishak 1978; Lack et al. 1991; Yedibela et al. 2000; Uchiyama et al. 2001; Hung et al. 2007; Yu et al. 2009), or rupture following abdominal trauma (Ida et al. 2009). Exceptionally, UES is associated with paraneoplastic syndromes or disorders. In a single reported patient, UES was associated with peripheral eosinophilia (Zaheer et al. 1994), and another adult patient revealed an erythropoietin-secreting tumor (Lin et al. 2013). One patient experienced fever of unknown origin, secretory diarrhea, refractory long QT syndrome, and torsades de pointes, resolved following surgical resection (Fricchione et al. 2013).
Imaging Features
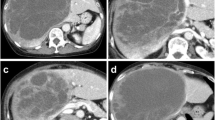
The imaging features of UES have been studied in detail, also with respect to radiologic-pathologic correlations and treatment response. Ultrasonography reveals large masses which are hyperechoic or isoechoic. Predominantly solid variants with many small anechoic spaces may be seen (Ros et al. 1986). On the other hand, a true cystic change of the tumor has already been described for one patient in the original series of 31 cases as of 1978, whereas in more than half of the cases of this series, there were multiple cystic areas containing necrotic debris, clotted blood, or gelatinous material (Stocker and Ishak 1978). Overall, CT usually shows large solid low-attenuating masses with cystic areas, with varying degrees of enhancement of the solid component and formation of hyperdense septa of variable thickness and a dense peripheral rim. Irregular high-density lesions exhibiting hemorrhage may occur. Cystic UES may show multiple internal septations (Ros et al. 1986; Joshi et al. 1997). The cystic changes may vary considerably both in size and shape and may cause a pitfall diagnosis in endemic hydatidosis areas (Joshi et al. 1997; Aggarwal et al. 2001; Charfi et al. 2008; Faraj et al. 2010). Large cysts in UES may also mimic complicated hepatic cysts at imaging (Tsukada et al. 2010). The cystic changes in UES may be the result of liquefied hemorrhage and/or necrosis, because hemorrhagic fluid in the cyst cavities suggested that cystic “degeneration” was related to hemorrhage. However, the finding that the inner wall of the cysts in UES may be rather smooth led to the view that the cysts develop spontaneously (Dai et al. 2005). At MRI, T1-weighted images reveal well-defined hypointense masses, sometimes with scattered high signal intensities, while T2-weighted images show high signal intensity in most of the tumor volumes. MRI clearly displays the cystic space of the tumors, the septations, and often substantial central necrosis (Marti-Bonmati et al. 1993; Buetow et al. 1997; Woong et al. 1997; Yoon et al. 1997; Psatha et al. 2004). Angiographically, UESs are usually hypovascular tumors, although hypervascular areas may also occur (Stocker and Ishak 1978; Ros et al. 1986).
Selected References
Ros et al. 1986; Marti-Bonmati et al. 1993; Urban et al. 1993; Moon et al. 1994, 1995; Sano et al. 1995; Buetow et al. 1997; Yoon et al. 1997; Helmberger et al. 1999; Chuang et al. 2002; Psatha et al. 2004; Goncalves-Matoso et al. 2005; Kim et al. 2008; Iqbal et al. 2008; Yu et al. 2008; Zhao et al. 2008; Crider et al. 2009; Lashkari et al. 2009; Yang et al. 2009; Kowalczyk and Carr 2010; Sodhi et al. 2010
Pathology
Macroscopy
Similar to other malignant hepatic tumors, UES develops more frequently in the right liver lobe (right lobe localization in 11/16 patients in one study, with contiguous involvement of the left lobe in three; Lack et al. 1991). UESs are usually large or even huge tumors that present as solitary and mostly well-delineated masses with variable areas of hemorrhage, confluent necrosis and sometimes macrocytic changes, resulting in a highly variegated cut surface. UES often grows to a very impressive size. In the series of Stocker and Ishak, tumor size was assessed in 20 cases, among which four were less than 10 cm in diameter, 10 were between 10 and 20 cm, and 6 exceeded 20 cm in diameter, the largest measuring 30 cm. The weight of the tumors varied from 90 to 3,375 g (average of 16 tumors, 1,310 g). In a study of 28 cases, the mean transverse diameter of the tumors was 14 cm (range, 10–25 cm). In a study of 16 patients, the average tumor diameter was 21 cm (range, 10–35 cm; Lack et al. 1991). The masses were predominantly solid (83 % of tumor volume), and pathologic and sonography findings were concordant (Buetow et al. 1997). The solid variants are globular in shape and are usually well demarcated from the rest of the liver. Typically, the tumors are nonencapsulated. Some tumors may show a pseudocapsule, but this impression is usually produced by compression and atrophy of adjacent liver substance.
The neoplasms are soft or fluctuant, with a glistening or variegated cut surface, colors ranging from gray-reddish to yellowish or even tan. Hemorrhage and necrosis may comprise up to 80 % of the tumor (Stocker and Ishak 1978; Ros et al. 1986; Leuschner et al. 1990; Lack et al. 1991; Parham et al. 1991; Kadomatsu et al. 1992; Walker et al. 1992; Buetow et al. 1997). The issue of macrocytic changes in UES has already been addressed above. In a minority of cases, “true” cystic cavities containing a mucoid fluid are in fact encountered. Mucoid or gelatinous areas are also observed. Together with necrosis cavities and spaces containing liquid or clotted blood, these can produce multiple cyst-like areas, seen in more than half of the cases in the series of 31 cases described by Stocker and Ishak (1978), but such lesions should not be confounded with the much rarer “true” cysts. Smaller cysts often contain a brownish mucoid fluid. UES may present as a single (solitary, unilocular) cyst of the liver (Chowdhary et al. 2004; Kim et al. 2009; Massani et al. 2010). Intravascularly growing tumors show a glistening, transparent quality with bosselations. Pedunculated tumors are very uncommon lesions, in contrast to mesenchymal hamartoma (Stanley et al. 1973; Stocker and Ishak 1978). In the case of Stanley and coworkers (1973), a 10-year-old boy had a partially cystic and solid pedunculated mass (17 cm diameter) that was attached to the posterior inferior surface of the left liver lobe.
Histopathology
The histologic features of UES have been described in detail (Stocker and Ishak 1978; O’Sullivan et al. 2001; Bliuznikov et al. 2007). The tumors are often surrounded by a compressed fibrous pseudocapsule of varying thickness, with atrophy of the adjacent liver parenchyma. The interior of the masses is composed by various types of tissue components (Table 1).
The bulk of the tumor consists of a loose but cellular tissue consisting of immature-looking or undifferentiated mesenchymal cells. These appear in several types, dominated by stellate and spindle cells, and followed in frequency by giant and polymorphic cells and by round cells. Most of these cells display very indistinct cell borders, sometimes suggesting the formation of syncytia. These types of cell seem to reflect various pathways of differentiation (Aoyama et al. 1991). Most of the stellate and spindle cells are scattered loosely in a matrix rich in Alcian blue-positive mucopolysaccharides, but part of these cells may also form fascicles or whorls or are packed in sheets. Such cases resemble malignant fibrous histiocytoma or fibrosarcoma (Keating and Taylor 1985), or hemangiopericytoma/solitary fibrous tumor. Spindle cells with myoid differentiation occur (Walker et al. 1992) (Figs. 1, 2, 3, 4, 5, 6, 7, and 8).
In a minority of cases, a contribution of anaplastic or small cells is seen (Walker et al. 1992). It was suggested that an anaplastic component is more commonly encountered in UES arising in adults (Nishio et al. 2003). Osteoid formation in UES has been described (Lack et al. 1991). This abnormal mesenchyme of UES often surrounds abnormal and dilated intratumoral, CK 19-positive bile duct-like profiles, and the cellularity of the tissue may be higher close to the epithelial lining, forming a “cambium.” This predominant component of UES shows high mitotic activity. The giant cells are either multinucleated or contain highly polyploidy and in part bizarre nuclei, with segregation of micronuclei, formation of nuclear bridges, and complex lobulations. Part of the large cells or giant cells contain few to numerous intracytoplasmic eosinophilic globules, measuring 2–40 μm in diameter, and sometimes hiding the nucleus. These globules are variably PAS-positive, but resistant to diastase digestion. In the Masson trichrome stain, they are blue to red, purple with PTAH, and positive with the Danielli staining method (Stocker and Ishak 1978). Globule-containing cells release the globules into the extracellular space upon cell death. The tumor tissue contains a fine network of reticulin fibers. Focally, dense bundles of partly hyalinized collagen are in evidence. Mainly in regions of necrosis, an infiltrate composed of lymphocytes, macrophages, and plasma cells is often present. UES has been diagnosed by the use of fine-needle aspiration cytology (Pieterse et al. 1985; Sola-Pérez et al. 1995; Krishnamurthy et al. 1996; Garcia-Bonafé et al. 1997; Allen et al. 1998; Pollono and Drut 1998; Sharifah et al. 1999; Anavi et al. 2001; Gupta et al. 2010; Kaur et al. 2010). Metastases of UES usually show the same histopathologic presentation as the primary lesion, but there are deviations from this presentation. Peritoneal metastases of UES in an adult patient were found to present as leiomyosarcomatous nodules (Fievez et al. 1983). This phenomenon may be linked to the fact that UES has been shown to undergo smooth muscle cell differentiation (Nishio et al. 2003), and such a cell lineage may then also evolve in metastatic disease.
A distinct feature of some UES is the focal differentiation along myoid (leiomyoid and/or rhabdomyoid) lineages or along an adipocyte-like lineage. Ultrastructural studies suggested a leiomyoblastic differentiation (Gonzalez-Crussi 1983), and features suggesting a leiomyoid cell lineage have been observed by previous investigators as well (Lagacé et al. 1974; Cozzutto et al. 1981 9) and supported by more recent findings (Walker et al. 1992; Nishio et al. 2003). A differentiation along a lipoblastoid lineage has chiefly been suggested based on the presence of oil red O staining (Stocker and Ishak 1978) and ultrastructural presence of lipid droplets in subsets of tumor cells (Cozzutto et al. 1981; Chang et al. 1983; Ellis and Cotton 1983; Gallivan et al. 1983; Parham et al. 1991). Lipid droplets in UES cells were detected in six out of seven neoplasms in one analysis (Parham et al. 1991). Morphologically, typical lipoblasts in UES have also been described (Lagacé et al. 1974). Whether this phenotype reflects liposarcomatous features of some, UES is not ascertained. In rare instances, aberrant differentiation patterns can occur, e.g., chondroid differentiation with formation of chondrosarcoma-like lesions (Kinjo et al. 2010), production of tumor osteoid (Chen et al. 2013), or lesions resembling telangiectatic hepatic adenoma (Tanaka et al. 2012). In one adult patient, UES arising from mesenchymal hamartoma exhibited peripheral angiosarcomatous differentiation (Tucker et al. 2012).
At the interface between the tumor and the liver, trapped and damaged hepatocytes, and abnormal and in part cystic bile ducts are seen (Figs. 9, 10, 11, and 12; Stanley et al. 1973; Stocker and Ishak 1978; Walker et al. 1992). The duct-like structures are sometimes markedly dilated and deformed; they may contain an eosinophilic material, but bile is consistently lacking. Only rarely were parenchymal cells seen deeper than 0.5–1.0 cm from the edge of the tumor (Stocker and Ishak 1978). The dilated bile duct-like structures are separated from the tumor tissue by a thin, PAS-positive lamella which may represent a basement membrane, but focally this lamella is broached by sarcoma cells. The cholangiocyte lining is typically not atrophic, the cells being cuboid instead of flat, but some cells show signs of damage, with irregular arrangement of the cell within the single layer and hyperchromatic nuclei as a frequent feature (Stanley et al. 1973). As the sarcoma tissue directly encroaches upon these ducts, the absence of atrophy is striking. The marked cystic dilatation also requires the production of more cholangiocytes to cover this large surface. In a thin zone immediately surrounding the ducts, round tumor cells are sometimes more frequent, a phenomenon also depicted in Figs. 11 and 12 of the Stanley et al. publication (1973). Stocker and Ishak (1978) noted that there are tumor areas where the epithelial cells seem to be separated from one another and blended with the sarcomatous cells. This phenomenon may be striking and may either represent and transient contact between malignant mesenchymal and nonneoplastic epithelial cells or eventually epithelial-mesenchymal transition. Whether these bile duct-like profiles are really entrapped bile ducts, as suggested by several authors (Stanley et al. 1973; Stocker and Ishak 1978), or represent neoplastic elements themselves has not been clarified (see below). An argument for entrapped bile ducts is their concentration in the area of the tumor closest to the interface between the involved liver and the tumor mass. Sections from central parts revealed only an occasional duct (Stanley et al. 1973).
Hepatocytes located within the tumor occur as single cells, small clusters, or even cell plates resembling those in the normal liver. The cells can show signs of damage or regeneration, and sometimes the hepatocytes are in direct contact with bile duct cells, suggesting differentiation of cholangiocytes from hepatocytes or their precursors.
The gelatinous center of the tumor may contain thrombosed vessels, sometimes with organization of the thrombi. Autopsy observations specifying the histology of metastases are available from the study of Stocker and Ishak (1978). All extrahepatic tumor manifestations contained sarcomatous cells, some of which had the typical PAS-positive globules. In one case, epithelial elements (bile duct-like structures) were found in the tumor adjacent to the stomach and bowel but in continuity with the hepatic lesion. But another case showed epithelium-lined structures in metastatic lesions in the lung. Stocker and Ishak (1978) argued that the epithelium-lined structures found in a tumor adjacent to the stomach and diaphragm, though still in continuity with the primary liver tumor, represent entrapped bile ducts carried along with the tumor mass as it extended beyond the boundaries of the liver. Furthermore, the epithelial structures these authors found in pulmonary metastases were interpreted as entrapped bronchioles. Hepatocytes (solitary cells, clusters, or even cords/plates) are sometimes found in UES. Stocker and Ishak (1978) observed parenchymal cells within the tumor’s pseudocapsule and at the periphery of the neoplasm, but only rarely were hepatocytes found deeper than 0.5–1.0 cm from the edge of the tumor. Such intratumoral hepatocytes have been interpreted as entrapped parenchymal cells, because of their position near the tumor margin (Walker et al. 1992; their patient 2). However, we have found that morphologically abnormal hepatoid cells also occur in central parts of large tumors, these cells not showing signs of damage or atrophy caused by entrapment.
Electron Microscopy Findings
Relatively few UESs have been examined ultrastructurally (Lagacé et al. 1974; Cozzutto et al. 1981; Chang et al. 1983; Keating and Taylor 1985; Pieterse et al. 1985; Chou et al. 1990; Parham et al. 1991; Agaram et al. 2006). At electron microscopy, spindle and stellate cells including both single and multinucleated forms are embedded in a loose collagenous stroma. Neighboring cells may be connected by poorly formed desmosome-like junctions. The cytoplasm of the tumor cells contains relatively few mitochondria, but is rich in free ribosomes and RER profiles. The lumens of the RER may be dilated and contain an amorphous material. Golgi apparatus is rare and not prominent. The presence of tonofilament-like bundles of intermediate filaments and cell junctions in some cases has suggested epithelial differentiation of a subset of tumor cells (Miettinen and Kahlos 1989). Some of the cells contain lipid droplets. In some cases, a myoid differentiation (“leiomyoblastic” cells) has been detected (Gallivan et al. 1983; Gonzalez-Crussi 1983), rarely with signs of rhabdomyoblastic differentiation (Vetter et al. 1989).
Immunohistochemistry
UES usually displays a complex immunohistochemical pattern, with many markers being positive in a variable pattern (Parham et al. 1991; Table 2).
The mesenchymal tumor cells are consistently reactive for vimentin (Lack et al. 1991; Diedhiou et al. 2002; Kiani et al. 2006; Zheng et al. 2007; Wei et al. 2008; Shehata et al. 2011). In one study, all tumors were vimentin positive, and part of the cases were reactive for Bcl-2 (Kiani et al. 2006), cytokeratin AE1/AE3, CD10, calponin, p53, and exceptionally desmin (Kiani et al. 2006), illustrating the polyantigenic features of this immature neoplasm. Focal cytokeratin expression has been documented in some reports (Chou et al. 1990; Lack et al. 1991), sometimes with formation of a paranuclear dot-like staining pattern (Pérez-Gomez et al. 2010). Part of the tumor cells is reactive for alpha-1-antitrypsin (Abramowsky et al. 1980; Miettinen and Kahlos 1989; Shehata et al. 2011). In particular, the typical PAS-positive intracellular globules were shown to contain alpha-1-antitrypsin, but these structures are ultrastructurally different from the inclusions found in hepatocytes of some forms of alpha-1-antitrypsin deficiency (Abramowsky et al. 1980). Diffuse membranous immunostaining of tumor cells for CD56 has been found (Pérez-Gomez et al. 2010). Rarely, clusters of spindle cells are positive for smooth muscle actin and desmin, suggesting muscle cell differentiation (Diedhiou et al. 2002; Nishio et al. 2003). In contrast, myogenin and myogenic regulatory protein D1 (MyoD1) were uniformly negative in UES (Nicol et al. 2007). The tumor cells do not express epithelial membrane antigen (EMA; Miettinen and Kahlos 1989), and nuclear accumulation of beta-catenin was not detected in UES (Yamaoka et al. 2006). UES, similar to mesenchymal hamartoma, shows cytoplasmic expression of glypican-3 (Levy et al. 2012). A part of the cells appear to share features with hepatic stellate cells, e.g., positivity for alpha-SMA and adipophilin-positive vesicles (Tanaka et al. 2012).
Cytogenetic Features
DNA ploidy studies revealed highly aneuploidy patterns as a sign of marked genomic instability (Chou et al. 1990; Leuschner et al. 1990). A DNA ploidy study demonstrated a single G0/G1 peak indicating a diploid DNA content, and only one tumor showed a hypodiploid DNA content (Leuschner et al. 1990). Another investigation found an aneuploidy cellular component (Chou et al. 1990). UES exhibits complex cytogenetic alterations. Both gains and losses of components of several chromosomes were detected, indicating extensive chromosomal rearrangements in UES (Iliszko et al. 1998). In one case, near-triploid and near-hexaploid clones with several chromosomal rearrangements were detected (Iliszko et al. 1998); this polyploidization probably reflected in the bizarre giant nuclei typically seen in this tumor. Aneuploidy of the cell population, usually with high S phase, is a typical feature of UES (Chou et al. 1990). Comparative genomic hybridization revealed multiple chromosomal amplifications and deletions in UES, including gains of 1q, 5p, 6q, 8p, and 12q and losses of 9p, 11p, and 14 (Sowery et al. 2001). UES is associated with a recurrent translocation t(11;19)(q11;q13.3–q13.4) or add(19)(q13.4) (see below).
Differential Diagnosis
As the clinical, radiological, and histologic presentations are highly characteristic and the tumor predominantly occurs in children beyond the hepatoblastoma age, this neoplasm can hardly be confounded with other tumors.
Biology of UES
UESs are aggressive tumors that so far implied a dismal outcome. However, new combined surgical and chemotherapy treatments have improved prognosis (Harris et al. 1984; Horowitz et al. 1987; Perilongo et al. 1987; Ware et al. 1988; Steiner et al. 1989; Orozco et al. 1991; Walker et al. 1992; Urban et al. 1993; Douglass 1997; Webber et al. 1999; Bisogno et al. 2002; Kim et al. 2002; Almogy et al. 2004, 2005; Baron et al. 2007; Kim et al. 2007; McCarthy et al. 2007; Pachera et al. 2008; May et al. 2012; Gao et al. 2013; Geel et al. 2013; Ismail et al. 2013; Plant et al. 2013). In a study of 17 patients enrolled by the Italian and German Soft Tissue Sarcoma Cooperative Groups and treated by surgery at diagnosis followed by multi-agent chemotherapy and second-look surgery in case of residual disease, 12 patients were alive with follow-up ranging from 2.4 to 20 years (Bisogno et al. 2002). Liver transplantation has been successfully performed in pediatric patients with UES (Kelly et al. 2009; Okajima et al. 2009; Plant et al. 2013; Walther et al. 2013).
Relation to Mesenchymal Hamartoma of the Liver and to Other Tumors
In few cases, UES seems to have arisen from preexisting hepatic mesenchymal hamartoma (see below; de Chadarévian et al. 1994; Lauwers et al. 1997; Begueret et al. 2001; O’Sullivan et al. 2001; Rajaram et al. 2007; Lahmar-Boufaroua et al. 2008; Shehata et al. 2011). In the case of a 15-year-old girl, mapping of the tumor demonstrated a typical mesenchymal hamartoma transforming gradually into UES. Cytometrically, the hamartoma component was diploid, while the UES component showed a prominent aneuploid peak. Karyotypic analysis revealed structural alterations of chromosome 19 (Lauwers et al. 1997). More than ten cases have been reviewed from the literature, and in part of them, transition zones between mesenchymal hamartoma and UES have been identified (Shehata et al. 2011). UES has been found to be associated with tumors other than hepatic mesenchymal hamartoma. A teenage girl with UES developed metachronous vaginal rhabdomyosarcoma, and a middle-aged woman developed metachronous B-acute lymphoblastic leukemia (Gasljevic et al. 2011).
Pathogenesis
A pathogenic or histogenetic link between UES and mesenchymal hamartoma of the liver has been suggested as early as 1978 (Stocker and Ishak 1978), but has been challenged previously or later (Stanley et al. 1973; Dehner et al. 1975; Donnelly et al. 1978; Cozzutto et al. 1981; Gallivan et al. 1983; Finegold 1986; Lack 1986; Chou et al. 1990; Aoyama et al. 1991). However, there are in fact few observations suggesting that UES may develop within mesenchymal hamartoma of the liver (de Chadarévian et al. 1994; Lauwers et al. 1997; Ramanujam et al. 1999; Begueret et al. 2001). In a 15-year-old female patient, mapping of the hepatic tumor demonstrated an atypical mesenchymal hamartoma transforming gradually into UES composed on anaplastic stromal cells. Flow cytometry revealed that the cells of the mesenchymal hamartoma were diploid, while UES cells showed a prominent aneuploidy peak, and cytogenetic analysis of UES exhibited structural alterations (translocation) of chromosome 19q13.4 (Lauwers et al. 1997), implicated as a potential marker of mesenchymal hamartoma (Speleman et al. 1989; Mascarello and Krous 1992; Bove et al. 1998; Rakheja et al. 2004). Based on these constellations, a pathogenetic link between these two tumors was suggested (Lauwers et al. 1997). Mesenchymal hamartoma of the liver is associated with a distinct chromosomal translocation, i.e., t(11;19q)(q11;q13.4) (Mascarello and Krous 1992; Bove et al. 1998; Hu et al. 2012), and this abnormality is thought to play a role in the pathogenesis of both this benign tumor and UES which may develop from it. Analysis of the translocation breakpoints showed that, in one case, the breakpoint occurred in the MALAT1 gene, and the breakpoint was termed MHLB1, for mesenchymal hamartoma of the liver breakpoint 1 (Rajaram et al. 2007).
MALAT1 (metastasis-associated lung adenocarcinoma transcript 1 ; NEAT2, noncoding nuclear-enriched abundant transcript 2; gene located on chromosome 11q13.1) is a long noncoding RNA species which is highly expressed in nuclei (Hutchinson et al. 2007) and whose expression correlates with the development and invasive behavior of several tumors (Ji et al. 2003; Diederichs 2010; Tani et al. 2010; Tano et al. 2010; Lin et al. 2011; Xu et al. 2011). In hepatocellular carcinoma, MALAT1 overexpression predicts tumor recurrence (Lai et al. 2012). MALAT1-associated small cytoplasmic RNA (also termed mascRNA) is roughly 53–61 nucleotides in length and is processed from the much longer noncoding (nc) RNA, MALAT1, by the enzyme RNAase P. MALAT1 regulates alternative splicing by controlling the activity of the SR protein family or splicing factors via phosphorylation (Tripathi et al. 2010; Ankö and Neugebauer 2010). Alternative splicing of pre-mRNA is employed to achieve increased transcriptome and proteome complexity. Alternative splicing utilizes specific serine/arginine (SR) splicing factors, which act in a cell-specific phosphorylation-dependent manner. SRs and several SR-related proteins interact with and process mRNA precursors and thus are master regulators of gene expression (reviews: Blencowe et al. 1999; Long and Caceres 2009). MALAT1 is localized to nuclear speckles and does not shuttle between the nucleus and cytoplasm, the localization to speckles being regulated by RNPS1, SRm160, and IBP160 (Miyagawa et al. 2012).
There is evidence that changes in p53 are involved in UES (Chuang et al. 2002; Lepreux et al. 2005; Sangkhathat et al. 2006). At least part of UES shows nuclear immunohistochemical reactivity for p53 protein (Chuang et al. 2002). Mutation analysis of UES revealed missense mutations of TP53 (Kusafuka et al. 1997; Hu et al. 2012). In one study of UES in adults, immunohistochemistry showed overexpression of p53 protein in more than 80 % of tumor cells, and mutations of the TP53 gene were observed in two cases, involving the sequence-specific DNA-binding domain (Lepreux et al. 2005). Missense mutations of TP53 were found in three tumors of another study, and the mutations were found specifically in tumor tissue and not detected in the surrounding normal hepatic tissue and were associated with strong p53 immunoreactivity. Mutation points were localized in exon 7 (Gly245Ser), exon 6 (Arg196Pro), and exon 8 (Arg273Pro) (Sangkhathat et al. 2006).
References
Abramowsky CR, Cebelin M, Choudhury A, Izant RJ (1980) Undifferentiated (embryonal) sarcoma of the liver with alpha-1-antitrypsin deposits: immunohistochemical and ultrastructural studies. Cancer 45:3108–3113
Agaram NP, Baren A, Antonescu CR (2006) Pediatric and adult hepatic embryonal sarcoma: a comparative ultrastructural study with morphologic correlations. Ultrastruct Pathol 30:403–408
Aggarwal S, Gularia S, Dinda AK, Kumar L, Tarique S (2001) Embryonal sarcoma of the liver mimicking a hydatid cyst in an adult. Trop Gastroenterol 22:141–142
Aghajanzadeh M, Riazi H, Kohsari MR, Jafroodi M, Hoda S, Ashtiani MN (2003) Undifferentiated (embryonal) sarcoma of the liver: case report and review of the literature. Saudi J Gastroenterol 9:139–141
Alagiozian-Angelova V, Jennings L, Jani J, Weisenberg E (2005) Arch Pathol Lab Med 129:947–949
Allen EA, Clark DP, Ali SZ (1998) Primary hepatic undifferentiated embryonal sarcoma: cytopathologic findings in peritoneal washings. Acta Cytol 42:449–451
Almogy G, Lieberman S, Gips M, Pappo O, Edden Y, Jurim O, Simon SB, Uzieli B, Eid A (2004) Clinical outcomes of surgical resections for primary liver sarcoma in adults: results from a single centre. Eur J Surg Oncol 30:421–427
Almogy G, Pappo O, Gips M, Lieberman S, Edden Y, Eid A (2005) Improved survival with surgery and systemic chemotherapy for undifferentiated embryonal sarcoma of the liver. Isr Med Assoc J 7:672–673
Anavi BL, Moumdjiev IN, Stoyanova AA, Sapunarova KG, Tashev PV, Dureva-Popova MK (2001) A case of undifferentiated (embryonal) sarcoma of the liver: fine needle aspiration cytology diagnosis by one cell type. Folia Med (Plovdiv) 43:53–58
Anderson DH (1951) Tumors of infancy and childhood: I. A survey of those seen in the pathology laboratory of the babies hospital during the years 1935–1950. Cancer 4:890–906
Ankö ML, Neugebauer KM (2010) Long noncoding RNAs add another layer to pre-mRNA splicing regulation. Mol Cell 39:833–834
Aoyama C, Hachitanda Y, Sato JK, Said JW, Shimada H (1991) Undifferentiated (embryonal) sarcoma of the liver. A tumor of uncertain histogenesis showing divergent differentiation. Am J Surg Pathol 15:615–624
Baron PW, Majlessipour F, Bedros AA, Zuppan CW, Ben-Youssef R, Yanni G, Ojogho ON, Concepcion W (2007) Undifferentiated embryonal sarcoma of the liver successfully treated with chemotherapy and liver resection. J Gastrointest Surg 11:73–75
Bassly S, Wang L, Han B, Jubinsky PT (2008) Undifferentiated hepatic sarcoma with cardiac extension. Pediatr Blood Cancer 50:732
Begueret H, Trouette H, Vielh P, Laurent C, MacGrogan G, Delsol M, Belleannee G, Masson B, De Mascarel A (2001) Hepatic undifferentiated embryonal sarcoma: malignant evolution of mesenchymal hamartoma? Study of one case with immunohistochemical and flow cytometric emphasis. J Hepatol 34:178–179
Bisogno G, Pilz T, Perilongo G, Ferrari A, Harms D, Ninfo V, Treuner J, Carli M (2002) Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer 94:252–257
Blattner WA, Henson DE, Young RC, Fraumeni JF (1977) Malignant mesenchymoma and birth defects. Prenatal exposure to phenytoin. JAMA 238:334–335
Blencowe BJ, Bowman JA, McCracken S, Rosonina E (1999) SR-related proteins and the processing of messenger RNA precursors. Biochem Cell Biol 77:277–291
Bliuznikov OP, Shabanov MA, Perevozchikov AG (2007) Embryonic hepatic sarcoma in children: aspects of morphological diagnosis (in Russian). Arkh Patol 69:40–47
Bove KE, Blough TI, Soukup S (1998) Third report of t(19q)(13.4) in mesenchymal hamartoma of liver with comments on link to embryonal sarcoma. Pediatr Dev Pathol 1:438–442
Boybeyi O, Karnak I, Orhan D, Akçören Z, Tanyel FC (2009) Undifferentiated embryonal sarcoma of the liver: an intriguing diagnosis in a child. Eur J Pediatr Surg 19:328–330
Buetow PC, Buck JL, Pantongrag-Brown L, Marshall WH, Ros PR, Levine MS, Goodman ZD (1997) Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology 203:779–783
Chang WWL, Farooq PA, Winfield SM (1983) Primary sarcoma of the liver in the adult. Cancer 51:1510–1517
Charfi S, Ayadi L, Toumi N, Frikha F, Daoud E, Makni S, Frikha M, Beyrouti MI, Sellami-Boudawara T (2008) Cystic undifferentiated sarcoma of the liver in children: a pitfall diagnosis in endemic hydatidosis areas. J Pediatr Surg 43:E1–E4
Chen et al (2006) http://www.ncbi.nlm.nih.gov/pubmed/17033524
Chen JH, Lee CH, Wei CK, Chang SM, Yin WY (2013) Undifferentiated embryonal sarcoma of the liver with focal osteoid picture – a case report. Asian J Surg 36:174–178
Chou P, Mangkornkanok M, Gonzalez-Crussi F (1990) Undifferentiated (embryonal) sarcoma of the liver: ultrastructure, immunohistochemistry, and DNA ploidy analysis of two cases. Pediatr Pathol 10:549–562
Chowdhary SK, Trehan A, Das A, Marwaha RK, Rao KL (2004) Undifferentiated embryonal sarcoma in children: beware of the solitary liver cyst. J Pediatr Surg 39:E9–E12
Chuang WY, Lin JN, Hung IJ, Hsueh C (2002) Undifferentiated sarcoma of the liver. Chang Gung Med J 25:399–404
Corapçioglu F, Sarper N, Demir H, Güvenç BH, Sözübir S, Akansel G, Berk FS (2004) A child with undifferentiated sarcoma of the liver complicated with bronchobiliary fistula and detected by hepatobiliary scintigraphy. Pediatr Hematol Oncol 21:427–433
Cornut-Sipido P (1979) Généralisation d’un mesenchymome hépatique à predominance liposarcomateuse chez un jeune enfant. Ann Anat Pathol 24:65–72
Cozzutto C, De Bernardi B, Comelli A et al (1981) Malignant mesenchymoma of the liver in children: a clinicopathologic and ultrastructural study. Hum Pathol 12:481–485
Crider MH, Hoggard E, Manivel JC (2009) Undifferentiated (embryonal) sarcoma of the liver. Radiographics 29:1665–1668
Dai CL, Xu F, Shu H, Xu YQ, Huang Y (2005) Undifferentiated (embryonal) sarcoma of the liver in adult: a case report. World J Gastroenterol 11:926–929
de Chadarévian JP, Pawel BR, Faerber EN, Weintraub WH (1994) Undifferentiated (embryonal) sarcoma arising in conjunction with mesenchymal hamartoma of the liver. Mod Pathol 7:490–493
Dehner LP, Ewing SL, Sumner HW (1975) Infantile mesenchymal hamartoma of the liver. Arch Pathol 99:379–382
Diederichs S (2010) Non-coding RNA in malignant tumors. A new world of tumor biomarkers and target structures in cancer cells (in German). Pathologe 31(suppl 2):258–262
Diedhiou A, Genin P, Terris B, Sauvanet A, Belghiti J, Degott C (2002) Undifferentiated embryonal sarcoma of the liver in an adult: a case report (in French). Ann Pathol 22:134–136
Donnelly WH, Talbert JL, Miale T (1978) Malignant undifferentiated stromal tumor of the liver (mesenchymoma): an ultrastructural study. Lab Invest 38:385–386
Donovan EJ, Santulli TV (1946) Resection of the left lobe of the liver for mesenchymoma. Report of a case. Ann Surg 124:90–93
Douglass EC (1997) Hepatic malignancies in childhood and adolescence (hepatoblastoma, hepatocellular carcinoma, and embryonal sarcoma). Cancer Treat Res 92:201–212
Ellis IO, Cotton RE (1983) Primary malignant mesenchymal tumor of the liver in an elderly female. Histopathology 7:113–121
Esposito R, Pollavini G, de Lalla F (1977) A case of primary undifferentiated sarcoma of the liver. Endoscopy 8:108–110
Faraj W, Mukherji D, El Majzoub N, Shamseddine A, Shamseddine A, Khalife M (2010) Primary undifferentiated embryonal sarcoma of the liver mistaken for hydatid disease. World J Surg Oncol 8:58
Fievez M, Jacques P, Guiot P, Golaire MC (1983) Malignant mesenchymatous hamartoma of the liver in adults. Apropos of a case (in French). Sem Hop 59:1625–1629
Finegold M (1986) Pathology of neoplasia in children and adolescents. W.B. Saunders, Philadelphia
Forster JH, Berman MM (1977) Solid liver tumors. W.B. Saunders, Philadelphia
Fricchione MJ, Glenn N, Follmer R, Kent PM (2013) Life-threatening paraneoplastic syndrome in a child with sarcoma of the liver cured by emergency resection. J Pediatr Hematol Oncol 35:153–155
Gallivan MV, Lack EE, Chun B, Ishak KG (1983) Undifferentiated (“embryonal”) sarcoma of the liver: ultrastructure of a case presenting as a primary intracardiac tumor. Pediatr Pathol 1:291–300
Gao J, Fei L, Li S, Cui K, Zhang J, Yu F, Zhang B (2013) Undifferentiated embryonal sarcoma of the liver in a child: a case report and review of the literature. Oncol Lett 5:739–742
Garcia-Bonafé M, Allende H, Fantova MJ, Tarragona J (1997) Fine needle aspiration cytology of undifferentiated (embryonal) sarcoma of the liver. A case report. Acta Cytol 41(suppl):1273–1278
Gasljevic G, Lamovec J, Jancar J (2011) Undifferentiated (embryonal) liver sarcoma: synchronous and metachronous occurrence with neoplasms other than mesenchymal liver hamartoma. Ann Diagn Pathol 15:250–256
Geel JA, Loveland JA, Pitcher GJ, Beale P, Kotzen J, Poole JE (2013) Management of undifferentiated embryonal sarcoma of the liver in children: a case series and management review. S Afr Med J 103:728–731
Goncalves-Matoso et al (2005) http://www.ncbi.nlm.nih.gov/pubmed/16198096
Gonzalez-Crussi F (1983) Undifferentiated (embryonal) liver sarcoma of childhood: evidence of leiomyoblastic differentiation. Pediatr Pathol 1:281–290
Gourgiotis S, Moustafellos P, Germanos S (2008) Undifferentiated embryonal sarcoma of the liver in adult. Surgery 143:568–569
Grazi GL, Gallucci A, Masetti M, Jovine E, Fiorentino M, Mazziotti A, Gozzetti G (1996) Surgical therapy for undifferentiated (embryonal) sarcomas of the liver in adults. Am Surg 62:901–906
Gupta C, Iyer VK, Kaushal S, Agarwala S, Mathur SR (2010) Fine needle aspiration cytology of undifferentiated embryonal sarcoma of the liver. Cytopathology 21:414–416
Harris MB, Shen S, Weiner MA, Bruckner H, Dasgupta I, Bleicher M, Fortner JG, Leleiko NS et al (1984) Treatment of primary undifferentiated sarcoma of the liver with surgery and chemotherapy. Cancer 54:2859–2862
Helmberger TK, Ros PR, Mergo PJ, Tomczak R, Reiser MF (1999) Pediatric liver neoplasms: a radiologic-pathologic correlation. Eur Radiol 9:1339–1347
Horowitz ME, Etcubanas E, Webber BL, Kun LE, Rao BN, Vogel RJ, Pratt CB (1987) Hepatic undifferentiated (embryonal) sarcoma and rhabdomyosarcoma in children. Results of therapy. Cancer 59:396–402
Houry S, Gharbi L, Huguier M, Callard P, André T (1998) Undifferentiated embryonal sarcoma in the liver of adults (in French). Presse Med 27:518–520
Hu X, Chen H, Jin M, Wang X, Lee J, Xu W, Zhang R, Li S, Niu J (2012) Molecular cytogenetic characterization of undifferentiated embryonal sarcoma of the liver: a case report and literature review. Mol Cytogenet 5:26
Hung TY, Lu D, Liu MC (2007) Undifferentiated (embryonal) sarcoma of the liver complicated with rupture in a child. J Pediatr Hematol Oncol 29:63–65
Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A (2007) A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8:39
Ida S, Okajima H, Hayashida S, Takeichi T, Asonuma K, Baba H, Inomata Y (2009) Undifferentiated sarcoma of the liver. Am J Surg 198:e7–e9
Iliszko M, Czauderna P, Babinska M, Stoba C, Roszkiewicz A, Limon J (1998) Cytogenetic findings in an embryonal sarcoma of the liver. Cancer Genet Cytogenet 102:142–144
Iqbal K, Xian ZM, Yuan C (2008) Undifferentiated liver sarcoma – rare entity: a case report and review of the literature. J Med 2:20, Case Reports
Ismail H, Dembrowska-Baginska B, Broniszczak D, Kalicinski P, Maruszewski P, Kluge P, Dwieszkowska E et al (2013) Treatment of undifferentiated embryonal sarcoma of the liver in children – single center experience. J Pediatr Surg 48:2202–2206
Ji P, Diedrichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H et al (2003) MALAT-1, a novel non-coding RNA, and thymosin beta4 predict metastasis an survival in early-stage non-small cell lung cancer. Oncogene 22:8031–8041
Jia C, Zhao W, Dai C, Wang X, Bu X, Peng S, Xu F, Xu Y, Zhao Y (2013) Undifferentiated embryonal sarcoma of the liver in a middle-aged adult with systemic lupus erythematosus. World J Surg Oncol 11:244
Jiménez-Fuertes M, Lopez AR, de Juan Burgueño M, Moya HA, Sanjuan RF et al (2008) Hepatic undifferentiated (embryonal) sarcoma in an adult: a case report and literature review (in Spanish). Gastroenterol Hepatol 31:12–17
Johnson JA, White JG, Thompson AR (1995) Undifferentiated (embryonal) sarcoma of the liver in adults. Am Surg 61:285–287
Joshi SW, Merchant NH, Jambhekar NA (1997) Primary multilocular cystic undifferentiated (embryonal) sarcoma of the liver in childhood resembling hydatid cyst of the liver. Br J Radiol 70:314–316
Kadomatsu K, Nakagawara A, Zaizen Y, Nagoshi M, Tsuneyoshi M, Fukushige T, Haigou A, Suita S (1992) Undifferentiated (embryonal) sarcoma of the liver: report of three cases. Surg Today 22:451–455
Kalra N, Vyas S, Jyoti DP, Kochhar R, Srinivasan R, Khandelwal N (2011) Undifferentiated embryonal sarcoma of the liver in an adult masquerading as complicated hydatid cyst. Ann Hepatol 10:81–83
Kanamaru R, Wakui A, Kambe M, Ouchi K, Kobari M, Matsuno S, Kimura N, Takahashi T et al (1991) Undifferentiated sarcoma of the liver in a 21-year-old woman: case report. Jpn J Clin Oncol 21:227–232
Kaur J, Dey P, Das A (2010) Fine needle aspiration cytology of undifferentiated (embryonal) sarcoma of liver in an adult male. Diagn Cytopathol 38:547–548
Kchir N, Chatti S, Limam R, Kacem M, Haouet S, Cherif R, Boubaker S, Zitouna MM (1991) Undifferentiated “embryonal” sarcoma of the liver. Apropos of a case with immunohistochemical study (in French). Arch Anat Cytol Pathol 39:162–165
Keating S, Taylor GP (1985) Undifferentiated (embryonal) sarcoma of the liver: ultrastructural and immunohistochemical similarities with malignant fibrous histiocytoma. Hum Pathol 16:693–699
Keeling JW (1971) Liver tumours in infancy and childhood. J Pathol 103:69–85
Kelly MJ, Martin L, Alonso M, Altura RA (2009) Liver transplant for relapsed undifferentiated embryonal sarcoma in a young child. J Pediatr Surg 44:e1–e3
Kiani B, Ferrell LD, Qualman S, Frankel WL (2006) Immunohistochemical analysis of embryonal sarcoma of the liver. Appl Immunohistochem Mol Morphol 14:193–197
Kim DY, Kim KH, Jung SE, Lee SC, Park KW, Kim WK (2002) Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg 37:1419–1423
Kim KT, Han SY, Park EH, Jang JS, Roh MH, Lee SW, Jeong JS (2007) A case of the treatment in an adult with hepatic undifferentiated (embryonal) sarcoma (in Korean). Korean J Hepatol 13:96–102
Kim M, Tireno B, Slanetz PJ (2008) Undifferentiated embryonal sarcoma of the liver. AJR Am J Roentgenol 190:W261–W262
Kim HS, Kim GY, Lim SJ, Lee SM, Kim YW (2009) Undifferentiated pleomorphic sarcoma of the liver presenting as a unilocular cyst. Hepatobiliary Pancreat Dis Int 8:541–543
Kinjo M, Shimokama T, Tanaka K, Enjoji M, Arima T, Ikeda K (1980) Undifferentiated (embryonal) sarcoma of the liver. Acta Pathol Jpn 30:459–464
Kinjo S, Sakaurai S, Hirato J, Sunose Y (2010) Embryonal sarcoma of the liver with chondroid differentiation. World J Gastrointest Oncol 2:247–250
Kowalczyk N, Carr Z (2010) Undifferentiated embryonal sarcoma: a case report. Radiol Technol 81:329–334
Krishnamurthy SC, Datta S, Jambhekar NA (1996) Fine needle aspiration cytology of undifferentiated (embryonal) sarcoma of the liver: a case report. Acta Cytol 40:567–570
Kullar P, Stonard C, Jamieson N, Huguet E, Praseedom R, Jah A (2009) Primary hepatic embryonal sarcoma masquerading as metastatic ovarian cancer. World J Surg Oncol 7:55
Küpeli S, Yalçin B, Cil BE, Akçören Z, Büyükpamukçu M (2008) Undifferentiated embryonal sarcoma of the liver in a child complicated by haemorrhage. Pediatr Radiol 38:1259–1261
Kusafuka T, Fukuzawa M, Oue T, Komoto Y, Yoneda A, Okada A (1997) Mutation analysis of p53 gene in childhood malignant solid tumors. J Pediatr Surg 32:1175–1180
Lack EE (1986) Mesenchymal hamartoma of the liver. A clinical and pathologic study of nine cases. Am J Pediatr Hematol Oncol 8:91–98
Lack EE, Schloo BL, Azumi N, Travis WD, Grier HE, Kozakewich HP (1991) Undifferentiated (embryonal) sarcoma of the liver. Clinical and pathologic study of 16 cases with emphasis on immunohistochemical features. Am J Surg Pathol 15:1–16
Lagacé R, Delage C, Robert J (1974) Le mésenchymome primitif du foie. Etude ultrastructurale. Ann Anat Pathol 19:275–286
Lahmar-Boufaroua A, Giutallier C, Yacoubi MT, Mania S, Mestiri H, Mzabi Regaya S (2008) Hepatic undifferentiated (embryonal) sarcoma arising in a mesenchymal hamartoma: association or malignant transformation (in French). Tunis Med 86:628–629
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY, Zhang F, Wu LM, Chen LM, Zheng SS (2012) Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol 29:1810–1816
Lashkari HP, Khan SU, Ali K, Sashikumar P, Mukherjee S (2009) Diagnosis of undifferentiated embryonal sarcoma of the liver – importance of combined studies of ultrasound and CT scan. J Pediatr Hematol Oncol 31:797–798
Lauwers GY, Grant LD, Donnelly WH, Meloni AM, Foss RM, Sanberg AA, Langham MR (1997) Hepatic undifferentiated sarcoma arising in a mesenchymal hamartoma. Am J Surg Pathol 21:1248–1254
Lee JA, Kim TW, Min JH, Byon SJ, Jang SH, Choi SY, Kim HJ (2010) A case of undifferentiated (embryonal) liver sarcoma mimicking Klatskin tumor in an adult (in Korean). Korean J Gastroenterol 55:144–148
Lenze F, Birkfellner T, Lenz P, Hussein K, Länger F, Kreipe H, Domschke W (2008) Undifferentiated embryonal sarcoma of the liver in adults. Cancer 112:2274–2282
Lepreux S, Rebouissou S, Le Bail B, Saric J, Balabaud C, Bloch B, Martin-Négrier ML, Zucman-Rossi J, Bioulac-Sage P (2005) Mutation of TP53 gene is involved in carcinogenesis of hepatic undifferentiated (embryonal) sarcoma of the adult, in contrast to Wnt or telomerase pathways: an immunohistochemical study of three cases with genomic relation in two cases. J Hepatol 42:424–429
Leuschner I, Schmidt D, Harms D (1990) Undifferentiated sarcoma of the liver in childhood: morphology, flow cytometry, and literature review. Hum Pathol 21:68–76
Levy M, Trivedi A, Zhang J, Miles L, Mattis AN, Kim GE, Lassman C, Anders RA, Misdraji J et al (2012) Expression of glypican-3 in undifferentiated embryonal sarcoma and mesenchymal hamartoma of the liver. Hum Pathol 43:695–701
Li XW, Gong SJ, Song WH, Zhu JJ, Pan CH, Wu MC, Xu AM (2010) Undifferentiated liver embryonal sarcoma in adults: a report of four cases and literature review. World J Gastroenterol 16:4725–4732
Lightfoot N, Nikfarjam M (2012) Embryonal sarcoma of the liver in an adult patient. Case Rep Surg 2012:382723
Lin R, Roychowdhury-Saha M, Black C, Watt AT, Marcusson EG, Freier SM, Edgington TS (2011) Control of RNA processing by a large non-coding RNA over-expressed in carcinomas. FEBS Lett 585:671–676
Lin JM, Heath JE, Twadell WS, Castellanti RJ (2013) Undifferentiated sarcoma of the liver: a case study of an erythropoietin-secreting tumor. Int J Surg Pathol
Long JC, Caceres JF (2009) The SR protein family of splicing factors: master regulators of gene expression. Biochem J 417:15–27
Lorimer WS (1955) Right hepatolobectomy for primary mesenchymoma of the liver. Ann Surg 151:246–250
Ma L, Geng CZ, Liu YP, Wu GX, Wang XL, Tian ZH (2008) Undifferentiated embryonal sarcoma of liver in an old female: case report and review of the literature. World J Gastroenterol 14:7267–7270
Marti-Bonmati L, Ferrer D, Menor F, Galant J (1993) Hepatic mesenchymal sarcoma: MRI findings. Abdom Imaging 18:176–179
Martins CC, Marcao I, Belo AI, Rolo J, Cabrita F, Partidario A, Castano J (1992) Undifferentiated sarcoma of the liver in the adult (in Portuguese). Acta Med Port 5:31–34
Mascarello JT, Krous HF (1992) Second report of a translocation involving 19q13.4 in a mesenchymal hamartoma of the liver. Cancer Genet Cytogenet 58:141–142
Massani M, Caratozzolo E, Baldessin M, Bonariol L, Bassi N (2010) Hepatic cystic lesion in adult: a challenging diagnosis of undifferentiated primary embryonal sarcoma. G Chir 31:225–228
May LT, Wang M, Albano E, Garrington T, Dishop M, Macy ME (2012) Undifferentiated sarcoma of the liver: a single institution experience using a uniform treatment approach. J Pediatr Hematol Oncol 34:e114–e116
McCarthy FP, Harris M, Kornman L (2007) Management of undifferentiated embryonal sarcoma of the liver in pregnancy. Obstet Gynecol 109:558–560
McFadden DW, Kelley DJ, Sigmund DA, Barrett WL, Dickson B, Aron BS (1992) Embryonal sarcoma of the liver in an adult treated with preoperative chemotherapy, radiation therapy, and hepatic lobectomy. Cancer 69:39–44
Miettinen M, Kahlos T (1989) Undifferentiated (embryonal) sarcoma of the liver. Epithelial features as shown by immunohistochemical analysis and electron microscopic examination. Cancer 64:2096–2103
Mistry RC, Deshpande RK, Chinoy R, Sampat M, Desai PB (1995) Undifferentiated embryonal sarcoma of the liver in childhood. Indian J Cancer 32:175–178
Miyagawa R, Tano K, Mizuno R, Nakamura Y, Ijiri K, Rakwal R, Shibato J, Masuo Y, Mayeda A et al (2012) Identification of cis- and trans-acting factors involved in the localization of MALAT-1 noncoding RNA to nuclear speckles. RNA 18:738–751
Mohapatra N, Krisnanand BR (2007) Embryonal sarcoma of liver: an undifferentiated rare primary solid and mucoid to cystic tumour. Indian J Pathol Microbiol 50:811–813
Moon WK, Kim WS, Kim IO, Yeon KM, Yu IK, Choi BI, Han MC (1994) Undifferentiated embryonal sarcoma of the liver. US and CT findings. Pediatr Radiol 24:500–503
Moon WK, Kim WS, Choi BI, Kim IO, Yeon KM, Han MC (1995) Undifferentiated embryonal sarcoma of the liver treated with chemotherapy: CT imaging in four patients. Abdom Imaging 20:133–137
Nicol K, Savell V, Moore J, Teot L, Spunt SL, Qualman S, Children’s Oncology Group, Soft Tissue Sarcoma Committee (2007) Distinguishing undifferentiated embryonal sarcoma of the liver from biliary tract rhabdomyosarcoma: a Children’s Oncology Group study. Pediatr Dev Pathol 10:89–97
Nishio J, Iwasaki H, Sakashita N, Haraoka S, Isayama T, Naito M, Miyayama H, Yamashita Y, Kikuchi M (2003) Undifferentiated (embryonal) sarcoma of the liver in middle-aged adults: smooth muscle differentiation determined by immunohistochemistry and electron microscopy. Hum Pathol 34:246–252
O’Sullivan MJ, Swanson PE, Knoll J, Taboada EM, Dehner LP (2001) Undifferentiated embryonal sarcoma with unusual features arising within mesenchymal hamartoma of the liver: report of a case and review of the literature. Pediatr Dev Pathol 4:482–489
Okajima H, Ohya Y, Lee KJ, Yamamoto H, Asonuma K, Nagaoki Y, Ohama K, Korogi M et al (2009) Management of undifferentiated sarcoma of the liver including living donor liver transplantation as a backup procedure. J Pediatr Surg 44:e33–e38
Okuda H, Saito A, Shiratori K, Yamamoto M, Takasaki K, Nakano M (2005) Clinicopathologic features of patients with primary malignant hepatic tumors seropositive for alpha-fetoprotein-L3 alone in comparison with other patients seropositive for alpha-fetoprotein-L3. J Gastroenterol Hepatol 20:759–764
Orozco H, Mercado MA, Takahashi T, Chan C, Quintanilla L, Jiménez M, Sosa R, Esquivel E (1991) Undifferentiated (embryonal) sarcoma of the liver. Report of a case (in Spanish). Rev Invest Clin 43:255–258
Pachera S, Nishio H, Takahashi Y, Yokoyama Y, Oda K, Ebata T, Igami T, Nagino M (2008) Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J Hepatobiliary Pancreat Surg 15:536–544
Parham DM, Kelly DR, Donnelly WH, Douglass EC (1991) Immunohistochemical and ultrastructural spectrum of hepatic sarcomas of childhood: evidence for a common histogenesis. Mod Pathol 4:648–653
Parichatikanond P, Parichatikanond P (1985) Undifferentiated (embryonal) sarcoma of the liver in childhood: report of two cases. J Med Assoc Thai 68:99–105
Pathirana A, Siriwardana RC, Deen KI, Rupasinghe Y (2010) A case of embryonal sarcoma of the liver. Ceylon Med J 55:90
Pérez-Gomez RM, Soria-Céspedes D, de Leon-Bojorge B, Ortiz-Hidalgo C (2010) Diffuse membranous immunoreactivity of CD56 and paranuclear dot-like staining pattern of cytokeratins AE1/3, CAM5.2, and OSCAR in undifferentiated (embryonal) sarcoma of the liver. Appl Immunohistochem Mol Morphol 18:195–198
Perilongo G, Carli M, Sainati L, Cecchetto G, Mancini A, Cordero di Montezemolo L, Cornelli A et al (1987) Undifferentiated (embryonal) sarcoma of the liver in childhood: results of a retrospective Italian study. Tumori 73:213–217
Pieterse AS, Smith M, Smith LA, Smith P (1985) Embryonal (undifferentiated) sarcoma of the liver. Fine-needle aspiration cytology and ultrastructural findings. Arch Pathol Lab Med 109:677–680
Plant AS, Busuttil RW, Rana A, Nelson SD, Auerbach M, Federman NC (2013) A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): success of combined therapy and the use of orthotopic liver transplant. J Pediatr Hematol Oncol 35:451–455
Pollono DG, Drut R (1998) Undifferentiated (embryonal) sarcoma of the liver: fine-needle aspiration cytology and preoperative chemotherapy as an approach to diagnosis and initial treatment. A case report. Diagn Cytopathol 19:102–106
Psatha EA, Semelka RC, Fordham L, Firat Z, Woosley JT (2004) Undifferentiated (embryonal) sarcoma of the liver (USL): MRI findings including dynamic gadolinium enhancement. Magn Reson Imaging 22:897–900
Rajaram V, Knezevich S, Bove KE, Perry A, Pfeifer JD (2007) DNA sequence of the translocation breakpoints in undifferentiated embryonal sarcoma arising in mesenchymal hamartoma of the liver harboring the t(11;19)(q11;q13.4) translocation. Genes Chromosomes Cancer 46:508–513
Rakheja D, Margraf LR, Tomlinson GE, Schneider NR (2004) Hepatic mesenchymal hamartoma with translocation involving chromosome band 19q13.4: a recurrent abnormality. Cancer Genet Cytogenet 153:60–63
Ramanujam TM, Ramesh JC, Goh DW, Wong KT, Ariffin WA, Kumar G, Taib NA (1999) Malignant transformation of mesenchymal hamartoma of the liver: case report and report of the literature. J Pediatr Surg 34:1684–1686
Reichel C, Fehske W, Fishcer HP, Hartlapp JH (1994) Undifferentiated (embryonal) sarcoma of the liver in an adult patient with metastasis in the heart and brain. Clin Investig 72:209–212
Ros PR, Olmsted WW, Dachman AH, Goodman ZD, Ishak KG, Hartman DS (1986) Undifferentiated (embryonal) sarcoma of the liver: radiologic-pathologic correlation. Radiology 161:141–145
Sakellaridis T, Panagiotou I, Georgantas T, Micros G, Rontogianni D, Antiochos C (2006) Undifferentiated embryonal sarcoma of the liver mimicking acute appendicitis: case report and review of the literature. World J Surg Oncol 4:9
Sangkhathat S, Kusafuka T, Nara K, Yoneda A, Fukuzawa M (2006) Non-random p53 mutations in pediatric undifferentiated (embryonal) sarcoma of the liver. Hepatol Res 35:229–234
Sano N, Takehara H, Udaka H, Izumi K (1995) Undifferentiated sarcoma of the liver. Tokushima J Exp Med 42:37–40
Scudiere JR, Jakate S (2006) A 51-year-old woman with a liver mass. Undifferentiated embryonal sarcoma of the liver. Arch Pathol Lab Med 130:e24–e26
Sharifah NA, Muhaizan WM, Rahman J, Zulfikar A, Zahari Z (1999) Fine needle aspiration cytology of undifferentiated embryonal sarcoma of the liver: a case report. Malays J Pathol 21:105–109
Shehata B, Gupta NA, Katzenstein H, Steelman CK, Wulkan ML, Gow KW, Bridge JA et al (2011) Undifferentiated embryonal sarcoma of the liver is associated with mesenchymal hamartoma and multiple chromosomal abnormalities: a review of eleven cases. Pediatr Dev Pathol 14:111–116
Shufaro Y, Uzieli B, Pappo O, Abramov Y (2002) Pregnancy and delivery in a patient with metastatic embryonal sarcoma of the liver. Obstet Gynecol 99:951–953
Sodhi KS, Bekhitt E, Rickert C (2010) Paradoxical hepatic tumor: undifferentiated embryonal sarcoma of the liver. Indian J Radiol Imaging 20:69–71
Sola-Pérez J, Pérez-Guillermo M, Giménez-Bascunana A, Garre-Sanchez C (1995) Cytopathology of undifferentiated (embryonal) sarcoma of the liver. Diagn Cytopathol 13:44–51
Sowery RD, Jensen C, Morrison KB, Horsman DE, Sorensen PH, Webber EM (2001) Comparative genomic hybridization detects multiple chromosomal amplifications and deletions in undifferentiated embryonal sarcoma of the liver. Cancer Genet Cytogenet 126:128–133
Speleman F, De Telder V, De Potter KR, Dal CP, Van Daele S, Benoit Y, Leroy JG et al (1989) Cytogenetic analysis of a mesenchymal hamartoma of the liver. Cancer Genet Cytogenet 40:29–32
Stanley RJ, Dehner LP, Hesker AE (1973) Primary malignant mesenchymal tumors (mesenchymoma) of the liver in childhood. Cancer 32:973–984
Steiner M, Bostrum B, Leonard AS, Dehner LP (1989) Undifferentiated (embryonal) sarcoma of the liver. A clinicopathologic study of a survivor treated with combined technique therapy. Cancer 64:1318–1322
Stocker JT, Ishak KG (1978) Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer 42:336–348
Suarez Y, De Lacy AM, Llovet JM (2000) Images in hepatology. Intrahepatic bleeding due to undifferentiated (embryonal) hepatic sarcoma. J Hepatol 32:361
Tanaka S, Takasawa A, Fukasawa Y, Hasegawa T, Sawada N (2012) An undifferentiated embryonal sarcoma of the liver containing adipophilin-positive vesicles in an adult with massive sinusoidal invasion. Int J Clin Exp Pathol 5:824–829
Tani H, Nakamura Y, Ijiri K, Akimitsu N (2010) Stability of MALAT-1, a nuclear long non-coding RNA in mammalian cells, varies in various cancer cells. Drug Discov Ther 4:235–239
Tano K, Mizuno R, Okada T et al (2010) MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett 584:4575–4580
Tokunaga Y, Ryo J, Hoppou T, Kitaoka A, Tokuka A, Osumi K, Tanaka T (2000) Hepatic undifferentiated (embryonal) sarcoma in an adult: a case report and review of the literature. Eur J Gastroenterol Hepatol 12:1247–1251
Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A et al (2010) The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell
Tsukada A, Ishizaki Y, Nobukawa B, Kawasaki S (2010) Embryonal sarcoma of the liver in an adult mimicking complicated hepatic cyst: MRI findings. J Magn Reson Imaging 31:1477–1480
Tucker SM, Cooper K, Brownschidle S, Wilcox R (2012) Embryonal (undifferentiated) sarcoma of the liver with peripheral angiosarcoma differentiation arising in a mesenchymal hamartoma in an adult patient. Int J Surg Pathol 20:297–300
Uchiyama M, Iwafuchi M, Yagi M, Iinuma Y, Kanada S, Yamazaki S, Ohtaki M, Shirai Y (2001) Treatment of ruptured undifferentiated sarcoma of the liver in children: a report of two cases and review of the literature. J Hepatobiliary Pancreat Surg 8:87–91
Urban CE, Mache CJ, Schwinger W, Pakisch B, Ranner G, Riccabona M, Schimpl G, Brandesky G et al (1993) Undifferentiated (embryonal) sarcoma of the liver in childhood. Successful combined-modality therapy in four patients. Cancer 72:2511–2516
Varol U, Karaca B, Muslu U, Degirmenci M, Uslu R, Göker E (2012) Undifferentiated embryonal sarcoma of the liver in an adult patient: case report. Turk J Gastroenterol 23:279–283
Vetter D, Bellocq JP, Amaral D, Boilletot A, Bockel PY, Paris F, Jaeck D, Batzenschlager A et al (1989) Hepatic undifferentiated (or embryonal) sarcoma. Diagnostic and therapeutic problems apropos of botryoid rhabdomyosarcoma (in French). Gastroenterol Clin Biol 13:98–103
Walker NI, Horn MJ, Strong RW, Lynch SV, Cohen J, Ong TH, Harris OD (1992) Undifferentiated (embryonal) sarcoma of the liver. Pathologic findings and long-term survival after complete surgical resection. Cancer 69:52–59
Walther A, Geller J, Coots A, Towbin A, Nathan J, Alonso M, Sheridan R, Tiao G (2013) Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transplant
Ware R, Friedman HS, Filston HC, Chaffee S, Kurtzberg J, Kinney TR, Falletta JM (1988) Childhood hepatic mesenchymoma: successful treatment with surgery and multiple-agent chemotherapy. Med Pediatr Oncol 16:62–65
Webber EM, Morrison KB, Pritchard SL, Sorenson PH (1999) Undifferentiated embryonal sarcoma of the liver: results of clinical management in one center. J Pediatr Surg 34:1641–1644
Wei ZG, Tang LF, Chen ZM, Tang HF, Li MJ (2008) Childhood undifferentiated embryonal liver sarcoma: clinical features and immunohistochemistry analysis. J Pediatr Surg 43:1912–1919
Weinberg AG, Finegold MJ (1983) Primary hepatic tumors of childhood. Hum Pathol 14:512–537
Willeford G, Stembridge VA (1950) Primary sarcoma of liver. Report of a case. AMA Am J Dis Child 80:404–407
Woong Y, Jae KK, Heoung KK (1997) Hepatic undifferentiated embryonal sarcoma: MR findings. J Cent Adv Telev 21:100–102
Xu HF, Mao YL, Du SD, Chi TY, Lu X, Yang ZY, Sang XT, Zhong SX, Huang JF (2010) Adult primary undifferentiated embryonal sarcoma of the liver: a case report. Chin Med J (Engl) 123:250–252
Xu C, Yang M, Tian J, Wang X, Li Z (2011) MALAT-1: a long non-coding RNA and its important 3′ end functional motif in colorectal cancer metastasis. Int J Oncol 39:169–175
Yamaoka H, Ohtsu K, Sueda T, Yokoyama T, Hiyama E (2006) Diagnostic and prognostic impact of beta-catenin alterations in pediatric liver tumors. Oncol Rep 15:551–556
Yang L, Chen LB, Xiao J, Han P (2009) Clinical feature and spiral computed tomography analysis of undifferentiated embryonic liver sarcoma in adults. J Dig Dis 10:305–309
Yedibela S, Reck T, Ott R, Müller V, Papadopoulos T, Hohenberger W (2000) Undifferentiated, embryonal sarcoma as a rare cause of spontaneous liver rupture in adults (in German). Chirurg 71:101–105
Yoon W, Kim JK, Kang HK (1997) Hepatic undifferentiated embryonal sarcoma. MR findings. J Comput Assist Tomogr 21:100–102
Yoon JY, Lee JM, Kim do Y, Choi GH, Park YN, Chung JW, Kim EY, Park JY, Ahn SH et al (2010) A case of embryonal sarcoma of the liver mimicking a hydatid cyst in an adult. Gut Liver 4:245–249
Yu RS, Chen Y, Jiang B, Wang LH, Xu XF (2008) Primary hepatic sarcomas: CT findings. Eur Radiol 18:2196–2205
Yu DC, Tandon R, Bohlke AK, Steiner RB, Haque S, Florman SS (2009) Resection of a large, ruptured undifferentiated embryonal sarcoma of the liver in a child: a case report and review of the literature. J La State Med Soc 161:41–44
Zaheer W, Allen SL, Ali SZ, Kahn E, Teichberg S (1994) Primary multicystic undifferentiated embryonal sarcoma of the liver in an adult presenting with peripheral eosinophilia. Ann Clin Lab Sci 24:495–500
Zhao H, Cai JQ, Bi XY, Zhao JJ, Huang Z, Lu HZ, Zhou HT (2008) Diagnosis, treatment, and prognostic of liver sarcoma: analysis of 16 cases (in Chinese). Zhonghua Yi Xue Za Zhi 88:1537–1539
Zheng JM, Tao X, Xu AM, Chen XF, Wu MC, Zhang SH (2007) Primary and recurrent embryonal sarcoma of the liver: clinicopathological and immunohistochemical analysis. Histopathology 51:195–203
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this entry
Cite this entry
Zimmermann, A. (2017). Undifferentiated (Embryonal) Sarcoma (UES). In: Tumors and Tumor-Like Lesions of the Hepatobiliary Tract. Springer, Cham. https://doi.org/10.1007/978-3-319-26956-6_105
Download citation
DOI: https://doi.org/10.1007/978-3-319-26956-6_105
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26954-2
Online ISBN: 978-3-319-26956-6
eBook Packages: MedicineReference Module Medicine