Abstract
The long-term predictability of dental implants is directly associated with the quality and quantity of the available bone for implant placement. Many times, clinicians have to face situations where implants cannot be placed in an ideal position. For different reasons, soft and hard tissues do not present an adequate volume that is required to achieve an ideal situation to ensure the survival, function, and aesthetics of our implants. To solve these problems, bone grafting procedures may be indicated in order to prepare the site in advance or sometimes at the time of implant surgery. When the alveolar ridges lack appropriate volume, reconstructive surgery is needed.
Several surgical techniques and bone grafting materials are available for that purpose. For that, the surgeon needs a critical evaluation of all these techniques and biomaterials to be able to select the most appropriate procedure and graft type.
The ultimate aim of the surgeon is to maximize success and minimize morbidity.
The use of bone grafts in the repair of defects in dentistry has a long history of success, primarily with the use of autogenous bone. There is an increase in the demand for reconstructive surgery and thus bone substitutes, principally due to the increase in life expectancy, changes in the lifestyles with expectation of a good life quality, and the wide acceptance of minimally invasive surgery.
Bone graft is placed to sustain coagulum stabilization and reduces the risk of soft tissue collapse into bone defects. The graft would thus support the maturation and remodeling of the coagulum into an osseous tissue. Ideally, bone substitute materials should be biocompatible, not antigenic, sterilizable, sufficiently strong to maintain space, and easy to manipulate.
A great number of different treatment options have been proposed to achieve an adequate bone volume. Success rates of different bone grafting techniques are high and often have the same success rates as in native pristine bone. However, disadvantages such as morbidity, prolonged treatment times, increased costs, and technically demanding procedures that require an expert surgeon are associated with these techniques.
In this chapter, meta-analyses, systematic reviews, and clinical trials were the main source of data obtained to compare different therapeutic alternatives and materials. This evidence-based approach assures clinicians that their therapeutic decisions are supported by solid research that will help standardize implant protocols.
This chapter will summarize the most common reconstructive options and the different grafting materials available for bone augmentation, focusing on its indications, advantages, limitations, complications, and survival and success rates.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biphasic Calcium Phosphate
- Autogenous Bone
- Alveolar Ridge
- Guide Bone Regeneration
- Bone Substitute Material
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
Osseointegrated implant is a highly predictable method to restore an incomplete dentition and rehabilitate function, phonation, and aesthetics.
In the past, conventional removable dentures and/or fixed bridges over natural teeth were the only available prosthetic rehabilitations. Nowadays, implantology has introduced a new era of treatments.
Many times, clinicians have to face situations where implants cannot be placed in an ideal position. For different reasons (trauma, prolonged edentulism, congenital anomalies, periodontal disease, and infection), soft and hard tissues do not present an adequate volume that is required to achieve an ideal situation to ensure the survival, function, and aesthetics of our implants.
When a tooth is lost, the alveolar ridge resorbs in width and height very fast; it can reach as much as 50 % loss in width in the first 12 months, two-thirds of which occurs in the first 3 months [1].
To solve these problems, bone grafting procedures may be indicated in order to prepare the site in advance or sometimes at the time of implant surgery. The ideal and most appropriate approach is first to plan the prosthetic reconstruction and then place the implants in the optimal 3D position, evaluating the necessary bone to osseointegrate the implants [2]. A great number of different treatment options have been proposed to achieve an adequate bone volume and overcome these limitations.
However, this is not always possible due to different patient-related variables. These variables include patient expectations, finances, compliance, aesthetics, and medical history.
There are different important factors that must be known in advance in order to achieve a good prognosis of the procedure. These include soft tissue closure, vascularization, immobilization of the graft, space maintenance, absence of infection, type of defect, graft materials used, growth factors, etc. The clinician should analyze the planned recipient site for the keratinized gingiva, tissue thickness, high muscle attachments, frenum, and scarring, among others. Also, the bone augmentation technique employed to reconstruct different ridge defects depends on the horizontal and vertical extent of the defect.
Success rates of different bone grafting techniques are high and often have the same success rates as in native pristine bone. However, disadvantages such as morbidity, prolonged treatment times, increased costs, and technically demanding procedures that require an expert surgeon are associated with these techniques. Each treatment has its own indications and contraindications, as well as advantages and disadvantages.
Therefore, many clinicians and patients are willing to perform the easiest and less invasive procedure to solve their situation, avoiding graft surgery. In this sense, many other options are also available such as zygomatic implants, short implants, tilted implants, computerized implantology, etc., which also have their indications, limitations, and risks.
There are different reasons for the number of complications and failures experienced by the clinicians. One is that the total number of implants placed has increased during the last 10 years, and consequently a more number of dentists started to place implants and perform reconstructing procedures, often without the necessary academic background and training. Moreover, many practitioners currently receive their surgical training from continuing education courses, many times given by implant and material companies. These trainings usually lack of the sufficient clinical practice and enough patient follow-up that will enable the clinician to become familiar with intraoral surgical complications as well as postoperative and long-term complications.
Many courses and lectures often show high implant and grafting success and survival rates; although it may be true, this data should be analyzed with care as they may not have enough long-term basis, the inclusion and exclusion criteria may eliminate patients with high risk of complications, or the lecturers may have a great experience with a specific type of material or implant and will show their most successful cases.
Another reason is that nowadays due to patient expectations or clinician desires, more aggressive protocols such as extraction, grafting, and immediate implant placement with immediate loading are used. Also many times implants are placed in compromised sites where there is inadequate bone and soft tissue that requires augmentation procedures before implant placement.
Considerable controversy still exist regarding the choice of the most reliable technique and materials. However, the clinician should be aware that the most important thing and the end goal of treatment is to provide the patient a functional and aesthetic restoration in harmony with the natural dentition. The easiest procedure could be the first choice, provided it offers the same success rates as the more complex one, and the clinician has the required experience to perform it.
This chapter will summarize in an evidence-based approach the most common reconstructive options available, materials used, and their outcomes, focusing on its limitations, complications, and success rates (Fig. 10.1).
10.2 General Review of the Literature
Implantology has become a main field in dentistry, but most of the research that flows into the clinician’s office is sponsored by companies, which means that their studies are based on new products and technologies.
Many dentists still adopt a self-experience-based approach in their practices. Their decisions are mostly based on intuitive comparisons with other patients, making their accumulated knowledge and reliance on standard practices to be of great importance. As a result, many clinicians are slow and reticent to welcome a change.
Decision making in evidence-based implant dentistry involves diagnostic and therapeutic uncertainties, clinicians’ heuristics and biases, patients’ preferences and values, as well as cost considerations [3].
Information derived from clinical trials is considered more reliable than information based on intuition, authority, or custom. There is a hierarchy when considering the levels of evidence. Systematic reviews of randomized controlled trials are considered to be at the highest level, whereas expert opinion is considered the lowest level of evidence [3].
Several treatment choices are analyzed for different surgical situations as well as indications, advantages, complications, survival and success rates, and limitations.
It is important to differentiate between implant survival and implant success. Implant survival refers to implants still in function, but may be aesthetically compromised or may have periimplant bone resorption. Implant success should be evaluated as good function, good aesthetics, and absence of pathology. Most of the articles refer to success if the following criteria, previously defined by Albrektsson et al. [4] and adapted by Buser and coworkers [5] as well as Karoussis et al. [6], are fulfilled:
-
Absence of persistent subjective complaints, such as pain, foreign body sensation, and/or dysphasia
-
Absence of periimplant infection with suppuration
-
Absence of mobility
-
Absence of a continuous radiolucency around the implant
-
Vertical bone loss less than 1.5 mm in the first year of function and less than 0.2 mm annually in subsequent years of function [7]. Although these criteria would need to be updated in the following years according to the new implant surfaces and designs.
In this chapter, meta-analyses, systematic reviews, and clinical trials were the main source of data obtained to compare different therapeutic alternatives and materials. This evidence-based approach assures clinicians that their therapeutic decisions are supported by solid research that will help standardize implant protocols.
Heterogeneity of studies is important in a meta-analysis or systematic review because data from multiple studies are pooled based on the assumption that studies are similar enough to be compared with confidence, and thus, the results may be more generalizable.
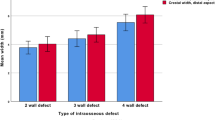
Studies evaluate different parameters which may have an impact on the final outcome of the treatment. These parameters include the initial clinical alveolar ridge situation (which traditionally has been classified as horizontal (class I), vertical (class II), or combined (class III) defects) [8, 9] (Fig. 10.2), patient-related factors (age, gender, smoking, medical history), implant characteristics (dimension, micro-/macrostructure, surgical technique, loading protocol), prosthetic characteristics (type and fixation, crown-implant ratio, occlusal situation), materials used, graft harvesting sites, and outcomes (success criteria, survival rates, follow-up time, complications).
Not all studies evaluate the same parameters, and not all parameters are defined the same way. There is a lack of comparative effective research to guide decision making in oral grafting surgery and no long-term investigation comparing all available treatment options could be identified.
It has been suggested that priority may be given to procedures that appear less invasive and carry a lower risk of complications [10].
This chapter will evaluate different preimplant surgery options and materials available to help clinicians to make decisions based on the available scientific evidence literature.
10.3 Materials Used in Pre-Implant Surgery
10.3.1 Classification of Grafting Materials According to Their Origin
10.3.1.1 Autogenous Bone
The bone is transferred from one position to another within the same individual. Autogenous bone is the “gold standard” in bone grafting as it is osteogenic (has bone-forming cells [11]), osteoinductive (actively promotes bone formation [12, 13]), osteoconductive (facilitates the colonization and ingrowth of new bone cells and sprouting capillaries on its surface [14]), and osteotransductive (degradability to be replaced by new bone).
Extraoral and intraoral donor sites have been established as donor sites that will be compared afterward (Figs. 10.3 and 10.4).
10.3.1.2 Allografts
The bone is obtained from an individual and placed in another individual of the same species [15]. Two main types of allogeneic bone grafts are clinically used, freeze-dried bone allografts (FDBAs) and demineralized (decalcified) freeze-dried bone allografts (DFDBAs).
They are available in particulate, block, and injectable forms and are composed of cortical bone, cancellous bone, or a composite of both [16, 17].
The use of this source of bone substitute materials varies between countries. For example, it is the most frequently used as an alternative to autogenous bone in the USA [18]. The FDBA is both an osteoconductive and osteotransductive bone substitute material.
The acid (0.5–0.6 molar hydrochloric acid) demineralization of bone samples to obtain the DFDBA would preserve and expose bone morphogenetic proteins (osteoinductive proteins). Urist has been the first to describe the osteoinductivity of DFDBA [13]. However, the osteoinductivity of DFDBA shows great variation and is highly dependent on the manufacturing procedure [19, 20].
10.3.1.3 Xenografts
The bone is harvested from an individual and placed in another individual of different species. Three main sources could be identified: bovine, coral, and porcine. Bio-Oss (Osteohealth Co., Shirley, NY), Bio-Oss Collagen (Osteohealth Co., Shirley, NY), OsteoGraf/N (VeraMed Dental, LLC, Lakewood, CO), PepGen P-15 (Dentsply Friadent, Mannheim, Germany), and Endobon ® (Biomet 3i, Palm Beach Gardens, FL) are examples of commercially available bovine-derived bone substitute materials.
OsteoBiol® Gen-Os (Tecnoss Dental, Turin, Italy) is a hydrophilic porcine-derived bone substitute that contains both porous hydroxyapatite structure and collagen. Biocoral (Inoteb, Saint Connery, France) and porous fluorohydroxyapatite (FRIOS® Algipore®) (Friadent GmbH, Mannheim, Germany) are examples of commercially available products of coral and algae origin. The mineral composition of all these grafts is hydroxyapatite as shown in Fig. 10.5.
Deproteinized, anorganic bovine bone (Bio-Oss®, Geistlich Pharma AG, Wolhusen, Switzerland) is one of the most studied bone substitute materials [21]. Bio-Oss® is an anorganic bovine bone that is chemically and thermally treated to extract all organic components, maintaining the 3D structure of the bone [22].
There is evidence that Bio-Oss® undergoes resorption by giant multinucleated cells (like osteoclasts). However, the in vivo resorption of Bio-Oss® is very slow, which explains its presence in biopsies obtained after 10 years of placement [23, 24]. It is available in cancellous and cortical granules and blocks. A 10 % of highly purified porcine collagen is added to produce Bio-Oss® collagen (Fig. 10.6).
10.3.1.4 Alloplastic Materials
The use of synthetic biomaterials for bone grafting presents several advantages like unlimited quantity, avoidance of surgical morbidity associated with the harvesting of autogenous bone, and no risk of disease transmission [25].
Most alloplastic materials are composed of calcium and phosphate ions due to its chemical similarity to the mineral component of the bone [26]. The most common calcium phosphates that are commercially available are ceramic in nature (synthesized at high temperature) like hydroxyapatite (HA), tricalcium phosphate (TCP), and biphasic calcium phosphates (sintered HA and β-TCP) [27]. Synthetic, nonceramic resorbable hydroxyapatite materials are also available.
Calcium phosphates have the ability to promote bone growth and an appropriate three-dimensional geometry to promote cellular viability. β-tricalcium phosphate (β-TCP) is one of the most frequently used synthetic grafts in implant dentistry [28]. Tatum in 1986 was the first to successfully apply a bone substitute (tricalcium phosphate) for sinus floor augmentation [28].
Various randomized clinical trials have been conducted to study the efficiency of biphasic calcium phosphate (BCP) and beta-tricalcium phosphate (β-TCP) in bone regeneration. New bone formation with BCP ranged between 21 and 30 % after 5–6 months postoperatively [29–31]. In one study, new bone formation reached a value of 41 % after 8 months [32]. The β-TCP promotes 30–36 % of new bone formation after 6 months of sinus floor augmentation [33, 34]. Both bone substitutes are resorbable with a residual bone graft between 10 and 28 % for BCP and between 13.95 and 28.4 % for TCP [29–32].
Calcium sulfate has been used in craniofacial surgery for more than 100 years [27]. However, calcium sulfate has a relatively high rate of degradation that may not be compatible with the rate of bone formation. It is completely resorbable in 5–7 weeks in vivo [35]. Calcium sulfate has been used as a bone substitute, a graft binder/extender, and a barrier in guided tissue regeneration [36].
Calcium sulfate is also used as a delivery vehicle for growth factors and antibiotics, although this application has not been thoroughly exploited in the clinical setting [36]. Osteoset® (Wright Medical Technology, Arlington, TN, USA) is an example of the use of calcium sulfate as a vehicle for the delivery of an antibiotic (tobramycin in the case of Osteoset) for the treatment of osteomyelitis. Calcium sulfate has never attracted the same degree of research interest as have other biomaterials. Recently, however, it has enjoyed a resurgence of sorts in the areas of periodontology, sinus augmentation, and orthopedic surgery [36].
Bioglass or bioactive glass is a calcium-substituted silicon dioxide. The bioactivity as property for bioglass refers to its ability to promote the precipitation of biological hydroxyapatite on its surface that would result in its bonding to the hosting bone. The leakage of sodium and potassium ions from the bioglass would result in the formation of a silica gel layer that would promote the precipitation of a hydroxyapatite layer on its surface [37]. It has been shown to experience severe resorption during the first 2 weeks after implantation; however, beyond this point, its resorption rate stabilizes [38].
The in vivo solubility of the biomaterial is proportional to the content of sodium oxide [37]. Successful outcomes in bone augmentation procedures and as filler for intra-bony defects have been reported [27]. However, its bone regeneration capacity has not been superior to calcium phosphate biomaterials [39].
10.3.2 Which Is the Best Bone Graft for Alveolar Bone Augmentation?
10.3.2.1 Alveolar Ridge Augmentation
In one systematic review, a mean implant survival rate of 98.6 ± 2.9 % for autogenous bone alone (healing period before implant placement 5.1 ± 1.4 months), 100 % for bone substitute material + autogenous bone (healing time before implant placement 5.25 ± 1.9 months), and 97.4 ± 2.5 % for bone substitute material alone (healing time before implant placement 4.7 ± 1.1 months) for a follow-up period of 30.6 ± 27.1 months has been reported. Implant success was indicated in five studies and ranged from 90.3 to 100 % [14].
Five studies (from 2000 to Jan 2014) that compared the clinical outcome of ridge augmentation procedure using bone substitute material or autogenous bone have been included in the meta-analysis. The meta-analysis has shown no statistically significant difference between both types of grafts. Three studies compared implant survival after ridge augmentation using bone substitute material + autogenous bone or autogenous bone alone. All these studies showed a survival rate of 100 % for both grafting materials [14] (Fig. 10.7).
When autogenous bone blocks (alone or in combination with membrane, grafting materials) have been utilized for horizontal bone augmentation, the mean gain in ridge width was reported to be 4.4 mm, the percentage of cases that had no additional grafting was 97.2 %, and the complication rate was 3.8 % [40]. When no autogenous block graft was used, the corresponding figures were 2.6 mm and 75.6 and 39.6 %. The survival of implants placed in augmented area with autogenous block from intraoral site ranged from 96.9 to 100 % [40]. The same RCT reported that augmented sites that were covered with nonresorbable membranes showed a mean gain in ridge width of 2.9 mm. When resorbable membranes were used, a mean gain in ridge width was 4.3 mm, and when no membrane was used, the results were 4.5 mm [40]. When the complication rate was calculated for studies with the use of nonresorbable membranes, resorbable membranes, or no membranes, the corresponding rates were 23.6, 18.9, and 9.4 % [40].
In a RCT, anorganic bovine bone with resorbable and nonresorbable membranes has been evaluated in horizontal ridge augmentation [40, 41]. The outcomes have indicated that both groups experience high frequencies of membrane exposures of 64 and 71 %, respectively [40, 41]. The most predictable horizontal bone augmentation seems to involve an autogenous block graft alone or in combination with a particulate bone graft of bone substitute material [40]. The best documented grafting protocol included intraorally harvested autogenous bone block alone or in combination with anorganic bovine bone and with or without coverage of barrier membrane [40].
In the same systematic review, regarding vertical bone augmentation, the use of autogenous block graft has resulted in a height gain of 3.7 mm, 83.1 % of the cases did not require second grafting and the complication rate was 29.8 % [40]. However, when particulate autograft or bone substitute material has been utilized, the corresponding figures were 3.6 mm, 67.4 and 21.0 % [40]. Comparing the data whether a membrane was used or not, the gain in ridge height was 3.5 mm vs. 4.2 mm, and the complication rate was 23.2 % vs. 25.3 % [40].
The most frequent grafting materials used were intraorally harvested autogenous block graft and autogenous particulate. The grafts were harvested from the mandibular chin or body/ascending ramus [40]. The use of block grafts seemed to yield more gain in ridge height and a greater reduction in the need of additional grafting procedures than the use of granular grafts. However, the rate of dehiscence seemed to increase with their use in comparison with horizontal bone augmentation [40].
10.3.2.2 Treatment of Fenestrations and Dehiscences: Guided Bone Regeneration
Autogenous and nonautogenous options are available. The source of autogenous particulate is the same as the one that has been discussed for block harvest. For small amounts of bone, local sites can be used, including the symphysis, ramus, and maxillary tuberosity. They have the advantage of a more rapid vascularization [42].
Nonautogenous bone substitutes, such as ABBM, may be also a valid alternative to autogenous bone (particularly when harvested from extraoral locations), since they are associated with less postoperative morbidity. They can also be used in combination with autogenous bone [42, 43].
The best documented augmentation protocols are anorganic bovine bone covered with a membrane, particulate autograft with or without a resorbable membrane [40].
Jensen et al. [40] in their systematic review found that the use of autogenous bone grafts, harvested intraorally, resulted in a defect fill of 83.8 %, 68.8 % of the cases showing a complete defect fill. Membrane or graft dehiscence was reported in 15.5 % of the cases. The use of anorganic bovine bone with or without membrane resulted in a mean defect fill of 88.9 % and a complete defect fill was achieved in 67.7 % of the cases. The rate of dehiscence was 12 %. Implant survival rates of 93–100 % have been reported.
Use of Membranes
Successful vertical and horizontal ridge augmentation with the GBR technique, using both resorbable and nonresorbable membranes, was shown in human studies [8, 44, 45].
Nonresorbable barriers are available as e-PTFE, titanium-reinforced e-PTFE, high-density PTFE, or titanium mesh [8].
e-PTFE is a synthetic polymer with a porous structure, which does not induce immunologic reactions and resists enzymatic degradation by host tissues and microbes. Titanium-reinforced e-PTFE membranes increase their mechanical stability and allow the membranes to be shaped [2]. However an increased rate of soft tissue complications after premature membrane exposure has been reported [2]. Once exposed to the oral cavity, the porous surface of e-PTFE membranes is soon colonized by bacteria, which can lead to infections and to the subsequent need for early membrane removal. This could interfere with bone regeneration. Another disadvantage of e-PTFE membranes is the need for a second surgery to remove the membrane [2] (Fig. 10.8).
Bioabsorbable membranes can be classified into natural or synthetic. Natural products are made of various types of collagen of animal origin, while synthetic ones are made of aliphatic polyesters (polylactic and polyglycolic acid polymers). The difference is their mode of resorption; collagen products undergo enzymatic degradation, whereas synthetic barriers are degraded by hydrolysis [8].
The main advantage of resorbable membranes is that a second procedure to remove the membrane is not needed; thus, patient morbidity is decreased. However, the difficulty of maintaining the barrier function for an appropriate length of time is considered a major disadvantage. Depending on the material, the resorption process and length can vary [2]. Also, because of a lack of rigidity, in all but the smallest defects, most of these bioabsorbable membranes must be used in combination with a graft material for space maintenance in bone augmentation applications [8].
Some studies suggested that the volume of regenerated bone achieved was higher using nonresorbable membranes in comparison with resorbable membranes [8], although this did not affect the outcome of the treatment and implant survival rates.
In one RCT, regarding the use of nonresorbable membranes and resorbable membranes, the percentages of defect fill were 75.7 and 87 %; the percentages of cases with complete defect fill were 75.5 and 75.4 %; the rates of membrane/graft dehiscences were 26.3 and 14.5 %; and the implant survival rates were 92.9–100 % (median 96.5 %) with nonresorbable membranes and 94–100 % (median 95.4 %) with resorbable membranes [40].
Products that are available to stabilize membranes include nonresorbable mini-screws and bioabsorbable tacks made from polylactic acid [8].
The choice of membrane will depend on the required duration of membrane function for achieving the desired tissue regeneration (generally 6 months) [8]. In general, the criteria required to select appropriate barrier membranes for guided bone regeneration include biocompatibility, integration by the host tissue, cell occlusiveness, space-making ability, and adequate clinical manageability [2].
Addition of bone graft material to the GBR technique increases the amount of achievable vertical regeneration [8, 46].
10.3.2.3 Lateral Sinus Floor Augmentation
10.3.2.3.1 Histomorphometric Total Bone Volume
There are two systematic reviews that discussed the available scientific evidence regarding the histomorphometric parameter of total bone volume in maxillary sinuses augmented with different grafting materials [47, 48]. In the study by Handschel et al. [48], a meta-analysis has been performed for those studies that report sinus elevation with various grafting materials (autogenous bone, Bio-Oss® + autogenous bone, Bio-Oss®, and β-TCP). The study has demonstrated the presence of significantly higher mineralized bone during the early healing phase (the first 9 months after surgery) for autogenous bone compared to various bone substitutes.
However, the differences in the total bone volume between grafts disappeared over time, and after 9 months, no statistically significant differences were detected. Klijn et al. [47] have performed a meta-analysis of the total bone volume present in biopsies obtained from augmented maxillary sinus with autogenous bone. The study indicated that the bone harvested from an intraoral site would result in higher total bone volume than the bone harvested from the iliac crest [47].
In the systematic review by Pjetursson et al., it is suggested that maxillary sinuses grafted with autogenous bone may receive dental implants earlier than sinuses grafted with bone substitute materials [49] (Fig. 10.9).
10.3.2.3.2 Complications
Nkenke et al. demonstrated in their review that the partial or total graft loss related to the event of sinusitis is independent of the grafting materials used [50]. For that, the use of bone substitutes would not increase the risk of developing sinusitis and graft loss [14].
10.3.2.3.3 Implant Survival
Autogenous bone alone or autogenous bone mixed with bone substitute materials are the most common grafting materials used for sinus floor augmentation [40].
When autogenous bone block grafts from the iliac crest are applied, the overall implant survival rate has been reported from 61.2 to 94.4 % (median 84.9 %) after a period of function of up to 102 months [40] (Table 10.1). The use of particulate autologous bone grafts resulted in a survival rate with a range of 82.4–100 % up to 52 months of follow-up.
Implants placed into sinuses elevated with particulate autografts have shown higher survival rates than those placed in sinuses that had been augmented with block grafts [51, 52].
The relatively lower implant survival rates reported when autogenous bone blocks from the iliac crest were used can be explained due to the fact that most of the placed implants had a machined surface [51, 52].
The lateral and anterior wall of the maxillary sinus and maxillary tuberosity has also been employed as a donor site of autogenous bone but in particulate form [53]. The lateral wall of the maxillary sinus has also been used as a donor site for onlay bone graft to gain alveolar width [54].
The use of a mixture of a composite graft consisting of particulate and a bone substitute has also been evaluated, showing an implant survival rate ranging from 84.2 to 100 %. In one systematic review [40], allograft was added to autogenous bone in four studies that completed the inclusion criteria. All these studies have reported a 100 % survival rate up to 42 months. In another nine studies, autograft was mixed with DBBM. The implant survival rate ranged between 89 and 100 % (median 94.3 %) with a follow-up of 12–60 months after loading [40].
When comparing autogenous bone alone and bone substitute material mixed with autogenous bone, the meta-analysis results indicated the absence of statistically significant differences in implant survival between the two groups [14]. The meta-analysis showed trends toward a higher implant survival when using bone substitute materials compared to autogenous bone; however, the difference was not statistically significant [14].
When DBBM alone was used for maxillary sinus floor elevation, implant survival rates ranged from 85 to 100 % (median 95 %) [40]. When the xenograft was mixed with PRP/PRGF, the results ranged from 90 to 96 %. When using alloplastic materials after a period of function up to 134 months, the survival rate ranged from 81 to 100 % (median 93 %) (Table 10.1).
In different systematic reviews, it was reported that when only rough-surface implants were included, bone substitutes, a combination of autogenous bone and bone substitutes, and autogenous bone blocks all showed similar annual failure rates [49, 51, 55] (Table 10.2). The lowest annual failure rate of rough-surface implants was observed using autogenous particulate bone graft (Table 10.2).
When the overall machined implant survival rates were compared with rough-surface implant survival rates, the results were lower for machined than for rough-surface implants [40, 51, 52].
Consequently, the use of autogenous bone alone or in a combination with other bone substitutes would not affect implant survival. Klinge et al. in the consensus report on tissue augmentation and aesthetics have indicated that the evidence neither supports nor refutes the superiority of any specific graft material for sinus augmentation [56]. A Cochrane review has concluded that bone substitute materials may replace autografts in this indication [43]. Furthermore, Nkenke et al. concluded that graft resorption seems not to affect implant survival [50].
For lateral sinus floor augmentation, the following grafting protocols may be considered well documented: autogenous particulate alone or in combination with either anorganic bovine bone or DFDBA, anorganic bovine bone alone or in combination with DFDBA, and an alloplastic HA alone [40].
10.3.2.3.4 Graft Stability
To overcome the inherent problem with resorption of an autogenous bone graft, it was suggested that particulate autogenous bone in a mixture with xenografts or alloplastic materials or bone substitutes alone may reduce the risk of bone resorption and sinus re-pneumatization [44, 57]. The slow resorption capacity of the bone substitute can minimize the amount of resorption [57]. In one histological analysis of a sinus grafted with ABBM, particles were found surrounded by a new bone even 10 years after the grafting procedure, still showing osteoclastic activity [24] (Figs. 10.10, 10.11, and 10.12).
Cross section of a core sample with ABBM and PRGF showing newly formed bone (nb) around particles of ABBM (b). Vital bone formation is apparent between the residual ABBM particles. (HE, 40×) (Reprinted from Pardiñas López et al. [24]. Copyright © 2015, with permission from Wolters Kluwer Health, Inc.)
High-power image showing immature, newly formed bone (nb) around particles of ABBM (b), along with osteoid (os). Note the osteocytes (ocy) inside the ABBM (b). (HE, 100×) (Reprinted from Pardiñas López et al. [24]. Copyright © 2015, with permission from Wolters Kluwer Health, Inc.)
High-power view of vital bone formation (nb) directly on the residual ABBM particles (b). Note the presence of osteoclast (oc) around ABBM. (HE, 400×) (Reprinted from Pardiñas López et al. [24]. Copyright © 2015, with permission from Wolters Kluwer Health, Inc.)
In a systematic review, volume changes of maxillary sinus augmentation using different bone substitutes were evaluated [58]. The study has included only those studies that report on volume changes using 3D imaging (like CT or CBCT). The analysis has been performed for only 12 articles that fulfill the inclusion criteria. Regarding autogenous bone, no statistically significant difference in average volume reduction was reported when comparing autogenous bone in either particulate or block form. The weighted average volume reduction was 48 ± 23 %. There was no statistically significant difference when autogenous block from the iliac crest was compared to allografts. A weighted average volume reduction of 30.3 % was calculated for allografts.
The use of bovine bone mineral showed a volume change of 15–20 % suggesting better volume stability over time compared to autogenous bone. The addition of particulate autogenous bone to bovine bone mineral in 70:30 and 80:20 resulted in volume reduction of 19 and 20 %, respectively, with no significant differences when compared to bovine bone mineral alone [58, 59].
The average volume reduction for allogeneic bone and for allogeneic bone + bovine mineral was 41 and 37 %, respectively [58, 60]. In one randomized clinical trial, the volume reduction after 6 months of sinus grafting with biphasic calcium phosphate (BCP) was 15.2 % [58, 61]. The study also found no significant effect on volume preservation by adding autogenous bone in 1:1 ratio to BCP graft [58, 61].
10.3.2.4 Extraction Socket Preservation
In the review by Horowitz et al. [62], it has been concluded that although extraction socket grafting has consistently demonstrated to reduce the alveolar ridge resorption, there is no superiority of one grafting material over the another. The biopsies of multiple studies that used mineralized grafting materials demonstrated the presence of 17–27 % of vital bone following 3–6 months of healing [62]. Nonmineralized products tend to demonstrate the presence of 28–53 % of vital bone [62]. However, there is a negative effect on alveolar ridge preservation for the use of collagen plug alone [63].
In one review [64], the freeze-dried bone allograft performed best with a gain in height and concurrent loss in width of 1.2 mm. In one meta-analysis, the use of xenogeneic and allogeneic grafts has resulted in lesser height loss at the middle of the buccal wall when compared with the use of alloplastic grafts [65]. However, another review [66] has indicated that the scientific evidence did not provide clear guidelines with regard to the type of biomaterials to use for alveolar ridge preservation (Fig. 10.13).
10.3.3 The Use of Autologous Growth Factors and Bone Morphogenetic Proteins to Enhance the Regenerative Capacity of Bone Substitute Materials
The use of platelet-rich plasma would improve the handling and placement of particulate grafts providing stability and avoid the need for compaction [27]. A positive effect on soft tissue healing has been observed in most of the studies that were included in a systematic review on the use of platelet-rich plasma in extraction sockets [67].
A beneficial effect of plasma rich in growth factors on postoperative quality of life could be evidence, as a consequence of the enhanced soft tissue healing [67–71]. The use of plasma rich in growth factors has also been reported to reduce bleeding, edema, scarring, and pain levels [67, 71–74]. In the systematic review by Del Fabbro et al. [75], few clinically controlled studies are available on the effect of autologous growth factors (platelet concentrate) when combined with a bone graft for sinus augmentation. Even more, those few studies have significant heterogeneity [75].
Regarding implant survival, the authors concluded that no evident benefit of the use of platelet concentrates can be drawn from the included studies [75]. However, there is a suggestion that in critical clinical conditions, the addition of plasma rich in growth factors could be beneficial [75]. Regarding histomorphometric analysis of biopsies obtained from grafted maxillary sinuses, the review indicated the possible advantage of using platelet-derived growth factors during the early phases of healing (3–6 months) [75]. In a RCT, the addition of plasma rich in growth factors (Endoret (PRGF)) could enhance the bone formation supported by anorganic bovine bone with a slow healing dynamics [75–77].
Titanium grids have been used alone or with a graft material for the correction of height/width of the alveolar ridge. In the review by Ricci et al. [78], it has been shown that titanium grid exposure occurred in 22.78 % of patients. In a RCT, the effect of using plasma rich in growth factors (Endoret (PRGF)) on the outcomes of titanium mesh exposure has been evaluated [42]. While 28.5 % of the cases in the control group suffered from mesh exposure, no exposures were registered in the Endoret (PRGF) group. Radiographic analysis revealed that bone augmentation was higher in the Endoret (PRGF) group than in the control group.
Overall, 97.3 % of implants placed in the control group and 100 % of those placed in the Endoret (PRGF) group were successful during 2 years after placement [42]. The authors have suggested that the positive effect of Endoret (PRGF) on the Ti-Mesh technique could be related to its capacity to improve soft tissue healing, thereby protecting the mesh and graft material secured beneath the gingival tissues [42]. Plasma rich in growth factors employs fibrin scaffold and endogenous growth factors that orchestrate tissue healing to promote adequate tissue regeneration and to reduce tissue inflammation [79, 80]. These growth factors promote cellular growth, proliferation, and migration [79, 80] (Figs. 10.14 and 10.15).
10.3.4 Bone Morphogenetic Proteins (BMPs)
In a systematic review on the effect of using rhBMP-2 in maxillary sinus floor augmentation, only three human clinical articles were eligible for analysis [81]. Two of these studies were randomized clinical trials and one was a prospective study, evidencing the need for more randomized clinical trials with a long-term follow-up to provide evidence-based criteria for the use of rhBMP-2 for alveolar bone augmentation. The review has indicated that a higher level of initial bone gain and density was observed for the group of autogenous bone than for the group of rhBMP-2 [81]. Furthermore, higher cell activity, osteoid lines, and vascular richness have been observed in the rhBMP-2 groups [81].
A 1.50 mg/mL rhBMP-2/ACS has been compared to autogenous graft for maxillary sinus augmentation [82]. One hundred and sixty patients with less than 6 mm of residual bone height were treated. A significant amount of new bone was formed after 6 months postoperatively in each group. At 6 months after dental restoration, the induced bone in the rhBMP-2/ACS group was significantly denser than that in the bone graft group [82]. No significant differences were found histologically. The new bone was comparable to the native bone in terms of density and structure in both groups. 201 over a total of 251 implants placed in the bone graft group and 199 over a total of 241 implants placed in the rhBMP-2/ACS group were integrated and functional 6 months after loading. No adverse events were deemed related to the rhBMP-2/ACS treatment. However, when rhBMP-2 has been added to Bio-Oss (anorganic bovine bone), less bone formation has been observed [81, 83].
Moreover, no significant differences have been found when comparing autogenous bone and rhBMP-2/ACS after 6 months postsurgery (although initially higher bone gain was observed only subcrestally in the rhBMP-2 group) [84]. In another clinical study, guided bone regeneration with xenogeneic bovine bone and collagen membrane was performed with and without rhBMP-2 [85]. There were no significant differences between the groups after 3 and 5 years of evaluation [85]. In both groups, the implant survival and periimplant tissue stability were not affected by the use rhBMP-2 [85].
Based on these data, a comprehensive assessment of the cost-effectiveness of using rhBMP-2 for alveolar bone augmentation should be performed.
10.4 Options for Preimplant Reconstructive Surgery
10.4.1 Ridge Augmentation
10.4.1.1 Block Grafts
10.4.1.1.1 Autogenous
Autogenous block grafts have been used for many years for ridge augmentation procedures for implant placement since it was first described by Branemark et al. [86].
Treatments with autogenous block grafts include different extraoral and intraoral harvest sites such as the iliac crest, calvarium, tibia, mandibular symphysis, and ramus, among others.
The election depends on the volume of graft required, the location of the recipient site, and the type of graft needed.
10.4.1.1.1.1 Intraoral
10.4.1.1.1.1.1 Symphysis
One study carried out by Pikos that reviewed more than 500 block grafts concluded that block grafts harvested from the symphysis show a predictable bone augmentation up to 6 mm in both horizontal and vertical aspects [87]. An average block size of 20.9 × 9.9 × 6.9 mm can be harvested [88], which means that up to a three-tooth edentulous site can be treated [87].
This type of graft is considered as corticocancellous with a density D-1 or D-2, with an average of 65 % of cortical bone and 35 % of cancellous bone [87, 89]. The corticocancellous nature of bone provides faster angiogenesis, achieving a more rapid integration and less potential resorption during healing [90, 91] (Fig. 10.16).
10.4.1.1.1.1.2 Ramus
The ramus block graft can be used for horizontal or vertical augmentation of 3–4 mm. An average block thickness of 2–4.5 mm can be harvested from the ramus, with a length enough to treat a defect involving a one- to four-tooth edentulous area [87, 92].
This type of graft has almost all cortical nature [89]. The cortical nature of bone exhibits less volume loss and maintains their volumes significantly better than cancellous bone [92, 93] (Fig. 10.17).
Bone blocks harvested from sites derived from intramembranous bone (intraoral) have been shown to revascularize faster and have less resorption than those from an endochondral (extraoral) source. This fact is related to a faster angiogenesis and greater inductive capacity, due to a higher concentration of bone morphogenetic proteins and growth factors [90, 94, 95].
Resorption
In the past, before the era of osseointegrated implants, the use of onlay bone grafts to reconstruct atrophic ridges was criticized because of the important resorption that they suffered.
Nevertheless, these drawbacks were mostly due to the use of removable dentures, which negatively affected the grafted jaws and also the nongrafted edentulous ridges.
The capacity of bone grafts to resist remodeling is variable, and results reported in the literature have a great variability. Different aspects can affect these results: differences in observation periods, type and site of reconstruction, timing of implant loading, use of provisional dentures on reconstructed sites, site of bone harvesting, type of implant, type of material, etc. Moreover, there is a paucity of information, as many studies only report implant survival rates and do not report changes in grafts [96].
Several studies have analyzed resorption rates of autogenous block grafts and most of them reported similar rates of resorption (Table 10.3).
Intraoral block graft resorption ranges from 0 to 42 % for vertical augmentation and from 9 to 24 % for horizontal augmentation, but can be excessive if graft dehiscence occurs [87, 97].
One study showed that the contraction in the horizontal bone augmentation with a bone block from the ramus of the mandible was from 4.6 ± 0.73 mm to 4.0 ± 0.77 mm [98]. Using the same graft source, other studies (using a CBCT scan) have reported a decrease in width from 6.1 ± 2.0 to 5.6 ± 2.1 mm of augmented alveolar ridges after 4 months of healing [99].
Vertical augmentation appears to be more problematic with both block and particulate grafts, with higher resorption rates than for horizontal augmentations [100–103].
One reason could be that the forces exerted on the graft when the soft tissue envelope expands vertically are higher than in horizontal augmentations [92].
Bone resorption is reported to be higher in the first year after the grafting procedure and in the first year postloading of implants [104]. Some studies have reported that resorption of the bone graft consolidates after implant placement. Furthermore, implant placement shortly after graft consolidation could have a stimulating effect on the bone, maintaining the graft volume and preventing further loss [96, 97]. Maintenance of the periimplant bone volume may also be due to occlusal stimuli to the implants [104, 105].
Wound dehiscence and/or infection is also related to partial or total loss of the graft, which in one study was reported in 3.3 and 1.4 % of the cases, respectively [44] (Fig. 10.18).
Use of Membranes
Membranes are often used in combination with block grafts and/or particulate graft materials to maximize the regenerative outcome and minimize graft resorption [8, 106, 107].
The use of membranes has shown a significant difference in width and height graft resorption, suffering less bone resorption in cases when a membrane was used than without membrane, 13.5 % vs. 34.5 %. However, this benefit was reduced when the membrane was exposed [104, 108].
Both nonresorbable and resorbable membranes reported good results, but the nonresorbable appears to have better results in terms of bone formation [40, 103].
One disadvantage is that resorbable membranes resorb relatively fast, so the block graft becomes unprotected. To avoid this, covering the block graft with ABBM particles will prevent surface resorption [103]. Regarding the disadvantages of the nonresorbable membranes, it makes more difficult the surgical technique (needs to be adapted and fixated to the underlying bone to prevent micromotion) and presents a higher risk of exposure which can lead to wound infection and complications [40, 106].
On the other hand, other authors only recommend the use of a membrane if a large quantity of particulate graft is used, as they report that no membrane is necessary for predictable block grafting [87] (Figs. 10.19, 10.20, 10.21, and 10.22).
Advantages, Disadvantages, and Complications
Different aspects have to be taken into account regarding the success rates of the different techniques.
The time of evaluation, the type of graft, the residual alveolar ridge present before surgery, the use of barrier membranes, the exposure of the membrane or graft, the tension-free closure, and the lag screw fixation, among others, can influence in the treatment results.
Autogenous block grafts have shown evidence of bone augmentation and high success rates. On the other hand, these techniques are associated with morbidity [92].
It is important to remark that there is heterogeneity in the literature in terms of what is considered a complication.
Complications must be differentiated in between donor sites and recipient sites. Many studies refer to complications only on the recipient site or only in the harvesting area; also other studies mix these complications. Although neural disturbances and graft exposure are the most important complications that must be taken into account, all complications should be considered as the final treatment will be affected by the whole range of procedures performed.
Complications have been reported up to 80 % in different studies. The most common surgical complications include neural disturbances. Temporary nerve disturbances involving the mental nerve have been reported as 10–80 % and 0–37.5 % when grafts were harvested from the mandibular ramus [97, 102, 108–110]. Permanent neural disturbances were reported to 0 % for ramus and 13 % for symphysis in one systematic review [96]. The most common postsurgical complications reported include mucosal dehiscences with or without exposure of the grafts or membranes, swelling, inflammation, and hematoma [92, 97, 102, 103, 108, 111] (Table 10.4).
Diabetes and smoking are common factors that were associated with a high rate of complications and graft failure in different studies [97, 102, 103].
Regarding the type of defect, vertical bone grafting is also associated with more complications rates than horizontal augmentations [102].
The mandibular ramus donor site results in fewer complications and morbidity and appears to have fewer difficulties in managing postoperative edema and pain in comparison with other donor sites [90, 97, 101].
Ramus graft has some advantages as compared to the chin area, the quality of bone is similar (mainly cortical), the amount of harvested bone may be higher, and the risk of neural disturbances is lower [44, 112]. However, surgical access in some patients is more difficult [97] (Figs. 10.23, 10.24, 10.25, and 10.26).
One important advantage of intraoral grafts is that the donor and recipient sites are in the same operating field, so surgical and anesthesia times are reduced as well as morbidity [92]. Also these grafts require a short healing period, in comparison to other techniques like GBR or particulate allografts or xenografts [97]. Moreover, fast osseointegration of the autogenous block grafts allows an early reentry for implant placement, often in 3–4 months, in comparison to the 6–8 months required for the particulate GBR techniques.
Cortical nature of the grafts results in optimal bone density for primary implant stability. Block grafts are also good space maintainers during healing, preventing collapse and allowing the bone to form [90].
Success of the grafting procedure has been reported as 87.5 % [102], but the most relevant data is regarding implant success, which is the final goal of the treatment and will be discussed further on.
10.4.1.1.1.2 Extraoral
Distant site bone harvesting has been suggested as an indication when a large graft is needed. The iliac crest, calvarium, and tibia have been reported as reliable sources of grafts and are the most common extraoral harvest sites [113].
10.4.1.1.1.2.1 Ilium
The hip offers an area where large amounts of bone can be harvested, but these grafts usually have a thin cortical layer and a thick cancellous part [114].
However the main disadvantage is the morbidity associated with bone graft harvest (Figs. 10.27, 10.28, and 10.29).
Iliac harvesting site (Reprinted from Kademani and Keller [211]. Copyright (2006), with permission from Elsevier)
Iliac harvesting site Reprinted from Kademani and Keller [211]. Copyright (2006), with permission from Elsevier)
Iliac block graft Reprinted from Kademani and Keller [211]. Copyright (2006), with permission from Elsevier)
The most frequently reported complications include temporary gait disturbance, paresthesia, infections, hematoma/seroma, fracture, scaring, and persistent pain [115–117].
The reported complication incidence is higher than with other donor sites, from 1 to 63.6 %, and can be higher if postoperative pain/gait is considered.
Pain/gait disturbances were reported from 2 to 97 % of the cases [44]. This variability is also related with the personal resistance to pain, so it is important to note that complications should also be evaluated regarding patient feelings. A descriptive analysis of the visual analog scale (VAS) demonstrated that 70 % of the patients reported more severe pain from the harvest site than oral pain, 20 % reported more intense oral pain compared to hip pain, and 5 % reported that oral and hip pain were similar [116, 118].
The advantages of the iliac crest as a donor site are the simple accessibility and the potential abundant amount and quality of bone [118]. However, a second surgical site (donor site) is related with higher morbidity [118]. The mean hospital stay reported in the literature after an iliac crest bone harvest is 3–5 days [118, 119].
Resorption rates of the initial graft height 1–5 years postloading of implants have been reported in a range from 12 to 60 % [40, 44, 120].
Another study showed that at the 5-year examination, the mean bone resorption was 4.8 mm, although these results were attributed to the design of the 3.6-mm conical unthreaded part of the implant, which may produce more initial resorption [55]. Other authors reported a mean resorption of 2.75 mm [121] and 0.85 mm (range of 0–4.5 mm) [122] before implant placement.
When the resorption of iliac crest bone grafts used for vertical or horizontal onlay augmentation was compared between the maxilla and mandible, the resorption in the maxilla was significantly more pronounced after 2 years. After 6 years, 87 % of resorption was found in the mandible, while the grafts were completely resorbed in the maxilla [111].
10.4.1.1.1.2.2 Tibia
The tibia serves mainly as a source of cancellous bone and a small quantity of cortical bone [113].
When cancellous bone is needed, the tibia is one of the indicated harvest sites because it provides an abundant amount of bone with a low morbidity [123, 124].
Advantages include a low complication rate; a large quantity of cancellous bone can be harvested (1 × 2-cm block), and it is a technically simple and quick surgical procedure [116] (Figs. 10.30 and 10.31).
Tibia harvesting site (Reprinted from Tiwana et al. [212]. Copyright (2006), with permission from Elsevier)
Tibia harvesting site (Reprinted from Tiwana et al. [212]. Copyright (2006), with permission from Elsevier)
Although this procedure is relatively simple and safe, it also presents some complications. Reported complications include prolonged pain, gait disturbance, wound dehiscence, infection, scarring, hematoma, infection, paresthesia, and fracture, in a range of 1.4–5.5 % [116].
10.4.1.1.1.2.3 Calvarial
Calvarial grafts are usually harvested from the parietal bone, which has an average thickness of 7.45 mm [125] (Fig. 10.32).
Calvarial harvesting site (Reprinted from Ruiz et al. [213]. Copyright (2005), with permission from Elsevier)
Large amounts of bone can be harvested from the skull with the advantage that the operative field is in proximity to the recipient site. The main advantage is the presence of a dense cortical structure that can better resist resorption [126] (Fig. 10.33).
Calvarial block grafts (Reprinted from Ruiz et al. [213]. Copyright (2005), with permission from Elsevier)
On the other hand, this procedure usually requires general anesthesia in a hospital and requires a close postoperative care. Also minor and severe complications may occur, such as trepanation of the inner table, hemorrhage, superior sagittal sinus laceration, brain injury, air embolism, hematoma, infection, subgaleal seroma, meningitis, depression of the skull, altered sensation, and pain [127, 128].
Another point of interest is that the scar on the scalp can be visible and can also lead to localized alopecia [116].
Different studies reported a range of 0–57.7 % of both major and minor complications. Among which 0.4–4 % correspond to hematomas, 2 % to dura mater exposures, 2.3 % to alopecia, and 0–12 % to neurosurgical complications [128, 129].
Also another study reported an 82.1 % of cases in which skull depression could be observed [129]. A reconstruction by means of biomaterials is necessary to avoid this sequel.
These contrasts in complication rates may reflect differences in study designs and populations, harvesting techniques, and levels of surgical skill [128].
A close neurologic monitoring is required during the first 24 h postoperatively [129], and a mean of 5.1 days of hospital stay is reported in the literature [128].
Regarding graft resorption, most of the studies showed less resorption of calvarial grafts when compared to other donor sites. Two studies compared the resorption of calvarial grafts, ramus grafts, and iliac grafts. The resorption for calvarial grafts ranged from 0.18 mm ± 0.33 to 0.28 mm at the time of implant placement [122, 130], while at a mean follow-up of 19 months was 0.41 mm ± 0.67 mm [130]. Regarding ramus block grafts, the resorption was 0.42 ± 0.39 mm [130] and 0.35 mm (range of 0–1.25 mm) [122] at the time of implant placement and at a mean follow-up of 19 months was 0.52 ± 0.45 mm (range 0–1.75 mm) [130]. In the case of iliac grafts, the resorption was 0.85 mm (range of 0–4.5 mm) at the time of implant placement [122].
Some studies reported resorption rates of calvarial grafts ranging from 0 to 15 % of the initial graft height at a mean follow-up of 19.3 months (range 6–42 months) [44, 131]. However other authors reported that although at 10 months follow-up calvarial bone had significantly less resorption than iliac grafts, after 30 months the difference was no longer statistically significant [132].
Implant Placement
Implants placed in regenerated autogenous block grafts are predictable operations that have shown high survival and success rates [92].
Implant survival and periimplant bone levels have shown no significant differences between implants placed in block-grafted areas and implants placed in nongrafted native bone [133]. Implant survival and success rates have been reported in the literature in a mean range from 76.8 to 100 % for autogenous onlay bone grafts [44], although the majority of articles reported survival rates of more than 90 %.
Regarding the harvesting site, the least implant survival rates occurred in patients reconstructed with iliac grafts, followed by implants placed in calvarial grafts, and lastly for implants placed in areas grafted with intraoral grafts (Table 10.5).
Data were more insufficient in terms of success rates of implants according to well-defined criteria [96]. Also, a less number of implants were analyzed for success rates in comparison to the number of implants analyzed for survival rates.
Regarding intraoral harvesting sites, with no statistically significant differences between the ramus and symphysis, reported survival rates ranged from 92.3 to 100 %, while success rates ranged from 89.5 to 100 %. Implant survival rates placed in grafted areas with iliac bone grafts range from 60 to 100 %, and implant success rates vary from 72.8 to 95.6 % (Table 10.5). Implant survival rates placed in grafted areas with calvarial bone grafts range from 86 to 100 % and success rates range from 90.3 to 97.6 % (Table 10.5).
Survival rates of implants placed in grafted mandibles are reported to be better than in grafting maxillae (Table 10.6).
Time of Implant Placement
Many different characteristics and situations can influence the osseointegration of implants, such as waiting times for implant placement and loading, macro- and micro-implant geometry and materials, and quality of bone [44].
The level of evidence for implant survival and success rates is better for the delayed implant placement and may be preferable to simultaneous placement, although much controversy still exists [44, 105, 108, 134] (Table 10.6).
It has been suggested that immediate implant placement is exposed to some risks like wound dehiscence (which can expose the graft and lead to infection with the inherent risk of losing the graft partially or totally) and that immediate implants are placed into a nonvascular bone, which increases the risk of no osseointegration [44].
In addition, with the two-stage protocol, the operator can achieve prosthetically better implant placement and superior aesthetics [105].
Authors that rely on immediate implant placement suggest that resorption of the onlay graft is more pronounced after transplantation; therefore, immediate implant placement would shorten the waiting time before the prosthetic rehabilitation, thus preventing resorption [44].
Loading of Implants
The majority of studies suggest to wait similar times as to implants placed in native bone (3–6 months) [44], although there is also evidence that an early or immediate loading in grafted areas may have a stimulating effect on the bone and thus prevent bone resorption [96]. Research has shown that primary implant stability is crucial to the success of implant therapy and to determine the election of immediate or delayed loading.
10.4.1.1.1.3 How to Select the Donor Site of Autogenous Bone for Implant Site Preparation?
Based on the available scientific evidence, there are several factors that may help the surgeon to decide on the different donor sites of autogenous bone.
Due to the high heterogeneity of the studies available which analyze many different variables, it is very difficult to compare results of the different treatment options. General conclusions can be drawn but must be analyzed with care.
-
Graft volume:
Extraoral donor sites are usually selected when a large amount of bone is needed for jawbone reconstruction [54].
However, reconstruction of alveolar bone for the placement of dental implants is usually localized to a small area and requires smaller amounts of bone which makes feasible the selection of an intraoral donor site [54]. A recent study in cadavers showed that grafts harvested from the symphysis had higher thickness than grafts harvested from the ramus [135]. Bone blocks retrieved from the symphysis could provide sufficient bone to achieve a horizontal augmentation of 4–6 mm [90, 136], whereas a block from the mandibular ramus provides sufficient bone to thicken the alveolar ridge by 3–4 mm [90].
-
Surgical morbidity:
Harvest of iliac crest bone is associated with the highest percentage of complications, followed by intraoral and calvarial grafts. Intraoral harvest site complications have been reported up to 80 % in different studies (Table 10.7). The most common surgical complications include neural disturbances. Temporary nerve disturbances and morbidity have been reported more in grafts harvested from the symphysis area than grafts harvested from the ramus area. Also regarding the type of defect, vertical bone grafting is associated with more complication rates than horizontal augmentations.
Table 10.7 Complications of extraoral grafts Intraoral grafts have the advantage that donor and recipient sites are in the same operating field, so surgical and anesthesia times are reduced as well as morbidity, and mainly cortical bone can be harvested.
Main disadvantages of bone harvesting from extraoral areas include morbidity and hospitalization for general anesthesia and requires a longer surgical procedure [109, 137]. Studies have reported that the most frequent complaints of bone harvesting from the iliac crest were the temporary pain/gait disturbance [96]. Long-standing pain/gait disturbances were reported from 2 to 97 % in the literature. The surgical morbidity when calvarium was used was reported to be lower, from 0 to 57.7 %, although one study reported an 86 % of cases presenting skull depression (Table 10.7).
-
Promotion of new bone formation:
One meta-analysis was performed in relation to the total bone volume present in biopsies obtained from augmented maxillary sinus with autogenous bone [47]. The study indicated that bone harvested from an intraoral site would result in higher total bone volume than the bone graft from the iliac crest [47].
Different studies reported that the mean gain at the time of implant placement (4–6 months after grafting) ranges from 2.2 to 7 mm for intraoral autogenous grafts (Table 10.3).
-
Stability of augmented bone:
The embryonic origin is different between the extraoral harvested bone (endochondral ossification) and the alveolar bone (intramembranous ossification) [138, 139]. This is a factor that could influence the success of bone augmentation surgery as intramembranous bone graft seems to maintain better its volume, whereas endochondral bone graft undergoes a variable degree of resorption over a variable period of time [94, 140, 141].
Symphysis grafts have a corticocancellous nature, which provides faster angiogenesis, achieving a more rapid integration and less potential resorption during healing, while the ramus has almost all cortical nature which exhibits less volume loss and maintains its volume significantly better than cancellous bone.
Intraoral block graft resorption ranges from 0 to 42.5 %, and vertical augmentation appears to show higher resorption rates than horizontal augmentations.
The hip offers an area where large amounts of bone can be harvested, but it usually has a thin cortical layer and a thick cancellous part which is prone to more resorption. Initial resorption rates appear to be more significant in comparison with intraoral grafts. Also resorption is more pronounced in the maxilla than in the mandible.
Large amounts of bone can be harvested from the skull with the advantage that the operative field is in proximity to the recipient site and that presents a dense cortical structure that can better resist resorption. It has been reported that calvarial grafts show less initial resorption when compared to other donor sites; however, at long-term follow-up, differences may not be significant [122]. Extraoral resorption rates of 0–15 % in calvarial grafts and up to 60 % in iliac bone grafts after the prostheses connection were documented with the use of extraoral autogenous block grafts [8, 126, 142].
These data would indicate the importance of taking measures to compensate the loss in graft volume. Overaugmentation and the use of bone substitutes could be useful tools to compensate graft remodeling [99].
Also, the use of membranes has shown less bone resorption in comparison to cases when a membrane was not used.
-
Healing:
If a bone block is needed, then it is highly recommended to use corticocancellous bone blocks [96]. Cancellous bone alone and particulate bone, if not associated with titanium mesh membranes or titanium-reinforced membranes, do not provide sufficient rigidity to withstand tensions from the overlying soft tissues or from the compression by provisional removable dentures and may undergo almost complete resorption [86, 96]. Wound dehiscence and/or infection is related to partial or total loss of the graft.
Uneventful healing/consolidation of both intraoral and extraoral grafts could be expected [96]. One systematic review reported that wound dehiscence/infection occurred in 3.3 % of the cases of alveolar ridge augmentation, while total graft loss occurred in 1.4 % of the cases, the majority being related to extensive reconstruction with iliac grafts [96].
Regarding the harvesting area, the least implant survival and success rates occurred in patients reconstructed with iliac grafts, followed by implants placed in calvarial grafts, and lastly for implants placed in intraoral grafts. Also implant survival rates placed in grafted mandibles are reported to be better than in grafting maxillae (Table 10.6).
The level of evidence for implant survival and success is better for the delayed implant placement and may be preferable to simultaneous placement, although much controversy still exists.
10.4.1.1.2 Allogeneic
Although for many clinicians, autogenous bone grafts (as block or particulate form) still remain the gold standard for ridge augmentation, donor site morbidity associated with block graft harvesting has changed directions to the use of allogenic materials.
Different studies demonstrated success with FDBA and DFDBA block graft material in horizontal ridge augmentation procedures [8].
The behavior of an allograft depends not only on the harvested bone but also on the method in which the harvested bone is prepared and also on the quality of the source.
Allograft bone grafts have the advantage of permitting the selection of blocks with a predefined configuration and corticocancellous composition [143]. Also, morbidity discomfort and operation time are reduced [144].
Clinical evidence for allogeneic block grafting is mainly limited to case series and reports, and many different aspects have to be taken into account like defect selection, treatment approaches, and follow-up period. Also many of the analyzed cases focused on anterior graft sites having little information in posterior alveolar ridge augmentation.
In terms of block graft failure rates, a range of 2–8.5 % was reported in one case series and a systematic review [144, 145].
Graft failures most often involved mandibular posterior defects (71 %), and as with autogenous onlay, graft wound dehiscence and membrane exposure appear to be the most common complications [143–145]. Sites yielded an average of 2–3.5-mm vertical gain and an average horizontal gain of 3.92–4.79 mm [143, 144].
In one study, only one of the 57 allogenic block grafts presented a resorption of 2.5 mm; none was observed in the others after 3–4 months after grafting. They remained stable after implant placement during the 26 months of follow-up [146].
In another study, allogeneic block graft resorption ranged from 10 ± 10 % to 52 ± 25.97 % at 6 months after grafting [144].
The studies examined reported evidence that successful alveolar ridge augmentation using allogeneic onlay grafts has a high (92.8–99 %) short-term (less than 5 years) implant success rates [143, 144]. Success rates in a range of 86.9–90.0 % have also been reported in another study [111].
The use of allogeneic bone block grafts represents a reliable alternative to autogenous block grafts for augmenting the atrophic maxilla. Furthermore, implants placed in areas grafted with allogeneic blocks can achieve similar implant survival rates as implants placed in areas grafted with autogenous block grafts. However, these conclusions should be interpreted with caution due to the limitation of studies [144].
10.4.1.2 Particulate Graft: Guided Bone Regeneration
The concept of guided bone regeneration (GBR) was first described in 1959 when cell-occlusive membranes were employed for spinal fusions [147]. This principle is based on that cells which first populate a wound area determine the type of tissue that ultimately occupies the original space.
This technique is used for space maintenance over a vertical or horizontal defect, enabling the ingrowth of osteogenic cells and preventing migration of undesired cells coming from the soft tissue. Therefore, osteogenesis can occur without the interference of other competing types of tissue cells [148–150] (Fig. 10.34).
Different space maintainers have been described, such as particulate grafts, block grafts, resorbable and nonresorbable membranes, and screws, among others.
Guided bone regeneration (GBR) and guided tissue regeneration (GTR) are often used to describe the same procedure, which is inappropriate. GTR is referred to the regeneration of the periodontium, including the cementum, periodontal ligament, and alveolar bone, whereas GBR refers to the promotion of bone formation alone. GBR and GTR are based on the same principles [2, 8].
Studies reported a mean augmentation from 2 to 4.5 mm for horizontal augmentations and from 2 to 7 mm for vertical augmentations [8, 44, 45] (Figs. 10.35, 10.36, 10.37, and 10.38).
Implant Survival/Success
It has been documented that guided bone regeneration is a successful method for augmenting the bone in situations where there is inadequate bone volume for the placement of endosseous dental implants [151, 152].
Studies reported implant survival rates in a range of 76.8–100 %, while success rates ranged from 61.5 to 100 % in a period of 6–133 months. Most of them showed survival/success rates higher than 90 %, which is comparable to implants placed in native bone [44, 153]. However bone resorption was more pronounced in sites with GBR treatment [154]. No differences were found regarding vertical or horizontal augmentation, while procedures in the maxilla tend to have lower implant survival rates than those performed in the mandible (Table 10.8).
The type of graft material (or without material) and the use of resorbable or nonresorbable membranes (including titanium meshes) do not seem to affect the clinical survival/success of the implants (Table 10.8). However, this conclusion must be analyzed with care; no conclusive recommendations can be given to clinicians as it is difficult to correlate the survival/success rate of implants to the type of grafting materials used in association with membranes, because of the wide range of different materials used, the wide range of initial defects, and the paucity of comparative, controlled, split-mouth studies comparing different grafting materials and different membranes [44].
Also, the time of implant placement (staged versus simultaneous) does not seem to affect the survival/success of the treatment [7], so no indications regarding the choice of simultaneous vs. delayed implant placement have yet been defined [44], although some authors reported that a staged approach may have a lower risk for crestal bone loss as compared with a simultaneous approach, but not affecting the treatment final outcome [44].
Resorption
Different studies report a range of 0.3–2.9-mm resorption in a mean of 65 months of follow-up (Table 10.8).
It was demonstrated that the initial bone gain undergoes contraction over time (40 % of the initial bone gain) [44].
The greatest amount of bone loss is reported to be within the first year after loading and thereafter seems to remain stable [7].
Cancellous bone alone and particulate bone, if not associated with membranes of titanium meshes, may not provide sufficient rigidity to support tension from the overlying soft tissues or from the compression by provisional removable dentures and may suffer from partial or total resorption [96].
Complications, Advantages, and Disadvantages
This technique can be applied to extraction socket defects, localized defects, horizontal and vertical ridge augmentation, and correction of dehiscence and fenestration defects around implants [8, 155].
Like the nonresorbable membranes, bioabsorbable membranes can experience premature soft tissue dehiscences and exposures.
Communication with the oral cavity accelerates their resorption rate and contamination of the regenerated bone matrix [8, 156], augmenting the chances of partial or total loss of the graft.
Although collagen barriers offer improved soft tissue response, they have less ability to maintain an adequate defect space than a nonresorbable one, which is more rigid [8]. Following this reason, when a particulate graft is selected for vertical augmentation, a rigid membrane may be used to protect the graft [156].
Failures are mainly reported to be related to premature membrane exposure. Rates of exposure have been reported up to 50 %, particularly when large vertical augmentations are performed, and can lead to infection and eventually partial or total loss of the regenerated bone [8, 156, 157].
One study showed that in 16 % of the cases where GBR was performed using resorbable membranes and in 24 % of cases where nonresorbable membranes were used, membrane exposure was present at the time of suture removal, and 44 % of the nonresorbable had to be removed prematurely [8].
10.4.2 Alveolar Split Osteotomy
Alveolar split osteotomy can be used to widen a horizontally narrow mandibular ridge. It is classically admitted that there must be at least 2–3 mm of crestal bone width and a certain amount of cancellous bone present to perform it [8, 113, 158, 159].
The procedure consists in splitting the alveolar bone longitudinally provoking a greenstick fracture, using chisels, osteotomes, or piezosurgical devices. With the use of sequentially expanding osteotomes, the bone can be forced buccally with the possibility of interposition (inlay) bone grafts or simultaneous implants to keep the segments separated and to shorten the treatment time, reducing morbidity and costs [8, 113, 158, 160] (Fig. 10.39).
The gap created by sagittal osteotomy/expansion follows a spontaneous ossification with a mechanism similar to that occurring in fractures.
Success rates of the surgical procedure ranged from 87.5 to 100 % in the studies analyzed. Fracture of the buccal plate during the ridge expansion is reported to be the most common complication, from 4 to 22 % [96, 161–164] (Table 10.9).
The limitations of this technique are the presence of highly compact bone and the lack of a cancellous bone layer between the two cortical plates [159].
Implant Placement
In the studies reviewed, survival rates of implants ranged from 86 to 100 % in a 6–144-month period, which is consistent with placement in native bone. Implant success rates ranged from 86.2 to 97.5 % [90, 165, 166]. However, the few available homogenous data shows that a slightly more marginal bone loss can be expected compared with implants placed in intact bone [159].
The primary advantages of the ridge split technique using particulate graft, block graft, or GBR are that treatment time and morbidity are reduced, resulting from avoiding the necessity to obtain a graft from a separate donor site [8] (Figs. 10.40 and 10.41).
Intraoral picture showing alveolar ridge splitting with implants placed (Reprinted from Gonzalez-Garcıa and Monje [214]. Copyright (2010), with permission from Elsevier)
Intraoral picture showing alveolar ridge splitting with implants placed and space filled with particulate bone graft (Reprinted from Gonzalez-Garcıa and Monje [214]. Copyright (2010), with permission from Elsevier)
Resorption
In different studies, the mean ridge width augmentation was 3.5 ± 0.93 mm–4.03 ± 0.67 mm [167, 168]. The mean vertical bone loss was 1.77 + −1.1 mm (ranging from 0.35 to 4.35 mm) in a mean time of 52.4 months [167].
In one retrospective study, the mean marginal bone loss during the first year was reported as 0.69–0.43 mm followed by an annual loss of 0.065 mm in the following years. 1.61 mm of vertical bone loss between postoperation and loading was also reported [161].
10.4.3 Distraction Osteogenesis
Distraction osteogenesis relies on the long-standing biologic principle that a new bone fills in the gap defect created when two pieces of bone are separated slowly under tension. Distraction of the segment can be achieved in a vertical and/or a horizontal direction. The process involves cutting an osteotomy in the alveolar ridge. Then an appliance is screwed directly into the bone segments. The classic basic principles suggest an initial latency period of 5–7 days; the appliance is gradually activated to separate the bony segments at approximately 1 mm per day. The gradual tension placed on the distracting bony interface produces continuous bone formation, and the adjacent tissue expands and adapts to this gradual tension (histogenesis). A consolidation phase is needed for 3–4 months in order to allow bone regeneration. The distraction appliance is then removed, and implants are usually placed at the time of distractor removal [8] (Fig. 10.42).
The mean bone gain reported ranges from 3 to 15 mm, and the overall success rate of the procedure is reported to be in a range from 96.7 to 100 % [44, 96] (Table 10.10).
Implant survival rates reported ranges from 88 to 100 % after a period between 6 and 72 months after prosthetic rehabilitation, which are similar to implants placed in native bone [96, 133, 165]. The cumulative success rate of implants was reported as more than 90 % (Table 10.10). Data on implant success in distracted bone at 3–5 years postloading showed favorable results compared to other grafting approaches [165, 169] (Figs. 10.43, 10.44, 10.45, 10.46, and 10.47).
Bone resorption before implant placement was reported as 0.3 mm and 1.3 + −0.4 mm after 4 years postloading [133].
Advantages, Disadvantages, and Complications
This technique presents a low postoperative morbidity because there is no need of bone harvesting. Another advantage is that the soft tissues overlying the distracted area also grow. Moreover, there is a low risk of infection of the surgical site.
However, this procedure has some limitations, bone gain may not be well controlled and an adequate thickness has to be present. Also, making a provisional prosthesis while the device is in use is very difficult or impossible, and after removing the device, patients cannot wear any removable provisional prostheses for 2 months [133]. A second surgery is also needed to remove the distractor [112]. Other disadvantages include the need for daily activation, compromised speech, eating, and appearance.
Smaller ridge defects of one or two teeth in width were associated with higher rates of complications in different studies when treated with the distraction technique; for this reason, it is recommended for an edentulous ridge span of at least three missing teeth [8, 169].
Complications include fracture of the moveable segment, increase in patient discomfort during activation of the device, damage to basal bone (2.7 %), incorrect direction of the distraction leading to excessive bone on the lingual aspect (8.3–35.4 %), resorption of the moveable segment (7.7 %), inadequate bone formation (2, 2 %), fracture of the distraction device (1.6 %), and transient paresthesia in the innervation area of the mandibular nerve (1.6 %). Total failure of the procedure was reported in only 1.1 % of the cases [96, 112, 165].
Histologic results seem to demonstrate that distraction osteogenesis allows formation of adequate quality and quantity of bone tissue, which can provide primary stability of implants and favorably withstand the biomechanical demands of loaded implants.
Survival and success rates of implants placed in distracted areas are consistent with those reported in the literature for implants placed in native intact bone [96].
The majority of authors reported some relapse of initial bone gain before implant placement, due to marginal bone loss of the most coronal part of the distracted segment. Therefore, an overcorrection was suggested. However, crestal bone changes around implants after the start of prosthetic loading seem to be similar to those occurring in cases of implants placed in native nonreconstructed bones [96].
10.4.4 Interpositional Bone Grafts: LeFort I Osteotomy and Inlay Bone Grafts
Interpositional bone grafts (also known as sandwich grafts) are mainly performed for reconstructing vertical defects. The osteotomized bone segment is secured in its final position.
Big differences exist between augmenting the mandible or maxilla with interpositional grafts (inlay) and performing a LeFort I osteotomy in the maxilla.
10.4.4.1 LeFort I
The overall complication rate of this surgical procedure was reported to be 3.1 % (range of 0–10 %). The most common complications include wound dehiscences (3–4 %), postoperative sinusitis (3 %), partial graft loss (3 %), and midpalatal fracture (2 %) [96].
Reported success rates of the grafting procedure range from 95.8 to 99.5 % [96].
Different studies reported implant survival rates in a range from 60 to 96.1 % (Table 10.11). Also more implants were lost when placed at the same time of the osteotomy (6.96 %) than when placed in two stages (4.62 %) [96]. Few publications reported implant success rates according to well-defined criteria in a range of 82.9–91 % [96]. Implant survival rates, although lower, can be compared with those of implants placed in native maxillary bone.
None of the publications reviewed proposed immediate loading of implants placed in the reconstructed maxillae [96].
The analysis of the available publications demonstrated an average poor methodological quality with regard to the completeness of follow-up and success criteria of implants. Despite these limits, some conclusions can be drawn.
LeFort I osteotomy in association with interpositional bone grafts and immediate or delayed implant placement is a reliable and demanding procedure that should be limited to severe maxillary atrophy cases which are associated with an unfavorable intermaxillary relationship. In these cases, onlay grafting, although it could create an adequate scenario for implant placement, may not be enough to achieve a correct intermaxillary relationship [44, 57, 96] (Figs. 10.48 and 10.49).
Intraoral picture showing a LeFort I osteotomy (Reprinted from van der Mark et al. [215]. Copyright (2011), with permission from Elsevier)
Intraoral picture showing a LeFort I osteotomy with inlay particulate graft placed (Reprinted from van der Mark et al. [215]. Copyright (2011), with permission from Elsevier)
10.4.4.2 Inlay
When compared with the onlay technique, the inlay technique is associated with lower bone resorption values (10.2–14.2 % at 4 months postsurgery) [121] and produces more predictable outcomes, but requires an experienced surgeon. However, once implant placement is performed, the outcomes are similar for both graft procedures (Fig. 10.50) (Table 10.12).
The most common reported complications include dehiscences (10–20 %) and neural disturbances in cases of posterior mandible. Up to 40 % of the patients reported altered sensation in the lip during the first weeks after surgery [121].
For inlay grafting in the mandible, the overall survival rate of implants ranged from 90 to 95 %, while the success rate of implants (95 %) was reported only in one article [44].
The inlay technique provides superior bone graft incorporation than the onlay method by assuring blood supply by the cranially displaced segment [121] (Fig. 10.51).
10.4.5 Sinus Augmentation Procedures
The edentulous posterior maxilla is often a challenging site for implant placement because of atrophy of the alveolar ridge, poor bone quality, and increased pneumatization of the maxillary sinus [24].
The maxillary sinus augmentation procedure, initially published by Boyne and James [170] and described by Tatum [28], was introduced to restore this anatomic deficiency, placing graft material between the sinus membrane and the residual alveolar ridge. Since then, sinus augmentation procedure has been shown to be a predictable technique to increase available bone height in deficient posterior maxillary ridges prior to implant placement. Various approaches have been proposed in order to achieve the necessary bone dimensions for the insertion of implants in the atrophic posterior maxilla [24, 171].
The lateral wall sinus augmentation approach is considered as one of the most versatile pre-prosthetic surgical techniques. Recent systematic literature reviews have demonstrated that the sinus augmentation procedure is well documented with an overall implant survival rate well beyond 90 %. Evidence-based reviews have reported positive outcomes using different graft materials for maxillary sinus augmentation, such as autogenous bone, allografts, xenograft, alloplasts, and combinations of these graft materials [24, 171] (Figs. 10.52, 10.53, 10.54, and 10.55).
10.4.5.1 Lateral Approach
It is important to mention that results of the analyzed studies must be reviewed with caution, as many variables influence the outcomes, such as type of implant (machined vs. rough), residual crestal bone, immediate vs. delayed, use of membranes, and type of grafting material.
Overall, survival rates of implants were reported in a range from 52.5 to 100 % in the analyzed studies, with the majority of articles reporting values higher than 90 %, which is comparable and even higher to implants placed in native bone in the posterior maxilla with poor quality of bone, but adequate quantity for implant placement [172, 173]. Success rates of implants according to well-defined criteria range from 74.7 to 100 % [96], although few studies reported information on this (Fig. 10.56).
The most statistically significative parameters related to implant survival are the preoperative residual crestal bone, the implant surface (machined or rough), and the use of block grafts.
Regarding the type of material, no statistical differences were found between different bone grafts in implant survival rates except block grafts. Block grafts seem to reduce the survival rate of implants compared to particulate autografts, although resorption is higher in autologous particles than in block particles (Table 10.1). But again, comparison of survival rates is difficult because of the many variables existing [52] (Figs. 10.57, 10.58, 10.59, and 10.60).
In one meta-analysis of the volume changes after maxillary sinus augmentation, the weighted mean average resorption was 48 ± 23 % when calculated for controlled studies, and a wide variation in graft resorption was observed between individuals [58].
As far as the timing of implant placement is concerned, different studies show no statistically significant difference in the survival rate of implants placed in simultaneously or delayed. Rates were reported to be 61.2 % to 100 % (mean 95 %) for the simultaneous implants and 72.7–100 % (mean 93.7 %) in the case of a staged approach (Table 10.1) [49]. However another study reported that delayed implant placement had a 2–3-fold higher hazard risk of implant failure in comparison with simultaneous implant placement (HR = 2.37, 95 % CI 1.02–5.50) [171].
Moreover, some authors demonstrated that the survival of implants placed at the time of sinus augmentation using the lateral window approach is increased with crestal ridge heights >3 mm [8, 44, 96, 174]. In one study, a 55 % reduction in hazard risk of failure was observed comparing 3–4- to <3-mm residual crest and an 86 % reduction comparing ≥5- to <3-mm residual crest [171].
Although it is impossible to determine a clear indication, the majority of authors agree in suggesting immediate implant placement when the residual alveolar bone presents adequate quality and quantity to allow primary stability of implants [96] (Figs. 10.61 and 10.62).
Immediate Loading
Immediate loading in the posterior maxilla following sinus augmentation procedure is particularly controversial. Few studies have been published on immediate loading in the posterior maxilla, due to the low bone density, reduced bone volumes, and risk for low primary stability [171, 175]. However, some authors have suggested that immediate loading is applicable for implants placed in previously augmented sites with high implant survival rates (100 % in 13–24 months of postloading follow-up) [171, 176, 177]. They suggest that early functional loading could positively influence the rapidity of bone mineralization also during the early modeling phase of new bone formation [177].
However, another study reported a reduced implant survival with the immediate loaded implants. Implants that were immediately loaded had a 4–6-fold higher hazard risk of implant failure than those with delayed loading [171] (Fig. 10.63).
Use of Membranes
The placement of resorbable or nonresorbable barrier membranes over the lateral sinus window and graft material is reported to have a positive effect in terms of implant survival and new bone formation [8, 96] (Fig. 10.64).
Different studies reported implant survival rates ranging from 93.1 to 100 % when membranes were used and a survival of 78.1–96.3 % when no membrane was used [40, 49, 51]. However, it was shown in a systematic review that when smooth implants and iliac blocks were not considered (which reduce implant survival rates), the survival rates with and without the use of a barrier membrane were almost identical [40]. When the percentage of vital bone was analyzed, one study showed that results were higher when a membrane was placed over the window [51]. However, one meta-analysis indicated that there was no evidence whether the use of a resorbable membrane over the lateral window would have a positive or negative effect on the amount of total bone volume [47] (Fig. 10.65).
Advantages, Disadvantages, and Complications
Although the sinus augmentation surgery is a predictable treatment, it also carries some risks.
Complications include infection, bleeding, cyst formation, membrane perforations, ridge resorption, sinusitis, and wound dehiscence, among others [8].
The most frequent reported intraoperative complication is Schneiderian membrane perforation, which is reported to be in a range of 4.8–58 % (Table 10.13) (Fig. 10.66).
Controversy exists regarding the implant success rate and perforation of the Schneiderian membrane. Some studies suggest a lower success rate (70 % perforated vs. 100 % nonperforated) when the membrane is perforated (and repaired) [178, 179]. However, other studies show no statistically significant differences in implant survival in the nonperforated versus perforated sinus [180–182].
When a piezoelectric surgical device is used, the incidence of perforation is reported to be lower, 3.6–17.5 % [183–185] (Figs. 10.67, 10.68, and 10.69).
Another important intraoperative complication that can occur is an excessive bleeding due to the trauma to the intraosseous branches of the posterior superior alveolar artery, which can be occasionally encountered during the lateral window procedure in 2 % of the cases [186]. To prevent this, preoperative CT scan evaluations to detect the path of the artery should be performed [187].
The most common reported postoperative complications are sinusitis (2.5 %, ranging from 0 to 27 %) [44, 96, 180] and wound infection (2.9 %, ranging from 0 to 7.4 %) [40, 49, 181], followed by exposures and total or partial loss of the graft (less than 1.9 %) [44, 49, 96, 188].
Also some studies suggest that a history of sinusitis may be related to higher complication rates [171, 188].
The type of graft (particulate vs. block) is suggested to be related as more prone to total or partial loss of the graft, which is considered a postoperative complication [40, 51, 96, 173].
10.4.5.2 Transalveolar
Implant survival rates are reported in a range from 83 to 100 % with the majority of articles reporting values higher than 92 % (Table 10.1), while success rates are reported to be 93.5–97.8 % after a mean of 36 months after prosthetic loading (range 6–93 months) (Table 10.1) (Fig. 10.70).
Intraoral picture showing an osteotome used for performing a transalveolar sinus lift (Reprinted from Patel et al. [216]. Copyright (2015), with permission from Elsevier)
The survival rate of implants placed in conjunction with the augmentation procedure is reported to show no statistical differences in comparison to implants placed in a staged approach [44].
Also the available data did not demonstrate significant differences in survival rates of implants according to different grafting materials [44].
Maxillary sinus floor elevation using the transalveolar approach may be a valid and less invasive supplement to the lateral window technique. A prerequisite for using this technique is that primary implant stability can be achieved [40].
One systematic review showed that implant survival rates were more than 96 and 85.7 % with pretreatment bone heights of ≥5 mm and 3 to 4 mm, respectively [172].
The average measured bone gain was 2.9–3.25 mm (range 2–7 mm) [172].
Membrane perforations are reported to be low, and most of them occurred when the membrane was lifted more than 5 mm [172]. Some authors recommend an endoscopic control when the sinus membrane is lifted >3 mm [172].
Sinus grafting is now considered a safe and well-documented procedure to prepare an environment in which dental implants may have an excellent prognosis [52]. Different sinus elevation techniques do not seem to affect the implant success rates [172].
10.4.6 Socket Preservation
Preclinical and clinical studies have demonstrated that after a tooth extraction, the socket and alveolar ridge suffer a physiological healing process that results in a reduction of its dimension [65], as previously described in Chap. 4.
Therefore, socket preservation techniques have been proposed with the objective of maintaining the hard and soft tissue dimensions after a tooth is extracted, which is particularly important in cases of anterior aesthetic and posterior implant placement, in order to have the best bone and soft tissue availability for achieving a successful final treatment [65, 66, 189] (Fig. 10.71).
If a tooth is nonrestorable and extraction is needed, simultaneous preservation of the socket using different bone materials can help in the maintenance of alveolar height and width [190].
It is evident that, regardless of the surgical procedures and biomaterials used, socket preservation techniques minimized the amount of postextraction bone loss [62, 66, 191] (Fig. 10.72).
Some studies reported an implant survival rate ranging from 90.3 to 100 % after 6–144 months, which is consistent with implant survival rates placed in native bone [192] (Table 10.14). There is no evidence to support the fact that implant placement survival is increased following socket preservation procedures in comparison with unassisted socket healing. The survival, success, and marginal bone levels of implants placed in alveolar ridges following socket preservation procedures are comparable to that of implants placed in untreated sockets [193]. Therefore, the positive influence of the socket preservation therapy may be attributed more to achieving enhanced restorative and aesthetic outcomes, as well as better maintenance of healthy periimplant soft tissues [66].
Different studies showed that immediate implant placement had a failure rate of less than 5 %, which is comparable to delayed placement [8]. However, one systematic review suggested that the insertion of dental implants in fresh extraction sockets affects the implant failure rates (4.75 % in fresh socket and 1.59 % in healed sockets) [189].
Also, the immediate placement of implants into fresh extraction sockets in conjunction with bone augmentation has shown comparable success to that observed in delayed implant placement [8].
No high-level evidence was found in the literature regarding contraindications specific for ridge preservation and if socket preservation requires primary closure [62, 63] (Figs. 10.73, 10.74, 10.75, 10.76, 10.77, 10.78, 10.79, and 10.80).
Conclusions
Many techniques exist for effective bone augmentation. The approach largely is dependent on the extent of the defect and specific procedures to be performed for the implant reconstruction. It is most appropriate to use an evidenced-based approach when a treatment plan is being developed for bone augmentation cases [8]. The capacity of bone grafts to restore original bone volume varies, and the results reported in the literature are contradictory due to differences in observation periods, type and site of reconstruction, timing of implant loading, and, last but not least, site of bone harvesting [133].
A second concern is the adequate adaptation, stabilization, and vascularization of the bone graft, which are critical for graft success [133].
In this chapter, when analyzing the effect of bone grafting materials, specific commercially available bone substitutes have not been assessed. The clinician should be critical and evaluate the available studies in the scientific literature regarding the effectiveness of the product that he/she would use. Doing that, the clinician should ask for randomized clinical trials.
Oversized grafts should be harvested to maintain enough graft volume after the initial resorption phase. If autogenous bone grafts are used, it is highly suggested to use corticocancellous bone blocks. Cancellous bone alone or particulate bone, if not associated with membranes of titanium meshes, does not provide sufficient rigidity to withstand tension from the overlying soft tissues or from the compression by provisional removable dentures and may undergo partial or complete resorption [44].
More surgical challenges arise from vertical augmentations, which can be particularly difficult to reconstruct due to soft tissue collapse over the graft if the space is not maintained; thus, short implants may be a feasible option [108, 113].
Although autogenous bone has been considered the gold standard in the past, more recent studies have shown that vertical and horizontal augmentation can be successfully performed with allogeneic and xenogeneic grafts when properly protected with the appropriate membrane. In this sense, by eliminating the need for a second surgical site to harvest the graft, the morbidy is significantly reduced. Extraoral harvesting sites are related with an increased morbidity and prolonged treatment times in comparison to intraoral harvesting sites [201]. Moreover, the use of growth factors can enhance the success of grafts and provide a faster soft tissue healing [113].
Survival and success rates of implants placed in horizontally and vertically resorbed edentulous ridges reconstructed with block bone grafts are similar to those of implants placed in native bone, in grafted sockets, in distracted sites, in grafted sinus, or with guided bone regeneration [108].
Other surgical options such as LeFort I osteotomy with interpositional bone grafts and microvascular free flaps present even more morbidity and should be limited to extreme atrophy or severe intermaxillary discrepancy not susceptible to be treated with onlay grafts [44].
In conclusion, the clinician should have the enough knowledge and evidence-based data in order to choose the most appropriate technique and materials. The practitioner should also have the capacity to analyze the patient needs and expectations and be aware of his/her skill limitations, being able to develop a comprehensive treatment plan in order to provide the patient with the most proper solution.
References
L. Schropp, A. Wenzel, L. Kostopoulos, T. Karring, Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int. J. Periodontics Restorative Dent. 23(4), 313–323 (2003)
G.I. Benic, C.H. Hammerle, Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 66(1), 13–40 (2014)
T.F. Flemmig, T. Beikler, Decision making in implant dentistry: an evidence-based and decision-analysis approach. Periodontol. 2000 50, 154–172 (2009)
T. Albrektsson, G. Zarb, P. Worthington, A.R. Eriksson, The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1(1), 11–25 (1986)
D. Buser, R. Mericske-Stern, J.P. Bernard, A. Behneke, N. Behneke, H.P. Hirt et al., Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin. Oral Implants Res. 8(3), 161–172 (1997)
I.K. Karoussis, U. Bragger, G.E. Salvi, W. Burgin, N.P. Lang, Effect of implant design on survival and success rates of titanium oral implants: a 10-year prospective cohort study of the ITI Dental Implant System. Clin. Oral Implants Res. 15(1), 8–17 (2004). PubMed PMID: 9586460
M. Nevins, J.T. Mellonig, D.S. Clem 3rd, G.M. Reiser, D.A. Buser, Implants in regenerated bone: long-term survival. Int. J. Periodontics Restorative Dent. 18(1), 34–45 (1998)
B.S. McAllister, K. Haghighat, Bone augmentation techniques. J. Periodontol. 78(3), 377–396 (2007)
J.S. Seibert, Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. Technique and wound healing. Compend. Contin. Educ. Dent. 4(5), 437–453 (1983)
M. Esposito, M.G. Grusovin, P. Felice, G. Karatzopoulos, H.V. Worthington, P. Coulthard Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane. Database. Syst. Rev. (4), CD003607 (2009)
P.V. Giannoudis, I. Pountos, Tissue regeneration. The past, the present and the future. Injury 36 Suppl 4, S2–S5 (2005)
G.M. Calori, E. Mazza, M. Colombo, C. Ripamonti, The use of bone-graft substitutes in large bone defects: any specific needs? Injury 42(Suppl 2), S56–S63 (2011)
M.R. Urist, Bone: formation by autoinduction. Clin. Orthop. Relat. Res. 2002(395), 4–10 (1965)
B. Al-Nawas, E. Schiegnitz, Augmentation procedures using bone substitute materials or autogenous bone – a systematic review and meta-analysis. Eur. J. Oral Implantol. 7(Suppl 2), S219–S234 (2014)
B.L. Eppley, W.S. Pietrzak, M.W. Blanton, Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J. Craniofac. Surg. 16(6), 981–989 (2005)
E. Rosenberg, L.F. Rose, Biologic and clinical considerations for autografts and allografts in periodontal regeneration therapy. Dent. Clin. N. Am. 42(3), 467–490 (1998)
H.F. Nasr, M.E. Aichelmann-Reidy, R.A. Yukna, Bone and bone substitutes. Periodontol. 2000 19, 74–86 (1999)
M.A. Reynolds, M.E. Aichelmann-Reidy, G.L. Branch-Mays, Regeneration of periodontal tissue: bone replacement grafts. Dent. Clin. N. Am. 54(1), 55–71 (2010)
Z. Schwartz, J.T. Mellonig, D.L. Carnes Jr., J. de la Fontaine, D.L. Cochran, D.D. Dean et al., Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J. Periodontol. 67(9), 918–926 (1996)
G.I. Drosos, K.I. Kazakos, P. Kouzoumpasis, D.A. Verettas, Safety and efficacy of commercially available demineralised bone matrix preparations: a critical review of clinical studies. Injury 38(Suppl 4), S13–S21 (2007)
N. Van Assche, S. Michels, I. Naert, M. Quirynen, Randomized controlled trial to compare two bone substitutes in the treatment of bony dehiscences. Clin. Implant Dent. Relat. Res. 15(4), 558–568 (2013)
R.E. Cohen, R.H. Mullarky, B. Noble, R.L. Comeau, M.E. Neiders, Phenotypic characterization of mononuclear cells following anorganic bovine bone implantation in rats. J. Periodontol. 65(11), 1008–1015 (1994)
S. Sartori, M. Silvestri, F. Forni, A. Icaro Cornaglia, P. Tesei, V. Cattaneo, Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin. Oral Implants Res. 14(3), 369–372 (2003)
S. Pardinas Lopez, S. Froum, I. Khouly, Histomorphometric analysis of a biopsy harvested 10 years after maxillary sinus augmentation with anorganic bovine bone matrix and plasma rich in growth factors: a case report. Implant Dent. 24(4), 480–486 (2015)
J.H. Bae, Y.K. Kim, S.G. Kim, P.Y. Yun, J.S. Kim, Sinus bone graft using new alloplastic bone graft material (Osteon)-II: clinical evaluation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109(3), e14–e20 (2010)
J.W. Hwang, J.S. Park, J.S. Lee, U.W. Jung, C.S. Kim, K.S. Cho et al., Comparative evaluation of three calcium phosphate synthetic block bone graft materials for bone regeneration in rabbit calvaria. J. Biomed. Mater. Res. B Appl. Biomater. 100(8), 2044–2052 (2012)
M. Hallman, A. Thor, Bone substitutes and growth factors as an alternative/complement to autogenous bone for grafting in implant dentistry. Periodontol. 2000 47, 172–192 (2008)
H. Tatum Jr., Maxillary and sinus implant reconstructions. Dent. Clin. N. Am. 30(2), 207–229 (1986)
C.M. Schmitt, H. Doering, T. Schmidt, R. Lutz, F.W. Neukam, K.A. Schlegel, Histological results after maxillary sinus augmentation with Straumann(R) BoneCeramic, Bio-Oss(R), Puros(R), and autologous bone. A randomized controlled clinical trial. Clin. Oral Implants Res. 24(5), 576–585 (2013)
S.J. Froum, S.S. Wallace, S.C. Cho, N. Elian, D.P. Tarnow, Histomorphometric comparison of a biphasic bone ceramic to anorganic bovine bone for sinus augmentation: 6- to 8-month postsurgical assessment of vital bone formation. A pilot study. Int. J. Periodontics Restorative Dent. 28(3), 273–281 (2008)
L. Cordaro, D.D. Bosshardt, P. Palattella, W. Rao, G. Serino, M. Chiapasco, Maxillary sinus grafting with Bio-Oss or Straumann Bone Ceramic: histomorphometric results from a randomized controlled multicenter clinical trial. Clin. Oral Implants Res. 19(8), 796–803 (2008)
C. Lindgren, L. Sennerby, A. Mordenfeld, M. Hallman, Clinical histology of microimplants placed in two different biomaterials. Int. J. Oral Maxillofac. Implants 24(6), 1093–1100 (2009)
G. Szabo, L. Huys, P. Coulthard, C. Maiorana, U. Garagiola, J. Barabas et al., A prospective multicenter randomized clinical trial of autogenous bone versus beta-tricalcium phosphate graft alone for bilateral sinus elevation: histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implants 20(3), 371–381 (2005)
J. Wiltfang, K.A. Schlegel, S. Schultze-Mosgau, E. Nkenke, R. Zimmermann, P. Kessler, Sinus floor augmentation with beta-tricalciumphosphate (beta-TCP): does platelet-rich plasma promote its osseous integration and degradation? Clin. Oral Implants Res. 14(2), 213–218 (2003)
W.H. Bell, Resorption characteristics of bone and bone substitutes. Oral Surg. Oral Med. Oral Pathol. 17, 650–657 (1964)
M.V. Thomas, D.A. Puleo, M. Al-Sabbagh, Calcium sulfate: a review. J. Long Term Eff. Med. Implants 15(6), 599–607 (2005)
L.L. Hench, J. Wilson, Surface-active biomaterials. Science 226(4675), 630–636 (1984)
V.V. Valimaki, H.T. Aro, Molecular basis for action of bioactive glasses as bone graft substitute. Scand. J. Surg. SJS 95(2), 95–102 (2006)
F.A. Santos, M.T. Pochapski, M.C. Martins, E.G. Zenobio, L.C. Spolidoro, E. Marcantonio Jr., Comparison of biomaterial implants in the dental socket: histological analysis in dogs. Clin. Implant Dent. Relat. Res. 12(1), 18–25 (2010)
S.S. Jensen, H. Terheyden, Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bonesubstitute materials. Int. J. Oral Maxillofac. Implants 24(Suppl), 218–236 (2009)
A. Friedmann, F.P. Strietzel, B. Maretzki, S. Pitaru, J.P. Bernimoulin, Histological assessment of augmented jaw bone utilizing a new collagen barrier membrane compared to a standard barrier membrane to protect a granular bone substitute material. Clin. Oral Implants Res. 13(6), 587–594 (2002)
J. Torres, F. Tamimi, M.H. Alkhraisat, A. Manchon, R. Linares, J.C. Prados-Frutos et al., Platelet-rich plasma may prevent titanium-mesh exposure in alveolar ridge augmentation with anorganic bovine bone. J. Clin. Periodontol. 37(10), 943–951 (2010)
M. Esposito, M.G. Grusovin, P. Felice, G. Karatzopoulos, H.V. Worthington, P. Coulthard, The efficacy of horizontal and vertical bone augmentation procedures for dental implants – a Cochrane systematic review. Eur. J. Oral Implantol. 2(3), 167–184 (2009)
M. Chiapasco, M. Zaniboni, M. Boisco, Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral Implants Res. 17(Suppl 2), 136–159 (2006)
M. Simion, P. Trisi, A. Piattelli, Vertical ridge augmentation using a membrane technique associated with osseointegrated implants. Int. J. Periodontics Restorative Dent. 14(6), 496–511 (1994)
C. Tinti, S. Parma-Benfenati, G. Polizzi, Vertical ridge augmentation: what is the limit? Int. J. Periodontics Restorative Dent. 16(3), 220–229 (1996)
R.J. Klijn, G.J. Meijer, E.M. Bronkhorst, J.A. Jansen, A meta-analysis of histomorphometric results and graft healing time of various biomaterials compared to autologous bone used as sinus floor augmentation material in humans. Tissue Eng. Part B Rev. 16(5), 493–507 (2010)
J. Handschel, M. Simonowska, C. Naujoks, R.A. Depprich, M.A. Ommerborn, U. Meyer et al., A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 5, 12 (2009)
B.E. Pjetursson, W.C. Tan, M. Zwahlen, N.P. Lang, A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 35(8 Suppl), 216–240 (2008)
E. Nkenke, F. Stelzle, Clinical outcomes of sinus floor augmentation for implant placement using autogenous bone or bone substitutes: a systematic review. Clin. Oral Implants Res. 20(Suppl 4), 124–133 (2009)
S.S. Wallace, S.J. Froum, Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann. Periodontol. Am. Acad. Periodontol. 8(1), 328–343 (2003)
M. Del Fabbro, T. Testori, L. Francetti, R. Weinstein, Systematic review of survival rates for implants placed in the grafted maxillary sinus. Int. J. Periodontics Restorative Dent. 24(6), 565–577 (2004)
M. Peleg, A.K. Garg, C.M. Misch, Z. Mazor, Maxillary sinus and ridge augmentations using a surfacederived autogenous bone graft. J. Maxillofac. Surg. 62(12), 1535–1544 (2004)
E. Anitua, M.H. Alkhraisat, A. Miguel-Sanchez, G. Orive, Surgical correction of horizontal bone defect using the lateral maxillary wall: outcomes of a retrospective study. J. Maxillofac. Surg. 72(4), 683–693 (2014)
E. Nystrom, J. Ahlqvist, J. Gunne, K.E. Kahnberg, 10-year follow-up of onlay bone grafts and implants in severely resorbed maxillae. Int. J. Oral Maxillofac. Surg. 33(3), 258–262 (2004)
B. Klinge, T.F. Flemmig, G. Working, Tissue augmentation and esthetics (Working Group 3). Clin. Oral Implants Res. 20(Suppl 4), 166–170 (2009)
W. Att, J. Bernhart, J.R. Strub, Fixed rehabilitation of the edentulous maxilla: possibilities and clinical outcome. J. Maxillofac. Surg. 67(11 Suppl), 60–73 (2009)
S. Shanbhag, V. Shanbhag, A. Stavropoulos, Volume changes of maxillary sinus augmentations over time: a systematic review. Int. J. Oral Maxillofac. Implants 29(4), 881–892 (2014)
C. Dellavia, S. Speroni, G. Pellegrini, A. Gatto, C. Maiorana, A new method to evaluate volumetric changes in sinus augmentation procedure. Clin. Implant Dent. Relat. Res. 16(5), 684–690 (2014)
E.S. Kim, S.Y. Moon, S.G. Kim, H.C. Park, J.S. Oh, Three-dimensional volumetric analysis after sinus grafts. Implant Dent. 22(2), 170–174 (2013)
S. Kuhl, M. Payer, R. Kirmeier, A. Wildburger, S. Acham, N. Jakse, The influence of particulated autogenous bone on the early volume stability of maxillary sinus grafts with biphasic calcium phosphate: a randomized clinical trial. Clin. Implant Dent. Relat. Res. 17(1), 173–178 (2015)
R. Horowitz, D. Holtzclaw, P.S. Rosen, A review on alveolar ridge preservation following tooth extraction. J. Evid. Based Dent. Pract. 12(3 Suppl), 149–160 (2012)
C.H. Hammerle, M.G. Araujo, M. Simion, C.G. Osteology, Evidence-based knowledge on the biology and treatment of extraction sockets. Clin. Oral Implants Res. 23(Suppl 5), 80–82 (2012)
J.M. Ten Heggeler, D.E. Slot, G.A. Van der Weijden, Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: a systematic review. Clin. Oral Implants Res. 22(8), 779–788 (2011)
G. Avila-Ortiz, S. Elangovan, K.W. Kramer, D. Blanchette, D.V. Dawson, Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J. Dent. Res. 93(10), 950–958 (2014)
F. Vignoletti, P. Matesanz, D. Rodrigo, E. Figuero, C. Martin, M. Sanz, Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implants Res. 23 Suppl 5, 22–38 (2012)
M. Del Fabbro, S. Corbella, S. Taschieri, L. Francetti, R. Weinstein, Autologous platelet concentrate for post-extraction socket healing: a systematic review. Eur. J. Oral Implantol. 7(4), 333–344 (2014)
M. Mozzati, G. Gallesio, S. di Romana, L. Bergamasco, R. Pol, Efficacy of plasma-rich growth factor in the healing of postextraction sockets in patients affected by insulin-dependent diabetes mellitus. J. Maxillofac. Surg. 72(3), 456–462 (2014)
R. Alissa, M. Esposito, K. Horner, R. Oliver, The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur. J. Oral Implantol. 3(2), 121–134 (2010)
O.K. Ogundipe, V.I. Ugboko, F.J. Owotade, Can autologous platelet-rich plasma gel enhance healing after surgical extraction of mandibular third molars? J. Maxillofac. Surg. 69(9), 2305–2310 (2011)
E. Anitua, A. Murias-Freijo, M.H. Alkhraisat, G. Orive, Clinical, radiographical, and histological outcomes of plasma rich in growth factors in extraction socket: a randomized controlled clinical trial. Clin. Oral Investig. 19(3), 589–600 (2015)
E. Anitua, Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implants 14(4), 529–535 (1999)
S.E.M.R. Lynch, M. Nevins, L.A. Winsley-Lynch, Platelet-rich plasma: a source of multiple autolgous growth factors for bone grafts in: tissue engineering: applications in maxillofacial surgery and periodontics (Quintessence, Chicago, 1999)
A. Rodriguez, G.E. Anastassov, H. Lee, D. Buchbinder, H. Wettan, Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J. Maxillofac. Surg. 61(2), 157–163 (2003)
M. Del Fabbro, M. Bortolin, S. Taschieri, R.L. Weinstein, Effect of autologous growth factors in maxillary sinus augmentation: a systematic review. Clin. Implant Dent. Relat. Res. 15(2), 205–216 (2013)
J. Torres, F. Tamimi, P.P. Martinez, M.H. Alkhraisat, R. Linares, G. Hernandez et al., Effect of platelet-rich plasma on sinus lifting: a randomized-controlled clinical trial. J. Clin. Periodontol. 36(8), 677–687 (2009)
S. Taschieri, T. Testori, S. Corbella, R. Weinstein, L. Francetti, A. Di Giancamillo et al., Platelet-rich plasma and deproteinized bovine bone matrix in maxillary sinus lift surgery: a split-mouth histomorphometric evaluation. Implant Dent. 24(5), 592–597 (2015)
L. Ricci, V. Perrotti, L. Ravera, A. Scarano, A. Piattelli, G. Iezzi, Rehabilitation of deficient alveolar ridges using titanium grids before and simultaneously with implant placement: a systematic review. J. Periodontol. 84(9), 1234–1242 (2013)
E. Anitua, M.H. Alkhraisat, G. Orive, Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control. Release 157(1), 29–38 (2012)
E. Anitua, M. Sanchez, G. Orive, Potential of endogenous regenerative technology for in situ regenerative medicine. Adv. Drug Deliv. Rev. 62(7–8), 741–752 (2010)
L. Torrecillas-Martinez, A. Monje, M.A. Pikos, I. Ortega-Oller, F. Suarez, P. Galindo-Moreno et al., Effect of rhBMP-2 upon maxillary sinus augmentation: a comprehensive review. Implant Dent. 22(3), 232–237 (2013)
R.G. Triplett, M. Nevins, R.E. Marx, D.B. Spagnoli, T.W. Oates, P.K. Moy et al., Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Maxillofac. Surg. 67(9), 1947–1960 (2009)
D.W. Kao, A. Kubota, M. Nevins, J.P. Fiorellini, The negative effect of combining rhBMP-2 and Bio-Oss on bone formation for maxillary sinus augmentation. Int. J. Periodontics Restorative Dent. 32(1), 61–67 (2012)
R.M. de Freitas, C. Susin, R. Spin-Neto, C. Marcantonio, U.M. Wikesjo, L.A. Pereira et al., Horizontal ridge augmentation of the atrophic anterior maxilla using rhBMP-2/ACS or autogenous bone grafts: a proof-of-concept randomized clinical trial. J. Clin. Periodontol. 40(10), 968–975 (2013)
R.E. Jung, S.I. Windisch, A.M. Eggenschwiler, D.S. Thoma, F.E. Weber, C.H. Hammerle, A randomized-controlled clinical trial evaluating clinical and radiological outcomes after 3 and 5 years of dental implants placed in bone regenerated by means of GBR techniques with or without the addition of BMP-2. Clin. Oral Implants Res. 20(7), 660–666 (2009)
P.I. Branemark, J. Lindstrom, O. Hallen, U. Breine, P.H. Jeppson, A. Ohman, Reconstruction of the defective mandible. Scand. J. Plast. Reconstr. Surg. 9(2), 116–128 (1975)
M.A. Pikos, Mandibular block autografts for alveolar ridge augmentation. Atlas Oral Maxillofac. Surg. Clin. North Am. 13(2), 91–107 (2005)
A. Montazem, D.V. Valauri, H. St-Hilaire, D. Buchbinder, The mandibular symphysis as a donor site in maxillofacial bone grafting: a quantitative anatomic study. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 58(12), 1368–1371 (2000)
R.F. Neiva, R. Gapski, H.L. Wang, Morphometric analysis of implant-related anatomy in Caucasian skulls. J. Periodontol. 75(8), 1061–1067 (2004)
N.S.N. Toscano, D. Holztclaw, The art of block grafting. A review of the surgical protocol for reconstruction of alveolar ridge deficiency. J. Implant. Adv. Clin. Dent. 2(2), 45–66 (2010)
H. Burchardt, The biology of bone graft repair. Clin. Orthop. Relat. Res. 174, 28–42 (1983)
C.M. Misch, Use of the mandibular ramus as a donor site for onlay bone grafting. J. Oral Implantol. 26(1), 42–49 (2000)
W. Ozaki, S.R. Buchman, Volume maintenance of onlay bone grafts in the craniofacial skeleton: micro-architecture versus embryologic origin. Plast. Reconstr. Surg. 102(2), 291–299 (1998)
J.E. Zins, L.A. Whitaker, Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast. Reconstr. Surg. 72(6), 778–785 (1983)
C.M. Misch, Ridge augmentation using mandibular ramus bone grafts for the placement of dental implants: presentation of a technique. Pract. Periodontics. Aesthet. Dent. PPAD 8(2), 127–135 (1996); quiz 38
M. Chiapasco, P. Casentini, M. Zaniboni, Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implants 24(Suppl), 237–259 (2009)
C.M. Misch, Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int. J. Oral Maxillofac. Implants 12(6), 767–776 (1997)
A. Acocella, R. Bertolai, M. Colafranceschi, R. Sacco, Clinical, histological and histomorphometric evaluation of the healing of mandibular ramus bone block grafts for alveolar ridge augmentation before implant placement. J. Craniomaxillofac. Surg. Off. Publ. Eur. Assoc. Craniomaxillofac. Surg. 38(3), 222–230 (2010)
E. Anitua, A. Murias-Freijo, M.H. Alkhraisat, Implant site under-preparation to compensate the remodeling of an autologous bone block graft. J. Craniofac. Surg. 26(5), e374–e377 (2015)
L. Cordaro, D.S. Amade, M. Cordaro, Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin. Oral Implants Res. 13(1), 103–111 (2002)
M. Roccuzzo, G. Ramieri, M.C. Spada, S.D. Bianchi, S. Berrone, Vertical alveolar ridge augmentation by means of a titanium mesh and autogenous bone grafts. Clin. Oral Implants Res. 15(1), 73–81 (2004)
D. Schwartz-Arad, L. Levin, L. Sigal, Surgical success of intraoral autogenous block onlay bone grafting for alveolar ridge augmentation. Implant Dent. 14(2), 131–138 (2005)
T. von Arx, D. Buser, Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin. Oral Implants Res. 17(4), 359–366 (2006)
M. Roccuzzo, G. Ramieri, M. Bunino, S. Berrone, Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: a controlled clinical trial. Clin. Oral Implants Res. 18(3), 286–294 (2007)
P. Proussaefs, J. Lozada, A. Kleinman, M.D. Rohrer, The use of ramus autogenous block grafts for vertical alveolar ridge augmentation and implant placement: a pilot study. Int. J. Oral Maxillofac. Implants 17(2), 238–248 (2002)
M. Chiapasco, S. Abati, E. Romeo, G. Vogel, Clinical outcome of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin. Oral Implants Res. 10(4), 278–288 (1999)
H. Antoun, J.M. Sitbon, H. Martinez, P. Missika, A prospective randomized study comparing two techniques of bone augmentation: onlay graft alone or associated with a membrane. Clin. Oral Implants Res. 12(6), 632–639 (2001)
A. Aloy-Prosper, D. Penarrocha-Oltra, M. Penarrocha-Diago, M. Penarrocha-Diago, The outcome of intraoral onlay block bone grafts on alveolar ridge augmentations: a systematic review. Med. Oral Patol. Oral Cir. Bucal 20(2), e251–e258 (2015)
J. Clavero, S. Lundgren, Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin. Implant Dent. Relat. Res. 5(3), 154–160 (2003)
T. von Arx, J. Hafliger, V. Chappuis, Neurosensory disturbances following bone harvesting in the symphysis: a prospective clinical study. Clin. Oral Implants Res. 16(4), 432–439 (2005)
E. Nkenke, F.W. Neukam, Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur. J. Oral Implantol. 7(Suppl 2), S203–S217 (2014)
J.H. Fu, H.L. Wang, Horizontal bone augmentation: the decision tree. Int. J. Periodontics Restorative Dent. 31(4), 429–436 (2011)
P.J. Louis, Bone grafting the mandible. Dent. Clin. N. Am. 55(4), 673–695 (2011)
R. Crespi, R. Vinci, P. Cappare, E. Gherlone, G.E. Romanos, Calvarial versus iliac crest for autologous bone graft material for a sinus lift procedure: a histomorphometric study. Int. J. Oral Maxillofac. Implants 22(4), 527–532 (2007)
R.E. Marx, Bone harvest from the posterior ilium. Atlas Oral Maxillofac. Surg. Clin. North Am. 13(2), 109–118 (2005)
K.J. Zouhary, Bone graft harvesting from distant sites: concepts and techniques. Oral Maxillofac. Surg. Clin. North Am. 22(3), 301–316, v (2010)
M. Almaiman, H.H. Al-Bargi, P. Manson, Complication of anterior iliac bone graft harvesting in 372 adult patients from may 2006 to may 2011 and a literature review. Craniomaxillofac. Trauma. Reconstr. 6(4), 257–266 (2013)
T. Fretwurst, L. Wanner, S. Nahles, J.D. Raguse, A. Stricker, M.C. Metzger et al., A prospective study of factors influencing morbidity after iliac crest harvesting for oral onlay grafting. J. Craniomaxillofac. Surg. Off. Publ. Eur. Assoc. Craniomaxillofac. Surg. 43(5), 705–709 (2015)
R. Dimitriou, G.I. Mataliotakis, A.G. Angoules, N.K. Kanakaris, P.V. Giannoudis, Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury 42(Suppl 2), S3–S15 (2011)
M. Clementini, A. Morlupi, C. Agrestini, L. Ottria, Success rate of dental implants inserted in autologous bone graft regenerated areas: a systematic review. ORAL Implantol. 4(3–4), 3–10 (2011)
P. Felice, R. Pistilli, G. Lizio, G. Pellegrino, A. Nisii, C. Marchetti, Inlay versus onlay iliac bone grafting in atrophic posterior mandible: a prospective controlled clinical trial for the comparison of two techniques. Clin. Implant Dent. Relat. Res. 11(Suppl 1), e69–e82 (2009)
M. Chiapasco, P. Casentini, M. Zaniboni, Implants in reconstructed bone: a comparative study on the outcome of Straumann(R) tissue level and bone level implants placed in vertically deficient alveolar ridges treated by means of autogenous onlay bone grafts. Clin. Implant Dent. Relat. Res. 16(1), 32–50 (2014)
J.M. Marchena, M.S. Block, J.D. Stover, Tibial bone harvesting under intravenous sedation: morbidity and patient experiences. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 60(10), 1151–1154 (2002)
R.M. O'Keeffe Jr., B.L. Riemer, S.L. Butterfield, Harvesting of autogenous cancellous bone graft from the proximal tibial metaphysis. A review of 230 cases. J. Orthop. Trauma 5(4), 469–474 (1991)
A. Moreira-Gonzalez, F.E. Papay, J.E. Zins, Calvarial thickness and its relation to cranial bone harvest. Plast. Reconstr. Surg. 117(6), 1964–1971 (2006)
W. Ozaki, S.R. Buchman, S.A. Goldstein, D.P. Fyhrie, A comparative analysis of the microarchitecture of cortical membranous and cortical endochondral onlay bone grafts in the craniofacial skeleton. Plast. Reconstr. Surg. 104(1), 139–147 (1999)
M.T. Iturriaga, C.C. Ruiz, Maxillary sinus reconstruction with calvarium bone grafts and endosseous implants. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 62(3), 344–347 (2004)
L.M. Scheerlinck, M.S. Muradin, A. van der Bilt, G.J. Meijer, R. Koole, E.M. Van Cann, Donor site complications in bone grafting: comparison of iliac crest, calvarial, and mandibular ramus bone. Int. J. Oral Maxillofac. Implants 28(1), 222–227 (2013)
S. Touzet, J. Ferri, T. Wojcik, G. Raoul, Complications of calvarial bone harvesting for maxillofacial reconstructions. J. Craniofac. Surg. 22(1), 178–181 (2011)
M. Chiapasco, P. Casentini, M. Zaniboni, E. Corsi, Evaluation of peri-implant bone resorption around Straumann Bone Level implants placed in areas reconstructed with autogenous vertical onlay bone grafts. Clin. Oral Implants Res. 23(9), 1012–1021 (2012)
R. Adell, B. Eriksson, U. Lekholm, P.I. Branemark, T. Jemt, Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implants 5(4), 347–359 (1990)
F. Carinci, A. Farina, U. Zanetti, R. Vinci, S. Negrini, G. Calura et al., Alveolar ridge augmentation: a comparative longitudinal study between calvaria and iliac crest bone grafrs. J. Oral Implantol. 31(1), 39–45 (2005)
M. Chiapasco, M. Zaniboni, L. Rimondini, Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: a 2-4-year prospective study on humans. Clin. Oral Implants Res. 18(4), 432–440 (2007)
U. Kuchler, T. von Arx, Horizontal ridge augmentation in conjunction with or prior to implant placement in the anterior maxilla: a systematic review. Int. J. Oral Maxillofac. Implants 29(Suppl), 14–24 (2014)
D.M. Yates, H.C. Brockhoff 2nd, R. Finn, C. Phillips, Comparison of intraoral harvest sites for corticocancellous bone grafts. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 71(3), 497–504 (2013)
G.M. Raghoebar, L. Meijndert, W.W. Kalk, A. Vissink, Morbidity of mandibular bone harvesting: a comparative study. Int. J. Oral Maxillofac. Implants 22(3), 359–365 (2007)
R.M. Wood, D.L. Moore, Grafting of the maxillary sinus with intraorally harvested autogenous bone prior to implant placement. Int. J. Oral Maxillofac. Implants 3(3), 209–214 (1988)
R. Koole, H. Bosker, F.N. van der Dussen, Late secondary autogenous bone grafting in cleft patients comparing mandibular (ectomesenchymal) and iliac crest (mesenchymal) grafts. J. Craniomaxillofac. Surg. Off. Publ. Eur. Assoc. Craniomaxillofac. Surg. 17(Suppl 1), 28–30 (1989)
J.F. Kusiak, J.E. Zins, L.A. Whitaker, The early revascularization of membranous bone. Plast. Reconstr. Surg. 76(4), 510–516 (1985)
U. Breine, P.I. Branemark, Reconstruction of alveolar jaw bone. An experimental and clinical study of immediate and preformed autologous bone grafts in combination with osseointegrated implants. Scand. J. Plast. Reconstr. Surg. 14(1), 23–48 (1980)
F. Brugnami, A. Caiazzo, C. Leone, Local intraoral autologous bone harvesting for dental implant treatment: alternative sources and criteria of choice. Keio J. Med. 58(1), 24–28 (2009)
G. Widmark, B. Andersson, C.J. Ivanoff, Mandibular bone graft in the anterior maxilla for single-tooth implants. Presentation of surgical method. Int. J. Oral Maxillofac. Surg. 26(2), 106–109 (1997)
J. Waasdorp, M.A. Reynolds, Allogeneic bone onlay grafts for alveolar ridge augmentation: a systematic review. Int. J. Oral Maxillofac. Implants 25(3), 525–531 (2010)
A. Monje, M.A. Pikos, H.L. Chan, F. Suarez, J. Gargallo-Albiol, F. Hernandez-Alfaro et al., On the feasibility of utilizing allogeneic bone blocks for atrophic maxillary augmentation. Biomed. Res. Int. 2014, 814578 (2014)
J.D. Keith Jr., P. Petrungaro, J.A. Leonetti, C.W. Elwell, K.J. Zeren, C. Caputo et al., Clinical and histologic evaluation of a mineralized block allograft: results from the developmental period (2001-2004). Int. J. Periodontics Restorative Dent. 26(4), 321–327 (2006)
M. Peleg, Y. Sawatari, R.N. Marx, J. Santoro, J. Cohen, P. Bejarano et al., Use of corticocancellous allogeneic bone blocks for augmentation of alveolar bone defects. Int. J. Oral Maxillofac. Implants 25(1), 153–162 (2010)
L.A. Hurley, F.E. Stinchfield, A.L. Bassett, W.H. Lyon, The role of soft tissues in osteogenesis. An experimental study of canine spine fusions. J. Bone Joint Surg. Am. 41-A, 1243–1254 (1959)
S. Nyman, Bone regeneration using the principle of guided tissue regeneration. J. Clin. Periodontol. 18(6), 494–498 (1991)
T. Karring, S. Nyman, J. Lindhe, Healing following implantation of periodontitis affected roots into bone tissue. J. Clin. Periodontol. 7(2), 96–105 (1980)
S. Nyman, T. Karring, J. Lindhe, S. Planten, Healing following implantation of periodontitis-affected roots into gingival connective tissue. J. Clin. Periodontol. 7(5), 394–401 (1980)
C. Dahlin, U. Lekholm, W. Becker, B. Becker, K. Higuchi, A. Callens et al., Treatment of fenestration and dehiscence bone defects around oral implants using the guided tissue regeneration technique: a prospective multicenter study. Int. J. Oral Maxillofac. Implants 10(3), 312–318 (1995)
C. Dahlin, A. Linde, J. Gottlow, S. Nyman, Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 81(5), 672–676 (1988)
D. Buser, K. Dula, N.P. Lang, S. Nyman, Longterm stability of osseointegrated implants in bone regenerated with the membrane technique. 5-year results of a prospective study with 12 implants. Clin. Oral Implants Res. 7(2), 175–183 (1996)
N.U. Zitzmann, P. Scharer, C.P. Marinello, Longterm results of implants treated with guided bone regeneration: a 5-year prospective study. Int. J. Oral Maxillofac. Implants 16(3), 355–366 (2001)
C. Dahlin, U. Lekholm, A. Linde, Membraneinduced bone augmentation at titanium implants. A report on ten fixtures followed from 1 to 3 years after loading. Int. J. Periodontics Restorative Dent. 11(4), 273–281 (1991)
P.J. Louis, R. Gutta, N. Said-Al-Naief, A.A. Bartolucci, Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 66(2), 235–245 (2008)
M.S. Block, C.J. Haggerty, Interpositional osteotomy for posterior mandible ridge augmentation. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 67(11 Suppl), 31–39 (2009)
T. Vercellotti, Piezoelectric surgery in implantology: a case report – a new piezoelectric ridge expansion technique. Int. J. Periodontics Restorative Dent. 20(4), 358–365 (2000)
M.A. Bassetti, R.G. Bassetti, D.D. Bosshardt, The alveolar ridge splitting/expansion technique: a systematic review. Clin. Oral Implants Res. 27(3), 310–324 (2016)
M. Simion, M. Baldoni, D. Zaffe, Jawbone enlargement using immediate implant placement associated with a split-crest technique and guided tissue regeneration. Int. J. Periodontics Restorative Dent. 12(6), 462–473 (1992)
Y.L. Tang, J. Yuan, Y.L. Song, W. Ma, X. Chao, D.H. Li, Ridge expansion alone or in combination with guided bone regeneration to facilitate implant placement in narrow alveolar ridges: a retrospective study. Clin. Oral Implants Res. 26(2), 204–211 (2015)
N. Donos, N. Mardas, V. Chadha, Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J. Clin. Periodontol. 35(8 Suppl), 173–202 (2008)
O.T. Jensen, D.R. Cullum, D. Baer, Marginal bone stability using 3 different flap approaches for alveolar split expansion for dental implants: a 1-year clinical study. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 67(9), 1921–1930 (2009)
D.S. Sohn, H.J. Lee, J.U. Heo, J.W. Moon, I.S. Park, G.E. Romanos, Immediate and delayed lateral ridge expansion technique in the atrophic posterior mandibular ridge. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 68(9), 2283–2290 (2010)
J.P. Fiorellini, M.L. Nevins, Localized ridge augmentation/preservation. A systematic review. Ann. Periodontol. Am. Acad. Periodontol. 8(1), 321–327 (2003)
A. Sethi, T. Kaus, Maxillary ridge expansion with simultaneous implant placement: 5-year results of an ongoing clinical study. Int. J. Oral Maxillofac. Implants 15(4), 491–499 (2000)
R. Kolerman, J. Nissan, H. Tal, Combined osteotome-induced ridge expansion and guided bone regeneration simultaneous with implant placement: a biometric study. Clin. Implant Dent. Relat. Res. 16(5), 691–704 (2014)
D.J. Holtzclaw, N.J. Toscano, P.S. Rosen, Reconstruction of posterior mandibular alveolar ridge deficiencies with the piezoelectric hinge-assisted ridge split technique: a retrospective observational report. J. Periodontol. 81(11), 1580–1586 (2010)
O.T. Jensen, R. Cockrell, L. Kuhike, C. Reed, Anterior maxillary alveolar distraction osteogenesis: a prospective 5-year clinical study. Int. J. Oral Maxillofac. Implants 17(1), 52–68 (2002)
P.J. Boyne, R.A. James, Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 38(8), 613–616 (1980)
I. Khouly, S. Pardiñas López, I. Aliaga, J. Pardiñas Arias, S. Froum. Long-term implant survival following 100 maxillary sinus augmentations using plasma rich in growth factors. Under Review in Implant Dentistry. 2016
D. Emmerich, W. Att, C. Stappert, Sinus floor elevation using osteotomes: a systematic review and meta-analysis. J. Periodontol. 76(8), 1237–1251 (2005)
D.C. Tong, K. Rioux, M. Drangsholt, O.R. Beirne, A review of survival rates for implants placed in grafted maxillary sinuses using meta-analysis. Int. J. Oral Maxillofac. Implants 13(2), 175–182 (1998)
S. Corbella, S. Taschieri, M. Del Fabbro, Longterm outcomes for the treatment of atrophic posterior maxilla: a systematic review of literature. Clin. Implant Dent. Relat. Res. 17(1), 120–132 (2015)
G. Luongo, R. Di Raimondo, P. Filippini, F. Gualini, C. Paoleschi, Early loading of sandblasted, acid-etched implants in the posterior maxilla and mandible: a 1-year follow-up report from a multicenter 3-year prospective study. Int. J. Oral Maxillofac. Implants 20(1), 84–91 (2005)
C.Y. Lee, M.D. Rohrer, H.S. Prasad, Immediate loading of the grafted maxillary sinus using platelet rich plasma and autogenous bone: a preliminary study with histologic and histomorphometric analysis. Implant Dent. 17(1), 59–73 (2008)
G. Cricchio, M. Imburgia, L. Sennerby, S. Lundgren, Immediate loading of implants placed simultaneously with sinus membrane elevation in the posterior atrophic maxilla: a two-year follow-up study on 10 patients. Clin. Implant Dent. Relat. Res. 16(4), 609–617 (2014)
P. Proussaefs, J. Lozada, J. Kim, M.D. Rohrer, Repair of the perforated sinus membrane with a resorbable collagen membrane: a human study. Int. J. Oral Maxillofac. Implants 19(3), 413–420 (2004)
F. Hernandez-Alfaro, M.M. Torradeflot, C. Marti, Prevalence and management of Schneiderian membrane perforations during sinus-lift procedures. Clin. Oral Implants Res. 19(1), 91–98 (2008)
D. Schwartz-Arad, R. Herzberg, E. Dolev, The prevalence of surgical complications of the sinus graft procedure and their impact on implant survival. J. Periodontol. 75(4), 511–516 (2004)
A. Barone, S. Santini, L. Sbordone, R. Crespi, U. Covani, A clinical study of the outcomes and complications associated with maxillary sinus augmentation. Int. J. Oral Maxillofac. Implants 21(1), 81–85 (2006)
S.J. Froum, I. Khouly, G. Favero, S.C. Cho, Effect of maxillary sinus membrane perforation on vital bone formation and implant survival: a retrospective study. J. Periodontol. 84(8), 1094–1099 (2013)
D.S. Weitz, A. Geminiani, D.E. Papadimitriou, C. Ercoli, J.G. Caton, The incidence of membrane perforation during sinus floor elevation using sonic instruments: a series of 40 cases. Int. J. Periodontics Restorative Dent. 34(1), 105–112 (2014)
S.S. Wallace, Z. Mazor, S.J. Froum, S.C. Cho, D.P. Tarnow, Schneiderian membrane perforation rate during sinus elevation using piezosurgery: clinical results of 100 consecutive cases. Int. J. Periodontics Restorative Dent. 27(5), 413–419 (2007)
N.J. Toscano, D. Holtzclaw, P.S. Rosen, The effect of piezoelectric use on open sinus lift perforation: a retrospective evaluation of 56 consecutively treated cases from private practices. J. Periodontol. 81(1), 167–171 (2010)
P. Fugazzotto, P.R. Melnick, M. Al-Sabbagh, Complications when augmenting the posterior maxilla. Dent. Clin. N. Am. 59(1), 97–130 (2015)
N. Elian, S. Wallace, S.C. Cho, Z.N. Jalbout, S. Froum, Distribution of the maxillary artery as it relates to sinus floor augmentation. Int. J. Oral Maxillofac. Implants 20(5), 784–787 (2005)
J.C. Moreno Vazquez, A.S. Gonzalez de Rivera, H.S. Gil, R.S. Mifsut, Complication rate in 200 consecutive sinus lift procedures: guidelines for prevention and treatment. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 72(5), 892–901 (2014)
D.P. Tarnow, R.N. Eskow, Preservation of implant esthetics: soft tissue and restorative considerations. J. Esthet. Dent. 8(1), 12–19 (1996)
B.K. Bartee, Extraction site reconstruction for alveolar ridge preservation. Part 1: rationale and materials selection. J. Oral Implantol. 27(4), 187–193 (2001)
G. Vittorini Orgeas, M. Clementini, V. De Risi, M. de Sanctis, Surgical techniques for alveolar socket preservation: a systematic review. Int. J. Oral Maxillofac. Implants 28(4), 1049–1061 (2013)
N.P. Lang, L. Pun, K.Y. Lau, K.Y. Li, M.C. Wong, A systematic review on survival and success rates of implants placed immediately into fresh extraction sockets after at least 1 year. Clin. Oral Implants Res. 23(Suppl 5), 39–66 (2012)
N. Mardas, A. Trullenque-Eriksson, N. MacBeth, A. Petrie, N. Donos, Does ridge preservation following tooth extraction improve implant treatment outcomes: a systematic review: Group 4: therapeutic concepts & methods. Clin. Oral Implants Res. 26(Suppl 11), 180–201 (2015)
T.L. Aghaloo, P.K. Moy, Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int. J. Oral Maxillofac. Implants 22(Suppl), 49–70 (2007)
W.C. Tan, N.P. Lang, M. Zwahlen, B.E. Pjetursson, A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. Part II: transalveolar technique. J. Clin. Periodontol. 35(8 Suppl), 241–254 (2008)
M.A. Pikos, Facilitating implant placement with chin grafts as donor sites for maxillary bone augmentation – part I. Dent. Implantol. Update 6(12), 89–92 (1995)
M.A. Pikos, Chin grafts as donor sites for maxillary bone augmentation – part II. Dent. Implantol. Update 7(1), 1–4 (1996)
M.A. Pikos, Block autografts for localized ridge augmentation: part I. The posterior maxilla. Implant Dent. 8(3), 279–285 (1999)
C. Maiorana, M. Beretta, S. Salina, F. Santoro, Reduction of autogenous bone graft resorption by means of bio-oss coverage: a prospective study. Int. J. Periodontics Restorative Dent. 25(1), 19–25 (2005)
F.A. Alerico, S.R. Bernardes, F.N. Fontao, G.F. Diez, J.H. Alerico, M. Claudino, Prospective tomographic evaluation of autogenous bone resorption harvested from mandibular ramus in atrophic maxilla. J. Craniofac. Surg. 25(6), e543–e546 (2014)
R. Adell, U. Lekholm, K. Grondahl, P.I. Branemark, J. Lindstrom, M. Jacobsson, Reconstruction of severely resorbed edentulous maxillae using osseointegrated fixtures in immediate autogenous bone grafts. Int. J. Oral Maxillofac. Implants 5(3), 233–246 (1990)
L. Levin, D. Nitzan, D. Schwartz-Arad, Success of dental implants placed in intraoral block bone grafts. J. Periodontol. 78(1), 18–21 (2007)
M. Penarrocha-Diago, A. Aloy-Prosper, D. Penarrocha-Oltra, J.L. Guirado, M. Penarrocha-Diago, Localized lateral alveolar ridge augmentation with block bone grafts: simultaneous versus delayed implant placement: a clinical and radiographic retrospective study. Int. J. Oral Maxillofac. Implants 28(3), 846–853 (2013)
G.M. Calori, M. Colombo, E.L. Mazza, S. Mazzola, E. Malagoli, G.V. Mineo, Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury 45(Suppl 6), S116–S120 (2014)
J. Blanco, A. Alonso, M. Sanz, Long-term results and survival rate of implants treated with guided bone regeneration: a 5-year case series prospective study. Clin. Oral Implants Res. 16(3), 294–301 (2005)
S. Bernstein, J. Cooke, P. Fotek, H.L. Wang, Vertical bone augmentation: where are we now? Implant Dent. 15(3), 219–228 (2006)
M. Chiapasco, U. Consolo, A. Bianchi, P. Ronchi, Alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: a multicenter prospective study on humans. Int. J. Oral Maxillofac. Implants 19(3), 399–407 (2004)
S.A. Zijderveld, J.P. van den Bergh, E.A. Schulten, C.M. ten Bruggenkate, Anatomical and surgical findings and complications in 100 consecutive maxillary sinus floor elevation procedures. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 66(7), 1426–1438 (2008)
L. Schwarz, V. Schiebel, M. Hof, C. Ulm, G. Watzek, B. Pommer, Risk factors of membrane perforation and postoperative complications in sinus floor elevation surgery: review of 407 augmentation procedures. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 73(7), 1275–1282 (2015)
K. Shiffler, D. Lee, T. Aghaloo, P.K. Moy, J. Pi-Anfruns, Sinus membrane perforations and the incidence of complications: a retrospective study from a residency program. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 120(1), 10–14 (2015)
D. Kademani, E. Keller, Iliac crest grafting for mandibular reconstruction. Atlas. Oral. Maxillofac. Surg. Clin. N. Am. 14, 161–170 (2006)
P.S. Tiwana, G.M. Kushner, R.H. Haug. Maxillary sinus augmentation. Dent. Clin. N. Am. 50, 409–424 (2006)
R.L. Ruiz et al., Cranial bone grafts: craniomaxillofacial applications and harvesting techniques. Atlas. Oral. Maxillofac. Surg. Clin. North. Am. 13, 127–137 (2005)
R. Gonzalez-Garcıa, F. Monje, Alveolar split osteotomy for the treatment of the severe narrow ridge maxillary atrophy: a modified technique. Int. J. Oral Maxillofac. Surg. 40, 57–64 (2011)
E.L. van der Mark et al., Reconstruction of an atrophic maxilla: comparison of two methods. Br.J. Oral Maxillofac. Surg. 49, 198–202 (2011)
P. Sarav et al., Resonance frequency analysis of sinus augmentation by osteotome sinus floor elevation and lateral window technique. J. Oral. Maxillofac. Surg. 73, 1920–1925 (2015)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pardiñas López, S., Anitua, E., Alkhraisat, M.H. (2016). Pre-Implant Reconstructive Surgery. In: Iocca, O. (eds) Evidence-Based Implant Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-319-26872-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-26872-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26870-5
Online ISBN: 978-3-319-26872-9
eBook Packages: MedicineMedicine (R0)