Abstract
The participation of myeloid cells in tumor progression and metastasis has been known for a long time. The role of M2 macrophages, tolerogenic DCs, and N2 neutrophils in tumor immunology has been researched extensively. About 10 years ago, a “re-discovered” new myeloid player named myeloid-derived suppressor cell (MDSC) was put on the spot. However, its precise origin and nature was a subject of some scientific debate. MDSCs turned out to be highly heterogeneous, especially in humans, and exhibiting cancer type-specific properties and characteristics. And despite all recent advances in MDSC research, many questions remain unanswered. In this chapter we will summarize the main subjects addressed in this book and point out the questions that remain unanswered.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Inmunosuppression
- PDL1
- PD1
- Therapeutic antibodies
- Signaling pathways
- Kinase inhibitors
- Chemotherapy
- Immunotherapy
7.1 Myeloid Cells and Cancer
The tumor microenvironment is composed not only by cancerous cells, but also other associated cell types including fibroblasts, endothelial cells, and infiltrating immune cells. Within the tumor, there is a balance between cells with antitumor capacities and immunosuppressive properties. The outcome is usually favorable for immunosuppressive cells, which also exert strong pro-angiogenic effects and accelerate tumor growth and metastasis.
-
Do MDSCs comprise a distinct myeloid lineage?
As discussed in the first chapter, the infiltration of tumors by myeloid cells was observed and described before the 70s [1]. In fact, infiltration of tumors with these cells was a sign of poor prognosis rather than proof of antitumor responses. However, cells of the myeloid lineage are quite heterogeneous and include dendritic cells, macrophages, and granulocytes. These cell types can also possess either stimulatory properties or immunosuppressive capacities. Thus, when MDSCs were defined according to the expression of CD11b and GR1 (in mice), there was some opposition in considering them as a lineage apart [2]. Even more, monocytic MDSCs show a phenotype that closely resembles inflammatory monocytes. Granulocytic MDSCs are phenotypically closely similar to neutrophils [3, 4].
Therefore, the main question that remains to be answered is whether MDSCs are truly a lineage apart, they are “alternative” forms of monocytes or granulocytes or they convert into one another [5–7]. Whether they are considered as a bona fide myeloid lineage or not, their role in tumor progression is not questioned. Infiltrating myeloid cells are present within the tumor and protect cancer against both conventional and immunotherapies.
-
What is the relationship between MDSCs and other regulatory cell lineages?
The tumor environment can be conserved as a complex “organ” under chronic inflammatory conditions which favor the infiltration of regulatory cells [8]. These strongly immunosuppressive cells play an important role in tumor biology, as they suppress antitumor immune responses, favor tumor progression, tissue repair and neoangiogenesis. These tumor-promoting functions accelerate cancer metastasis. Immunosuppressive infiltrating cells comprise tumor-associated M2 macrophages, tumor-associated neutrophils, tolerogenic DCs, and regulatory T and B cells. Recently, there has been growing experimental evidence that MDSCs do not function on their own, but cooperate with other tumor-associated regulatory cells. This includes crosstalk with macrophages, induction of regulatory T cells, and with regulatory tumor-associated B cells [9, 10]. Interestingly, all these cell types share many of the suppressive pathways, including TGFβ and IL10 production, consumption of essential amino acids, and cell-to-cell contact dependent immunosuppression [8]. Thus, not only MDSCs cooperate with other tumor-associated cells, but they also share common procarcinogenic mechanisms. The identification of their interactions will surely open new opportunities for therapeutic intervention by simultaneously targeting several of these cell types within the tumor.
7.2 Differentiation of Myeloid-Derived Suppressor Cells
As mentioned above, the specific nature and ontogeny of MDSCs are still under debate, possibly due to their phenotypic plasticity and heterogeneity. Therefore, the study of the MDSC differentiation pathways will help to understand whether MDSCs can be considered a lineage of its own right or just a collection of heterogenous myeloid cells at a various differentiation stages.
-
Murine MDSC differentiation
Without any doubt, murine systems are usually way ahead of their human counterparts. This is also true for MDSCs, which can be easily obtained from mice by inducing tumor growth in vivo, or by differentiating MDSCs from bone marrow cells in vitro. As exposed in various parts of this book, there is a somewhat “strong” consensus on murine MDSC phenotype [1, 11]. These cells express CD11b and high levels of GR1. Then, according to their pattern of ly6C-Ly6G expression, they can be further classified as monocyte (Ly6Chigh, Ly6Glow/neg) or granulocytic (Ly6C+ Ly6Ghigh) [12–14]. Unfortunately, these phenotypes are equivalent to those of inflammatory monocytes and neutrophils, respectively. Thus, at the end only the immunosuppressive properties define them. Recent data has shown that melanoma MDSCs present a kinase signature that controls their suppressive activities [15, 16]. Nevertheless, although these kinase signatures explain the nature of MDSCs at least functionally, all these data does not clarify their ontogeny yet.
In vitro systems have not shed much light on this subject, as it would have been expected. Each system has its advantages and limitations, but so far the MDSC differentiation pathway (if there is a single one) is still poorly understood [11]. Therefore, even though some steps have been undertaken toward the development of efficacious ex vivo MDSC production methods [7, 13–15, 17], the faithful replication of the MDSC differentiation pathways in vitro and in vivo is a pending subject.
-
Human MDSC differentiation
Compared to murine systems, very little is known about human MDSCs. This is directly caused by the intrinsic difficulties of working with samples from patients with cancer. Most of the studies are centered on peripheral blood cells, and the in vitro MDSC systems are highly inefficient as they do not use fully pluripotent hematopoietic precursors [11]. In addition to these important drawbacks, the human MDSC phenotype is still largely undefined [18, 19]. Some attempts have been made at classifying MDSC types in humans according to phenotype, tumor models, and sources of cells [20]. Thus, in the human system we might have three possibilities. First, it might be intrinsically heterogenous with several types of co-existing MDSCs. Second, there might not be MDSCs at all (as we understand from the murine system) but a collection of myeloid cells at different differentiation stages. Or third, we are studying mainly circulating MDSCs from peripheral blood rather than homogeneous cell populations derived from bone marrow.
Efficient in vitro systems should be developed for human MDSCs, and this will surely help deciding whether human MDSCs are comparable to their murine counterparts. This is also a key issue, as most cancer therapies are tested first in murine systems. Although major advances have been made toward this goal, these human MDSC differentiation systems are still poorly efficient.
7.3 Targeting MDSC-Specific Pathways for Therapy
While there are still many open questions on MDSC biology, in practical terms their elimination from a tumor-bearing subject improves anticancer therapies. Thus, obviating the fact whether these cells comprise a specific myeloid lineage or not, much is being understood on their immunosuppressive mechanisms. This knowledge uncovers opportunities for therapeutic interventions. From early studies, it was observed that MDSCs could exert suppressive activities by secreting factors, or by cell-to-cell contact mechanisms. Apart from the classical secretion of immunosuppressive cytokines such as TGFβ or IL10, similarly to M2 macrophages the arginine metabolism was shown to play a very important role in their activities [7, 21]. Arginine is processed in MDSCs by two enzymes, iNOS and arginase-1. Blocking the activity of both enzymes improves antitumor activities in mouse models [7].
-
The tumor environment as a target
The tumor environment as a whole is also a therapeutic target. Cancer cells and tumor-infiltrating cells are under a very strong oxidative stress, and upregulate detoxifying enzymes and ROS scavenging proteins. MDSCs have been shown to selectively upregulate the P450 reductase, and this upregulation explains the anti-MDSC properties of Paclitaxel [22]. This chemotherapy drug needs to be activated by P450R to acquire cytotoxic activities. As conventional immunogenic DCs express lower levels of P450R, these cells are by far less sensitive to Paclitaxel than MDSCs [14]. This is just but one example on how to exploit these tumor-induced cellular targets.
-
MDSC signaling pathways as a target
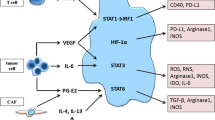
Interestingly, there is a growing field of research on MDSC signaling, as tyrosine kinase inhibitors and other chemotherapy drugs eliminate MDSCs both in murine models and human patients. Again, pathways shared by cancer cells and tumor-associated cells are also present in MDSCs [14–16]. Therefore, and unknowingly, many of the anticancer drugs that were designed to directly attack cancer cells, also have anti-MDSC properties. Thus, all these shared pathways are susceptible of therapeutic intervention in a straightforwardly manner. As already discussed in a previous chapter, one of those is the STAT3-dependent signaling pathway [23, 24]. This pathway regulates cell growth, survival, and inflammation. It is also activated by IL6, a cytokine known to contribute to MDSC differentiation [25]. STAT3 is constitutively activated in cancer cells, tumor cells (which includes cancerous and associated cells), and in tumor infiltrating cells of the immune system [26]. Extensive work has been performed on STAT3 in macrophages. IL10 is also a potent induction of STAT3, and its phosphorylation in macrophages leads to their polarization toward immunosuppressive subsets [27, 28]. This pathway together with others such as PI3 K/AKT acts as a safeguard against uncontrolled inflammation. However, cancer can turn on this pathway to inhibit antitumor immune responses [29, 30]. MDSCs seem to activate the STAT3 pathway in cancer, and therapeutic strategies devised to act upon tumor-infiltrating macrophages and DCs will probably be successful in counteracting MDSC-suppressive activities.
Apart from STAT3, the implication of several intracellular pathways on MDSC biology has also been described [14, 15]. These pathways are linked to cell survival, anti-inflammatory responses and stress responses against oxidative stress. Thus, other tumor-associated cells also share them. Moreover, there is a specific kinase profile in MDSCs that separates them from other conventional myeloid immunogenic cell types. The PI3 K, AKT, and the SRC family of kinases are highly upregulated in murine melanoma MDSCs and their expression differentiates them from conventional myeloid DCs [15]. MDSCs obtained from other tumor backgrounds, especially breast cancer MDSCs, also show increased levels of AKT, and a gene expression profile characteristic of the activity of SRC family members, particularly HCK and FYN kinases [15]. AKT and PI3 K are also highly activated in MDSCs. Interestingly, tumor-infiltrating melanoma MDSCs specifically activate ERK1 and PKC kinases, which are also known to be activated in tumor cells [8, 15, 31].
There is currently a wide range of small molecules that target these pathways, activated both in cancer cells and MDSCs. The Ras-Raf-MEK-ERK signaling axis is probably one of the most studied for the development of anticancer treatments [32]. MDSC differentiation has been found to be particularly affected by AKT and MEK inhibitors, while their immunogenic myeloid DC counterparts were largely unaffected [15]. Moreover, MEK inhibition enhances DC differentiation and activates DC-mediated antitumor activities [15, 33–35].
Thus, many anti-neoplastic treatments also have “beneficial collateral” effects on MDSCs. The assessment of these anticancer drugs over MDSCs will surely shed light on their multiple mechanisms of action over the immune system [11]. Furthermore, the specific kinase signature found in MDSCs will facilitate the development of efficacious MDSC-targeted therapies that do not affect immunogenic cells such as DCs.
-
Interfering with negative co - stimulation of T cells as a target
Similarly to DCs and other myeloid cells, MDSCs are also antigen-presenting cells. However, after antigen presentation by MDSCs, T cells get inactivated, suppressed, or differentiate toward regulatory T cells [13, 36]. There are multiple mechanisms by which MDSCs can exert T cell inhibitory effects, and those include secretion of anti-inflammatory cytokines, consumption of essential amino acids, production of NO and use of negative co-stimulation during antigen presentation to T cells [8].
During antigen presentation, the antigen-presenting cell (APC) presents to T cells complexed to major histocompatibility molecules on their surface (Fig. 7.1). These pMHC complexes are recognized and bind to specific T cell receptors (TCRs) present on the surface of CD4 or CD8 T cells. This recognition sends a signal (signal 1) to the T cells. However, this signal is not sufficient to activate a T cell and leads to T-cell anergy instead [37]. Further interactions between these two cells are required within the immunological synapse. These interactions take place between antigenic peptides receptors on the T cells and their respective ligands on the APC. Some of these interactions will lead to T-cell activation while others will dampen T cells. The integration between all these differing interactions provides a second signal during antigen presentation. This signal 2 will determine whether T cells get activated or not, and the extent of T-cell activation. Positive co-stimulation is represented by the classical interaction between CD80 and CD28, on the surfaces of APCs and T cells, respectively. However, there are a high number of interactions that regulate T-cell activation by sending inhibitory signals. For example, CD80-CTLA4 or PDL1-PD1 (Fig. 7.1).
Physiological antigen presentation to T-cells. The scheme represents a DC as an antigen-presenting cell to a T lymphocyte through the MHC-TCR complex as indicated in the immunological synapse between the two cell types. Both positive and negative receptor–ligand interactions take place, as indicated in the picture. These interactions will transmit signals (activatory and inhibitory signal 2, as shown) that together with antigen recognition (signal 1), will regulate T-cell activation or its effector functions. In addition, a third signal is provided within the immunological synapse in the form of secreted cytokines (top of the figure). The integration of these three signals within the T cell will determine the level of T-cell activation and its polarization
Thus, antibodies which block these interactions have been developed to strengthen T-cell activation by interfering with these interactions. Preventing CTLA4 binding to CD80 has been one of the first to be applied to human therapy and showing success [38]. In fact, MDSCs express very high levels of CD80, which seems to be required for their suppressive functions (Fig. 7.2) [14, 17, 39, 40]. Recently, blocking PDL1-PD1 interactions with antibodies is demonstrating to be a very successful immunotherapy anticancer strategy [41, 42]. While it is widely thought that the mechanism of action takes place at the tumor site by facilitating the attack of the effector T cell, their efficacy in some PDL1-negative tumors indicates that there are other mechanisms of action. In fact, PDL1-PD1 interactions play a key role in antigen presentation. Their interaction following antigen recognition by the T cells facilitates ligand-induced TCR down-modulation while the T cell gets activated and proliferates [33, 43, 44]. TCR expression recovers after one week, and this is a safeguard mechanism that ensures that T cells do not attack their targets until they reach a critical number [45, 46]. Interfering with this interaction leads to hyperproliferative TCRhigh polyfunctional effector CD8 T cells with strong antitumor activities [33, 47]. Additionally, interference with PDL1 expression also leads to a low level expansion of polyclonal CD8 T cells which probably contribute to anticancer activities [13]. Tumor-infiltrating MDSCs express very high levels of PDL1 (Fig. 7.2) [7, 12–14, 17]. Interference with PDL1 expression on MDSCs converts these cells in T-cell stimulators [48]. It is highly likely that current blocking antibodies used in human therapy are converting MDSCs to efficient immunstimulatory APCs. As there are an increasing number of positive and negative co-stimulatory molecules and antibodies targeting their interaction partners [49, 50], it is highly likely that immunotherapy will become a first-line treatment for cancer. These immunotherapy approaches directly target MDSCs by converting them in immunostimulatory myeloid cells.
The MDSC as an immunosuppressive antigen-presenting cell. The scheme represents a MDSC presenting antigen to a T lymphocyte through the MHC-TCR complex as indicated in the immunological synapse between the two cell types. Negative receptor–ligand interactions take place primarily when MDSCs present antigen, by upregulating PDL1 binding to PD1 on the T-cell surface, and expressing high levels of CD80 which binds CTLA4 on the T cell, as shown in the figure. These interactions together with antigen recognition (signal 1), inhibits either T-cell activation, or its effector functions. In addition, MDSCs produce high levels of immunosuppressive cytokines, as indicated in the figure. These cytokines will polarize T cells toward tolerogenic subsets such as inducible regulatory T cells (Tregs)
Conversion of MDSCs to efficient APCs with antitumor properties
While specific targeting and depletion of MDSCs improves antitumor immune responses [51, 52], an interesting approach that will certainly have a future in anticancer therapies is the conversion of MDSCs into proinflammatory APCs. MDSCs have been shown to possess the potential of differentiation toward other myeloid cell types such as DCs, macrophages, and inflammatory granulocytes. While several cytokines and factors may drive this differentiation, IL12 is proving to be quite efficacious in converting MDSCs to immunogenic myeloid APCs. Thus, direct treatment with IL12 transforms MDSCs into activated antigen-presenting cells [13, 53, 54]. Within the tumor environment, IL12 production leads to a collapse of the tumor stroma, which helps regression and improves antitumor capacities of T cells [55]. It is highly likely that the method of IL12 administration will likely have an impact in its efficacy. So far, local IL12 production within the tumor environment is proving the method of choice as it will surely reduce cytotoxicity from systemic administration.
7.4 Summary and Conclusions
Although the participation of myeloid cells on tumor progression and metastasis has been known for a long time, only recently another “subset” of myeloid cells has been added to this picture. This has raised some controversy on their nature and relationship with other myeloid cell types. Nevertheless, whether they represent a bona fide myeloid lineage, or another differentiation stage of highly plastic myeloid cells, they strongly possess procarcinogenic properties.
From a scientific point of view, their “true” ontogenetic nature needs to be clarified, especially for human MDSCs. From a practical point of view, tumor-associated myeloid immunosuppressive cells need to be eliminated.
Apart from controversies, their importance in cancer immunology is undeniable. Proof of this is the increasingly higher number of publications dealing with them. An important effort is being devoted to devise efficient differentiation methods for basic research or for cellular therapies. Obtaining MDSCs that resemble tumor-infiltrating subsets is still challenging, although encouraging steps have been recently taken toward this goal in murine systems. The human system is still a pending subject.
Immunotherapy will surely become a first-line anticancer treatment strategy, and MDSCs will surely occupy a central position in anticancer research.
Finally, a clearer view on MDSC biology is emerging from recent research, which highlights the metabolic changes and high differentiation plasticity of the “myeloid cell compartment”. However, this plasticity can be used to devise targeted therapies that will eliminate the procarcinogenic myeloid cells and shift differentiation toward immunogenic, protective cells.
References
Talmadge JE, Gabrilovich DI (2013) History of myeloid-derived suppressor cells. Nat Rev 13(10):739–752
Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H (2007) The terminology issue for myeloid-derived suppressor cells. Cancer Res 67 (1):425; author reply 426. doi:67/1/425 [pii] 10.1158/0008-5472.CAN-06-3037
Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Hornq W, Fridlender G, Bayuh R, Worthen GS, Albelda SM (2012) Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myloid-derived suppressor cells and normal neutrophils. PLoS ONE 7(2):e31524
Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI (2012) Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 91(1):167–181. doi:10.1189/jlb.0311177
Koffel R, Meshcheryakova A, Warszawska J, Henning A, Wagner K, Jorgl A, Gubi D, Moser D, Hladik A, Hoffmann U, Fischer MB, van der Berg W, Koenders M, Scheinecker C, Gesslbauer B, Knapp S, Strobl H (2014) Monocytic cell differentiation from band-stage neutrophils under inflammatory conditions via MKK6 activation. Blood 124(17):2713–2724
Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, Sarnaik A, Horna P, Sotomayor E, Gabrilovich DI (2013) Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 14(3):211–220. doi:10.1038/ni.2526ni 2526[pii]
Dufait I, Schwarze JK, Liechtenstein T, Leonard W, Jiang H, Law K, Verovski V, Escors D, De Ridder M, Breckpot K (2015) Ex vivo generation of myeloid-derived suppressor cells that model the tumor immunosuppressive environment in colorectal cancer. Oncotarget 6(14):12369–12382
Escors D (2014) Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy. New J Sci 2014. doi:10.1155/2014/734515 734515[pii]
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 22(4):275–281. doi:10.1016/j.semcancer.2012.01.011
Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, Araki Y, Miyoshi I, Yang L, Trinchieri G, Biragyn A (2015) Immune suppressive and pro-metastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Cancer Res. doi:10.1158/0008-5472.CAN-14-3077
Escors D, Liechtenstein T, Perez-Janices N, Schwarze J, Dufait I, Goyvaerts C, Lanna A, Arce F, Blanco-Luquin I, Kochan G, Guerrero-Setas D, Breckpot K (2013) Assessing T-cell responses in anticancer immunohterapy: dendritic cells or myeloid-derived suppressor cells? Oncoimmunology 12(10):e26148
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181(8):5791–5802
Liechtenstein T, Perez-Janices N, Blanco-Luquin I, Schwarze J, Dufait I, Lanna A, De Ridder M, Guerrero-Setas D, Breckpot K, Escors D (2014) Anti-melanoma vaccines engineered to simultaneously modulate cytokine priming and silence PD-L1 characterized using ex vivo myeloid-derived suppressor cells as a readout of therapeutic efficacy. Oncoimmunology 3:e29178
Liechtenstein T, Perez-Janices N, Gato M, Caliendo F, Kochan G, Blanco-Luquin I, Van der Jeught K, Arce F, Guerrero-Setas D, Fernandez-Irigoyen J, Santamaria E, Breckpot K, Escors D (2014) A highly efficient tumor-infiltrating MDSC differentiation system for discovery of anti-neoplastic targets, which circumvents the need for tumor establishment in mice. Oncotarget 5(17):7843–7857
Gato-Cañas M, Martinez de Morentin X, Blanco-Luquin I, Fernandez-Irigoyen J, Zudaire I, Liechtenstein T, Arasanz H, Lozano T, Casares N, Knapp S, Chaikuad A, Guerrero-Setas D, Escors D, Kochan G, Santamaria E (2015) A core of kinase-regulated interactomes defines the neoplastic MDSC lineage. Oncotarget In press
Aliper AM, Frieden-Korovkina VP, Buzdin A, Roumiantsev SA, Zhavoronkov A (2014) Interactome analysis of myeloid-derived suppressor cells in murine models of colon and breast cancer. Oncotarget 5(22):11345–11353
Van der Jeught K, Joe PT, Bialkowski L, Heirman C, Daszkiewicz L, Liechtenstein T, Escors D, Thielemans K, Breckpot K (2014) Intratumoral administration of mRNA encoding a fusokine consisting of IFN-beta and the ectodomain of the TGF-beta receptor II potentiates antitumor immunity. Oncotarget 5(20):10100–10113
Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22(2):238–244. doi:10.1016/j.coi.2010.01.021 S0952-7915(10)00022-1 [pii]
Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, Mandruzzato S (2015) Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry Part B, Clinical cytometry 88(2):77–91. doi:10.1002/cyto.b.21206
Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V (2014) Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 1319:47–65. doi:10.1111/nyas.12469
Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI (2009) Regulatory myeloid suppressor cells in health and disease. Cancer Res 69(19):7503–7506
Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, Shurin GV, Shurin MR, Umansky V (2013) Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol 190(5):2464–2471. doi:10.4049/jimmunol.1202781
Emeagi PU, Maenhout S, Dang N, Heirman C, Thielemans K, Breckpot K (2013) Downregulation of Stat3 in melanoma: reprogramming the immune microenvironment as an anticancer therapeutic strategy. Gene Ther. 20(11):1085–1092. doi:10.1038/gt.2013.35 gt201335 [pii]
Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, Abrams SI (2013) Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest 123(10):4464–4478
Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T (2004) IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 173(6):3844–3854
Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D (2004) Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol 172(1):464–474
Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Muller-Newen G (2003) Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol 170(6):3263–3272
O’Farrell AM, Liu Y, Moore KW, Mui AL (1998) IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J 17(4):1006–1018. doi:10.1093/emboj/17.4.1006
Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y (2013) Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem cells (Dayton, Ohio) 31(2):248–258. doi:10.1002/stem.1281
Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H (2004) Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 10(1):48–54
Arce F, Kochan G, Breckpot K, Stephenson H, Escors D (2012) Selective Activation of Intracellular Signalling Pathways In Dendritic Cells For Cancer Immunotherapy. Anti-Cancer Agents Med Chem 1:29–39
Samatar AA, Poulikakos PI (2014) Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discovery 13(12):928–942. doi:10.1038/nrd4281
Karwacz K, Bricogne C, Macdonald D, Arce F, Bennett CL, Collins M, Escors D (2011) PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8(+) T cells. EMBO Mol Med 3(10):581–592. doi:10.1002/emmm.201100165
Arce F, Breckpot K, Stephenson H, Karwacz K, Ehrenstein MR, Collins M, Escors D (2011) Selective ERK activation differentiates mouse and human tolerogenic dendritic cells, expands antigen-specific regulatory T cells, and suppresses experimental inflammatory arthritis. Arthritis Rheum 63:84–95
Escors D, Lopes L, Lin R, Hiscott J, Akira S, Davis RJ, Collins MK (2008) Targeting dendritic cell signalling to regulate the response to immunisation. Blood 111(6):3050–3061. doi:10.1182/blood-2007-11-122408 blood-2007-11-122408 [pii]
Luan Y, Mosheir E, Menon MC, Wilson D, Woytovich C, Ochando J, Murphy B (2013) Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant 13(12):3123–3131. doi:10.1111/ajt.12461
Yamamoto T, Hattori M, Yoshida T (2007) Induction of T-cell activation or anergy determined by the combination of intensity and duration of T-cell receptor stimulation, and sequential induction in an individual cell. Immunology 121(3):383–391. doi:10.1111/j.1365-2567.2007.02586.x
Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, O’Day SJ, Hoos A, Humphrey R, Berman DM, Lonberg N, Korman AJ (2013) Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 1291(1):1–13. doi:10.1111/nyas.12180
Dilek N, Vuillefroy de Silly R, Blancho G, Vanhove B (2012) Myeloid-derived suppressor cells: mechanisms of action and recent advances in their role in transplant tolerance. Front Immunol 3:208. doi:10.3389/fimmu.2012.00208
Maenhout SK, Van Lint S, Emeagi PU, Thielemans K, Aerts JL (2014) Enhanced suppressive capacity of tumor-infiltrating myeloid-derived suppressor cells compared to their peripheral counterparts. Int J Cancer 134(5):1077–1090. doi:10.1002/ijc.28449
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366(26):2455–2465
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Karwacz K, Arce F, Bricogne C, Kochan G, Escors D (2012) PD-L1 co-stimulation, ligand-induced TCR down-modulation and anti-tumor immunotherapy. Oncoimmunology 1(1):86–88
Escors D, Bricogne C, Arce F, Kochan G, Karwacz K (2012) On the mechanism of T cell receptor down-modulation and its physiological significance. J biosci med 1(1). 2011.5 [pii]
Liechtenstein T, Dufait I, Bricogne C, lanna A, Pen J, Breckpot K, Escors D (2012) PD-L1/PD-1 co-stimulation, a brake for T cell activation and a T cell differentiation signal. J Clin Cell Immunol S12(006):6. doi:10.4172/2155-9899.S12-006
Bricogne C, Laranga R, Padella A, Dufait I, Liechtenstein T, Breckpot K, Kochan G, Escors D (2012) Critical Mass Hypothesis of T-Cell Responses and its Application for the Treatment of T-Cell Lymphoma. In: Harvey WK, Jacobs RM (eds) Hodgkin’s and T-cell lymphoma: Diagnosis. Nova Publishers, Treatment Options and Prognosis
Pen JJ, Keersmaecker BD, Heirman C, Corthals J, Liechtenstein T, Escors D, Thielemans K, Breckpot K (2013) Interference with PD-L1/PD-1 co-stimulation during antigen presentation enhances the multifunctionality of antigen-specific T cells. Gene Ther 21(3):262–271
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211(5):781–790. doi:10.1084/jem.20131916
Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L (2007) Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev 7(2):95–106. doi:10.1038/nrc2051
Chen L, Flies DB (2013) Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13(4):227–242. doi:10.1038/nri3405
Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, Qian J, Hailemichael Y, Nurieva R, Dwyer KC, Roth J, Yi Q, Overwijk WW, Kwak LW (2014) Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 20(6):676–681
Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S (2012) Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS ONE 7(7):e40677. doi:10.1371/journal.pone.0040677
Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C (2011) The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology 133(2):221–238. doi:10.1111/j.1365-2567.2011.03429.x
Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, Rosenberg SA, Restifo NP (2011) IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 121(12):4746–4757
Kerkar SP, Leonardi AJ, van Panhuys N, Zhang L, Yu Z, Crompton JG, Pan JH, Palmer DC, Morgan RA, Rosenberg SA, Restifo NP (2013) Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Mol Ther 21(7):1369–1377. doi:10.1038/mt.2013.58 mt201358 [pii]
Acknowledgments
David Escors is funded by a Miguel Servet Fellowship (CP12/03114), a FIS project grant (PI14/00579) from the Instituto de Salud Carlos III, Spain, the Refbio transpyrenaic collaborative project grants (NTBM), a Sandra Ibarra Foundation grant, Gobierno de Navarra Grant (BMED 033-2014), and a Gobierno Vasco BioEf project grant (BIO13/CI/014). Grazyna Kochan is funded by a Caixa Bank Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Escors, D., Kochan, G. (2016). Future Perspectives. In: Myeloid-Derived Suppressor Cells and Cancer. SpringerBriefs in Immunology. Springer, Cham. https://doi.org/10.1007/978-3-319-26821-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-26821-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26819-4
Online ISBN: 978-3-319-26821-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)