Abstract
The gastrointestinal tract (TGI) has a multitude of functions in addition to digestion. One important function is the ability to serve as a barrier against living organisms and antigens within the lumen, the so-called intestinal barrier function. The breakdown of this barrier may result in the crossing of viable bacteria and their products to mesenteric lymph nodes and more distant sites, a process known as bacterial translocation (BT) (Gatt et al., Aliment Pharmacol Ther 25:741–57, 2007).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Key Points
-
Intestinal barrier dysfunction may allow the penetration of luminal antigens such as bacteria and their toxins, event known as bacterial translocation (BT).
-
l-Arginine is an important and versatile amino acid with several immunological and trophic properties under stressful situations.
-
It is believed that the main effects of l-arginine on bacterial translocation are due to nitric oxide synthase (NO) and arginase pathways.
-
The l-arginine can prevent bacterial translocation due its effects by intestinal mucosa preservation and enhancement of immune response.
-
l-Arginine supplementation is interesting for a number of critical clinical situations; however, in cases of sepsis, the use of l-arginine should be carefully evaluated, because an overproduction of NO can be deleterious to the patient.
- 99mTc-DTPA:
-
99m-technetium diethylene triamine pentaacetic acid
- 99mTc-EDTA:
-
99m-technetium ethylenediaminetetraacetic acid
- AIDS:
-
Acquired immune deficiency syndrome
- ARG:
-
l-Arginine
- BT:
-
Bacterial translocation
- CD4:
-
Cluster of differentiation 4
- CD8:
-
Cluster of differentiation 8
- DNA:
-
Deoxyribonucleic acid
- E. coli :
-
Escherichia coli
- eNOS:
-
Endothelial NOS
- IL:
-
Interleukin
- INF:
-
Interferon
- iNOS:
-
Inducible nitric oxide synthase
- IO:
-
Intestinal obstruction
- l-NAME:
-
NG- nitro-l-arginine methyl ester
- l-NMMA:
-
NG-monomethyl-l-arginine
- l-NNA:
-
NG-nitro-l-arginine
- LPS:
-
Lipopolysaccharide
- mRNA:
-
Messenger ribonucleic acid
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate-oxidase
- nNOS:
-
Neuronal NOS
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- ODC:
-
Ornithine decarboxylase
- SD:
-
Standard deviation
- sIgA:
-
Secretory immunoglobulin A
- SMCs:
-
Suppressor myeloid cells
- TCV:
-
Total caloric value
- TGI:
-
Gastrointestinal tract
- Th1:
-
T-helper cell type 1
- Th2:
-
T-helper cell type 2
- TNF-α:
-
Tumor necrosis factor
Introduction
The gastrointestinal tract (TGI) has a multitude of functions in addition to digestion. One important function is the ability to serve as a barrier against living organisms and antigens within the lumen, the so-called intestinal barrier function. The breakdown of this barrier may result in the crossing of viable bacteria and their products to mesenteric lymph nodes and more distant sites, a process known as bacterial translocation (BT) [1].
Localized and systemic disorders , such as ischemia, intestinal obstruction, shock, or sepsis, can damage the intestinal barrier, increasing mucosa permeability and allowing for BT. These disorders worsen the primary pathological event and may induce multiple organ failure and death [2, 3].
In order to avoid bacterial translocation, supplementation with immunomodulatory substrates seems essential [4]. In this context, l-arginine has been extensively studied.
l-Arginine or l-amino-5-guanidinovaleric acid (Fig. 46.1) is a basic conditionally essential amino acid with four nitrogen atoms that plays an important role in the transport, storage, and excretion of nitrogen and in the disposal of ammonia via the urea cycle . In catabolic states, l-arginine may become essential because of alterations in the overall metabolism [5]. l-Arginine provided by diet proteins is metabolized by the enterocytes and is responsible for various functions in the gut under stressful situations. It is well established that l-arginine can enhance morphometric aspects, such as the stimulation of enterocyte proliferation, the number of villi, and their height under such adverse conditions [6–9].
l-Arginine also plays a central role in the immune system, and it is especially important for macrophage and T-lymphocyte metabolism [10, 11]. Dietary l-arginine increases the activity of macrophages and enhances the CD4:CD8 ratio, the number of lymphocytes in Peyer’s patches, as well as the levels of secretory immunoglobulin A (sIgA) . It also increases the expression of the messenger ribonucleic acid (RNA) for the production of Th1 cytokines and Th2 cytokines, suggesting that l-arginine acts both in the cellular and humoral immune response [7, 10, 12].
l-Arginine is also a precursor for the synthesis of molecules with enormous biological importance including urea, ornithine, polyamines, nitric oxide, creatine, agmatine, and many others, besides being a major nitrogen carrier and a component of proteins [13]. It is believed that the main effects of l-arginine on bacterial translocation are due to nitric oxide (NO) and polyamines.
In this chapter we discuss bacterial translocation and the l-arginine mechanisms on this condition, emphasizing the study of the metabolites NO and polyamines.
Bacterial Translocation
Bacterial translocation involves the initial contact of bacteria with the intestinal wall , leading to cytokine production and subsequent inflammatory response. Once the bacteria enter the mucosa, they can be transported to distant organs through the circulation [1].
There are several evidences that BT is associated with the increased incidence of septic complications. Macfie et al. [13] showed 14 % of prevalence of BT, in 927 surgical patients, and a relationship with increased postoperative sepsis. In addition, Nieves et al. [14] observed a prevalence of 33 % of BT when evaluating lymph nodes of patient victims of abdominal trauma.
On the other hand, BT also occurs in healthy individuals. Low levels of bacterial translocation can be an important physiological event to prepare and alert the immune system of the host. Salzedas-Netto et al. [15] showed significant reduction of BT in animals previously challenged with the same bacteria used for BT induction. Therefore, it is possible that BT can occur to present lumen antigens to TGI, generating immunocompetent cells , a process known as oral tolerance [1].
There are three mechanisms involved in BT (Fig. 46.2): modified gut microbiota, reduced intestinal barrier function, and inadequate response of the host immune system [16].
Mechanisms of bacterial translocation. The main mechanisms of bacterial translocation. In cases of bacterial overgrowth, increase of the colonization leading to BT can occur; increases of paracellular permeability induce the opening of the tight junctions with the passage of bacteria, which may lead to the excessive inflammatory response. Lastly, the T cells induce the production of Th1 and Th2 cytokines with activation of B lymphocytes , thereby regulating the production of antibodies. When the system fails, BT can occur
Gut Microbiota
TGI is a dynamic organ with direct or indirect influences in the translocation of intestinal particles [16]. When the ecological balance is affected due to changes in the intestinal microbiota (e.g., use of antibiotics, decreased in gastric acidity and mucus production, obstructive jaundice, and changes in bowel motility), bacterial overgrowth is favored, increasing the colonization and leading to BT [16, 17]. Only a few strains of intestinal bacteria are able to translocate to the mesenteric lymph nodes, these include Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, enterococci, and some streptococci [16].
Parenteral nutrition with disuse of the TGI, malnutrition, diabetes, cirrhosis, and endotoxic shock also induce bacterial overgrowth, promoting subsequent translocation [17]. A recent study showed that 10 of 32 cirrhotic patients had bacterial DNA-positive in blood when intestinal bacterial overgrowth was positive. In a multivariate analysis, only the existence of intestinal bacterial overgrowth was the independent risk factor for bacterial DNA. The authors concluded that the increases in plasma endotoxin and bacterial DNA were directly associat ed with intestinal bacterial overgrowth in these patients [18].
Barrier Function
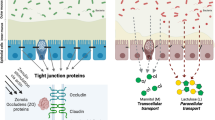
The normal intestinal epithelium acts as a selective barrier between the environment of the intestinal lumen and the lamina propria. This barrier consists of a single layer of epithelial cells, which are connected by firm junctions (tight junctions) [19]. The epithelium balance is also influenced by local factors such as mucus, stomach acid, pancreatic enzymes, bile, and the intestinal motility [17]. These factors together acting prevents the invasion of bacteria to the epithelium.
The tight junctions allow the selective paracellular permeability, which excludes passive movement of uncharged hydrophilic compounds, such as bacteria and macromolecules. The increase in paracellular permeability induces the opening of the tight junctions with the passage of bacteria, which may induce the excessive inflammatory response. Furthermore, damaged epithelia also allow the access of microorganisms to the bloodstream or lymph nodes through the transcellular pathway [20, 21].
These effects can be observed in critically ill patients and are associated with the high incidence of bacterial and toxin translocation from the intestinal lumen to the systemic circulation, causing infectious complications [22]. In a recent study, Founts et al. [23] showed increased intestinal permeability and bacteria translocation in mice with liver injury. These alterations were accompanied by decreased intestinal expression of the tight junctions and the protein occludin.
The evaluation of changes in intestinal permeability can be performed using specific tests designed to measure the intestinal barrier function, such as large molecules (i.e., sugars and drugs) to determine the paracellular passage from the intestine into the plasma and therefore into the urine [24]. Currently, substances labeled with radioactive isotopes, such as 99mTc-EDTA e 99mTc-DTPA, have also been used as an alternative, with good results [24]. In a recent murine study, intestinal permeability of mice undergoing intestinal obstruction was assessed by the determination of the percentage of 99mTc-DTPA found in the blood of the animals. There was g ood sensitivity of the method to evaluate changes in cell permeability [6].
Immune System
The intestinal tract is an active immune organ, containing several factors involved in the immune response [17]. The gut-associated lymphoid tissue (GALT) is the largest immune organ in the body containing 25 % of the total mucosal immune cell.
The GALT covers the epithelium, inside of the lamina propria and submucosa, including more than a half of lymphoid cells in Peyer’s patches, follicle-associated epithelium (consisting of M cells), intra-epithelial lymphocytes, macrophages, neutrophils, and dendritic cells [16]. The M cells are a major component of the GALT and often constitute the first defense line against the passage of microorganisms from the intestinal lumen into the epithelium. M cells are an unusual type of epithelial cells, because they don’t have on their surface microvilli or glycocalyx. The M cells have very long cytoplasmic extensions into the lamina propria forming a pocket within the antigens which are phagocytized by macrophages and then penetrate into Peyer’s patches [25].
When the intestinal immune system acts, the antigens are transported by M cells to the antigen-presenting cells (macrophages and dendritic cells) into the mesenteric lymph nodes. Then, the processing and presentation of antigens to CD4+ T lymphocytes and inactive B cells occur. These cells are the second line of defense against translocation and initiate the production of cytokines [26].
T cells induce the production of TH1 and TH2 cytokines. Th1 cytokines (IL-2, INF, and TNF-α) stimulate cellular immunity, resulting in activation of macrophages, neutrophils, and T lymphocytes, especially CD8+ T lymphocytes. Th2 cytokines (IL-4, IL-5, IL-6, IL-10, and IL-13) are responsible for the activation of B lymphocytes, thereby regulating the production of antibodies [26].
It is widely accepted that cytokine levels after an inflammatory insult, such as lipopolysaccharide (LPS), are characterized by an initial increase and subsequent decrease in TNF-α levels, followed by IL-1, IL-6, and IL-10, respectively. However many septic patients exhibit high levels of TNF-α, IL-1, and IL-6 until death [27]. The presence of sIgA immunoglobulin enhances barrier function of the intestine, playing a key role in the formation of the immune response to microbial colonization [28]. Mucosal secretions, rich in sIgA, can bind to bacteria preventing adherence and mucosal colonization [28].
Nutrient-Related Prevention and Therapy Against Bacterial Translocation
A variety of strategies have been investigated for the treatment of bacterial translocation. Most of them are linked with the ability of some compounds and nutrients to act in the immune system modulation or preventing the bacterial overgrowth , thus maintaining the intestinal barrier.
Aydocan et al. [29] showed that enteral diets supplemented with l-arginine, nucleotides, and omega-3 fatty acids reduce bacterial translocation. The investigators concluded that this effect might be related to improvement in the immune function resulting from the use of immunonutrients.
Enteral diets with glutamine resulted in less intestinal lesions and weight loss, improved nitrogen balance, and reduced bacterial translocation in a colitis model [30].
In a recent study, Sánches et al. [31] showed that treatment with probiotics decreases bacterial translocation, the pro-inflammatory state, and the ileal oxidative damage and increased ileal occludin expression in rats with experimental cirrhosis.
Data of our research group showed that immunomodulatory agents such as glutamine, citrulline, and l-arginine were able to reduce bacterial translocation in an animal model of intestinal obstruction. The probable mechanisms involved are related to the maintenance of the intestinal barrier and the regulation of the immune response [32–34].
Implications of l-Arginine Metabolic Pathways in Bacterial Translocation
l-Arginine is an important and versatile amino acid with several immunological and trophic properties under stressful situations. The l-arginine metabolism generates essential nitrogen compounds as creatine, polyamines, agmatine, and NO (Fig. 46.3). l-Arginine can be converted in the liver into creatine by l-arginine-glycine amidinotransferase. The creatine is transported into muscle tissue serving as a phosphate carrier and adenosine triphosphate regenerator [13].
The exact beneficial effects and mechanisms are still not well described. However, there are strong evidences indicating that l-arginine metabolites such as polyamines and NO are related to its role.
l-Arginine and the Nitric Oxide Pathway
Nitric oxide (NO) is a short-live free radical and a very small compound that diffuses freely within cells from its sites of formation to the sites of action. In solution, the NO has a half-life of 0.1–10 s before its transformation into nitrite (NO2) and nitrate (NO3). It is an important intracellular signaling molecule and it acts as a biological mediator similar to neurotransmitters in the neuronal system. NO can also regulate the blood vessel tone in vascular systems, and it is an important host defense effector in the immune system since it can act as a cytotoxic agent under pathological processes [35].
The biosynthesis of NO is carried out by l-arginine and the molecular oxygen, utilizing nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) as an electron donor. The overall reaction utilizes NADPH2 and Ca2+ as cofactors and consists of two steps. The conversion of l-arginine to NO and L-citrulline via Nw-hydroxy-l-arginine, that is an intermediate which may also function as a substrate for nitric oxide synthase (NOS) [36]. The produced citrulline can be used in the synthesis of l-arginine in the kidney, endothelial cells, macrophages, and cells of the peripheral nervous system [37].
NO is enzymatically produced by three different NO synthases (NOS). Neuronal NOS (nNOS) (NOS1) and endothelial NOS (eNOS) (NOS3) are constitutive enzymes expressed in the plexus myentericus and the vascular endothelium of the gut, respectively. They produce small amounts of NO in response to increases in intracellular calcium . The third enzyme, inducible NOS (iNOS) (NOS2), is normally not expressed, but is produced in larger amounts in macrophages and other tissues in response to pro-inflammatory mediators, such as bacterial membrane lipopolysaccharides, endotoxins, and inflammatory cytokines. It is also calcium-independent and produces NO over prolonged periods of time [38].
NOS enzymes produce NO from l-arginine, and thus competitive l-arginine analogues may prevent them from producing NO. These analogues include NG-monomethyl-l-arginine (l-NMMA), NG-nitro-l-arginine (l-NNA), and NG-nitro-l-arginine methyl ester (l-NAME). These are nonselective inhibitors. Aminoguanidine was the first class of specific iNOS inhibitors; however, it is not a potent inhibitor [39–42].
Nitric oxide is involved in a variety of biological functions throughout the body. It is a potent vasoactive regulator and the main factor of endothelium-derived relaxation. By promoting vasodilation, it increases blood flow to injured tissues [3].
Furthermore, NO plays an important role in the immune response acting on cells of the innate immune response such as monocytes, macrophages, microglia, Kupffer cells, eosinophils, and neutrophils. Therefore, during inflammation, it acts mediating cytotoxicity and supporting the nonspecific host defense [43].
Nitric oxide appears to play a dual function in the body, its beneficial or destructive effects depend on the amount produced [43]. The nitric oxide derived by activated macrophages is an important mediator of the inflammatory response ; however, when it acts as an oxidant, the excessive production of NO is detrimental to the tissues [44]. Increased expression of iNOS has been demonstrated in disorders such as destruction of the intestinal mucosa, sepsis, and clinical conditions associated with all these disorders [45].
Sepsis and Nitric Oxide
Sepsis is defined as a systemic response to an infection . It is a major health problem because of its significant morbidity and overall mortality rate of 30 % and it generally requires intensive care treatment [36]. Sepsis can be a consequence of bacterial translocation with bacteria after bacteria having penetrated the mucosa and being transported to distant organs through the circulation [20].
The role of nitric oxide on sepsis is controversial. Endotoxins and TH1 cytokines initiate the cascade that causes increased expression of NOS, especially iNOS, in several tissues (lung, liver, intestine) resulting in systemic hypotension. The reversal of hypotension has been the focus of septicemia treatment since it is associated with increased mortality in septic patients. Thus, it is important to evaluate the potential benefits of NOS inhibition in this context [40].
Different explanations may be suggested for the dual personality of NO during sepsis. First of all, there is no doubt about the detrimental effect of excessive NO on vasorelaxation, hypotension, and shock. The NO-mediated hypotension leads to severe hypoxia in peripheral vital organs, resulting in progressive organ failure. Second, increased NO may also provide certain benefits to the patient during sepsis. Increased NO release protects the kidney by causing local vasodilation and by inhibiting platelet aggregation and leukocyte adhesion. In addition, NO may also exert protective effects in other organs via its capacity to counteract oxidative stress, shut off apoptosis, prevent platelet aggregation and leukocyte adhesion, induce anti-inflammatory gene expression, and kill pathogens [46].
Sundrani et al. [39] in a sepsis model demonstrated that nonselective NOS inhibition with l-NMA actually reverses the hypotension but increases leukocyte adhesion and rolling. However, Petersson et al. [41] used the same inhibitor on a colitis model and showed that nonselective NOS inhibition caused a reduction in blood flow during acute inflammation. Thus, the NO was considered crucial for tissue perfusion during the inflammatory process and contributed to the maintenance of microvascular flow, adequate supply of oxygen and nutrients, as well as protection of the endothelium against oxidative stress.
Our group has assessed bacterial translocation (BT) in an intestinal obstruction model and seven day treatment with l-arginine and l-NAME led to absence of l-arginine beneficial effects with increased BT when NO is inhibited. These results point out that l-arginine acts on BT by the NO pathway [38]. Many authors claim that NO synthesis is part of the inflammatory response, to minimize ischemia and exacerbated coagulation while concomitantly fighting bacteremia. Side effects of inhibiting NO synthesis may be more pronounced than those caused by itself and its metabolites, as previously thought [11, 41].
Clinical studies were also performed to evaluate NO action. Avontur et al. [47] observed maintenance of vascular tone and blood pressure in septic patients undergoing nonselective inhibition (using l-NAME). However, there was no clinical improvement or reduction of mortality in these patients compared to the control group. In a randomized, double-blind, placebo-controlled study, Lopez et al. [48] evaluated the effects of the inhibitor 546C88, a nonselective NOS, and observed that septic patients treated with this inhibitor showed a higher mortality rate than patients in the placebo group. On the other hand, continuous supplementation of l-arginine (the NO precursor) in septic patients did not affect hemodynamic, cardiac, and pulmonary parameters [41]. Latter, in 2009, Luiking et al. [38] observed that septic patients had a reduction in NO synthesis because l-arginine was shifted to the urea synthesis. In this context, it should be considered that increasing NO synthesis is only one of the many factors that contribute to the septicemia process. The isolated inhibition of NO synthesis was not sufficient to stop or reduce the cascade of events that lead to the exacerbated activation of the immune system .
The l-arginine/NO controversy will remain until more studies have consistently confirmed either benefit or detriment of l-arginine supplementation. Zhou and Martindale [49], considering a review from animals and human available data, concluded that l-arginine appears to be safe and potentially beneficial for most all hemodynamically stable ICU populations, at doses delivered in immune modulation formulas.
Arginase-Polyamine Pathway
The enzyme arginase hydrolyzes l-arginine to l-ornithine and urea. There are two types of arginase: arginase-I and arginase-II. The arginase-I is a cytosolic enzyme present in the liver, related to the detoxification of ammonia and urea synthesis. The arginase-II is found in extrahepatic cells ’ mitochondria, such as macrophages, kidney, intestinal, and endothelial cells, and is involved in the regulation of ornithine, proline, and glutamate cell synthesis [50, 51].
The enzyme ornithine decarboxylase (ODC) , responsible for polyamines biosynthesis, is high in the small intestinal mucosa and plays an important role in polyamine metabolism. Polyamines are cationic molecules with low molecular weight. Putrescine, spermidine, and spermine are essential composites for cellular proliferation and differentiation [52].
The usual Western diet daily provides adequate polyamines supply. Meats are rich sources of spermine, while plant foods are high in putrescine and spermidine . Polyamines used by the human body can be also originated from TGI secretions, enterocyte desquamation, or bacterial synthesis [44, 53]. Polyamines, both exogenous as endogenous, are completely absorbed and directed to tissue growth or repair [53].
Polyamines regulate genic expression, signal transduction, ion channel function, DNA and proteins synthesis, and apoptosis. Thus, they are essential in cell proliferation, differentiation, and function. Under cell growth stimulation, the induction of polyamine synthesis is a key factor, preceding DNA replication and protein synthesis [44, 53].
Polyamines also act on fibroblasts , inducing wound healing and extracellular matrix proliferation [53].
There are few studies assessing the clinical effects of polyamines, especially in humans. Several studies have been carried out with animal models, which are presented in Table 46.1. Most of them have shown positive and encouraging results.
The correct l-arginine supply is essential to maintain the adequate immune function, considering the intense activity of arginase in the suppressor myeloid cells (SMCs; immature cells of the myeloid lineage that may differentiate into macrophages, dendritic cells, or granulocytes after stimulation). This reduces the availability of l-arginine, and therefore, it inhibits the proliferation of T cells, in addition to reducing the synthesis of IFN-γ and interleukin-2 (IL-2), growth factors, to T-lymphocyte function. The addition of l-arginine sharply increased the capacity for proliferation and the production of IFN-γ, IL-4, and IL-10 [8, 59, 60].
Thus, it is reasonable that the effects of the arginase-polyamine pathway in bacterial translocation are connected with the ability to maintain the integrity and regeneration of the intestinal barrier rather than the effects on the immune response, assuming that these are the two main mechanisms by which l-arginine helps avoid bacterial translocation [61].
l-Arginine in Bacterial Translocation
l-Arginine and Intestinal Barrier
The l-arginine effects in maintaining the integrity of the intestinal mucosa have been the focus of several investigations [9, 62]. In the ischemia/reperfusion model, l-arginine improved the weight of duodenal, jejunal, and ileal mucosa. The rate of jejunal cell proliferation in rats reduced or prevented the morphological and functional damage of the intestine and also worked on protecting the lipid peroxidation and maintenance of tissue levels of glutathione , a powerful free radical scavenger [8, 59, 60].
Chang et al. [61] observed that this amino acid supplementation increased the number of villi and reduced the intensity of the intestinal mucosa lesions in an intestinal obstruction model. l-Arginine also presented a protective effect on the intestinal mucosa during endotoxemia caused by LPS, inducing increased proliferation and maintenance of villus enterocytes [8]. In experimental models of radiation-induced enteritis, the number of animals with positive cultures and the number of bacteria present in mesenteric lymph nodes were decreased when the l-arginine was administered after radiation [9, 62].
A study conducted by our group [6] evaluated bacterial translocation and intestinal permeability in mice after treatment with l-arginine. Mice were divided into three groups, treated for seven days before surgical intervention with isocaloric and isoproteic diets. The l-arginine group (ARG) received a diet containing 2 % l-arginine, while animals in the intestinal obstruction (IO) received no supplementation and control groups (sham) received standard chow diet. In order to evaluate the intestinal permeability, after the seven days, the animals were gavaged with radiolabeled diethylenetriaminepentaacetic acid solution and, after 90 min, they were anesthetized and the ileum ligated. At 4, 8, and 18 h, the blood was collected for radioactivity determination and permeability analysis. In others to evaluate bacterial translocation, another group of animals, also treated for 7 days, was gavaged with 108 CFU/mL of 99m-technetium (99mTc) E. coli. After intestinal obstruction, BT was determined by the uptake of 99mTc E. coli in the mesenteric lymph nodes, blood, liver, spleen, and lungs, 18 h after the operation. The results are shown in Fig. 46.4 and Table 46.2. The data show that l-arginine supplementation reduced intestinal permeability and BT to physiological levels [63].
Intestinal permeability. Intestinal permeability after 4, 8, and 18 h of intestinal obstruction (IO) . The IO group showed enhancement in intestinal permeability in the times of 4–18 h. The treatment with l-arginine was able to prevent the increase of intestinal permeability. Errors bars show the SD. Data are expressed as mean ± SD (n = 5). * p < 0.05
l-Arginine and Immune Response
l-Arginine supplementation increases the immune function in humans and animal models acting in the host defense, inflammation, wound healing, and several other pathophysiological adaptations [8, 11].
l-Arginine has a central role in the immune system and its metabolism is important for macrophages and T-lymphocyte function. l-Arginine participates in the inflammatory response through two principal mechanisms: NO production (via iNOS) in the macrophages and l-arginine utilization for proliferation and activation of T lymphocytes. When TH2 immune response predominates, l-arginine may also follow the arginase pathway. In the latter, the inflammatory modulation is due to the production of ornithine (proline and polyamine precursors) and by the regulation of l-arginine availability, thus modulating NO synthesis and proliferation of T lymphocytes [63].
The exogenous l-arginine supply increases lymphocyte proliferation, especially T-helper cells which induce the appropriate cytokine production, and increases phagocytosis by enhancing the activity of macrophages and natural killer cells [11, 65].
The l-arginine effect on the intestinal mucosal immunity is evident. Shang and colleagues [7], in a study performed with septic rats, found that daily administration of enteral l-arginine, 2 % of the total caloric value (TCV) , increased the number of lymphocytes in Peyer’s patches, and secretory IgA levels tended to be higher in the groups treated with l-arginine before the induction of sepsis, suggesting the importance of l-arginine on the humoral immune response. The same research group showed, in another study, that l-arginine supplementation increases the expression of mRNA for the production of Th1 cytokines (INF-γ and IL-2) and Th2 cytokines (IL-4 and IL-10) [64].
Kang et al. [65] developed a meta-analysis enrolling data from 11 trials involving 321 patients, with the purpose to evaluate l-arginine effects on immune function in diseases like gastrointestinal malignancies, pressure ulcers, head and neck cancer, HIV/AIDS, head and neck cancer, unstable angina undergoing angioplasty, older people undergoing vaccination against streptococcus pneumonia, and burns. The data showed that the l-arginine-supplemented group had a significantly greater CD4+ T-cell proliferation; however, the CD4/CD8 ratio was not statistically significant between the l-arginine-supplemented and control groups. Furthermore, incidence of infectious complications was lower in the l-ARG with statistical significance. The group also showed that patients with l-arginine supplementation had a shorter length of hospital stay; however, this was not statistically significant.
Conclusions
Intestinal permeability changes are associated with higher bacterial translocation levels, commonly associated with sepsis. Local immune response and cytokines are involved in modulating intestinal permeability and BT to avoid increased inflammation. The host immune response plays a major role in the overall process. Thus, alternative treatment with immunomodulator agents would be beneficial in this clinical situation.
The l-arginine, due the arginase and NO pathways, can prevent bacterial translocation because of its potential effects mediated by mechanisms of intestinal mucosa preservation, reducing intestinal permeability and promoting tissue integrity and enhancement of immune response, considering its particular importance for macrophage and B lymphocyte production and the ability of l-arginine in modulating the immune response to balance the serum production of pro- and anti-inflammatory cytokines. In addition, l-arginine increases IgA secretion in the intestinal mucosa, contributing also for local immune response.
Furthermore, it is important to consider that one size does not fit all, and l-arginine supplementation should individually be assessed considering the several clinical situations.
In this way, the use of l-arginine is interesting for a number of clinical situations such as surgery, trauma, and burn patients, for example, potentially avoiding bacterial translocation. However, in cases of sepsis, an overproduction NO state, the use of l-arginine should be carefully evaluated, since the excesses of this metabolic intermediate can be deleterious to the patient.
References
Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill-evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25:741–57.
Ding L, Li J, Li Y, et al. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J Gastroenterol. 2004;10:2373–8.
Samel S, Keese M, Laning, et al. Supplementation and inhibition of nitric oxide synthesis influences bacterial transit time during bacterial translocation in rats. Shock. 2003;19:378–82.
Tsuei BJ, Bernard AC, Barksdale AR, et al. Supplemental enteral l-arginine is metabolized to ornithine in injured patients. J Surg Res. 2005;123:17–24.
Duggan C, Gannon J, Walker WA. Protective nutrients and functional foods for the gastrointestinal tract. Am J Clin Nutr. 2002;75:789–808.
Viana ML, Santos RG, Generoso SV, et al. Pretreatment with l-arginine preserves intestinal barrier integrity and reduces bacterial translocation in mice. Nutrition. 2010;26:218–23.
Shang HF, Wang YY, Lai YN, et al. Effects of l-arginine supplementation on mucosal immunity in rats with septic peritonitis. Clin Nutr. 2004;23:561–9.
Sukhotnik I, Mogilner J, Krausz MM, et al. Oral l-arginine reduces gut mucosal injury caused by lipopolysaccharide in rat. J Surg Res. 2004;122:256–62.
Ersin S, Tuncyurek P, Esassolak M, et al. The prophylactic and therapeutic effects of glutamine and l-arginine enriched diets on radiation induced enteritis in rats. J Surg Res. 2000;89:121–5.
Stechmiller BC, Childress B, Porter T. l-Arginine immunonutrition in critically ill patients: a clinical dilemma. Am J Crit Care. 2004;13:17–23.
Suchner U, Heyland DK, Peter K. Immune-modulatory actions of l-arginine in the critically ill. Br J Nutr. 2002;87:121–32.
Yeh CL, Yeh SL, Lin MT, et al. Effects of l-arginine-enriched total parenteral nutrition on inflammatory-related mediator and T-cell population in septic rats. Nutrition. 2002;18:631–5.
Macfie J, Reddy BS, Gatt M, et al. Bacterial translocation studied in 927 patients over 13 years. Br J Surg. 2006;93:87–93.
Nieves Jr C, Langkamp-Henken B. l-Arginine and immunity: a unique perspective. Biomed Pharmacother. 2002;56:471–82.
Salzedas-netto AA, Silva RM, Martins JL, et al. Can bacterial translocation be a beneficial event? Transplant Proc. 2006;38:1836–7.
Wiest R, Rath HC. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 2003;17:397–425.
Berg RD. Mechanisms promoting bacterial translocation from the gastrointestinal tract [Monograph]. Herborn-Diel: Old Herborn University Seminar, Department of Microbiology and Immunology; 2001.
Jun DW, Kim KT, Lee OY, et al. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci. 2010;55:1465–71.
Clavel T, Haller D. Molecular interactions between bacteria, the epithelium, and the mucosal immune system in the intestinal tract: implications for chronic inflammation. Curr Issues Intest Microbiol. 2007;8:25–43.
Macfie J. Enteral versus parenteral nutrition: the significance of bacterial translocation and gut-barrier function. Nutrition. 2000;16:606–11.
Balzan S, Quadros CA, Cleva R, et al. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22:464–71.
De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125–35.
Fouts DE, Torralba M, Nelson KE, et al. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–92.
Katouzian F, Sblattero D, Tarcisio N, et al. Dual sugar gut-permeability testing on blood drop in animal models. Clin Chem Acta. 2005;352:191–7.
Sawai T, Goldstone N, Drongowski RA, et al. Effect of secretory immunoglobulin A on bacterial translocation in an enterocyte-lymphocyte co-culture model. Pediatr Surg Int. 2001;17:275–9.
Kudsk KA. Glutamine: more evidence, more promise. J Parenter Enteral Nutr. 2008;32:492–4.
Marchiando AM, Granham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–44.
Sano T, Ajiki T, Takeyama Y, et al. Internal biliary drainage improves decreased number of gut mucosal T lymphocytes and MAdCAM-1 expression in jaundiced rats. Surgery. 2004;136:693–9.
Aydogan A, Kismet K, Kilicoglu B, et al. Effects of various enteral nutrition solutions on bacterial translocation and intestinal morphology during the postoperative period. Adv Ther. 2007;24:41–9.
Wang F, Zhao HY, Zhang ST, et al. Effect of enteral nutrition on dextran sulfate sodium induced colitis in rats. J Dig Dis. 2011;12:453–8.
Sánchez E, Nieto JC, Boullosa A, et al. VSL#3 probiotic treatment decreases bacterial translocation in rats with carbon tetrachloride-induced cirrhosis. Liver Int. 2014;35(3):735–45.
Batista MA, Nicoli JR, Martins FS. Pretreatment with citrulline improves gut barrier after intestinal obstruction in mice. JPEN J Parenter Enteral Nutr. 2012;36:69–76.
Viana ML, Dos Santos R, Generoso SV, et al. The role of l-arginine-nitric oxide pathway in bacterial translocation. Amino Acids. 2013;45:1089–96.
Quirino IE, Carneiro MB, Cardoso VN, et al. l-Arginine supplementation induces arginase activity and inhibits TNF-α synthesis in mice spleen macrophages after intestinal obstruction. JPEN. 2014.
Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6.
Cynober L, Le Boucher J, Vasson MP. l-Arginine metabolism in mammals. Nutr Biochem. 1995;6:402–13.
Hallemeesch MM, Lamers WH, Deutz NEP. Reduced l-arginine availability and nitric oxide production. Clin Nutr. 2002;21:273–9.
Luiking YC, Poeze M, Ramsay G, et al. Reduced citrulline production in sepsis is related to diminished de novo l-arginine and nitric oxide production. Am J Clin Nutr. 2009;89:142–52.
Sundrani R, Easington CR, Mattoo A, Parrillo JE, Hollenberg SM. Nitric oxide synthase inhibition increases venular leukocyte rolling and adhesion in septic rats. Crit Care Med. 2000;28:2898–903.
Luiking YC, Poeze M, Ramsay G, et al. The role of l-arginine in infection and septicemia. JPEN J Parenter Enteral Nutr. 2005;29:70–4.
Petersson J, Schreiber O, Steege A, et al. eNOS involved in colitis-induced mucosal blood flow increase. Am J Physiol Gastrointest Liver Physiol. 2007;293:1281–7.
Wu Y, Kudsk KA, Dewitt RC, et al. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229:662–8.
Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16.
Flynn NE, Meininger CJ, Haynes TE, et al. The metabolic basis of l-arginine nutrition and pharmacotherapy. Biomed Pharmacother. 2002;56:427–38.
Luiking YV, Deutz NEP. Isotopic investigation of nitric oxide metabolism in disease. Curr Opin Clin Nutr Metab Care. 2003;6:103–8.
Cauwels A. Nitric oxide in shock. Kidney Int. 2007;72:557–65.
Avontur J, Nolthenius RPT, Van Bodegom JW, et al. Prolonged inhibition of nitric oxide synthesis in severe shock—a clinical study. Crit Care Med. 1998;26:660–7.
López A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30.
Zhou M, Martindale RG. l-Arginine in the critical care setting. J Nutr. 2007;137:1687–92.
Zaloga GP, Siddiqui R, Terry C, Marik PE. l-Arginine: mediator or modulator of septicemia? Nutr Clin Pract. 2004;19:201–15.
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, et al. l-Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–68.
Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–51.
Moinard C, Cynober L, De Bandt JP. Polyamines: metabolism and implications in human diseases. Clin Nutr. 2005;24:184–97.
Witte MB, Vogt N, Stuelten C, et al. Arginase acts as an alternative pathway of l-arginine metabolism in experimental colon anastomosis. J Gastrointest Surg. 2003;7:378–85.
Lardy H, Mouillè B, Thomas M, et al. Enterocyte metabolism during early adaptation after extensive intestinal resection in a rat model. Surgery. 2004;135:649–56.
Duranton B, Schleiffer R, Gosse F, et al. Preventive administration of ornithine alpha-ketoglutarate improves mucosal repair after transient ischemia in rats. Crit Care Med. 1998;26:120–5.
De Oca J, Millat E, Dominguez MA, et al. Selective bowel decontamination, nutritional therapy and bacterial translocation after burn injury. Clin Nutr. 1993;12:355–9.
Gobert AP, Cheng Y, Akhtar M, et al. Protective role of arginase in a mouse model of colitis. J Immunol. 2004;173:2109–17.
Cintra AE, Martins JL, Patrício FR, et al. Nitric oxide levels in the intestines of mice submitted to ischemia and reperfusion: l-arginine effects. Transplant Proc. 2008;40:830–5.
Sayan H, Ozacmak VH, Altaner S, et al. Protective effects of l-arginine on rat terminal ileum subjected to ischemia/reperfusion. J Pediatr Gastroenterol Nutr. 2008;46:29–35.
Chang T, Lu R, Tsai L. Glutamine ameliorates mechanical obstruction-induced intestinal injury. J Surg Res. 2001;95:133–40.
Gurbuz AT, Kunzelman J, Ratzer EE. Supplemental dietary l-arginine accelerates intestinal mucosal regeneration and enhances bacterial clearance following radiation enteritis in rats. J Surg Res. 1998;74:149–54.
Bansal V, Ochoa JB. l-Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care. 2003;6:223–8.
Shang HF, Hsu CS, Yeh CL, Pai M, Yeh S. Effects of l-arginine supplementation on splenocyte cytokine mRNA expression in rats with gut-derived sepsis. World J Gastroenterol. 2005;11:7091–6.
Kang K, Shu XL, Zhong JX, Ting-Ting Y, Tao L. Effect of l-arginine on immune function: a meta-analysis. Asia Pac J Clin Nutr. 2014;23:351–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Viana, M.L., de Vasconcelos Generoso, S., dos Santos, R.d.G.C., Cardoso, V.N., Correia, M.I.T.D. (2017). l-Arginine and Bacterial Translocation: Implications for Health. In: Patel, V., Preedy, V., Rajendram, R. (eds) L-Arginine in Clinical Nutrition. Nutrition and Health. Humana Press, Cham. https://doi.org/10.1007/978-3-319-26009-9_46
Download citation
DOI: https://doi.org/10.1007/978-3-319-26009-9_46
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-26007-5
Online ISBN: 978-3-319-26009-9
eBook Packages: MedicineMedicine (R0)