Abstract
In biology, incompatibility is usually defined as restriction of mating competence controlled by genes other than those determining sexual differentiation. It has been long recognised that incompatibility concerns not only the sexual phase but also the vegetative phase. The latter becomes apparent especially in fungi and was first termed heterokaryon incompatibility. In both sexual and vegetative incompatibility, the action of the genetic traits involved precludes the exchange of genetic material. Thus, inhibition of recombination results by a lack of karyogamy (sexual incompatibility) as well as by an inability of the nuclei to coexist in a common cytoplasm and to undergo somatic recombination (vegetative incompatibility). Since recombination is of paramount importance in evolution, the biological significance of incompatibility as a factor controlling recombination is immediately apparent.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
I. Introduction
In biology, incompatibility is usually defined as restriction of mating competence controlled by genes other than those determining sexual differentiation. It has been long recognised that incompatibility concerns not only the sexual phase but also the vegetative phase. The latter becomes apparent especially in fungi and was first termed heterokaryon incompatibility. In both sexual and vegetative incompatibility, the action of the genetic traits involved precludes the exchange of genetic material. Thus, inhibition of recombination results by a lack of karyogamy (sexual incompatibility) as well as by an inability of the nuclei to coexist in a common cytoplasm and to undergo somatic recombination (vegetative incompatibility). Since recombination is of paramount importance in evolution, the biological significance of incompatibility as a factor controlling recombination is immediately apparent.
Nature has evolved two principal systems to control incompatibility. According to their mode of genetic determination, these have been called homogenic and heterogenic incompatibility (Esser 1962). The genetic basis of homogenic incompatibility consists in a sexual incompatibility of nuclei carrying identical incompatibility factors. Heterogenic incompatibility consists in a genetic difference of at least one single gene which inhibits the coexistence of the nuclei concerned in a common cytoplasm.
From these definitions, it follows that homogenic incompatibility enhances outbreeding and favours recombination and evolution of the species. Heterogenic incompatibility, however, restricts outbreeding and thereby favours the evolution of isolated groups within a single species. Both systems, despite controlling recombination in an antagonistic way, are integrated constituents of evolution.
Homogenic incompatibility has been known since Darwin’s time and its various mechanisms have been analysed in great detail, in both higher plants and fungi. Its actions and its distribution are the subject of many books and reviews. This type of incompatibility is described in Freihorst et al. (2016) and Dyer et al. (2016).
The genetics of heterogenic incompatibility was first revealed 60 years ago, in studies involving the ascomycete Podospora anserina (Rizet and Esser 1953; Esser 1954). Meanwhile, this subject has attracted much attention, and many cases of heterogenic incompatibility, dealing with the vegetative and/or the sexual phases of fungi, have been described and analysed (see Table 6.1).
It is understandable that heterogenic incompatibility was less intensively studied than homogenic incompatibility, since the former rarely occurs within true breeding laboratory strains, but rather between geographical races differing in their genetic constituency. In addition, heterogenic incompatibility has often been overlooked and sometimes misinterpreted as “sterility”, merely because of a failure to mate.
This review is a revised and updated version of the chapter on heterogenic incompatibility in the second edition of this volume (Esser 2006).
Other pertinent reviews on heterogenic incompatibility can be found in Esser and Kuenen (1967), Esser (1971), Lemke (1973), Carlile and Gooday (1978), Lane (1981), Esser and Meinhardt (1984), Jennings and Rayner (1984), Perkins and Turner (1988), Glass and Kuldau (1992), Leslie (1993), Bégueret et al. (1994), Leslie and Zeller (1996), Worall (1997), Glass et al. (2000), Saupe (2000) and Glass and Kaneko (2003).
II. Barrage Formation
More than 100 years ago, Reinhardt (1892) first observed that when certain fungal mycelia approached one another, sometimes an interaction phenotypically recognisable as repulsion occurred. This phenomenon was subsequently described by others (Cayley 1923, 1931; Nakata 1925), and it was probably Vandendries (1932) who introduced the term barrage to describe it. Certainly, older descriptions of barrages, based on quite different phenomena, were assigned different names. Since barrage is a phenotypic expression of heterogenic incompatibility, it is at first necessary to define the concept of barrage.
If fungal hyphae from different mycelia grow towards each other, in general four main types of interaction occur, and these can be easily demonstrated on agar media.
-
1.
Mutual Intermingling = Normal Contact
After approach, the hyphae intermingle in the zone of contact and show (with a few exceptions, as in Oomycota) numerous hyphal fusions via anastomosis. After a time, the border zone between the two mycelia becomes unrecognisable (Fig. 6.1a–d).
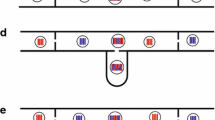
Fig. 6.1 (a–e) Compilation of mycelial interactions in fungi. (a) Podospora anserina. Barrage formation between different geographical races. Sexual reproduction is not affected. Perithecia are produced in any combination between different mating types. (b) Podospora anserina. Hyphal morphology. Above hyphae in the contact zone of an intra-race combination, below hyphae within the barrage zone. (c) Podospora anserina. Barrage formation linked with sexual incompatibility (for details, see Fig. 3 and text). (d) Intra- and interspecific interactions between monokaryons of Polyporus ciliatus (cil) and Polyporus brumalis (bru) showing normal contact (left), and barrage (bottom) and border line (top and right). All monokaryons are compatible in mating type. (e) Cross section of a log colonised by wood-destroying basidiomycetes. Dark zones indicate the aversion lines of mycelia. Further details are given at various sites in the text (adapted from Esser 1956, and Esser and Meinhardt 1984)
-
2.
Inhibition
When opposing hyphae approach each other, an inhibition zone free of hyphae is formed between the two mycelia. This phenomenon may be caused by unilateral or mutual interaction, due to the secretion and diffusion of inhibitory substances.
-
3.
Mutual Intermingling and Inhibition = Barrage Formation
When two mycelia grow into each other and intermingle, an antagonistic reaction ensues. In contrast to inhibition by diffusible substances, the barrage reaction requires cytoplasmic contact via hyphal fusions. The phenotype of the barrage varies depending on the species and mode of genetic control (Fig. 6.1). However, in all barrages known so far, nuclear exchange is not inhibited, but in most cases the two types of mycelia form abnormal and even lethal fusions. The hyphal tips may branch profusely. A clear line of contact appears with increasing age of the culture. Barrages are mainly found in intraspecific (interracial) matings. Depending on the species, the barrage may be colourless or pigmented (Fig. 6.1a, c). A recent study in Neurospora shows that different types of barrages may occur also within one and the same species (Micali and Smith 2003).
-
4.
Mutual Repulsion = Border Line, Demarcation Line
Especially noted in matings of wood-rotting basidiomycetes, a mutual repulsion and antagonistic reaction is evident which leads to the formation of a more or less strongly pigmented zone of intermingled hyphae. This demarcation line (Adams and Roth 1967) or border line (Esser and Hoffmann 1977) occurs mainly in interspecific matings. It is visible in nature on cuttings of logs (Rayner and Todd 1977; Esser and Meinhardt 1984; Fig. 6.1d) as well as in axenic cultures (Fig. 6.1c). Border lines are often used as criteria for species delineations. However, this commonly creates some problems in interpretation because, in most cases investigated to date, there are no analyses of the microscopic structure of these lines, nor of whether there is hyphal fusion allowing cytoplasmic contact and nuclear exchange. Therefore, it is often rather difficult to evaluate experimental reports with respect to barrage or border line formation and, consequently, to distinguish between intraspecific and interspecific matings.
Accordingly, I shall henceforth use the term barrage only if the microscopic observations show that a zone of aversion occurring between two mycelia is associated with hyphal fusions. I am well aware that this distinction is not always possible on the basis of exact data.
III. Heterogenic Incompatibility in the Ascomycete Podospora anserina
A. The Phenomenon
Although barrage formation is found in mycelial interactions with various higher fungi, the best analysed case concerns the ascomycete Podospora anserina. As in many ascomycetes, the mating competence of P. anserina is controlled by the bipolar mechanism of homogenic incompatibility (heterothallism) due to an interaction of the two idiomorphs + and − of the mating-type locus (see Dyer et al. 2016). In analogy to the Neurospora terminology, these alleles were later renamed and termed mat + and mat−, respectively.
In studying various races of P. anserina with different geographical origins, the mycelia of which showed no recognisable macroscopic differences, Rizet (1952, 1953) found that in interrace combinations a barrage was formed irrespective of mating type. As may be seen from Fig. 6.1a, this barrage is macroscopically characterised by a sharp white zone between two darkly pigmented (melanotic) paired mycelia. A microscopic examination revealed that in this zone the hyphae forming anastomoses become curled, swollen and degenerative (Fig. 6.1b). This barrage zone eventually consists of dead hyphae.
In some cases the barrage formation does not affect fruiting between different mating types, because perithecia are formed on both sides of the barrage (Fig. 6.1a).
This peculiar phenomenon is explained by the fact that the trichogynes of the + female sex organs (protoperithecia) fuse only with the − male gametes (spermatia), and vice versa. Spermatia, however, are never formed in the barrage zone. From this it follows that the trichogynes pass the barrage unimpaired, since no anastomoses involving trichogynes take place. Obviously, the fusion of the tip of the trichogyne with a spermatium does not necessarily bring about the incompatibility reaction occurring between two hyphae, although the reason for this remains obscure.
Nevertheless, as summarised in Fig. 6.2, a comprehensive study of the mating interactions between 19 geographical races revealed that in addition to the barrage formation, the fruit body formation (perithecia) also was quantitatively and/or qualitatively disturbed. In the first instance, the number of perithecia was drastically reduced on one or both sides of the barrage. In the second case, one or both of the reciprocal crosses between the two mates were incompatible. In the 13 (7.6 %) interracial combinations which showed no barrage, there was also no effect on fruiting—in other words, sexual incompatibility in interrace crosses is always linked with barrage formation.
Scheme of the mating reactions between various races of Podospora anserina isolated from different localities in France and Germany. +/− Mating type, uppercase letters designation of races, closed squares compatible in both vegetative and sexual phase, closed squares with inserted open squares sexual compatibility and vegetative incompatibility (barrage formation only), hatched squares vegetative incompatibility and reduced fruit body formation, open squares incompatibility in both vegetative and sexual phase (Adapted from Esser 1971)
In this context, it should be noted that at least one incompatibility mechanism may also lead to distortions in meiotic segregation due to spore killer effects (van der Gaag et al. 2003; Hamann and Osiewacz 2004).
B. Genetic Control
The genetic background of heterogenic incompatibility was revealed by the analysis of the two races s and M (Rizet and Esser 1953; Esser 1954, 1956). Six loci were identified as instrumental in two different mechanisms, as summarised in Fig. 6.3 and explained below:
Podospora anserina. Scheme of the action of the two mechanisms of heterogenic incompatibility operating in a cross of the races s and M. The different alleles are symbolised by lowercase letters. For further information, see text (Adapted from Blaich and Esser 1970)
-
1.
The allelic mechanism caused by alleles of the t and u loci does not interfere with sexual compatibility. If strains differ at either one (Fig. 6.1a) or both loci, a vegetative incompatibility is provoked, showing up as barrage formation.
-
2.
The non-allelic mechanism depends on the interaction of two specific alleles at two different loci. In the interracial cross of s and M, four loci a, b, c, v were identified showing an incompatibility of the alleles a1/b and c1/v, respectively, leading to barrage formation as well as to unilateral incompatibility (middle of Fig. 6.1c). If both mechanisms overlap in recombinants from the cross s × M in the combination a, b, c1, v1 × a1, b1, c, v, then a complete sexual incompatibility is brought about (Fig. 6.1c, right side).
C. Physiological Expression
As mentioned above, a prerequisite for the expression of heterogenic incompatibility is that two nuclei showing a specific allelic and/or non-allelic difference are brought together in a common cytoplasm. This occurs very frequently because in Podospora as in many other ascomycetes, hyphae fuse by anastomosis when they come into contact. This is followed by a mutual nuclear migration leading to heterokaryosis.
Incompatibility of heterogenic nuclei can be brought about by a unilateral or by a bilateral action. It was found that in those heterokaryons in which the allelic mechanism was effective, a destabilisation took place, leading to a formation of homokaryotic sectors of either nuclear type, separated by barrage formation. In heterokaryons in which the non-allelic mechanism was instrumental, however, no sectoring occurred, because one nuclear species was eliminated. For example, in the combination ab + a1b1, only the ab nuclei survived. Thus, it follows that the allelic mechanism brings about a mutual interaction, whereas the non-allelic mechanism is realised through unilateral gene action (Esser 1956, 1959a, b).
This explains as well the unilateral sexual incompatibility in the non-allelic mechanism present, for instance, in the crossing of strains ab × a1b1 (Fig. 6.3). It may be deduced from these heterokaryon experiments that b is the “aggressive” allele and that a1 is its target. Since during the mating procedure in Podospora in the differentiated trichogynes the nuclei degenerate in the combination ♀ab × ♂a1b1, the active b-nuclei are no longer present and there is no inhibition for the sensitive a1-nucleus, which may thus migrate into the ascogonial cell of the protoperithecium. In the alternate case, the active b-nucleus of the spermatium is able to initiate destruction when entering the trichogyne. As in the case of the allelic mechanism, there is also no explanation why the non-allelic mechanism does not become effective during the steps of sexual differentiation and is expressed only when the ascospores germinate, as demonstrated by the assay of heterokaryons.
If as a result of sexual propagation, two het-genes come together in one nucleus and in comparable heterokaryons, respectively, they are not viable (a phenomenon found also in other fungi). This has resulted to use in recent publications programmed cell death or apoptosis for heterokaryon incompatibility (Glass and Demnthon 2006; Goncalves and Videira 2014).
These findings were later confirmed by Bernet (1965), who studied the same races s and M. By analysis of other races, six more incompatibility loci were identified (Bernet 1967; Bourges et al. 1998), one being involved in the allelic and five in the non-allelic mechanism. Unfortunately, Bernet did not use our gene designations. All these genes were later summarised under the heading het-genes, e.g. het-c. In the following, I shall give the original names of these genes in parentheses.
D. Function of the het-Genes
During the last years, many efforts were undertaken to understand the function of the het-genes on the molecular and biochemical level.
1. The Non-allelic Mechanism
a) Application of Biochemical Techniques
In heterokaryons, specific proteins (Esser 1959b; Blaich and Esser 1970), catabolic enzymes (Blaich and Esser 1971) and new enzyme activities, such as for several proteases, phenoloxidases, malate and NADH dehydrogenase and amino acid oxidase (Boucherie and Bernet 1978; Boucherie et al. 1981; Paoletti et al. 1998), were found, but their involvement with the function of the het-genes was not evident.
b) Mutations Which Interfere with het-Genes
A series of modifier mutants (mod-genes) were found, most of which suppress in specific combinations the barrage formation caused by the allelic or by the non-allelic mechanism. In active combinations, these mutants also suppress the formation of female sex organs (protoperithecia). One of these mod-genes, mod-E (a), codes for a member of the Hsp90 family of heat-shock proteins acting in various types of stress responses (Loubradou et al. 1997). A second cloned gene, the mod-D gene (v), encodes a Gα protein. The gene displays functional interactions with mod-E and Pa AC, a gene for an adenylate cyclase. Although addition of cyclic AMP can partially suppress growth defects caused by mod-D mutations, a molecular connection of cAMP with the effect of mod-D mutations on the het-genes was not found (Loubradou et al. 1999).
c) Analyses of mRNA
During the incompatibility reaction, a strong decrease of mRNA synthesis and the appearance of a new set of proteins occur. Therefore, the vegetative incompatibility is regulated, at least in part, by variation of the mRNA content of specific genes. Genes induced during the incompatibility reactions have been termed idi.
-
idi-1 is a cell wall protein and resides in the septum during normal growth (Dementhon et al. 2003).
-
idi-4 is abZIP transcription factor regulating autophagy and cell fate and also expression of other idi-genes (Dementhon et al. 2004).
-
idi-6/pspA encodes a vacuolar protease involved in autophagy (Pinan-Lucarré et al. 2003).
-
idi-7 acts in the formation of autophagosomes, vesicles which target cytoplasmic material to the vacuole (Pinan-Lucarré et al. 2003).
Rapamycin treatment of Podospora anserina causes idi-gene expression and cellular effects typical for heterogenic incompatibility (Dementhon et al. 2003).
d) Molecular Analysis of the het-c, het-d and het-e Genes
Alleles of het-c and het-e genes correspond to the genes b/b1 and a/a1 in Esser’s studies. Alleles of the het-c locus have similar ORFs but lead to protein products with some amino acid differences (Saupe et al. 1995). The het-c gene products are members of a family of ancestral sphingolipid transfer proteins (Mattjus et al. 2003). By inactivation of the het-c gene, abnormal ascospores are formed. het-c alleles interact in different ways with the het-e and het-d alleles (Saupe et al. 1995). Bastiaans et al. (2014) identified 11 het-c alleles, which define 7 distinct incompatibility specifities.
Both the het-d and the het-e genes code for proteins which display a GTP-binding site and a WD40 repeat domain, typical for a β-subunit of a G-protein. Sequence comparison of different het-e alleles showed that het-e specificity is determined by the sequence of the WD40 domain, which may confer the incompatibility interactions (for references, see Espagne et al. 2002). Physical interactions with the het-c proteins need to be demonstrated to clarify this.
2. The Allelic Mechanism
The first het-genes of all described are the alleles het-s and het-S (Rizet 1952). They cause barrage formation by the allelic mechanism but do not interfere with fruit body production. Their co-expression in a common cytoplasm causes cell death. Both alleles encode 30-kd proteins consisting of 289 amino acids. The alleles differ in 43 amino acid positions (Turcq et al. 1990, 1991).
A disruption of either gene resulted in a lack of the 30-kd protein. When mated, these strains no longer formed a barrage. Sexual compatibility was not affected. It was further shown by detailed analyses of the het-s/S locus of 13 wild strains that the specificity of the s and S proteins to provoke heterogenic incompatibility depends on a single amino acid difference only (Deleu et al. 1993).
Strains with the genotype het-s exist in two phenotypic states: the neutral phenotype het-s* and the active phenotype het-s. The neutral phenotype is characterised by the fact that it does not show the barrage reaction, when crossed with het-S strains. The het-s phenotype is infective and is able to transform, via hyphal fusions, a neutral het-s* strain.
The het-s protein which provokes the transformation is a prion which adopts an amyloid structure and propagates in vivo as a self-perpetuating amyloid aggregate (Nazabal et al. 2003; Balguerie et al. 2004). Amyloid structures formed in vitro were shown to be infectious, in contrast to soluble het-s protein and amorphous aggregates, supporting the prion nature of the amyloid fibres (Maddelein et al. 2002).
The analysis of deletion constructs and site-directed mutants showed that a short C-terminal peptide (112 amino acids) allows the propagation of the prion analogue (Cousteau et al. 1997). This part of the protein contains the amyloid core regions of the het-s prion protein (Balguerie et al. 2003, 2004).
In conclusion: The many studies of heterogenic incompatibility performed with Podospora anserina have given deep insights into the genetic mechanism of the het-genes and shown many aspects of their action. However, a complete understanding of their function in causing their mutual antagonism still needs further research.
IV. Further Examples of Heterogenic Incompatibility
As one may suppose, the discovery of heterogenic incompatibility was not through focussed research. By contrast, in the ascomycetes it was observed as a “by-product” of genetic research on breeding competence. In the basidiomycetes, as discussed below, data on heterogenic incompatibility are very often a result of studies in population genetics dealing with intergeneric and interspecific delineation and evolution. In the literature, there is a diversity of names, definitions and gene symbols for effects which can be interpreted as manifestations of heterogenic incompatibility. Thus, it is understandable that in describing the antagonistic mycelial interactions and the various groups of natural isolates showing compatibility or incompatibility, different terms and expressions are used (as stated in the legend in Table 6.1), which I consider also as a source of information for a reader who is not familiar with this area of research and who wishes to gain more detailed information. Therefore, I shall discuss only some other cases of heterogenic incompatibility which have been studied in more detail.
Although the myxozoa are no longer grouped with the fungi, they have at least to be briefly mentioned, because these organisms have been the subject of intensive studies of heterogenic incompatibility. Vegetative incompatibility is widespread between geographical races of the genera and species analysed to date. In analogy with Podospora, plasmodial matings may lead to a visible zone of aversion, which does not allow nuclear migration, because there is either unilateral or mutual disintegration of the nuclei. Collectively from these studies, various genes acting according to the allelic mechanism have been identified. There are no data concerning the physiological actions of these genes.
For pertinent information, the reader is referred to literature on Didymium iridis and related species (Betterley and Collins 1984; Clark 1984, 2003), Physarum polycephalum (Knowles and Carlile 1978a,b; Lane and Carlile 1979; Schrauwen 1979; Lane 1981), and Dictyostelium discoideum (Robson and Williams 1979, 1980).
A. Oomycota
Oomycota have scarcely been used for genetic studies. This may be partially due to the fact that in contrast to ascomycetes and basidiomycetes, they are vegetative diploids, thus complicating genetic analysis of progeny. It is not surprising that our knowledge of genetic control of their breeding systems, and especially evidence for heterogenic incompatibility, is very limited. Furthermore, anastomoses between the coenocytic hyphae having cellulose walls do not occur. The only indication for the existence of heterogenic incompatibility in Oomycota concerns the genus Phytophthora (for details, this is referenced in Table 6.1).
B. Glomeromycota
In the recently defined Glomeromycota, heterogenic incompatibility has been reported between isolates of the arbuscular mycorrhizal fungus Glomus mosseae (Giovanetti et al. 2003).
C. Dikaryomycota
1. Ascomycotina
This class includes some of the most thoroughly studied saprophytic genera, for example, Neurospora, Aspergillus and Podospora. There are also numerous data proving the existence of heterogenic incompatibility in a great number of parasitic ascomycetes, but detailed genetic data are lacking in many cases.
a) Saprophytic Ascomycetes
Neurospora: Apart from Podospora, the genus Neurospora is the best analysed taxonomic entity for heterogenic incompatibility in fungi (Table 6.1).
Over 3900 isolates have been collected from nature from over 500 sampling sites. Perkins and his collaborators have classified and assessed this impressive dataset in terms of inter- and intraspecies mating relations (Perkins et al. 1976; Perkins and Turner 1988). The species most studied is Neurospora crassa, but the closely related species Neurospora sitophila is also used for investigating heterogenic incompatibility.
Heterogenic incompatibility in Neurospora has, according to my knowledge, been reported only as heterokaryon incompatibility concerning the vegetative phase. It is under polygenic control and involves several allelic mechanisms. According to Debets et al. (1994), mitochondrial plasmids might be involved in heterogenic incompatibility.
-
1.
The Mating-Type Locus
As early as 1933, Moreau and Moruzi reported “cross sterility” between opposite mating types in interracial crosses of N. sitophila. A comparable phenomenon of “cross sterility” was also described by Lindegren (1934) for N. crassa. In both cases, no genetic analyses were performed. A more substantial indication for the occurrence of heterogenic incompatibility originates from the classical “heterokaryon paper” of Beadle and Coonradt (1944), showing that strains of N. crassa must be of the same mating type in order to form a vigorous and stable heterokaryon.
Newmeyer and her collaborators (cf. Table 6.1) proved that the two mating types MAT A and MAT a are not able to coexist in a common cytoplasm. They found that the gene tol (linkage group IV), which is not linked with the mating-type locus, suppresses this vegetative incompatibility. tol does not interfere with the sexual compatibility initiated in an A/a cross. These authors proposed that the mating-type locus is a complex genetic trait controlling both heterokaryon formation and sexual compatibility.
The tol-gene encodes a putative reading frame for a 1011-amino-acid polypeptide with a coiled-coil domain and a leucine-rich repeat. It is suggested that the TOL-locus may interact with the mating-type proteins MAT A-1 and/or MAT a-1 to form a death-triggering complex (Shiu and Glass 1999).
The concept of complex mating-type loci was verified by molecular analyses. The MAT A gene consists of 5301 bp (Glass et al. 1990), whereas the MAT a gene is much smaller and comprises 3225 bp only (Staben and Yanofsky 1990). However, sexual compatibility and heterokaryon incompatibility were found to be inseparable. Thus, a single gene product in both mating types MAT A and MAT a is responsible for the completion of the sexual cycle and for heterogenic incompatibility in the vegetative phase (Shiu and GlassFdeets 2015). In order to emphasise the rather strong structural dissimilarity of the mating-type alleles, Metzenberg (1990) has proposed to use the term idiomorphs, rather than alleles.
The involvement of mating loci in heterogenic incompatibility is rather unusual in fungi. It seems to be restricted to some of the self-incompatible Neurospora species. Here again, the same question as in Podospora is raised: why do the genes responsible for heterokaryon incompatibility not interfere with the overall sexual process? It is not possible at present to answer this question. Maybe there are additional genes which, like the above-mentioned tol-gene, are able to act as switches to stop the interaction as soon the nuclei enter the sexual phase.
In this context, the spore killer genes of Neurospora should be mentioned (Raju 1979, 2002; Turner and Perkins 1979). These sk-genes are widely distributed in wild-type collections. They cause (albeit only in crosses with sensitive strains), after meiotic segregation, lethality of the four ascospores carrying the genes. This phenomenon does not occur in sk/sk matings. This observation also strengthens the idea that there are two different, genetically controlled phases in the sexual cycle of fungi: the bringing together of genetic material via cytoplasmic contact and the true sexual cycle leading eventually to recombination.
-
2.
The het-Genes
There are some other genes, apart from the mating idiomorphs, which control heterokaryon formation in Neurospora. This was earlier postulated by Gross (1952) and Holloway (1955). A detailed analysis of these so-called het-genes was performed by Garnjobst, Wilson and collaborators (cf. Table 6.1). At present, 11 het-genes are known.
Two unlinked allelic pairs (C/c and D/d) were identified which control heterokaryon formation according to the allelic mechanism. Heterokaryons are formed only if the two partners have identical alleles at both loci. If one or both factors are heterogenic, then there will be an incompatibility reaction, as in the barrage zone of Podospora, leading to a destruction of the hyphae which have anastomosed. The C and D genes are neither linked with the mating-type locus nor suppressed by the tol-gene, nor do they interfere with sexual compatibility of different mating types. A third locus (E/e) was identified which resembles in its effect the C and D genes. Subsequently, these genes were given the prefix het.
In analysing different geographical races, Perkins (1968) has detected multiple alleles at the het-C locus. The similarity of the action of the het-genes to that of the Podospora barrage genes is also supported by the observation that a clear barrage zone between the two lines of perithecia may be seen when strains of opposite mating types, but heteroallelic for the het-genes, meet (Griffith and Rieck 1981; Perkins 1988). Degradation of nuclear DNA indicating a form of programmed cell death has also been visualised (Marek et al. 2003).
Another het-gene, but not leading to cell death, was found by Pittenger (cf. Table 1). The allelic pair I/i controls the capacity of nuclei to divide. Allele I is weakly dominant over allele i. When the proportion of I nuclei in an (I + i) heterokaryon is more than 30 %, the i nuclei are lost and the heterokaryon becomes an I homokaryon. By use of different marker genes, it became evident that this incompatibility is independent of the genetic background and, hence, from the action of the C, D, E het-genes mentioned above.
In recent years, more detailed knowledge about the structure and function of the het-genes has accumulated. By deletions within het-genes, their action is suppressed and compatibility achieved (Smith et al. 1996). Two het-loci were studied in more detail.
The het-c gene encodes a 966-amino-acid polypeptide with a putative signal peptide, a coiled-coil motif and a C-terminal glycine-rich domain, found also in cell wall proteins. Deletions showed that this region is responsible for the activity of the het-c gene (Saupe et al. 1996). het-c specificities reside in a 38–48 aa domain at the N-terminal end (Saupe and Glass 1997; Wu and Glass 2001). Het-c alleles of N. crassa function also in Podospora anserina in cell death reaction related to heterokaryon incompatibility, whilst the P. abserina homolog Pahch causes no heterokaryon incompatibility in its host (Saupe 2000).
Three deletion mutants were identified within an open reading frame (named vib = vegetative incompatibility blocked). These mutants relieved growth inhibition and repression of conidiation caused by the het-c gene. Thus, it was suggested that the vib region is a regulator for conidiation (Xiang and Glass 2002). Rather often, these suppressor mutants exhibited chromosome rearrangements (Xiang and Glass 2004).
The het-6 gene maps to a region of 250 kbp. Within this region, two genes were identified which show incompatibility activity. One of these shows sequence similarity to the het-e product of Podospora anserina and the tol-gene product. The other encodes the large subunit of the ribonucleotide reductase. Both genes are inherited as a block. Thus, it was suggested that these genes act through a non-allelic mechanism to cause heterogenic incompatibility (Smith et al. 2000; Mir-Rashed et al. 2000).
Regarding the comprehensive new data obtained for Neurospora, like in Podospora, a breakthrough in understanding the molecular mechanism of the mutual interaction of the het-genes requires still further research.
Aspergillus: Some Aspergillus species were also studied for heterogenic incompatibility. The failure of heterokaryon formation between various natural isolates was already described by Gossop et al. (1940) for Aspergillus niger and by Raper and Fennell (1953) for Aspergillus fonsecaeus (both imperfect). Comprehensive studies with the perfect (teleomorphic) species Aspergillus nidulans were performed by Jinks and his co-workers (cf. Table 6.1). A synopsis of these observations and experiments, including numerous related and unrelated isolates from all over the world, allows the following conclusions:
-
1.
Heterokaryon incompatibility is not due to geographical isolation, since the various vegetative compatibility (v-c) groups comprise isolates from adjacent as well as from distant areas. Nor is it linked with minor morphological differences of the isolates.
-
2.
Vegetative incompatibility does not prevent heterokaryon formation. Heterokaryons seem to have selective disadvantage and are supposed to be overgrown by the homokaryons.
-
3.
Fruit body formation for teleomorphic species is not inhibited. However, the number of fruit bodies is reduced, as observed also in Podospora (Fig. 6.2).
-
4.
Eight het-loci were identified, two of which are multiallelic; het-B has four and het-C has three alleles. The interaction of the het-genes follows the allelic mechanism, as described above for Podospora and Neurospora.
-
5.
The physiological action of these genes is not yet understood. A “killing reaction” like the one in Podospora and Neurospora seems not to take place.
Comparable results were obtained with two other teleomorphic species, A. glaucus (Jones 1965) and A. heterothallicus (Kwon and Raper 1967).
Studies with some anamorphic species (A. versicolor, A. terreus, A. amstelodami) performed by Caten (cf. Table 6.1) in general confirmed the observations made on the teleomorphic species, although without having the opportunity to identify het-genes.
A comprehensive study of the species Aspergillus flavus, A. parasiticus and A. tamarii revealed an association of morphology and mycotoxin production with the various i-c groups within each species (Horn et al. 1996).
Ascobolus: In Ascobolus immersus (cf. Table 6.1) the mating pattern of 38 strains collected at various places in Europe and southern India has been determined. There were at least three compatibility groups: A (23 strains) and B (nine strains) comprise the European isolates, and C, the Indian isolates. Within each group sexual reproduction is, as expected, controlled by a bipolar mechanism of homogenic incompatibility. No fertile offspring are obtained in any intergroup crossing, showing that there is genetic separation by heterogenic incompatibility. However, the European group B seems to be more closely related to the Indian group (C) in that sterile fruit bodies are produced between + and − mating types. An indication for further subdivision is the occurrence of barrages between representatives of all three groups. These data thus indicate how speciation may be initiated in Ascobolus immersus by means of both spatial and genetic isolation, the latter mediated by heterogenic incompatibility.
b) Parasitic Ascomycetes
For a number of plant pathogenic fungi, heterokaryon tests between different isolates led to barrage or to border line formation and to the classification of the so-called vegetative compatibility groups (v-c). Partially due to the difficulty of breeding pathogens under laboratory conditions, genetic data are often not available (see Table 6.1). However, in some of the recently published papers, biochemical techniques such as RAPD analysis were used to characterise the v-c groups, e.g. Punja and Sun (2002).
More comprehensive data are available for Botrytis cinerea. It was found that heterokaryon incompatibility in this fungus is caused by the gene Bc-hch which is homolog to Nc-het-c and the Pa-hch loci of Neurospora crassa and Podospora anserina, respectively. A PCR-RFLP analysis on a 1171-bp section was used to screen for polymorphism for this locus among 117 wild isolates and revealed two allelic types, thus allowing scientists to structure the natural populations into two groups.
For some other parasites, more detailed studies are available. In the case of the chestnut blight, Cryphonectria (Endothia) parasitica, different v-c groups characterised by barrage formation of varied intensity were detected. Weak barrages did not inhibit heterokaryon formation. Over 75 v-c groups were identified, controlled by at least seven incompatibility loci, some with multiple alleles. The heterogenic incompatibility followed mostly an allelic mechanism, but a non-allelic mechanism was also observed. Sexual compatibility was not affected by these genes (Choi et al. 2012).
According to Nuss and Koltin (1990), hypovirulence is related to the presence of a virus-like double-stranded RNA which can be transmitted via heterokaryosis. The efficiency of the transfer is highly reduced between incompatible strains and leads therefore to a lack of horizontal transfer of this parasite and contributes to its biocontrol (Milgroom and Cortesi 2004; Smith et al. 2006).
In the Gibberella fujikuroi species complex, in addition to the mating-type genes (+/−), mating groups termed A, B, C and D are recognised. They are considered varieties according to their host specificity. Within each group, heterogenic incompatibility was found. This allelic mechanism is controlled by at least 10 loci in group A, five loci in group B and three loci each in both groups C and D.
Within parasitic ascomycetes, there are only two indications for heterogenic incompatibility which affect the sexual phase. Both are not linked with heterokaryon incompatibility. The genus Cochliobolus includes plant parasites causing leaf and inflorescence diseases in Gramineae (anamorphic: Helminthosporium). Nelson and collaborators have studied extensively the mating system within this genus. A detailed analysis of the mating reactions of nearly 10,000 isolates from North and South America, comprising more than 40,000 matings, has led to the following results:
-
1.
The bipolar mechanism of homogenic incompatibility is responsible for the basic control of mating, insofar as only the combination of the alleles A and a leads to fructification.
-
2.
Fertility in crosses between opposite mating types originating from different hosts or origin is about 29 %. Most of the infertile crosses produce perithecia with immature or sterile ascospores. The others show no fruit body formation.
-
3.
Several sterility genes blocking the normal ontogenesis at different stages were identified and were predominantly responsible for the formation of sterile perithecia.
-
4.
The fact that some strains exhibiting incompatibility in certain combinations were compatible in all others can be explained only by the action of heterogenic incompatibility, despite the fact that the appropriate genes have not yet been identified.
-
5.
The objection that the incompatibility might be provoked by gross genetic diversities or species differences could be excluded. Furthermore, Nelson was able to assign 92.2 % of his strains to five distinct morphological types which might correspond to a single species.
2. Basidiomycotina
The first phenomena which may be attributed to heterogenic incompatibility came from this group of fungi.
Apart from the description of barrage phenomena between geographical races of Fomes species (Mounce 1929; Mounce and Macrae 1938), Bauch (1927) found in the smut fungus, Microbotryum violaceum (Ustilago violacea), that in interracial crosses additional genes interfere with the bipolar mating system and cause unilateral or reciprocal incompatibility. Similar findings were later reported by Grasso (1955) who studied interracial crosses in two other species, U. avenae and U. levis, originating from Italy and the United States, respectively.
Many phenomena resulting in heterokaryon incompatibility or inhibition of fruit body formation were poorly understood in older publications. They were mostly referred to as demarcation lines, barrages and/or crossing barriers. Thus, it is understandable that in a very comprehensive review (Burnett 1965), the mating restrictions in 17 species of basidiomycetes were treated only under the general heading “restrictions of outbreeding”.
In this review, I distinguish between those cases in which the existence of heterogenic incompatibility is proved and supported by genetic data and cases in which antagonistic mycelial interactions may only be interpreted as the expression of heterogenic incompatibility. Only the better-analysed cases will be presented in detail here (for others, see Table 6.1).
In this context, it needs to be stressed that in contrast to ascomycetes where heterogenic incompatibility may concern either the vegetative and/or the sexual phase, in basidiomycetes it is instrumental only in the vegetative phase, because basidiomycetes do not form sex organs. From this it follows that if a heterokaryon incompatibility occurs, then the sexual propagation is automatically inhibited.
An impetus to study heterogenic incompatibility stems from the interest in the population structure of saprophytic basidiomycetes, involving matings between natural isolates in order to obtain information for classification of genera and species (cf. Boidin 1986). These studies have not only revealed basic mating systems but also indicate a variety of antagonistic mycelial interactions correlated with heterokaryon and/or sexual incompatibility.
However, during the last years the interest in heterogenic incompatibility of basidiomycetes seems to have decreased, because there have been fewer publications describing this phenomenon. Instead, many comprehensive studies were published in establishing intra- or interspecific relationships by using molecular techniques. Thus, there is not much progress in understanding the genetic and physiological control of heterogenic incompatibility within this group of fungi.
In wood-rotting fungi the antagonistic interaction is easily recognised in cross sections from logs, as a narrow zone of interwoven hyphae in a region of relatively undecayed wood. These border lines, also called interaction zones or demarcation lines, are usually darkly pigmented, in contrast to the adjacent decay zones (Fig. 6.1e). They also show up on agar-grown cultures, depending on the composition of the medium. Without microscopic examination, it is not possible to say whether hyphal interactions inhibiting heterokaryon formation take place, as is evident in the barrage zone of Podospora, or simply antagonistic repulsions occur. Thus, a distinction between delimitation of species or races is a priori not possible. In the literature, the terms biological races and intersterility groups (i-c) are often used to characterise the interacting mycelia.
In analogy to the evaluation of data concerning the ascomycetes, I shall start the discussion of the basidiomycetes with a subject for which information on both heterogenic incompatibility and genetic control of speciation has been obtained. This is the wood-rotting fungus Polyporus.
Macrae (1967) studied the mating reactions of 31 single spore isolates of the tetrapolar Polyporus abietinus (syn. Hirschioporus abietinus) collected in different places in North America and Europe. According to differences in the morphology of their hymenial surfaces, the isolates were assigned to three morphological groups. The North American strains could be subdivided into the two classes A and B which are incompatible with each other, but which are both compatible with a third class C comprising the European strains. Since geographical isolation could be excluded, Macrae concluded that genes additional to the mating-type factors were involved. Similar data were also reported for P. schweinitzii (Barrett and Uscuplic 1971). Unfortunately, in both cases no genetic data are available.
Comparable phenomena were found and could be interpreted after comprehensive studies of other species of the genus (Hoffmann and Esser 1978). We had chosen the wood-rotting genus Polyporus in order to investigate, by genetic parameters, the validity of the classical species concept based on typological characters. In performing these studies, we “accidentally” detected evidence for heterogenic incompatibility.
As a result of matings of single spore-derived mycelia from 26 races of different origin, all races could unequivocally be grouped into three separate entities corresponding with the typological species P. arcularius, P. brumalis and P. ciliatus, on the basis of the following results (Fig. 6.4):
Mating relations in intra- and interspecies combinations of monokaryons from 26 races of different species of Polyporus. ▪ Normal contact, clamp connections and fruit bodies formed when A ≠ B ≠;  barrage formation, fruit body production delayed;
barrage formation, fruit body production delayed;  barrage formation with unilateral nuclear migration (only in dark part);
barrage formation with unilateral nuclear migration (only in dark part);  border line, neither clamp connections nor dikaryotic fruit bodies formed in any combination (Adapted from Esser and Hoffmann 1977)
border line, neither clamp connections nor dikaryotic fruit bodies formed in any combination (Adapted from Esser and Hoffmann 1977)
-
1.
As expected, the basic breeding system in Polyporus is the tetrapolar mechanism of homogenic incompatibility controlled by multiple alleles of the mating-type factors A and B.
-
2.
All intraspecific combinations were fertile. A conspicuous barrage formed in those crosses where dikaryotisation and fruiting were impaired. This barrage is characterised by a clear zone, about 1–2 mm wide, free of aerial hyphae, and of reduced hyphal density in the medium (Fig. 6.1d).
-
3.
Using two races of P. ciliatus as an example, it was revealed that barrage formation is induced by the specific interaction of three unlinked genes (b+/b− = barrage initiation, bfI1/bfI2 and bfII1/bfII2 = barrage formation) in a way characteristic for systems of heterogenic incompatibility. Barrage formation requires the presence of the allele bi+ in at least one mating partner, in addition to heterogeneity of both bf-genes.
-
4.
Interspecific combinations were sterile. There is no hyphal fusion between mating partners, and because of the mutual repulsion, a sharp border line is formed in the area of contact. Its formation is independent of both mating type and the nuclear status (monokaryons or dikaryons) of the confronted mycelia (see also Silveira et al. 2002).
From the experimental data, the following conclusions may be drawn:
-
1.
The analysis of intraspecific matings has shown that within each species, the so-called biological races (intersterility groups, i-s) exist. They are delineated by barrage formation, which, as deduced from the genetic data, is an unequivocal example of heterogenic incompatibility. The unilateral inhibition of fruiting, not caused by the mating-type factors, can also be considered as an expression of heterogenic incompatibility, although genetic data for this are not yet available.
-
2.
The analysis of interspecific matings, all characterised by a strong macroscopic border line (Fig. 6.1d), is in good agreement with the species limits derived from morphological data. This indicates the validity of both the typological and the biological species concept. The latter, however, proved superior in compensating the variability of morphological characters, at least in higher fungi.
The biological species concept can thus be modified as follows: populations (races) belong to different species if the failure to interbreed and to produce viable offspring is caused by genetic mechanisms other than those operating upon completion of the sexual cycle.
There are some more examples where genetic control of heterogenic incompatibility is available.
In Sistotrema brinkmannii (syn. Corticium coronilla), strains obtained from different geographical locations exhibit three types of basic sexual control:
-
1.
Homokaryotic fruiting, i.e. homokaryons produce dikaryons and fruit bodies with viable spores.
-
2.
Homogenic incompatibility determined by the bipolar mechanism, i.e. one mating-type locus with multiple alleles.
-
3.
Homogenic incompatibility determined by the tetrapolar mechanism, i.e. two incompatibility factors, each with multiple alleles (Biggs 1937).
Lemke (1969) has confirmed and extended the work of Biggs by analysing the interstrain relations of 11 isolates from different parts of the world. In using the technique of forced heterokaryons between auxotrophs, he found that there are fertility barriers within each of the three above-mentioned fruiting classes as well as in interclass crosses. This phenomenon was interpreted by Lemke as heterogenic incompatibility for two reasons:
-
1.
In incompatible interracial matings, the two auxotrophic partners form unbalanced mycelia with poor vegetative vigour and no clamp connections. This points to an antagonistic reaction similar to that in Podospora heterokaryons.
-
2.
In one compatible interracial mating, recombinant types were obtained with an altered incompatibility pattern. This excluded the presence of sterility genes, which were sometimes found in other combinations.
In the oyster mushroom, Pleurotus ostreatus, 60 heterokaryons of a natural population were examined by pairwise matings for mycelial antagonisms (Kay and Vilgalys 1992). Most pairings (93–100 %) between sib-composed heterokaryons gave somatic incompatibility responses, showing that most isolates represent discrete individuals, with as many as 15 individuals occupying a single log. A total of 53 somatically distinct individuals were identified from the population, distributed among 21 logs. Test with homokaryons showed that genetic elements not identical with the incompatibility factors of the tetrapolar system are responsible for the formation of intersterility groups.
In Stereum hirsutum, mating is controlled by a bipolar mechanism of homogenic incompatibility (C-factor with multiple alleles). In analysing mating homokaryons from different geographical isolates, a mycelial aversion (bow-tie reaction) was observed. This involved the formation of a migrating or stationary band of suppressed mycelium. This was followed by partial or complete replacement of one homokaryon by another. This reaction is brought about by a heterozygosity at a single locus (B-factor), which is not linked with the mating-type locus. The fact that the heterogenic nuclei reject each other, reminiscent of the barrage formation of Podospora, is a further example for heterogenic incompatibility as an isolation mechanism within a single species. In four other species of Stereum, as in Polyporus, all interspecific matings were sterile and delineated by strong border lines.
In the litter-decomposing bipolar Collybia dryophila, collected from different continents, several intersterility groups (i-s) were identified, three of which are distributed over two or more continents. In some matings within one i-c, reduced sexual compatibility was found. Genetic diversity of some strains was proved by DNA-DNA hybridisation.
In the bipolar Heterobasidion annosum are at least three intersterility groups, P and S (from pine and spruce) and F (from firs), the representatives of which in general show no compatibility of different mating types. However, there are exceptions, because there is a significant degree of fertility in i-s matings. Five loci were identified controlling this system, superimposed upon the mating-type alleles. In contrast to the results obtained with Podospora, for instance, the heterogenic incompatibility between the i-s strains requires a heterogeneity of all five loci. A homogeneity at only one locus acts epistatically and suppresses the mating barrier. This does not exclude that under “fully” heterogenic conditions, interracial incompatibility is present. Comparable data for the Heterobasidion insulare complex were reported by Dai et al. (2002).
Hansen et al. (1993a, b) published data which lead to a contradictory interpretation. They suggested that mating between the incompatibility groups is controlled at 3–4 multiallelic loci. Each genotype acts independently in causing vegetative incompatibility. Thus, in accordance with the Podospora system, a single genetic difference would be sufficient to cause heterogenic incompatibility.
Perhaps the control of heterogenic incompatibility is not restricted to nuclear genes. In the tetrapolar Coprinus cinereus (Coprinopsis cinerea), barrage formation was observed in matings between heterokaryons from different geographical locations having different mitochondrial genomes but common nuclear genomes (May 1988). Unfortunately, no further details of this novel interaction were given.
Yet another example should be mentioned, which is caused by an interaction of nuclear genes and cytoplasmic genetic elements. In the tetrapolar Ustilago maydis, an antagonism between genetically different strains, which does not depend on the mating-type genes, was observed which is similar to the killer phenomenon of yeast (cf. Stark et al. 1990). There are three genotypes:
-
1.
Antagonistic strains, producing a heat-labile protein which inhibits the growth of sensitive strains but does not interfere with growth of the producing strain. Genetic configurations: a nuclear gene with the alleles s or s+, and cytoplasmic elements I and S. The s+ allele confers insensitivity, the s allele sensitivity which is suppressed by the cytoplasmic element S; the element I is responsible for the production of the killer substance.
-
2.
Sensitive strains, which do not produce inhibitor protein but are sensitive to it. Genetic configuration: gene s, but no cytoplasmic element O.
-
3.
Neutral strains, which do not produce inhibitor protein and are insensitive to it. Genetic configuration: s+ O, s S or s+ S.
There are phenotypic differences to the killer system in yeasts, since only growth inhibition of the sensitive cells occurs with no cell death, and there is no interference with the fusion of different mating types; hence, sexual propagation is not prevented. Thus, this phenomenon reveals similarity to heterogenic vegetative incompatibility in Neurospora and Podospora. Moreover, in the yeast killer system the genetic determinate for killer protein is a viral-related double-stranded RNA or DNA (cf. Tipper and Bostian 1984 and Stark et al. 1990, respectively).
Conclusion: The evaluation of the experimental data obtained with fungi with respect to the occurrence, distribution and mechanisms of heterogenic incompatibility allows one to make the following statements:
-
1.
The existence of heterogenic incompatibility is unequivocally proved among Dikaryomycota. Although het-genes were identified for a number of species, its genetic control certainly needs more experimental investigation.
-
2.
This holds even more true for an understanding of the expression and functions of the het-genes.
-
3.
The existence of many v-c and i-s groups in ascomycetes and basidiomycetes, respectively, shows the necessity to support taxonomic classification for speciation by means of comprehensive genetic data, and not just morphological criteria.
V. Correlations with Heterogenic Incompatibility in Plants and Animals, with DNA Restriction in Bacteria and with Histoincompatibility
As reviewed earlier (Esser and Blaich 1973), in plants there are also many examples for the existence of heterogenic incompatibility, manifesting as either unilateral or bilateral failures of matings between individuals of different isolates or races. Sometimes the genes responsible for homogenic incompatibility are involved, but mostly the action of other genes is superimposed. In addition, extrachromosomal genetic elements such as plastid-derived DNA have been found as determinative agents (de Nettancourt 1977; Barrett 1992). In plants, according to my knowledge, vegetative incompatibility has not been described.
In comparison to the predominantly hermaphroditic plants, sexual incompatibility of the homogenic type does not play a role in animal breeding systems. Increasing in outbreeding is in general achieved in animals by dioecism. There are some examples known in which karyogamy between female and male nuclei is prevented by genetic differences not identical with sex factors (cf. Esser and Blaich 1973).
The spectrum of heterogenic incompatibility comprises not only eukaryotes but also prokaryotes. The destruction of bacterial DNA by endonucleases, when brought into a genetically different host and as a defence mechanism to escape phage infection, is also a manifestation of this phenomenon.
Heterogenic incompatibility is not restricted to cell fusion and subsequent nuclear migration, because there is also a close correlation between heterogenic incompatibility and histoincompatibility, occurring after tissue transplantation. In the latter case, however, a complicated immune-response mechanism is involved. It seems justifiable to conclude that both heterogenic incompatibility and histoincompatibility, which seem to have convergently developed during evolution, exhibit one and the same effect, i.e. inability of genetically different material to coexist or tolerate a common physiological machinery, and simply represent different mechanisms of a fundamental biological process.
VI. Conclusions
As shown by this survey of the literature, most cases of heterogenic incompatibility can be supported by genetic data. A number of important special cases are known under different names. In other cases, however, effects indicative of heterogenic incompatibility have been attributed to other causes or relegated by investigators as inexplicable secondary effects. In any case, heterogenic incompatibility must be regarded as a basic biological phenomenon controlling the coexistence of different genetic determinants, whose impact may be summarised as follows.
-
1.
Occurrence
Heterogenic incompatibility is widespread in both prokaryotes and eukaryotes. Special cases such as DNA restriction and histoincompatibility may be considered different expressions of one basic biological phenomenon.
-
2.
Nature of Genetic Determinants
The widespread occurrence is also indicative of the general importance of heterogenic incompatibility, with a varied genetic basis ranging from single to multiple nuclear genes and even to extranuclear genetic elements.
-
3.
Biochemical Basis
There are not yet sufficient biochemical data regarding the action of the nuclear genes which bring about heterogenic incompatibility in fungi. By contrast, the molecular mechanisms of heterogenic incompatibility provoked by extranuclear genetic traits, such as bacterial DNA restriction, are well known. Evidently, many of the genetic mechanisms leading to heterogenic incompatibility have developed independently, and this may be reflected by a variety of mechanisms at the molecular level.
-
4.
Biological Impact
The effect of heterogenic incompatibility is threefold:
-
(a)
As stated in the Introduction, heterogenic incompatibility has to be considered a breeding system which, in contrast to homogenic incompatibility, favours inbreeding by restricting the exchange of genetic material. Since there is no fundamental difference between recombinational events in the sexual and the parasexual cycle, it is not surprising that heterogenic incompatibility influences both. This also applies to the initiation of plasmogamy, which may occur either by sexual processes or simply through heterokaryosis. This mode of isolating strains or races leads to further speciation. Thus, heterogenic incompatibility must have been and still is one of the basic genetic events acting in evolution.
-
(b)
Genetic isolation has a second effect which should not be overlooked. The suppression of cell fusion stops the transfer of harmful cytoplasmic components, such as mutated mitochondria, viruses or plasmids, between individuals and thereby inhibits the spread of cell diseases and favours the survival of uninfected cells or tissues. This is particularly important for organisms without strict cellular compartmentation, such as the majority of fungi.
-
(c)
Consequences for taxonomy: In many studies of natural isolates of fungi, the formation of antagonistic zones of mycelial aversion, such as barrages and/or border lines, is used by taxonomists as criteria for speciation. Although being a valuable tool, this parameter as a taxonomic criterion should be judged with great care to avoid creating taxonomic distinctions which are not valid and which could depend on only a single gene difference. This especially concerns the term “biological species”, often used without any genetic basis. It is better to use the terms “race” or “geographical isolate”, without a detailed evaluation of breeding patterns.
-
(a)
-
5.
Practical Implications
During the last decades, concerted breeding for biotechnologically relevant fungi has gained more and more importance (Esser 1985; Esser and Mohr 1990). Breeding techniques employing new isolates from nature in order to exploit varied genetic backgrounds require a profound knowledge of the breeding systems involved. The existence of heterogenic incompatibility could be a serious handicap for any genetic exchange via either sexual or parasexual matings. Detailed experimental work would allow one in most cases to reach the desired goal, if based on alternative genetic manipulations such as DNA-mediated transformation.
-
6.
Relation with Histoincompatibility and DNA Restriction
Both of these phenomena have the same effect: hostile interaction of different genetic material originating from closely related organisms. This brings up the question: will it be possible in the future, based on further experimental work, to interrelate these events and the many manifestations of heterogenic incompatibility in considering the diversity of the genetic mechanisms promulgating the failure of coexistence of genetically different material?
References
Adams DH, Roth LF (1967) Demarcation lines in paired cultures of Fomes cajanderi as a basis for detecting genetically distinct mycelia. Can J Bot 45:1583–1589
Anagnostakis SL (1977) Vegetative incompatibility in Endothia parasitica. Exp Mycol 1:306–316
Anagnostakis SL (1982a) The origin of ascogenous nuclei in Endothia parasitica. Genetics 100:413–416
Anagnostakis SL (1982b) Genetic analyses of Endothia parasitica: linkage data for four single genes and three vegetative compatibility types. Genetics 102:25–28
Anagnostakis SL (1983) Conversion to curative morphology in Endothia parasitica and its restriction by vegetative compatibility. Mycologia 75:777–780
Anderson JB (1986) Biological species of Armillaria in North America: redesignation of groups IV and VIII and enumeration of voucher strains for other groups. Mycologia 78:837–839
Anderson JB, Ullrich RC (1979) Biological species of Armillaria mellea in North America. Mycologia 71:402–414
Anderson JB, Korhonen K, Ullrich RC (1980) Relationship between European and North American biological species of Armillaria mellea. Exp Mycol 4:87–95
Anderson JB, Petsche DM, Herr FB, Horgien PA (1984) Breeding relationship among several species of Agaricus. Can J Bot 62:1884–1889
Balguerie A, Reis SD, Ritter C, Chaignepain S, Coulary-Salin B, Forge V, Bathany K, Lascu I, Schmitter JM, Riek R et al (2003) Domain organization and structure-function relationship of the HET-s prion of Podospora anserina. EMBO J 22:2071–2081
Balguerie A, Reis SD, Coulary-Salin B, Chaignepain S, Sabourain M, Schmitter JM, Saupe SJ (2004) The sequences appended to the amyloid core region of the HET-s prion protein determine higher-order aggregate organization in vivo. J Cell Sci 117:2599–2610
Barrett SCH (ed) (1992) Evolution and function of heterostyly, vol 15, Monographs on theoretical and applied genetics. Springer, Berlin
Barrett DK, Uscuplic M (1971) The field distribution of interacting strains of Polyporus schweinitzii and their origin. New Phytol 70:581–598
Bastiaans E, Debets AM, Aanen DK, Von Depeningen AD, Saupe SJ, Paoletti M (2014) Natural variation of heterokaryon incompatibility gene het-c in Podospora anserine reveals diversifying selection. Mol Biol Evol 3:962–974
Bauch R (1927) Rassenunterschiede und sekundäre Geschlechtsmerkmale beim Antherenbrand. Biol Zentralbl 47:370–383
Beadle GW, Coonradt VL (1944) Heterocaryosis in Neurospora crassa. Genetics 29:291–308
Bégueret J, Turc B, Clavé C (1994) Vegetative incompatibility in filamentous fungi – het genes begin to talk. Trends Genet 10:441–446
Bernet J (1965) Mode d’action des gènes de barrage et la relation entre l’incompatibilité cellulaire et l’incompatibilité sexuelle chez Podospora anserina. Ann Sci Nat Bot Biol Veg 6:611–768
Bernet J (1967) Les systèmes d’incompatibilité chez le Podospora anserina. C R Acad Sci Paris Ser D 256:1330–1333
Betterley DA, Collins OR (1984) Vegetative incompatibility and myxomycete biology. Mycologia 76:785–792
Biggs R (1937) The species concept in Corticium coronilla. Mycologia 29:686–706
Blaich R, Esser K (1970) The incompatibility relationships between geographical races of Podospora anserina. IV. Biochemical aspects of the heterogenic incompatibility. Mol Gen Genet 109:186–192
Blaich R, Esser K (1971) The incompatibility relationships between geographical races of Podospora anserina. V. Biochemical characterization of heterogenic incompatibility on cellular level. Mol Gen Genet 111:265–272
Boccas BR (1981) Interspecific crosses between closely related heterothallic Phytophthora species. Phytopathology 71:60–65
Boddy L, Rayner ADM (1982) Population structure, intermycelial interactions and infection biology of Stereum gausapatum. Trans Br Mycol Soc 78:337–351
Boddy L, Rayner ADM (1983) Mycelial interactions, morphogenesis and ecology of Phlebia radiata and P. rufa from oak. Trans Br Mycol Soc 80:437–448
Boidin J (1986) Intercompatibility and the species concept in the saprobic basidiomycotina. Mycotaxon 76:319–336
Boucherie H, Bernet J (1978) Protoplasmic incompatibility and self-lysis in Podospora anserina: enzyme activities associated with cell destruction. Can J Bot 56:2171–2176
Boucherie H, Dupont CH, Bernet J (1981) Polypeptide synthesis during protoplasmic incompatibility in the fungus Podospora anserina. Biochim Biophys Acta 653:18–26
Bourges N, Groppi A, Barreau C, Clavé C, Bégueret J (1998) Regulation of gene the expression during the vegetative incompatibility reaction in Podospora anserina: characterization of three induced genes. Genetics 150:633–641
Brasier CM (1984) Inter-mycelial recognition systems in Ceratocystis ulmi: their physiological properties and ecological importance. In: Jennings DH, Rayner ADM (eds) The ecology and physiology of the fungal mycelium. Cambridge University Press, Cambridge, pp 451–498
Brodie HJ (1970) Sexuality patterns in some new and little-known species of Cyathus. Sver Bot Tidskr 64:44–50
Bruehl GW, Machtmes R, Kiyomoto R (1975) Taxonomic relationships among Typhula species as revealed by mating experiments. Phytopathology 65:1108–1114
Burnett JH (1965) The natural history of recombination systems. In: Esser K, Raper JR (eds) Incompatibility in fungi. Springer, Berlin/New York, pp 98–113
Butcher AC (1968) The relationship between sexual outcrossing and heterokaryon incompatibility in Aspergillus nidulans. Heredity 23:443–452
Butcher AC (1969) Non-allelic interactions and genetic isolation in wild populations of Aspergillus nidulans. Heredity 24:621–631
Butcher AC, Croft J, Grindle M (1972) Use of genotype-environmental interaction analysis in the study of natural populations of Aspergillus nidulans. Heredity 29:263–283
Carlile MJ, Gooday GW (1978) Cell fusion in myxomycetes and fungi. In: Poste G, Nicholson GL (eds) Membrane fusion. Elsevier, Amsterdam, pp 219–265
Caten CE, Butcher AC, Croft JH (1971) Genetic control of heterokaryon formation in Aspergillus. Aspergillus Newslett 12:1–2
Cayley DM (1923) The phenomenon of mutual aversion between mono-spore mycelia of the same fungus (Diaporthe perniciosa Marchal) with a discussion of sex heterothallism in fungi. J Genet 13:353–370
Cayley DM (1931) The inheritance of the capacity for showing mutual aversion between monospore mycelia of Diaporthe perniciosa. J Genet 24:1–63
Chaisrisook C (2002) Mycelial reactions and mycelial compatibility groups of red rice mould (Monascus purpureus). Mycol Res 106:298–304
Chase TE, Ullrich RC (1990a) Genetic basis of biological species in Heterobasidion annosum: Mendelian determinants. Mycologia 82:67–72
Chase TE, Ullrich RC (1990b) Five genes determining intersterility in Heterobasidion annosum. Mycologia 82:73–81
Cherepennikova-Anikina MI, Savenkova LV, Dolgova AV, Shaw DS, Dyakov Yu T (2002) Vegetative incompatibility in Phytophthora infestans. J Russ Phytopathol Soc 3:19–28
Choi GH, Dawe AL, Churbanow A, Smith ML, Milgroom MG, Nuss D (2012) Molecular characterization of vegetative incompatibility genes in the chestnut blight Cryphonectria parasitica. Genetics 190:113–127
Clark J (1984) Three-clone Didymium iridis crosses and plasmodial incompatibility phenotype. Mycologia 76:810–815
Clark J (2003) Plasmodial incompatibility in the myxomycete Didymium squamulosum. Mycologia 95:24–26
Coates D, Rayner ADM (1985a) Genetic control and variation in expression of the “bow-tie” reaction between homokaryons of Stereum hirsutum. Trans Br Mycol Soc 84:191–205
Coates D, Rayner ADM (1985b) Heterokaryon-homokaryon interactions in Stereum hirsutum. Trans Br Mycol Soc 84:637–645
Coates D, Rayner ADM (1985c) Evidence for a cytoplasmically transmissible factor affecting recognition and somato-sexual differentiation in the basidiomycete Stereum hirsutum. J Gen Microbiol 131:207–219
Coates D, Rayner ADM, Todd NK (1981) Mating behaviour, mycelial antagonism and the establishment of individuals in Stereum hirsutum. Trans Br Mycol Soc 76:41–51
Coates D, Rayner ADM, Boddy L (1985) Interactions between mating and somatic incompatibility in the basidiomycete Stereum hirsutum. New Phytol 99:473–483
Correl JC, Puhalla JE, Schneider RW (1986) Identification of Fusarium oxysporum f. sp. apii on the basis of colony size, virulence, and vegetative compatibility. Phytopathology 76:396–400
Cortesi P, Milgroom MG (1998) Genetics of vegetative incompatibility in Cryphonectria parasitica. Appl Environ Microbiol 64:2988–2994
Cousteau V, Delau C, Saupe S, Bégueret J (1997) The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserine behaves as a prion analog. Proc Natl Acad Sci USA 94:9773–9778
Croft JH, Dales RBG (1984) Mycelial interactions and mitochondrial inheritance in Aspergillus. In: Jennings DH, Rayner ADM (eds) The ecology and physiology of the fungal mycelium. Cambridge University Press, Cambridge, pp 433–450
Cubeta MA, Briones-Ortega R, Vigalys R (1993) Reassessment of heterokaryon formation in Rhizoctonia solani anastomosis group 4. Mycologia 85:777–787
Dai YC, Vainio EJ, Hantula J, Niemelä T, Korhonen K (2002) Sexuality and intersterility within the Heterobasidion insulare complex. Mycol Res 106:1435–1448
Dales RBG, Croft JH (1977) Protoplast fusion and the isolation of heterokaryons and diploids from vegetatively incompatible strains of Aspergillus nidulans. FEMS Microbiol Lett 1:201–203
Dales RBG, Croft JH (1990) Investigation of the het genes that control heterokaryon incompatibility between members of heterokaryon compatibility (h-c) groups A and G1 of Aspergillus nidulans. J Gen Microbiol 136:1717–1724
De Nettancourt D (1977) Incompatibility in Angiosperms, vol 3, Monographs on theoretical and applied genetics. Springer, Berlin/New York
Debets F, Yang X, Griffiths AJF (1994) Vegetative incompatibility in Neurospora: its effect on horizontal transfer of mitochondrial plasmids and senescence in natural population. Curr Genet 26:113–119
Debuchy R, Coppin E (1992) The mating types of Podospora anserina: functional analysis and sequence of the fertilization domains. Mol Gen Genet 233:113–121
Deleu C, Clavé C, Bégueret J (1993) A single amino acid difference is sufficient to elicit vegetative incompatibility in the fungus Podospora anserina. Genetics 135:45–52
Dementhon K, Paoletti M, Pinan-Lucarré B, Loubradou-Bourges N, Sabourin M, Saupe SJ, Clavé C (2003) Rapamycin mimics the incompatibility reaction in the fungus Podospora anserina. Eukaryot Cell 2:238–246
Dementhon K, Saupe SJ, Clavé C (2004) Characterization of idi-4, a bZIP transcription factor inducing autophagy and cell death in the fungus Podospora anserina. Mol Microbiol 53:1625–1640
Dyer PS, Inderbitzin P, Debuchy R (2016) Mating-type structure, function, regulation and evolution in the pezizomycotina. In: Wendland J (ed) Growth, differentiation and sexuality. Springer, Cham, pp 351–385
Ennos RA, Swales KW (1987) Estimation of the mating system in a fungal pathogen Crumenulopsis sororia (Karst.) groves using isozyme markers. Hereditas 59:423–430
Espagne E, Balhadère P, Penin ML, Barreau C, Turcq B (2002) HET-E and HET-D belong to a new subfamily of WD40 proteins involved in vegetative incompatibility specificity in the fungus Podospora anserina. Genetics 161:71.81
Esser K (1954) Sur le déterminisme génétique d’un nouveau type d’incompatibilité chez Podospora. C R Acad Sci Paris 238:1731–1733
Esser K (1956) Die Incompatibilitätsbeziehungen zwischen geographischen Rassen von Podospora anserina (CES) REHM I. Genetische Analyse der Semi-Incompatibilität. Z Indukt Abstammungs Vererbungslehre 87:595–624
Esser K (1959a) Die Incompatibilitätsbeziehungen zwischen geographischen Rassen von Podospora anserina (CES) REHM II. Die Wirkungsweise der Semi-Incompatibilitäts-Gene. Z Vererbungslehre 90:29–52
Esser K (1959b) Die Incompatibilitätsbeziehungen zwischen geographischen Rassen von Podospora anserina (CES) REHM III. Untersuchungen zur Genphysiologie der Barragebildung und der Semi-Incompatibilität. Z Vererbungslehre 90:445–456
Esser K (1962) Die Genetik der sexuellen Fortpflanzung bei Pilzen. Biol Zbl 81:161–172
Esser K (1971) Breeding systems in fungi and their significance for genetic recombination. Mol Gen Genet 110:86–100
Esser K (1985) Potentials and prospects of genetics in biotechnology. In: Proceedings 3rd European congress biotechnology, München, 1984, vol IV. DECHEMA, Frankfurt/Main, pp 241–256
Esser K (2006) Heterogenic incompatibility in fungi. In: Kües U and Fischer R (eds) Growth, diferentiation and sexuality, 2nd edn. The mycota, vol I, Springer, Heidelberg, pp 141–165
Esser K, Blaich R (1973) Heterogenic incompatibility in plants and animals. Adv Genet 17:107–152
Esser K, Hoffmann P (1977) Genetic basis for speciation in higher basidiomycetes with special reference to the genus Polyporus. In: Clemencon H (ed) The species concept in hymenomycetes. Cramer, Vaduz, pp 189–214
Esser K, Kuenen R (1967) Genetics of fungi. Translated by Erich Steiner. Springer, Berlin/New York
Esser K, Meinhardt F (1984) Barrage formation in fungi, vol 17, Encyclopedia of plant physiology, new series. Springer, Berlin/New York, pp 350–361
Esser K, Mohr G (1990) Stammverbesserung von Hyphenpilzen durch die Gentechnik: Fakten und Perspektiven. Bioengineering 6:44–55
Fischer M (1994) Pairing tests in the Phellinus pini group. Mycologia 86:524–539
Fischer M, Bresinsky A (1992) Phellinus torulosus: sexuality and evidence of intersterility groups. Mycologia 84:823–833
Ford EJ, Miller RV, Gray, Sherwood JE (1995) Heterokaryon formation and vegetative incompatibility in Sclerotina sclerotium. Mycol Res 99:241–247
Fournier E, Levis C, Fortini D, Leroux P, Giraud T, Brygoo Y (2003) Characterization of Bc-hch, the Botrytis cinerea homolog of the Neurospora crassa het-c vegetative incompatibility locus, and its use as, a population marker. Mycologia 95:251–261
Freihorst D, Fowler TJ, Bartholomew K, Raudaskoski M, Horton JS, Kothe E (2016) The mating type genes of the basidiomycetes. In: Wendland J (ed) Growth, differentiation and sexuality. Springer, Cham, pp 351–385
Fries N (1985) Intersterility groups in Paxillus involutus. Mycotaxon 14:403–409
Fries N, Mueller GM (1984) Incompatibility systems, cultural features and species circumscriptions in the ectomycorrhizal genus Laccaria (agaricales). Mycologia 76:633–642
Garnjobst L, Wilson JF (1956) Heterokaryosis and protoplasmic incompatibility in Neurospora crassa. Proc Natl Acad Sci USA 42:613–618
Giovanetti M, Sbrana C, Strani P, Agnolucci M, Rinaudo V, Avio L (2003) Genetic diversity of isolates of Glomus mosseae from different geographic areas detected by vegetative compatibility testing and biochemical molecular analysis. Appl Environ Microbiol 69:616–624
Glass NL, Demnthon K (2006) Non self recognition and programmed cell death in filamentous fungi. Curr Opin Microbiol 9:553–558
Glass NL, Kaneko I (2003) Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot Cell 2:1–8
Glass NL, Kuldau GA (1992) Mating type and vegetative incompatibility in filamentous ascomycetes. Annu Rev Phytopathol 30:201–224
Glass NL, Grotelueschen J, Metzenberg RL (1990) Neurospora crassa a mating-type region. Proc Natl Acad Sci USA 87:4912–4916
Glass NL, Jacobson DJ, Shiu PKT (2000) The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycete fungi. Annu Rev Genet 34:165–186
Goh KM, Ganeson M, Subramaniam N (2014) Infection potential of vegetative incompatible Ganoderma boninense isolates with known ligninolytic enzyme production. Afr J Biotechnol 13:1056–1066
Goldstein D, Gilbertson RL (1981) Cultural morphology and sexuality of Inonotus arizonicus. Mycologia 73:167–180
Goncalves AP, Videira A (2014) Programmed cell death in Neurospora crassa. New J Sci 2014:7
Gordon SA, Petersen RH (1992) Interbreeding populations of some Marasmius species. Mycologia 84:204–208
Gossop GH, Yuill E, Yuill JL (1940) Heterogeneous fructifications in species of Aspergillus. Trans Br Mycol Soc 24:337–344
Grasso V (1955) Studio sulla genetica dei carboni dell’avena: Ustilago avenae e U. levis. Boll Staz Patol Veg Roma 12:115–126
Griffith AJF, Rieck A (1981) Perithecial distribution in standard and variant strains of Neurospora crassa. Can J Bot 59:2610–2617
Grindle M (1963a) Heterokaryon compatibility of unrelated strains in the Aspergillus nidulans group. Heredity 18:191–204
Grindle M (1963b) Heterokaryon compatibility of closely related wild isolates of Aspergillus nidulans. Heredity 18:397–405
Gross SR (1952) Heterokaryosis between opposite mating types in Neurospora crassa. Am J Bot 39:574–577
Guler P, Bicer H (2014) The somatic incompatibility in Trametes versicolor. J Appl Biol Sci 8:22–26
Hamann A, Osiewacz HD (2004) Genetic analysis of spore killing in the filamentous ascomycete Podospora anserina. Fungal Genet Biol 41:1088–1098
Hankin L, Puhalla JE (1971) Nature of a factor causing interstrain lethality in Ustilago maydis. Phytopathology 61:50–53
Hansen EM (1979) Sexual and vegetative incompatibility reactions in Phellinus weirii. Can J Bot 57:1573–1578
Hansen EM, Stenlid J, Johansson M (1993a) Somatic incompatibility and nuclear reassortment in Heterobasidion annosum. Mycol Res 97:1223–1228
Hansen EM, Stenlid J, Johansson M (1993b) Genetic control of somatic incompatibility in the root-rotting basidiomycete Heterobasidion annosum. Mycol Res 97:1229–1233
Hietala AM, Korhonen K, Sen R (2003) An unknown mechanism promotes somatic incompatibility in Ceratobasidium bicorne. Mycologia 95:239–250
Hoffmann P, Esser K (1978) Genetics of speciation in the basidiomycetous genus Polyporus. Theor Appl Genet 53:273–282
Holloway BW (1955) Genetic control of heterocaryosis in Neurospora crassa. Genetics 40:117–129
Horn BW, Greene RL, Sobolov VS, Dorner JW, Powell JH, Layton RC (1996) Association of morphology and mycotoxin production with vegetative compatibility groups in Aspergillus flavus, A. parasiticus and A. tamarii. Mycologia 88:574–587
Hornok L (2007) Sexual and vegetative compatibility/incompatibility in Fusarium species. Acta Phytopathologica and Entomologica Hungarica 42:2–11
Ikeda K, Nakamura H, Matsumato N (2003) Mycelial incompatibility operative in pairings between single basidiospore isolates of Helicobasidium mompa. Mycol Res 107:847–853
Jacobson DJ, Gordon TR (1988) Vegetative compatibility and self-incompatibility within Fusarium oxysporum f. sp. melonis. Phytopathology 78:668–672
Jamil N, Buck KW, Carlile MJ (1984) Sequence relationships between virus double-stranded RNA from isolates of Gaeumannomyces graminis in different vegetative compatibility groups. J Gen Virol 65:1741–1747
Jennings DH, Rayner ADM (eds) (1984) The ecology and physiology of the fungal mycelium. Cambridge University Press, Cambridge
Jinks JL, Caten CE, Simchen G, Croft JH (1966) Heterokaryon incompatibility and variation in wild populations of Aspergillus nidulans. Heredity 21:227–239
Jones DA (1965) Heterokaryon compatibility in the Aspergillus glaucus link group. Heredity 20:49–56
Katan T, Katan J (1988) Vegetative-compatibility grouping of Fusarium oxysporum f. sp. vasinfectum from tissue and the rhizosphere of cotton plants. Phytopathology 78:852–855
Kay E, Vilgalys R (1992) Spatial distribution and genetic relationships among individuals in a natural population of the oyster mushroom Pleurotus ostreatus. Mycologia 84:173–182
Kemp RFO (1989) Incompatibility in basidiomycetes – the heterogenic pentax. FORK Publ 1:1–19
Kiebacher J, Hoffmann GM (1981) Zur Entwicklung des Perfektstadiums Venturia inaequalis in vitro (Development of the perfect stage of Venturia inaequalis in vitro). J Plant Dis Protect 88:1–8
Knowles DJC, Carlile MJ (1978a) Growth and migration of plasmodia of the myxomycete Physarum polycephalum: the effect of carbohydrates, including agar. J Gen Microbiol 108:9–15
Knowles DJC, Carlile MJ (1978b) The chemotactic response of plasmodia of the myxomycete Physarum polycephalum to sugars and related compounds. J Gen Microbiol 108:17–25
Kohn E, Stasovcki F, Carbone I, Royer J, Anderson JB (1991) Mycelial incompatibility and molecular markers identify genetic variability in field populations of Sclerotinia sclerotiorum. Phytopathology 81:480–485
Kope HH (1992) Interactions of heterokaryotic and homokaryotic mycelium of sibling isolates of the ectomycorrhizal fungus Pisolithus arhizus. Mycologia 84:659–667
Kuhlmann EG (1982) Varieties of Gibberella fujikuroi with anamorphs in Fusarium section Liseola. Mycologia 74:759–768
Kwon KJ, Raper KB (1967) Heterokaryon formation and genetic analyses of color mutants in Aspergillus heterothallicus. Am J Bot 54:49–60
Lane EB (1981) Somatic incompatibility in fungi and myxomycetes. In: Gull K, Oliver SG (eds) The fungal nucleus. Cambridge University Press, Cambridge
Lane EB, Carlile MJ (1979) Post-fusion somatic incompatibility in plasmodia of Physarum polycephalum. J Cell Sci 35:339–354
Leach J, Yoder OC (1983) Heterokaryon incompatibility in the plant-pathogenic fungus, Cochliobolus heterostrophus. J Heredity 74:149–152
Lemke PA (1969) A reevaluation of homothallism, heterothallism and the species concept in Sistotrema brinkmanni. Mycologia 61:57–76
Lemke PA (1973) Isolating mechanisms in fungi – prezygotic, postzygotic, and azygotic. Persoonia 7:249–260
Leslie JF (1987) Heterokaryon incompatibility haplotypes on linkage group VII of Neurospora crassa (abstract). Mycol Soc Am Newslett 38:35
Leslie JF (1993) Fungal vegetative compatibility. Annu Rev Phytopathol 31:127–150
Leslie JF, Zeller KA (1996) Heterokaryon incompatibility in fungi: more than just a way to die. J Genet 75:416–424
Leslie JF, Zeller KA, Wohler M, Summerell BA (2004) Interfertility in two mating populations of Gibberella fujikuroi. Eur J Plant Pathol 90:1–8
Lindegren CC (1934) The genetics of Neurospora. VI. Bisexual and akaryotic ascospores from N. crassa. Genetica 16:315–320
Lombard FF, Larsen MJ, Dorworth EB (1992) Reassessment of the sexual incompatibility system and cultural characteristics of Bjerkandera fumosa. Mycologia 83:406–410
Loubradou G, Béguret J, Turcq B (1997) A mutation in aHSP90 gene affects the sexual cycle and suppresses vegetative incompatibility in the fungus Podospora anseria. Genetics 147:581–588
Loubradou G, Bégueret J, Turcq B (1999) Mod-D, a G α subunit of the fungus Podospora anserina, is involved in both regulation of development and vegetative incompatibility. Genetics 152:519–528
Macrae R (1967) Pairing incompatibility and other distinctions among Hirschioporus (Polyporus) abietinus, H. fusco-violaceus and H. laricinus. Can J Bot 45:1371–1398
Maddelein ML, Reis SD, Duvezin-Caubet S, Coulary-Salin B, Saupe SJ (2002) Amyloid aggregates of the HET-s prion protein are infectious. PNAS 99:7402–7407
Marek SM, Wu J, Glass NL, Gilchrist DG, Bostock RM (2003) Nuclear DNA degradation during heterokaryon incompatibility in Neurospora crassa. Fungal Genet Biol 40:126–137
Mattjus P, Turcq B, Pike HM, Molotkovsky JG, Brown RE (2003) Glycolipid intermembrane transfer is accelerated by HET-C2, a filamentous fungus gene product involved in the cell-cell incompatibility response. Biochemistry 42:535–542
May G (1988) Somatic incompatibility and individualism in the coprophilous basidiomycete Coprinus cinereus. Trans Br Mycol Soc 91:443–451
McKeen CG (1952) Studies of Canadian theleophoraceae. IX. A cultural and taxonomic study of three species of Peniophora. Can J Bot 30:764–787
Meinhardt F, Koch H, Esser K (1984) Compatibility groups in Ascobolus immersus, an indication of speciation. Theor Appl Genet 68:365–367
Metzenberg RL (1990) Anecdotal, historical and critical commentaries on genetics. In: Crow JF, Dove WF (eds). Genetics 125:457–462
Micali CO, Smith ML (2003) On the independence of barrage formation and heterokaryon incompatibility in Neurospora crassa. Fungal Genet Biol 38:209–219
Milgroom MG, Cortesi P (2004) The biological control of chestnut blight with hypervirulence: a critical analysis. Annu Rev Phytopathol 42:311–338
Mir-Rashed N, Jacobson DJ, Deghgany MR, Micali OC, Smith ML (2000) Molecular and functional analyses of incompatibility genes at het-6 in a population of Neurospora crassa. Fungal Genet Biol 30:197–205
Moreau F, Moruzi C (1933) Sur de nouvelles irrégularités de la bipolarité sexuelle chez les Ascomycetes du genre Neurospora. Bull Soc Bot Fr 80:574–576
Morrison RM (1960) Compatibility of several clonal lines of Erysiphe cichoracearum. Mycologia 52:786–794
Mounce I (1929) Studies in forest pathology. II. The biology of Fomes pinicola (Sw) Cooke. Can Dept Agric Bull 111:1–77
Mounce I, Macrae R (1938) Interfertility phenomena in Fomes pinicola. Can J Bot 16:354–376
Murphy JF, Miller OK Jr (1993) The population biology of two litter decomposing agarics on a southern Appalachian mountain. Mycologia 85:769–776
Mylyk OM (1975) Heterokaryon incompatibility genes in Neurospora crassa detected using duplication-producing chromosome rearrangements. Genetics 80:107–124
Mylyk OM (1976) Heteromorphism for heterokaryon incompatibility genes in natural populations of Neurospora crassa. Genetics 83:275–284
Nakata K (1925) Studies on Sclerotium rolfsii. I. The phenomenon of aversion and its relation to the biologic forms of the fungus. II. The possible cause of the phenomenon of aversion in the fungus and morphological features of the phenomenon. Bull Sci Fak Terkulturen Kjusa Imp Univ 1:4
Nazabal A, Reis SD, Bonneu M, Saupe SJ, Schmitter JM (2003) Conformational transition occurring upon amyloid aggregation of the HET-s prion protein of Podospora anserina analyzed by hydrogen/deuterium exchange and mass spectrometry. Biochemistry 42:8852–8861
Nelson RR (1963) Interspecific hybridization in the fungi. Annu Rev Microbiol 17:31–48
Nelson RR (1965a) Bridging interspecific incompatibility in the ascomycetous genus Cochliobolus. Evolution 18:700–704
Nelson RR (1965b) Assessing biological relationship in the fungi. Phytopathology 55:823–826
Nelson RR (1966) The genetic control of conidial morphology and arrangement in Cochliobolus carbonum. Mycologia 58:208–214
Nelson RR (1970) Variation in mating capacities among isolates of Cochliobolus carbonum. Can J Bot 48:261–263
Nelson RR, Kline DM (1964) Evolution of sexuality and pathogenicity. III. Effects of geographic origin and host association on cross-fertility between isolates of Helminthosporium with similar conidial morphology. Phytopathology 54:963–967
Newmeyer D (1968) A suppressor of mating type incompatibility in Neurospora. Genetics 60:207
Newmeyer D (1970) A suppressor of the heterokaryon-incompatibility associated with mating type in Neurospora crassa. Can J Genet Cytol 12:914–926
Newmeyer D, Taylor CW (1967) A pericentric inversion in Neurospora, with unstable duplication progeny. Genetics 56:771–791
Newton AC, Osbourn AE, Caten CE (1998) Heterokaryosis and vegetative incompatibility in Stagonospora nodorum. Mycologia 90:215–225
Nuss DI, Koltin Y (1990) Significance of dsRNA genetic elements in plant pathogenic fungi. Annu Rev Phytopathol 28:37–58
Padhaye AA, Carmichel JW (1971) Incompatibility in Microsporum cookei. Sabouraudia 9:27–29
Pal K (2005) Vegetative incompatibility in Aspergillus niger. Acta Biologica Szegediensis 49:57
Paoletti M, Clave C, Begueret J (1998) Characterization of a gene from the filamentous fungus Podospora anserina encoding an aspartyl protease induced upon carbon starvation. Gene 210:45–52
Papaioannou I, Typas M (2015) Barrage formation is independent from heterokaryon incompatibility. Eur J Plant Pathol 141:71–82
Perkins DD (1968) Multiple alleles at a heterokaryon-compatibility locus in Neurospora. Genetics 61:47
Perkins DD (1988) Main features of vegetative incompatibility in Neurospora. Fungal Genet Newslett 35:44–46
Perkins DD, Turner BC (1988) Neurospora from natural populations: toward the population biology of a haploid eukaryote. Exp Mycol 12:91–131
Perkins DD, Turner BC, Barry EG (1976) Strains of Neurospora collected from nature. Evolution 30:281–313
Pilotti CA, Sanderson FR, Aitken AB (2002) Sexuality and interactions of monokaryotic and dikaryotic mycelia of Ganoderma boninense. Mycol Res 106:1315–1322
Pinan-Lucarré B, Paoletti M, Dementhon K, Coulary-Salin B, Clavé C (2003) Autophagy is induced during cell death by incompatibility and is essential for differentiation in the filamentous fungus Podospora anserina. Mol Microbiol 47:321–333
Pittenger TH (1964) Spontaneous alterations of heterokaryon compatibility factors in Neurospora. Genetics 50:471–484
Pittenger TH, Brawner TG (1961) Genetic control of nuclear selection in Neurospora heterokaryons. Genetics 46:1645–1663
Ploetz RC, Shokes FM (1986) Evidence for homothallism and vegetative compatibility in southern Diaporthe phaseolorum. Can J Bot 64:2197–2200
Proffer TJ, Hart JH (1988) Vegetative compatibility groups in Leucocytospora kunzei. Phytopathology 78:256–260
Puhalla EJ (1968) Compatibility reactions on solid medium and interstrain inhibition in Ustilago maydis. Genetics 60:461–474
Puhalla EJ, Hummel M (1983) Vegetative compatibility groups within Verticillium dahliae. Phytopathology 73:1305–1308
Puhalla JE, Spieth PT (1983) Heterokaryosis in Fusarium moniliforme. Exp Mycol 7:328–335
Puhalla JE, Spieth PT (1985) A comparison of heterokaryosis and vegetative incompatibility among varieties of Gibberella fujikuroi (Fusarium moniliforme). Exp Mycol 9:39–47
Punja ZK, Grogan RG (1983a) Basidiocarp induction, nuclear condition, variability, and heterokaryon incompatibility in Athelia (Sclerotium) rolfsii. Phytopathology 73:1273–1278
Punja ZK, Grogan RG (1983b) Hyphal interactions and antagonism among field isolates and single-basidiospore strains of Athelia (Sclerotium) rolfsii. Phytopathology 73:1279–1284
Punja ZK, Sun LJ (2002) Genetic diversity among mycelial compatibility groups of Sclerotium rolfsii (teleomorph Athelia rolfsii) and S. delphinii. Mycol Res 105:537–546
Raju NB (1979) Cytogenetic behavior of spore killer genes in Neurospora. Genetics 93:607–623
Raju NB (2002) Spore killers: meiotic drive elements that distort genetic ratios. In: Osiewacz HD (ed) Molecular biology of the fungal development. Dekker, New York, pp 275–296
Raper KB, Fennell DL (1953) Heterokaryosis in Aspergillus. J Elisha Mitchell Sci Soc 69:1–29
Rayner ADM, Todd NK (1977) Intraspecific antagonism in natural populations of wood-decaying basidiomycetes. J Gen Microbiol 103:85–90
Rayner ADM, Turton MN (1982) Mycelial interactions and population structure in the genus Stereum: S. rugosum, S. sanguinolentum and S. rameale. Trans Br Mycol Soc 78:483–493
Reinhardt OM (1892) Das Wachstum der Pilzhyphen. Jahrb Wiss Bot 23:479
Rizet G (1952) Les phénomènes de barrage chez Podospora anserina. I. Analyse génétique des barrages entre souches S et s. Rev Cytol Biol Vég 13:51–92
Rizet G (1953) Sur la multiplicité des mécanismes génétiques conduisant à des barrages chez Podospora anserina. C R Acad Sci Paris 237:666–668
Rizet G, Esser K (1953) Sur des phénomènes d’incompatibilité entre souches d’origines différentes chez Podospora anserina. C R Acad Sci Paris 237:760–761
Rizzo DM, Rentmeester RM, Bursdall J (1996) Sexuality and somatic incompatibility in Phellinus gilvus. Mycologia 87:805–820
Robson GE, Williams KL (1979) Vegetative incompatibility and the mating-type locus in the cellular slime mold Dictyostelium discoideum. Genetics 93:861–875
Robson GE, Williams KL (1980) The mating system of the cellular slime mold Dictyostelium discoideum. Curr Genet 1:229–232
Saupe SJ (2000) Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol Mol Biol Rev 64:489–502
Saupe S, Glass NL (1997) Allelic specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa is determined by a highly variable domain. Genetics 146:1299–1309
Saupe S, Turcq B, Bégueret J (1995) Sequence diversity and unusual variability at the het-c locus involved in the vegetative incompatibility in the fungus Podospora anserina. Curr Genet 27:466–471
Saupe SJ, Kuldau GA, Smith ML, Glass NL (1996) The product of the het-C heterokaryon incompatibility gene of Neurospora crassa has characteristics of glycine cell rich wall protein. Genetics 143:1589–1600
Savage EJ, Clayton CW, Hunter JH, Brenneman JA, Laviola C, Gallegly ME (1968) Homothallism, heterothallism and interspecific hybridization in the genus Phytophthora. Phytopathology 58:1004–1021
Schrauwen JAM (1979) Post-fusion incompatibility in Physarum polycephalum. Arch Microbiol 122:1–7
Shiu PKT, Glass NL (1999) Molecular characterization of tol, a mediator of mating-type-associated vegetative incompatibility in Neurospora crassa. Genetics 154:545–555
Shiu PKT, Glass NL (2015) Sequence important for heterokaryon incompatibility function in MAT A-1 of Neurospora crassa. Fungal Genet Newslett 53:15–19
Sidhu GS (1986) Genetics of Gibberella fujikuroi. VIII. Vegetative compatibility groups. Can J Bot 64:117–121
Silveira RM, Borges DA, Wright JE (2002) Polyporus s. strictu in southern South America: mating tests. Mycol Res 106:1323–1330
Smith ML, Yang CJ, Metzenberg R, Glass NL (1996) Escape from het-6 incompatibility in Neurospora crassa. Partial diploids involves preferential deletion within ectopic segment. Genetics 144:523–531
Smith ML, Micali OC, Hubbard SP, Mir-Rashed N, Jacobson DJ, Glass NL (2000) Vegetative incompatibility in the het-6 region of Neurospora crassa is mediated by two linked genes. Genetics 155:1095–1104
Smith ML, Gibbs CC, Milgroom MC (2006) Heterokaryon incompatibility function of barrage-associated vegetative incompatibility genes (vic) in Cryphonectria parasitica. Mycologia 98:43–50
Staben C, Yanofsky C (1990) Neurospora crassa a mating-type region. Proc Natl Acad Sci USA 87:4917–4921
Stark JR, Boyd A, Mileham AJ, Romanos MA (1990) The encoded killer system of Kluyveromyces lactis: a review. Yeast 6:1–29
Stretton HM, Flentje NT (1972a) Inter-isolate heterokaryosis in Thanatephorus cucumeris. I. Between isolates of similar pathogenicity. Aust J Biol Sci 25:293–303
Stretton HM, Flentje NT (1972b) Inter-isolate heterokaryosis in Thanatephorus cucumeris. II. Between isolates of different pathogenicity. Aust J Biol Sci 25:305–318
Tipper DJ, Bostian KA (1984) Double stranded ribonucleic acid killer systems in yeasts. Microbiol Rev 48:125–156
Turcq B, Denayrolles M, Bégueret J (1990) Isolation of two allelic incompatibility genes s and S of the fungus Podospora anserina. Curr Genet 17:297–303
Turcq B, Deleu C, Denayrolles M, Bégueret J (1991) Two allelic genes responsible for vegetative incompatibility in the fungus Podospora anserina are not essential for cell viability. Mol Gen Genet 228:265–269
Turner BC, Perkins DD (1979) Spore killer, a chromosomal factor in Neurospora that kills meiotic products not containing it. Genetics 93:587–606
Turner BL, Taylor CW, Perkins DD, Newmeyer D (1969) New duplication-generating inversions in Neurospora. Can J Genet Cytol 11:622–638
Van der Gaag M, Debets AJM, Hoekstra RF (2003) Spore killing in the fungus Podospora anserina: a connection between meiotic drive and vegetative incompatibility? Genetics 117:59–65
Van Diepeningen AD, Debets JM, Hockstra RF (1997) Heterokaryon incompatibility blocks virus transfer among natural isolates of black Aspergilli. Curr Genet 32:209–217
Vandendries R (1932) La tétrapolarité sexuelle de Pleurotus columbinus. Démonstrations photographiques d’un tableau de croisement. Cellule 41:267–279
Vilgalys RJ, Johnson JL (1987) Extensive genetic divergence associated with speciation in filamentous fungi. Proc Natl Acad Sci USA 84:2355–2358
Vilgalys RJ, Miller OK (1987) Mating relationships within the Collybia dryophila group in Europe. Trans Br Mycol Soc 89:295–300
Webster RK, Nelson RR (1968) The genetics of Cochliobolus spiciferus. I. Genetic inhibition of perithecial and ascus formation. Can J Bot 46:197–202
Williams CA, Wilson JF (1968) Factors of cytoplasmic incompatibility. Neurospora Newslett 13:12
Wilson JF (1961) Microsurgical techniques for Neurospora. Am J Bot 48:46–51
Wilson JF (1963) Transplantation of nuclei in Neurospora crassa. Am J Bot 50:780
Wilson JF, Garnjobst L (1966) A new incompatibility locus in Neurospora crassa. Genetics 53:621–631
Wilson JF, Garnjobst L, Tatum EL (1961) Heterokaryon incompatibility in Neurospora crassa – microinjection studies. Am J Bot 48:299–305
Wong GJ (1993) Mating and fruiting studies of Auricularia delicata and A. fuscosuccinea. Mycologia 85:187–194
Worall JJ (1997) Somatic incompatibility in basidiomycetes. Mycologia 89:24–36
Wu J, Glass NL (2001) Identification of specificity determinants and generation of alleles with novel specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa. Mol Cell Biol 21:1045–1057
Xiang Q, Glass NL (2002) Identification of vib-1, a locus involved in vegetative incompatibility mediated by hetc in Neurospora crassa. Genetics 162:89–101
Xiang Q, Glass NL (2004) Chromosome rearrangement in isolates that escape from het-c heterokaryon incompatibility. Curr Genet 44:329–338
Yindeeyoungyeon W, Triratana S (1992) Hyphal interaction among dikaryotic pairings of Lentinula edodes. Micol Neotrop Apl 5:17–27
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Esser, K. (2016). 6 Heterogenic Incompatibility in Fungi. In: Wendland, J. (eds) Growth, Differentiation and Sexuality. The Mycota, vol 1. Springer, Cham. https://doi.org/10.1007/978-3-319-25844-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-25844-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25842-3
Online ISBN: 978-3-319-25844-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)