Abstract
Water moves across membranes through the lipid bilayer and through aquaporins, in this case in a regulated manner. Aquaporins belong to the MIP superfamily and two subfamilies are represented in yeasts: orthodox aquaporins considered to be specific water channels and aquaglyceroporins (heterodox aquaporins). In Saccharomyces cerevisiae genome, four aquaporin isoforms were identified, two of which are genetically close to orthodox aquaporins (ScAqy1 and ScAqy2) and the other two are more closely related to the aquaglyceroporins (ScFps1 and ScAqy3). Advances in the establishment of water channels structure are reviewed in this chapter in relation with the mechanisms of selectivity, conductance and gating. Aquaporins are important for key aspects of yeast physiology. They have been shown to be involved in sporulation, rapid freeze-thaw tolerance, osmo-sensitivity, and modulation of cell surface properties and colony morphology, although the underlying exact mechanisms are still unknown.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Water homeostasis is a fundamental requirement for survival and adaptation of all living beings. Although initially it was assumed that water diffuses only through the lipid bilayer, the first studies in the late 1950s on water transport in mammalian red blood cells (Paganelli and Solomon 1957; Vieira et al. 1970; Macey et al. 1972) demonstrated that water permeability in these cells was much higher than expected by diffusion across the bilayer, which provided clues for the existence of specialized water channels. Also, water transport exhibited low activation energy and was inhibited by mercurials (Macey et al. 1972; Farmer and Macey 1970; Sidel and Solomon 1957). Based on the ratio of osmotic to diffusion water permeabilities, a single file diffusion mechanism of water transport within the putative channel was proposed even before the molecular identification of water channels (Moura et al. 1984; Finkelstein 1984).
Only in the nineties, Agre and co-workers identified the first water channel protein, now known as aquaporin 1 (AQP1), in red blood cells (Preston et al. 1992). Meanwhile, numerous aquaporin and aquaporin-like sequences have been identified in nearly all living organisms (Nehls and Dietz 2014), including 2 in bacteria, 4 in yeasts (Kruse et al. 2006), 35 in plants and 13 in mammals (reviewed by Kaldenhoff and Fischer 2006).
Now it is generally accepted that water moves through membranes by two different pathways: through the lipid bilayer, in a high activation energy process, and through aquaporins in a low activation energy process, leading to a determinant role of temperature in the relative importance of both mechanisms in nature. Particularly in Saccharomyces cerevisiae, it was shown that water channels are important for cells osmotic adjustments at lower temperature (Soveral et al. 2006). In either case of lipid path or channel, the driving force for water movement is the gradient of chemical potential of water (osmotic and/or hydrostatic pressure) between both sides of the membrane and predicted to occur in either direction (Finkelstein 1984). Aquaporins provide regulation of the water status in the cells by different mechanisms. For example, in yeast and plants the activity of water channels is affected by phosphorylation, pH, pressure, tension, solute gradients and temperature (Chaumont et al. 2005; Maurel 2007; Tornroth-Horsefield et al. 2010; Soveral et al. 2008; Leitao et al. 2014).
Aquaporins belong to a highly conserved group of membrane proteins called the major intrinsic proteins (MIPs) that form a large family comprising more than 1700 integral membrane proteins (Abascal et al. 2014) and they are involved in the transport of water and small solutes such as glycerol (Maurel et al. 1994; Carbrey et al. 2003; Wang et al. 2005), H2O2 (Hooijmaijers et al. 2012), ammonia, boron (Dynowski et al. 2008), nitrate (Ikeda et al. 2002) and urea (Mitani-Ueno et al. 2011), and also gases like CO2 (Navarro-Ródenas et al. 2012). The MIP superfamily (MIP, 1.A.8) includes several subfamilies of proteins: (i) aquaporins 1.A.8.6. (called orthodox, ordinary, conventional or classical aquaporins) which are considered to be specific water channels (Takata et al. 2004), (ii) aquaglyceroporins 1.A.8.2. (called unconventional or heterodox aquaporins) and iii. aquaporins with unusual NPA (Asn-Pro-Ala) boxes (called unorthodox superaquaporins or subcellular aquaporins), a recently described third subfamily that is only present in animals but not in plants, fungi and bacteria (reviewed by Ishibashi et al. 2011; Ishibashi et al. 2014).
In S. cerevisiae genome, four aquaporin isoforms were identified, two of which are genetically close to orthodox aquaporins (ScAqy1 and ScAqy2) and the other more closely related to the aquaglyceroporins (YFL054Cp, recently reclassified as ScAqy3 (Patel 2013), and ScFps1) (Bonhivers et al. 1998; Carbrey et al. 2001). While Aqy- and Fps1-like aquaporins have been mainly localized in the plasma membrane, Aqy3-like aquaporins have been mainly localized in the vacuoles. Occurrence of water channels showed diverse patterns in yeasts. In some yeasts (S. cerevisiae strain Σ1278B and Candida glabrata) both functional orthodox aquaporins were found, while in some species (Schizosaccharomyces pombe, Zygosaccharomyces rouxii and Z. bailli), these genes were not present at all. In such cases, presence of the aquaglyceroporin (FPS1) was always observed. In several strains of S. cerevisiae, one of the genes (especially, AQY2) was truncated during evolution (Carbrey et al. 2001; Laizé et al. 2000). Whereas, strain S288C (the first strain used for sequencing of S. cerevisiae genome) carries inactivating mutations in both aquaporin genes and their deletion did not cause distinct growth phenotype (Laizé et al. 2000; Karpel and Bisson 2006).
Aquaporins are important for key aspects of yeast physiology. They are involved in sporulation (Sidoux-Walter et al. 2004; Will et al. 2010), rapid freeze-thaw tolerance (Tanghe et al. 2002; Fischer et al. 2009), osmo-sensitivity, and modulation of cell surface properties and colony morphology (Furukawa et al. 2009). These depicted roles of aquaporins are exclusively based on observed phenotypes caused by their deletion/overexpression or differential gene expression pattern, although the underlying exact mechanisms are still unknown.
5.2 Genes Coding for Water Channels in Yeasts
Protein sequences of orthodox aquaporins (Aqy1 and Aqy2) and aquaglyceroporin (Fps1) within the yeasts genome were searched by BLASTp and tBLASTn tool from the available genome database at Saccharomyces Genome Database (SGD, http://www.yeastgenome.org), Génolevures (http://www.genolevures.org) and NCBI (http://www.ncbi.nlm.nih.gov). These sequences were compared with sequences of functional versions of both Aqy1 and Aqy2 proteins of S. cerevisiae Σ1278B strain. In Table 5.1, we present a detailed compilation of obtained MIP sequences within the genome of 14 yeasts representing 8 species and 7 different strains of S. cerevisiae collected from different niches. Sequences annotated in this study were designated according to their highest percentage identity with ScAqy1, ScAqy2 (not shown in Table 5.1) and ScFps1. Among the different species, Aqy1 of S. paradoxus (SpAqy1) showed the highest identity (98 %), while Debaryomyces hansenii (DhAqy1) showed the lowest identity (34 %) with ScAqy1. An obvious high identity of sequences (up to 99 %) was observed within the strains of S. cerevisiae. ScAqy1 and ScAqy2 are highly similar (87 % identical) proteins, indicating the probable recent gene duplication during evolution (reviewed by Pettersson et al. 2005). Contrarily, ScFps1 showed very low similarity with ScAqy1 (20 %), suggesting that it belongs to a distinct subfamily (aquaglyceroporin) of MIPs (Abascal et al. 2014). It showed high variability (22–98 %) even within the alignment of Fps1 proteins of yeasts. Except for SpaFps1 (99 %) and SpFps1 (98 % identical), lower similarities of ScFps1 with other yeasts as well as within the strains of S. cerevisiae were found. The phylogenetic tree for sequences listed in Table 5.1 was constructed by MEGA5.1 software using neighbor-joining method (Tamura et al. 2011). It shows the distance among all obtained orthodox aquaporins (Fig. 5.1a) and among aquaglyceroporins (Fig. 5.1b). Most of the Aqy1 were grouped together, although CaAqy1, PpAqy1, KlAqy1 and DhAqy1, respectively, were more divergent from ScAqy1 (Fig. 5.1a). In the case of Fps1, the aquaglyceroporin of Sch. pombe was the most distant among different species, although Fps1 of S. cerevisiae RM11-1a strain was even more divergent than Fps1 of Sch. pombe (Fig. 5.1b).
Phylogenetic tree based on the protein sequences of yeast aquaporins. Dendrograms depicting the phylogenetic relationship of S. cerevisiae orthodox aquaporins (Aqy1 and Aqy2) (a), and Fps1 aquaglyceroporin (b) with the sequences detected in other yeasts. Dendograms were generated by neighbor-joining method (applied to 1000 bootstrap data sets) using the MEGA5.1 programme. Details of terminology and accession numbers are listed in Table 5.1
The number of genes of aquaporins/aquaglyceroporins family present in yeasts exhibits an interesting pattern. Almost all the selected yeasts possess at least one water/solute channel protein (either Aqy1, Aqy2 or Fps1). Yeasts (Sch. pombe, Z. rouxii and Z. bailii), which do not have any orthodox aquaporins, present at least one aquaglyceroporin, suggesting its putative versatile role as water/solute channel. In addition, even the orthodox aquaporins are also reported to be involved in transport of solutes other than water (reviewed by Pettersson et al. 2005).
5.3 PpAqy1 Structure and Gating
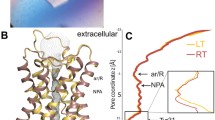
In the last decade several high resolution aquaporin structures have been solved, and currently structures of 14 aquaporins from 9 different organisms have been published. The overall structures of all aquaporins are essentially identical, regardless of the subfamily or the host organism (Klein et al. 2014). The reported structures revealed a tetrameric assembly of four identical monomers each behaving as an independent channel and sharing a conserved overall typical hourglass fold (Murata et al. 2000). Pichia pastoris Aqy1 is the only fungal AQP structure solved so far (Fischer et al. 2009). PpAqy1 along with ScAqy1 were used as reference sequences to compare the topology and important amino acid residues in the sequences of yeasts. All the yeast aquaporins in this study exhibited the typical topology of the MIP family. Figure 5.2a shows different views of PpAqy1 tetrameric structure (side, top and bottom). Similarly to all other AQP members, PpAqy1 monomers interact with two of their neighbours and form the tetramer with a central pore that excludes water molecules.
Structure of PpAqy1. (a) Tetrameric structure showing side, top and bottom views. (b) Monomeric structure highlighting the two half-helices entering from loops B and E to form a pseudo seventh transmembrane helix, and the selectivity filters regions ar/R and NPA. The red mesh represents the residues lining the channel, which is in the closed configuration, with Tyr31 protruding into the pore (Protein Data Bank code: 2W2E)
Figure 5.2b shows the typical topology of aquaporin monomers for PpAqy, where the pore region is indicated by the red mesh. Each monomer is comprised by six membrane-spanning helices (H1-6) and two elongated loops that form short half helices (loops B and E). These two half helices contain highly conserved NPA motifs (asparagine-proline-alanine) that are critical for water or other substrate permeation (Jung et al. 1994). The NPA repeats are located at the end of loops B and E that fold back into the membrane and form the seventh transmembrane pseudo-helix. The NPA signatures are oriented 180° to each other in the centre of the channel and form part of the surface of the aqueous pathway.
Both amino and carboxy termini are located in the cytoplasm, and similarly to other yeasts, the N-terminal of all selected AQPs is unusually long when compared with isoforms of other organisms (Tornroth-Horsefield et al. 2010), as depicted in Fig. 5.2a for PpAqy1. From the obtained crystal structure of PpAqy1 a functional role for the yeast aquaporin N terminus is inferred, which has been associated with mechanisms controlling the transport activity of the channel (gating) (Fischer et al. 2009).
5.3.1 Selectivity and Mechanism of Conductance
Classical aquaporins are still considered mostly specific for water. In the pore region, the water specificity is achieved by the presence of particular residues conferring size constrictions and/or charge characteristics that enable water molecules to pass through, while preventing permeation to protons or any solutes above 2.8 Å. The cytoplasmic and periplasmatic entry of the pore in the aquaporin monomer offers several water-wall residue interactions, mainly with carbonyl groups. After passing these first interactions, wall regions with different hydrophobic/hydrophilic characteristics determine selectivity, conduction rate and open/closed state of the pore. Two main constrictions sites that act as selectivity filters were identified in AQP channels: one is positioned on the extracellular face of the channel in the aromatic/arginine (ar/R) constriction region, whereas the other is located in the central part of the channel at the NPA region (Hub et al. 2009; Soveral et al. 2011; de Groot and Grubmuller 2001). The location of the two half helices and some of the residues lining the pore wall relevant for selectivity (ar/R and NPA filters) and gating are depicted in Fig 5.2b.
Beginning on the exoplasmic side and running along the pore, after passing the first interactions with the carbonyl groups (not shown), the narrowest region of the pore is found to be the aromatic/R constriction site (ar/R) containing highly conserved aromatic and arginine residues. In PpAqy1 the ar/R constriction is formed by phenylalanine/arginine with Arg227 and His212 creating the hydrophilic surface where the polar interactions water-protein occur, whereas Phe92 pushes away water molecules. Sequence alignment showed that these residues are highly conserved in all the yeasts selected in Table 5.1. Further down in the middle of the pore, water molecules find the second selective filter as they bypass the dual NPA motifs. In this region, water repulsion by a hydrophobic residue that intrudes into the pore, forces water molecules to interact strongly with Asn224 and Asn112 of the NPA loops, which are also conserved in all yeasts of Table 5.1. These residues act as hydrogen donors to the oxygen atoms of passing water molecules, compelling them to acquire a specific orientation (Murata et al. 2000; Sui et al. 2001; de Groot et al. 2003; de Groot and Grubmuller 2005). In addition, water molecules that enter this region are reoriented by the electrostatic field produced by the half helices HB and HE, resulting in hydrogen bonds disruption between neighboring water molecules (de Groot et al. 2003; de Groot and Grubmuller 2005). Additionally, hydrophobic residues lining the pore near the NPA filter provide a hydrophobic surface that prevents water-protein interaction (Soveral et al. 2011).
5.3.2 PpAqy1 Gating Mechanism
The resolution of multiple AQP structures has disclosed common patterns of gating among different isoforms. These mechanisms directly affect the protein conformation, which in turn impacts its transport activity.
Besides the described selectivity size filters, an additional and significantly narrower constriction site at the cytoplasmic entry and an insertion of a residue in a specific 3D position of the protein structure is a common feature to all gated AQPs (reviewed by Tornroth-Horsefield et al. 2010). This residue was found to be Tyr31 for PpAqy1 (Fig 5.2b).
Of notice, the existing PpAqy1 structure captures the channel in the closed configuration, depicting the N-terminal Tyr31 residue intruding and occluding the pore. In this structure, the kink introduced by Pro29 allows Tyr31 to be inserted into the channel and to participate in a network of hydrogen bonds with two water molecules and the backbone carbonyl oxygen atoms of Gly108 and Gly109. These interactions narrow the cytoplasmic side of the pore to around 0.8 Å diameter, hindering water permeation.
Fischer et al. (2009) reported that conformational changes able to dislodge Tyr31 from its blocking position could be attained by mechanical forces and/or by phosphorylation of Ser107. Ser107 locates at the cytoplasm end of helix 2 in loop B and connects to Tyr31 via a sequence of H-bonds, including Gly108 and a bridging water molecule thus helping the pore blockage. Through molecular dynamic simulation studies on PpAqy1, it was possible to resolve the question of how mechanical forces (bending the membrane towards the cytoplasmic side) are transmitted from membrane to the gate of the channel. The results pointed to Leu189, Ala190 and Val191 as responsible for this transmission via coupled movements of helices 4, 5 and 6 but this same opening mechanism can be triggered by Ser107 phosphorylation (Fischer et al. 2009). In agreement, functional studies have suggested the closure of yeast Aqy1 by membrane surface tension (stretching the membrane) (Soveral et al. 2008), an experimental approach in which the mechanical forces work in the inverse direction of the above-described mechanism thus producing the opposite effect.
Sequence alignment of PpAqy1 with other yeasts aquaporins showed that Tyr31 is not highly conserved in all selected yeasts, since it is only present in C. albicans and D. hansenii, whereas Pro29 was found to be conserved only in C. albicans, supporting the possibility of different gating mechanism in some yeasts (Cui and Bastien 2012). On the other hand, Gly108, Gly109, Ala190 and Val191 along with putative phosphorylation site Ser107 are conserved in all yeast species.
5.4 Assessment of Water Transport in Yeasts
A direct correlation between phylogeny and function is not obvious for fungal MIPs (Nehls and Dietz 2014). Therefore, prediction of MIP relevance is not possible without expression studies and further functional analysis.
Functional studies to access aquaporin activity are centred on following cell and/or vesicle volume changes resulting directly from water fluxes driven by osmotic and/or pressure gradients. The observed volume changes (swelling or shrinking) are directly proportional to water fluxes and consequently to the driving forces (the pressure gradients), with the osmotic permeability coefficient P f as the proportionality constant. The rate at which the volume changes occur depends on the fraction of water that follows the channel (aqueous pathway) versus the lipid pathway. In addition, compared with water flow across a hydrophobic lipid bilayer, water fluxes through a hydrophilic channel pore need lower activation energy E a . Thus, high P f and low E a values are an indication of an aqueous path (active aquaporins present), whereas low P f and high E a point mainly to lipid pathway (aquaporin absence or inactivation). For a comprehensive overview on the equations and parameters used to evaluate water permeability, see Soveral et al. (2011).
The techniques used to measure volume changes take advantage of volume dependent physical parameters based on optical properties, e.g. light transmission or absorbance, light scattering and fluorescence of volume sensitive dyes.
Different preparations involving different methodologies have been used for the functional characterization of yeast aquaporins. These include the heterologous expression of yeast aquaporins in Xenopus laevis oocytes (systems with low intrinsic water permeability) (Bonhivers et al. 1998; Carbrey et al. 2001), yeast protoplasts (Soveral et al. 2006; Laize et al. 1999), yeast secretory vesicles (Coury et al. 1999; Meyrial et al. 2001) and yeast intact cells (Soveral et al. 2007). In some laboratories permeability has been assessed by monitoring the kinetics of bursting of osmotically challenged protoplasts as a decrease in optical density (Pettersson et al. 2006), while in others the changes in cell volume by an image analysis system connected to an inverted light microscope have been followed (Prudent et al. 2005).
A frequently used method to follow rapid volume changes in a cell/vesicle suspension is the stopped flow spectroscopy. Here cell/vesicle suspensions are subjected to osmotic challenges by rapid mixing with an equal volume of hypo- or hyperosmotic solution. For vesicle or protoplast suspensions, light of a chosen wavelength is directed to the observation chamber through an optical fibre and the change in scattered light is followed until a stable signal is attained. An alternative approach uses cell/vesicles loaded with volume sensitive fluorescent dyes. Yeast cells are loaded with the membrane permeable nonfluorescent precursor carboxyfluorescein diacetate, which is cleaved intracellularly by non-specific esterases to form the fluorescent free form. Changes in fluorescence intensity resulting from osmotically induced volume changes can be monitored by stopped-flow fluorescence. Signals are then calibrated into volumes and analysed for permeability evaluation.
For protoplasts and vesicles, the signals obtained with small osmotic perturbations can be described by an exponential function whose rate constant allows the direct evaluation of the osmotic permeability (Soveral et al. 2006; Bonhivers et al. 1998). For walled cells however, evaluation of osmotic permeability must take into account possible gradients of hydrostatic pressure that arise when cells are in low osmotic buffers; this experimental situation was further used to disclose aquaporin gating by membrane surface tension (Soveral et al. 2008).
Gating of aquaporin function by pH, phosphorylation or specific inhibitors, can be screened through simple measurements of P f and E a . However, aquaporin gating by physical parameters such as membrane surface tension implies the design of specific protocols that can only be applied to vesicle systems (Soveral et al. 1997) or walled cells (Soveral et al. 2008) that can sustain membrane tensions without rupture. Using protocols that induce an increase in membrane surface tension just before the osmotic shock, Aqy1 from S. cerevisiae was found to be regulated by tension and to behave as a pressure regulated water channel (Soveral et al. 2008), supporting its combined involvement with the aquaglyceroporin Fps1 in yeast osmoregulation (Hohmann et al. 2007).
5.5 Aquaporins in Saccharomyces from Different Ecological Niches
In nature, wild S. cerevisiae strains exist in a wide range of environments, from cold (like oak soil) to hot climates, and are commonly found in sugar rich conditions (like in flowers, fruits, sap, grape must). The high sugar environment provides ideal conditions for their fermentation, reproduction and growth as single cell as well as pseudohyphae of attached cells. S. cerevisiae strains are also the most domesticated yeasts by human activity throughout history, due to their role in the production of important food and beverages such as bread, beer, wine and sake among several others. This role led to yeast improvement and selection for more efficient production and higher quality products, much before Pasteur reported its role in fermentation.
Evolutionary studies suggest that the whole genome of S. cerevisiae was duplicated around 100 million years ago, followed by partial loss of duplicated genes. The remaining 15 % of duplicated genes have evolved as functionally divergent from their parental genes, and have been maintained, providing selective benefits to the cells (Botstein and Fink 2011; Dujon 2010). The analysis of recently available genomes from wild and domesticated S. cerevisiae strains revealed the existence of two orthodox aquaporins, Aqy1 and Aqy2. Most of the laboratory and domesticated strains harbor at least one non-functional aquaporin. Exceptionally, S. cerevisiae Σ1278B laboratory strain contains both functional Aqy1 and Aqy2 (Carbrey et al. 2001).
The reason of the existence of aquaporins is still speculative, due to the lack of clear and direct correlation between yeast growth and overexpression/deletion of functional orthodox aquaporins (reviewed by Ahmadpour et al. 2014). Since microbial cells are of small size resulting in large surface-to-volume ratio, enhanced water permeability appeared irrelevant for water movement during osmotic adjustment (reviewed by Tanghe et al. 2006). So, what is the physiological role of microbial aquaporins? How does their presence correlate with adaptive evolution in different ecological niches?
Recently, important progress occurred in the answer to this issue and may provide the insight on physiological importance of aquaporins in yeasts (Will et al. 2010). Up to now, several purposes for aquaporins in yeasts have been pointed, namely: (1) adaptation to environmental conditions under which water flux through the lipid bilayer becomes more restrictive, in particular under low temperature (Soveral et al. 2006) and freeze-thaw cycles (Tanghe et al. 2002), (2) involvement in developmental stages where water transport becomes critical (Sidoux-Walter et al. 2004; Will et al. 2010), (3) control of water fluxes through the plasma membrane (as well as other small polar molecules) to adjust their internal osmotic pressure (Nehls and Dietz 2014; Soveral et al. 2008) and (4) modulation of cell surface properties for substrate adhesion and formation of cell biofilms (Furukawa et al. 2009). These findings are mostly supported by phenotypes observed in deletion mutants or aquaporin overexpressing strains as well as by the gene expression pattern during cell cycle and under various stress conditions (low temperature, osmotic shock and nutrients depletion), suggesting that their expression is differentially regulated and they appear to perform similar as well as different functions. The expression of AQY1 was up-regulated under starvation, contrarily, AQY2 was up-regulated in exponential phase of cells growing in rich medium (Meyrial et al. 2001). During cell cycle, only AQY1 expression appeared to be tightly linked with sporulation in S. cerevisiae SK1 (Sidoux-Walter et al. 2004) and YPS163 (Will et al. 2010) strains. Moreover, the level of aquaporins expression is highly correlated with rapid freeze-thaw tolerance. Higher expression provided improved tolerance (Tanghe et al. 2002), while deletion caused susceptibility to freeze/thaw cycles (Tanghe et al. 2002). Interestingly, Will et al (Will et al. 2010) reported that although the presence of a functional allele of aquaporins provides freeze-thaw tolerance (useful in oak soil), their absence offers them fitness during growth on high-sugar substrates. Additionally, together with the loss of functional aquaporins during evolution, their ancestral need of aquaporins for spore formation was also lost.
AQY2 appeared to behave as osmosensor in yeasts, since its expression was down regulated under hyperosmotic shock in a Hog1 dependent manner, and was recovered under lower osmotic conditions (reviewed by Pettersson et al. 2005). Moreover, overexpression of AQY1 and AQY2 also affected the cell surface properties and colony morphology of the yeasts. Their deletion enhanced the hydrophobicity of cell surface and cell flocculence (Carbrey et al. 2001), while their overexpression increased the plastic adhesion of cell surface, agar invasion and colony fluffiness (Furukawa et al. 2009; Št’ovíček et al. 2010).
In order to test the role of aquaporins during wine fermentation, Karpel and Bisson (Karpel and Bisson 2006) investigated five native wine yeast strains and found only a functional Aqy1. They observed that yeast adaptation to stress during wine fermentation was not dependent on aquaporins. Our recent study on the effect of ethanol on water fluxes on yeast demonstrated that a low concentration of ethanol (4 %) had a remarkable inhibitory effect on aquaporin activity (Madeira et al. 2010), supporting the idea that aquaporins play a poor role in wine fermentation.
Strains isolated from oak soil, harbouring functional aquaporins, probably represent an ancestral state of evolution. In association with humans, S. cerevisiae strains migrated worldwide. Human-facilitated migration may have significantly increased exposure of S. cerevisiae to various environments, imposing new selective pressures when strains occupied new ecological niches, where activity of functional aquaporins was deleterious and knock-out mutations in AQY genes brought benefits for their progeny.
References
Abascal F, Irisarri I, Zardoya R (2014) Diversity and evolution of membrane intrinsic proteins. Biochim Biophys Acta 1840:1468–1481
Ahmadpour D, Geijer C, Tamas MJ, Lindkvist-Petersson K, Hohmann S (2014) Yeast reveals unexpected roles and regulatory features of aquaporins and aquaglyceroporins. Biochim Biophys Acta 1840:1482–1491
Akao T, Yashiro I, Hosoyama A, Kitagaki H, Horikawa H et al (2011) Whole-genome sequencing of sake yeast saccharomyces cerevisiae Kyokai no. 7. DNA Res 18:423–434
Bonhivers M, Carbrey JM, Gould SJ, Agre P (1998) Aquaporins in Saccharomyces: genetic and functional distinctions between laboratory and wild-type strains. J Biol Chem 273:27565–27572
Botstein D, Fink GR (2011) Yeast: an experimental organism for 21st century biology. Genetics 189:695–704
Bussey H, Storms RK, Ahmed A, Albermann K, Allen E et al (1997) The nucleotide sequence of Saccharomyces cerevisiae chromosome XVI. Nature 387:103–105
Carbrey JM, Bonhivers M, Boeke JD, Agre P (2001) Aquaporins in Saccharomyces: characterization of a second functional water channel protein. Proc Natl Acad Sci U S A 98:1000–1005
Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S et al (2003) Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci 100:2945–2950
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97:749–764
Coury LA, Hiller M, Mathai JC, Jones EW, Zeidel ML et al (1999) Water transport across yeast vacuolar and plasma membrane-targeted secretory vesicles occurs by passive diffusion. J Bacteriol 181:4437–4440
Cui Y, Bastien DA (2012) Molecular dynamics simulation and bioinformatics study on yeast aquaporin Aqy1 from Pichia pastoris. Int J Biol Sci 8:1026–1035
Curtin CD, Borneman AR, Chambers PJ, Pretorius IS (2012) De-novo assembly and analysis of the heterozygous triploid genome of the wine spoilage yeast Dekkera bruxellensis AWRI1499. PLoS One 7:e33840
Daniels MJ, Wood MR, Yeager M (2006) In vivo functional assay of a recombinant aquaporin in Pichia pastoris. Appl Environ Microbiol 72:1507–1514
de Groot BL, Grubmuller H (2001) Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science 294:2353–2357
de Groot BL, Grubmuller H (2005) The dynamics and energetics of water permeation and proton exclusion in aquaporins. Curr Opin Struct Biol 15:176–183
de Groot BL, Frigato T, Helms V, Grubmuller H (2003) The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol 333:279–293
De Schutter K, Lin Y-C, Tiels P, Van Hecke A, Glinka S et al (2009) Genome sequence of the recombinant protein production host Pichia pastoris. Nat Biotechnol 27:561–566
Dujon B (2010) Yeast evolutionary genomics. Nat Rev Genet 11:512–524
Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S et al (2004) Genome evolution in yeasts. Nature 430:35–44
Dynowski M, Mayer M, Moran O, Ludewig U (2008) Molecular determinants of ammonia and urea conductance in plant aquaporin homologs. FEBS Lett 582:2458–2462
Farmer REL, Macey RI (1970) Perturbation of red cell volume: rectification of osmotic flow. Biochim Biophys Acta Biomembr 196:53–65
Finkelstein A (1984) Water movement through membrane channels. In: Felix B (ed) Current topics in membranes and transport. Academic Press, New York, pp 295–308
Fischer G, Kosinska-Eriksson U, Aponte-Santamaria C, Palmgren M, Geijer C et al (2009) Crystal structure of a yeast aquaporin at 1.15 angstrom reveals a novel gating mechanism. PLoS Biol 7:e1000130
Furukawa K, Sidoux‐Walter F, Hohmann S (2009) Expression of the yeast aquaporin Aqy2 affects cell surface properties under the control of osmoregulatory and morphogenic signalling pathways. Mol Microbiol 74:1272–1286
Hohmann S, Krantz M, Nordlander B (2007) Yeast osmoregulation. Methods Enzymol 428:29–45
Hooijmaijers C, Rhee JY, Kwak KJ, Chung GC, Horie T et al (2012) Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J Plant Res 125:147–153
Hub JS, Grubmuller H, de Groot BL (2009) Dynamics and energetics of permeation through aquaporins. What do we learn from molecular dynamics simulations? Handb Exp Pharmacol 190:57–76
Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P et al (2002) Characterization of aquaporin-6 as a nitrate channel in mammalian cells requirement of pore-lining residue threonine 63. J Biol Chem 277:39873–39879
Ishibashi K, Kondo S, Hara S, Morishita Y (2011) The evolutionary aspects of aquaporin family. Am J Physiol Regul Integr Comp Physiol 300:R566–R576
Ishibashi K, Tanaka Y, Morishita Y (2014) The role of mammalian superaquaporins inside the cell. Biochim Biophys Acta 1840:1507–1512
Johnston M, Hillier L, Riles L, Albermann K, André B et al (1997) The nucleotide sequence of Saccharomyces cerevisiae chromosome XII. Nature 387:87–90
Jung JS, Preston GM, Smith BL, Guggino WB, Agre P (1994) Molecular structure of the water channel through aquaporin CHIP the hourglass model. J Biol Chem 269:14648–14654
Kaldenhoff R, Fischer M (2006) Aquaporins in plants. Acta Physiol 187:169–176
Karlsson M, Fotiadis D, Sjövall S, Johansson I, Hedfalk K et al (2003) Reconstitution of water channel function of an aquaporin overexpressed and purified from Pichia pastoris. FEBS Lett 537:68–72
Karpel JE, Bisson LF (2006) Aquaporins in Saccharomyces cerevisiae wine yeast. FEMS Microbiol Lett 257:117–123
Klein N, Neumann J, O’Neil JD, Schneider D (2014) Folding and stability of the aquaglyceroporin GlpF: implications for human aqua(glycero)porin diseases. Biochim Biophys Acta 1848:622–633
Kruse E, Uehlein N, Kaldenhoff R (2006) The aquaporins. Genome Biol 7:206
Laize V, Gobin R, Rousselet G, Badier C, Hohmann S et al (1999) Molecular and functional study of AQY1 from Saccharomyces cerevisiae: role of the C-terminal domain. Biochem Biophys Res Commun 257:139–144
Laizé V, Tacnet F, Ripoche P, Hohmann S (2000) Polymorphism of Saccharomyces cerevisiae aquaporins. Yeast 16:897–903
Leitao L, Prista C, Loureiro-Dias MC, Moura TF, Soveral G (2014) The grapevine tonoplast aquaporin TIP2;1 is a pressure gated water channel. Biochem Biophys Res Commun 450:289–294
Macey R, Karan D, Farmer RL (1972) Properties of water channels in human red cells. In: Kreuzer F, Slegers JFG (eds) Biomembranes: passive permeability of cell membranes. Springer US, Boston, pp 331–340
Madeira A, Leitão L, Soveral G, Dias P, Prista C et al (2010) Effect of ethanol on fluxes of water and protons across the plasma membrane of Saccharomyces cerevisiae. FEMS Yeast Res 10:252–258
Maurel C (2007) Plant aquaporins: novel functions and regulation properties. FEBS Lett 581:2227–2236
Maurel C, Reizer J, Schroeder JI, Chrispeels MJ, Saier MH (1994) Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J Biol Chem 269:11869–11872
Meyrial V, Laize V, Gobin R, Ripoche P, Hohmann S et al (2001) Existence of a tightly regulated water channel in Saccharomyces cerevisiae. Eur J Biochem 268:334–343
Mitani-Ueno N, Yamaji N, Zhao F-J, Ma JF (2011) The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J Exp Bot 62:4391–4398
Moura T, Macey R, Chien D, Karan D, Santos H (1984) Thermodynamics of all-or-none water channel closure in red cells. J Membr Biol 81:105–111
Murata K, Mitsuoka K, Hirai T, Walz T, Agre P et al (2000) Structural determinants of water permeation through aquaporin-1. Nature 407:599–605
Navarro-Ródenas A, Ruíz-Lozano JM, Kaldenhoff R, Morte A (2012) The aquaporin TcAQP1 of the desert truffle Terfezia claveryi is a membrane pore for water and CO2 transport. Mol Plant-Microbe Interact 25:259–266
Nehls U, Dietz S (2014) Fungal aquaporins: cellular functions and ecophysiological perspectives. Appl Microbiol Biotechnol 98:8835–8851
Novo M, Bigey F, Beyne E, Galeote V, Gavory F et al (2009) Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc Natl Acad Sci U S A 106:16333–16338
Paganelli CV, Solomon AK (1957) The rate of exchange of tritiated water across the human red cell membrane. J Gen Physiol 41:259–277
Patel D (2013) Characterization of vacuole aquaporin function and its implication in membrane fission. Masters thesis Concordia University, Montreal, Quebec, Canada http://spectrum.library.concordia.ca/977797/1/Patel_MSc_F2013.pdf
Pettersson N, Filipsson C, Becit E, Brive L, Hohmann S (2005) Aquaporins in yeasts and filamentous fungi. Biol Cell 97:487–500
Pettersson N, Hagstrom J, Bill RM, Hohmann S (2006) Expression of heterologous aquaporins for functional analysis in Saccharomyces cerevisiae. Curr Genet 50:247–255
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
Prudent S, Marty F, Charbonnier M (2005) The yeast osmosensitive mutant fps1Delta transformed by the cauliflower BobTIP1;1 aquaporin withstand a hypo-osmotic shock. FEBS Lett 579:3872–3880
Sidel VW, Solomon AK (1957) Entrance of water into human red cells under an osmotic pressure gradient. J Gen Physiol 41:243–257
Sidoux-Walter F, Pettersson N, Hohmann S (2004) The Saccharomyces cerevisiae aquaporin Aqy1 is involved in sporulation. Proc Natl Acad Sci U S A 101:17422–17427
Soveral G, Macey RI, Moura TF (1997) Membrane stress causes inhibition of water channels in brush border membrane vesicles from kidney proximal tubule. Biol Cell 89:275–282
Soveral G, Veiga A, Loureiro-Dias MC, Tanghe A, Van Dijck P et al (2006) Water channels are important for osmotic adjustments of yeast cells at low temperature. Microbiology 152:1515–1521
Soveral G, Madeira A, Loureiro-Dias MC, Moura TF (2007) Water transport in intact yeast cells as assessed by fluorescence self-quenching. Appl Environ Microbiol 73:2341–2343
Soveral G, Madeira A, Loureiro-Dias MC, Moura TF (2008) Membrane tension regulates water transport in yeast. Biochim Biophys Acta 1778:2573–2579
Soveral G, Prista C, Moura TF, Loureiro-Dias MC (2011) Yeast water channels: an overview of orthodox aquaporins. Biol Cell 103:35–54
Št’ovíček V, Váchová L, Kuthan M, Palková Z (2010) General factors important for the formation of structured biofilm-like yeast colonies. Fungal Genet Biol 47:1012–1022
Sui H, Han BG, Lee JK, Walian P, Jap BK (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872–878
Takata K, Matsuzaki T, Tajika Y (2004) Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem 39:1–83
Tamura K, Peterson D, Peterson N, Stecher G, Nei M et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tanghe A, Van Dijck P, Dumortier F, Teunissen A, Hohmann S et al (2002) Aquaporin expression correlates with freeze tolerance in baker’s yeast, and overexpression improves freeze tolerance in industrial strains. Appl Environ Microbiol 68:5981–5989
Tanghe A, Carbrey JM, Agre P, Thevelein JM, Van Dijck P (2005) Aquaporin expression and freeze tolerance in Candida albicans. Appl Environ Microbiol 71:6434–6437
Tanghe A, Van Dijck P, Thevelein JM (2006) Why do microorganisms have aquaporins? Trends Microbiol 14:78–85
Tornroth-Horsefield S, Hedfalk K, Fischer G, Lindkvist-Petersson K, Neutze R (2010) Structural insights into eukaryotic aquaporin regulation. FEBS Lett 584:2580–2588
Vieira FL, Sha’afi RI, Solomon AK (1970) The state of water in human and dog red cell membranes. J Gen Physiol 55:451–466
Wang Y, Schulten K, Tajkhorshid E (2005) What makes an aquaporin a glycerol channel? A comparative study of AqpZ and GlpF. Structure 13:1107–1118
Will JL, Kim HS, Clarke J, Painter JC, Fay JC et al (2010) Incipient balancing selection through adaptive loss of aquaporins in natural Saccharomyces cerevisiae populations. PLoS Genet 6:e1000893
Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R et al (2002) The genome sequence of Schizosaccharomyces pombe. Nature 415:871–880
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sabir, F., Prista, C., Madeira, A., Moura, T., Loureiro-Dias, M.C., Soveral, G. (2016). Water Transport in Yeasts. In: Ramos, J., Sychrová, H., Kschischo, M. (eds) Yeast Membrane Transport. Advances in Experimental Medicine and Biology, vol 892. Springer, Cham. https://doi.org/10.1007/978-3-319-25304-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-25304-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25302-2
Online ISBN: 978-3-319-25304-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)